Abstract
Innate type 2 immune cells are activated in response to helminths, allergens, and certain types of proteases and particulates. Recently, innate type 2 immune pathways have also been implicated in protective host responses to homeostatic perturbations, such as metabolic dysfunction, atherosclerosis, and tissue injury. In this context, innate type 2 cytokines stimulate local tissues, recruit eosinophils, and alternatively activate macrophages to restore homeostasis. As the major source of innate interleukin (IL)-5 and IL-13, group 2 innate lymphoid cells are positioned to initiate and maintain homeostatic type 2 responses. The absence of exogenous stimuli in these processes implicates endogenous pathways in the activation of type 2 immunity and suggests an alternative evolutionary trajectory for type 2 immunity, apart from its role in response to helminths and allergens.
Introduction
Type 2 immunity describes cell and tissue processes driven by the cytokines IL-4, IL-5, IL-9, and IL-13, which are typically associated with adaptive T helper 2 (Th2) cells. The study of these pathways in innate immunity was greatly informed by the discovery of group 2 innate lymphoid cells, ILC2s, which are the major innate sources of IL-5 and IL-13, and which underpin the common association of these responses with eosinophils and alternatively activated macrophages (AAMs) [1].
Although typical agonists of innate type 2 immunity include helminths, allergens, and certain particles and proteases, we focus here on innate type 2 signaling in the absence of these exogenous agonists using models of metabolic dysregulation, atherosclerosis, and tissue repair. After a summary of recent findings in this field, we consider how ILC2s can initiate and maintain homeostatic type 2 responses and how endogenous pathways might contribute to ILC2 activation in the absence of exogenous stimuli. Finally, we consider evolutionary forces besides helminths that may have shaped innate type 2 responses. Although we focus on innate pathways here, it is important to realize that these innate responses positively regulate adaptive type 2 immunity [2] and are in turn positively regulated by adaptive responses [3].
Metabolic Homeostasis
The symptoms of human metabolic syndrome or pre-diabetes–including central obesity, glucose intolerance, and insulin resistance–are recapitulated in mice fed high-fat diet (HFD). Disease is exacerbated in the absence of IL-5 or IL-13, suggesting a protective role for type 2 responses. Consistently, transgenic mice overexpressing IL-5 are leaner and more glucose tolerant [4], while treating wild-type mice with IL-4 during HFD protects against insulin resistance [5].
Current models suggest that metabolic syndrome arises from disrupted immune homeostasis in the fat and liver. In particular, the activation status of macrophages in these sites influences the disease: an increase in classically activated macrophages exacerbates disease, whereas higher numbers of AAMs are associated with protection [6]. Induction of AAMs is likely a key mechanism by which IL-4 and/or IL-13 protect against metabolic syndrome. Accordingly, other pathways that promote AAMs, such as signaling through the fatty acid-sensing peroxisome proliferator-activated receptor gamma and delta (PPARγ/PPARδ), adenosine receptor 2B, or krüppel-like factor 4 (KLF4) are also associated with metabolic homeostasis [7–11]. Macrophage-specific deletion of Pparγ or Pparδ increases obesity, decreases glucose tolerance, and increases insulin resistance in mice fed HFD [8,10,11]. Mechanistically, AAMs were shown to secrete an unknown factor that promotes oxidative metabolism in hepatocytes [11]. AAMs may also improve metabolic homeostasis indirectly by dampening type I inflammation.
In addition to polarizing macrophages, IL-4 and IL-13 can act directly on hepatocytes to regulate metabolism. IL-13 signaling inhibits expression of gluconeogenesis genes [12], and IL-4 and/or IL-13 signals shift hepatocytes from fatty acid oxidation to glucose oxidation [5]. The metabolic phenotype in IL-5-deficient and -transgenic animals suggests a role for eosinophils as well. Indeed, increased eosinophilia in visceral adipose tissue (VAT) correlates with decreased obesity and improved glucose homeostasis during HFD [4,13]. AAMs are reduced in eosinophil-depleted fat, suggesting that eosinophils regulate metabolism in part by maintaining AAMs [4]. Whether eosinophils also act directly on hepatocytes or adipocytes remains unknown.
Atherosclerosis
Atherosclerosis, the thickening of arterial walls due to accumulation of lipids such as cholesterol and triglycerides in arterial plaques, is associated with an increased risk of heart attack and stroke. In mice, atherosclerosis is modeled by disrupting lipid clearance, either by deleting the lipoprotein transporter apolipoprotein E (Apoe) or the low-density lipoprotein receptor (Ldlr). These models suggest that IL-13 is protective against disease progression. Atherosclerosis is accelerated in Ldlr−/− mice reconstituted with Il-13−/− as compared to wild-type bone marrow cells [14]. In Ldlr−/− or Apoe−/− mice with established lesions, treatment with recombinant IL-13 improves plaque morphology by reducing total numbers of macrophages and shifting remaining macrophages to an AAM phenotype [14]. The role of IL-4 in atherosclerosis remains more controversial. Some studies have indirectly implicated IL-4 as pro-atherosclerotic [15,16], although others found little or no effect on disease in Apoe−/−;Il-4−/− mice reconstituted with Il-4−/− bone marrow [17].
As in metabolic syndrome, the polarization of tissue macrophages in atherosclerosis predicts pathology, with classically activated macrophages associated with disease progression and AAMs with protection [18]. Consistently, atherosclerosis is exacerbated when AAM-promoting pathways, such as signaling through thioredoxin-1 and KLF4, are deleted [19,20]. The mechanism by which AAMs or IL-13 protect against atherosclerosis is not defined. Macrophages treated with IL-13 in vitro increase uptake and efflux of cholesterol, suggesting an enhanced capacity to clear lipids [14]. AAMs may also act indirectly to dampen type I inflammatory phenotypes that promote atherosclerosis [18].
The role of eosinophils in atherosclerosis has not been studied directly, but plaque size is increased in Ldlr−/− mice reconstituted with Il-5−/− bone marrow [21]. The authors of this study link the phenotype to a deficiency of B1 cell-derived antibodies targeting oxidized lipids, but a role for eosinophils has not been excluded.
Tissue Repair
Adaptive type 2 responses to allergens or infection are associated with tissue remodeling, which not only serves to physically isolate worms or allergens, but also contributes to wound repair and recovery [22,23]. Recent studies have examined the role of type 2 pathways in wound healing and tissue regeneration in the absence of pathogenic infection. The experimental models for these studies include wound healing at barrier surfaces such as the skin and colon, and tissue regeneration in the liver or muscle following sterile injury.
As in the previous examples, primary evidence of a role for type 2 pathways in tissue repair comes from experiments in gene-targeted mice. Both Il-4/13- and Il4ra (IL4/13 receptor subunit)-deficient mice have reduced liver regeneration after partial hepatectomy and impaired skeletal muscle regeneration after cardiotoxin-mediated injury as compared to wild-type mice [24,25]. Mice deficient in IL-4 also show a defect in liver regeneration [26]. In the colon, healing of a punch biopsy wound is delayed in Stat6−/− mice, and when IL-4 and IL-13 are blocked [27]. In the lung, tissue repair following influenza infection is facilitated by ILC2s and the growth factor amphiregulin, for which ILC2s were suggested to be a primary source [23].
Macrophages are a dominant immune cell type in wounded tissues, where they are thought to be generally beneficial. Wound-associated macrophages adopt a range of phenotypes as healing progresses, and specific analysis of IL-4/13-induced AAMs in tissue repair has only begun. Using Arginase-1, Ym1, Cd206 (mannose receptor), and/or Fizz1 as markers, AAMs have been identified in wounded tissues, including skin, brain, peritoneum, and muscle [28–31].
Using genetic tools to inducibly deplete Lysozyme 2(Lyz2+) expressing cells, one study tested the roles of macrophages during the stages of wound repair in the skin. Depletion during the early and middle stages of healing led to a selective loss of Fizz1/Ym1+ macrophages, and this was associated with delayed wound closure, reduced proliferation of myofibroblasts, and poor revascularization [32]. Similarly, in Trem2−/− and Klf4−/− mice, where AAM polarization is inhibited, wound healing was delayed [9,27]. Mechanistically, AAMs have been proposed to secrete mitogens and pro-angiogenic signals, and to participate in debris clearance [33]. The AAM marker Arginase-1 has been implicated in collagen synthesis [34].
In some models, eosinophils have also been implicated in tissue repair. Both muscle repair following cardiotoxin administration and liver regeneration following partial hepatectomy are reduced in eosinophil-deficient dblGata1 mice [24,25], although contributions from other Gata1-expressing cell types have not been ruled out [35]. Eosinophils may provide IL-4 to damaged tissue and may secrete other factors that promote repair [24,25]. It is unknown whether eosinophils sustain AAMs during tissue repair, as they appear to do in the fat.
Type 2 cytokines also promote tissue repair through direct action on parenchymal cells. In fact, during liver regeneration, the IL-4/13 receptor subunit IL4Rα is dispensable in Lyz2+ myeloid cells (such as macrophages), but required in hepatocytes for proliferation [25]. IL4Rα signaling also drives proliferation of fibro/adipogenic progenitors, which contribute to debris clearance during muscle regeneration [5].
ILC2s: Initiating and Coordinating the Innate Type 2 Response
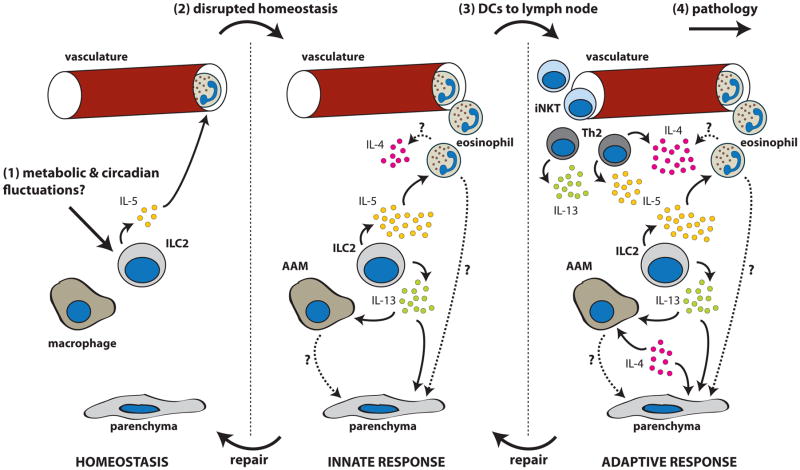
The three examples of homeostasis discussed above share several characteristics, including the IL-5-dependent accumulation of eosinophils and the maintenance of AAMs, which requires IL-4 or IL-13. ILC2s, which are systemically distributed in tissues at birth [36], represent the dominant innate source of IL-5 and IL-13 upon activation [1,37]. Thus, ILC2s can both initiate and maintain innate type 2 responses, suggesting that these cells may be important contributors to homeostasis (Figure 1).
Figure 1. Generic model of type 2 signaling in homeostasis.
(1) At rest, tonic activation of group 2 innate lymphoid cells (ILC2s) by as yet undefined signals drives expression of IL-5, which is required for systemic maintenance of eosinophils. (2) Disrupted homeostasis further activates ILC2s to increase IL-5 and IL-13 production, leading to eosinophil recruitment and alternatively activated macrophages (AAMs). Eosinophils, AAMs, and type 2 cytokines cooperate to restore tissue homeostasis. (3) If homeostatic disruptions are severe or prolonged, dendritic cells recirculate to lymph nodes and prime an adaptive type 2 response. Recruited helper type 2 (Th2) and type 2 invariant natural killer T (iNKT) cells provide IL-4, -5, and -13 to amplify the type 2 response. (4) Failure to resolve the type 2 response can lead to pathology, such as fibrosis or allergy.
In support of this hypothesis, ILC2s are constitutively present in the VAT of mice fed a normal chow diet [13]. Based on genetic lineage tracing, ~75% of VAT ILC2s express IL-5 and ~30% express IL-13. ILC2s comprise ~90% of all IL-5+ cells and ~80% of all IL-13+ cells, with CD4+ T cells accounting for the remainder. Deletion of IL-5- or IL-13-producing cells leads to a loss of both eosinophils and AAMs from the VAT. Since both eosinophils and AAMs are present in the VAT of Rag1−/− mice that lack CD4+ T cells, these results suggest that ILC2s are both necessary and sufficient for the homeostatic maintenance of eosinophils and AAMs in VAT.
Whether ILC2s similarly regulate AAMs and eosinophils during tissue injury or reparative responses to atherosclerosis remains unknown, but several findings suggest their involvement. Following tissue injury, induction of Arg1/Fizz1+ macrophages and recruitment of eosinophils are intact in Rag1−/− mice, suggesting an innate source of IL-4/13 and IL-5 [30]. Further, in atherosclerosis and heart disease, IL-33, a key activator of ILC2s, can have protective effects. Treating Apoe−/− mice with recombinant IL-33 blocks foam cell formation and reduces plaque size [38,39]. Conversely, elevated serum levels of the IL-33 decoy receptor (sST2) predict adverse cardiac outcomes in humans [40].
The Role of IL-4
While ILC2s are the primary source of innate IL-5 and IL-13, there is little evidence that they express IL-4 in mice. Indeed, analysis of IL-4 and IL-13 in vivo using reporter mice infected with the hookworm Nippostrongylus brasiliensis revealed a striking segregation of their expression[41]. In particular, IL-4 production was restricted to cells of the adaptive immune response (follicular helper T and Th2 cells) and to basophils, which require adaptive responses for recruitment to tissues or activation by IgE [42]. ILC2s were the predominant innate source of IL-13 in this model. Similar findings were established using an independently generated IL-13 reporter [43]. Together, these data suggest that ILC2-derived IL-13 initiates type 2 responses, whereas IL-4, produced primarily by recruited cells like Th2 cells and eosinophils, serves to amplify the response (Figure 1). Type 2 invariant natural killer T (iNKT) cells are another source of IL-4/5/13, and have been associated with protection in metabolic syndrome [44–46]. The steady-state presence of iNKT cells in tissue is, however, still unclear. While they are abundant in the liver, their residence in fat is variable, perhaps due to differences in animal housing conditions [13,44]. Finally, in several models it remains to be determined if phenotypes in Il-4/13−/− and Il4ra−/− mice are due to loss of IL-4, IL-13, or both.
ILC2s: Integrating Homeostatic Signals
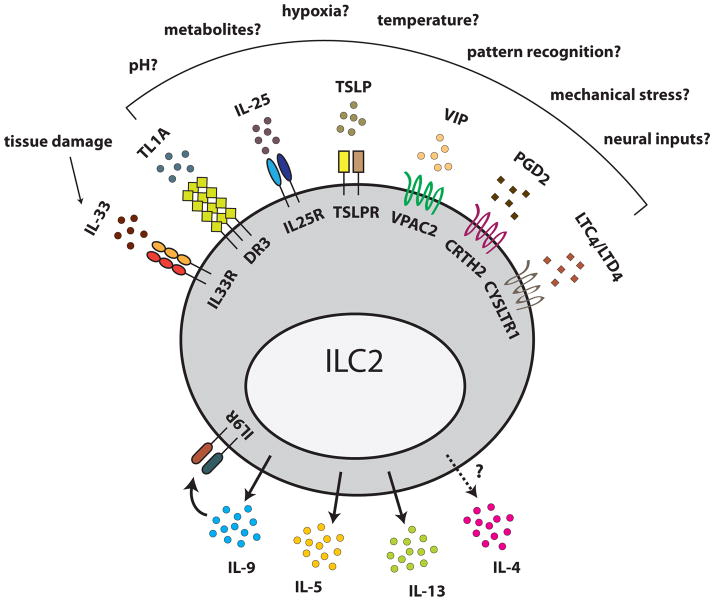
In the examples discussed, there is no apparent requirement for classical type 2 agonists such as helminths, allergens, or exogenous proteases. How then are ILC2s activated during homeostatic type 2 responses? Many endogenous signals, including IL-33, IL-25, thymic stromal lymphopoietin (TSLP), prostaglandin D2, leukotriene D4, vasoactive intestinal peptide, and TL1A can promote ILC2 cytokine secretion in vitro, and, in most cases, in vivo (Figure 2) [36,37,47–49]. Additionally, IL-9 may play roles in both auto-amplification and augmentation of Th9-derived signals to enhance ILC2 cytokines [50,51].
Figure 2. Integrating endogenous signals.
Group 2 innate lymphoid cells (ILC2s) are activated by diverse endogenous signals. One such signal, IL-33, is released by necrotic cell death and therefore signals tissue damage. The mechanisms that induce the other signals remain poorly understood, but we propose that they provide ILC2s with information about disrupted tissue homeostasis. When activated, ILC2s secrete IL-5, IL-13, IL-9, and perhaps IL-4. IL-9 enhances activation via an autocrine loop. Abbreviations: thymic stromal lymphopoietin (TSLP); vasoactive intestinal peptide (VIP); prostaglandin D2 (PGD2); cysteinyl leukotriene C4/D4 (LTC4/LTD4); death receptor 3 (DR3); chemoattractant receptor-homologous expressed on Th2 cells (CRTH2); cysteinyl leukotriene receptor 1 (CYSLTR1); vasoactive intestinal peptide receptor 2 (VPAC2).
We propose that by integrating these diverse signals, ILC2s monitor the homeostasis of their surrounding tissue. For example, IL-33, a potent ILC2 activator, is constitutively maintained in the nucleus of epithelial and endothelial cells as a pro-cytokine and released during necrosis or mechanical strain [52,53]. Therefore, by monitoring IL-33, ILC2s can promote eosinophil and AAM accumulation following local tissue damage, a response that the results discussed above suggest could mitigate tissue injury.
Much less is known about the regulation of other ILC2-activating signals. While some of the intracellular pathways required for IL-25, TSLP, or eicosanoid production are known, the upstream mechanisms that activate these responses in vivo remain incompletely defined. In our model, deviations from normal set points such as oxygen tension, pH, or temperature [54] might be involved. In addition, ILC2s might be activated directly or indirectly by changes in tissue metabolism [13] or neural inputs [55]. These remain active areas of investigation.
The diversity of signals to which ILC2s respond could also explain how ILC2s detect diverse agonists such as helminths, particles, and proteases. Rather than detecting a unique feature of each perturbation, ILC2s likely respond to the tissue disruptions common to them all. Nonetheless, the activation of type 2 signaling in the absence of exogenous stimuli suggests that type 2 pathways may not have emerged in direct response to helminths.
Innate Type 2 Immunity: Not Just for Worms?
The evolution of type I immunity to protect vertebrates from bacteria and viruses is well supported by human genetic deficiencies [56] and the complications induced by therapeutics that target these pathways (e.g. anti-TNFα). The same is not so apparent for type 2 immunity and worm infections [57–59]. While some worms cause significant disease, many humans and essentially all feral vertebrates are chronically parasitized by helminths with little consequence, despite type 2 immune responses. In fact, instances in which helminth parasites counteract more serious immune pathologies, such as allergy or ulcerative colitis, have been noted [60,61], as have instances in which host type 2 immunity is induced as a prerequisite for parasite maturation [62,63]. Taken together, the evidence suggests that multi-cellular parasites may not have been the earliest evolutionary drivers of type 2 responses. Perhaps type 2 pathways emerged to maintain homeostasis, as described above, and were later co-opted in response to chronic parasitism. For example, the roots of type 2 immunity may lie in primitive tissue repair responses, as others have noted [59]. Mucin induction, for example, is a target of the wounding response even in primitive cnidarians, Nematostella vectensis [64].
To understand the primordial role of type 2 pathways, it would be helpful to know when the genetic locus encoding IL-4, IL-5, IL-9, and IL-13 first emerged. While all bony vertebrates from zebrafish to humans encode IL-4/-13 and have eosinophils [65,66], the picture is less clear in more primitive lineages. Initial reports suggested that cartilaginous fish lack type 2 cytokines [67], but a more targeted analysis of the Elephant shark genome identified several IL-4, IL-5, and IL-13 candidate genes based on homology [68]. Moving further down the evolutionary tree, it will be interesting to examine if lamprey and other jawless vertebrates encode type 2 genes. Clearly, uncovering the early evolutionary drivers of type 2 pathways remains an important challenge.
Remaining Questions
The study of homeostatic type 2 responses is just beginning, and there are many unresolved questions. We have highlighted the importance of understanding pathways that lead to ILC2 activation, but our understanding of downstream mechanisms is similarly lacking. AAMs and eosinophils are important effectors, but how do these cells contribute to tissue repair, restriction of plaque formation, or metabolic homeostasis? Are there other tissues or processes in which these pathways are important? For example, IL-4/13 and AAMs sustain non-shivering thermogenesis in brown fat [69] and, together with eosinophils, drive cold or exercise-induced beiging of fat [54,70]. There is also a requirement for IL-4/13 during mammary gland development [71]. Are these ILC2-dependent processes? Much further work is necessary to address these questions.
Finally, what are the therapeutic implications of these findings? Mice treated with recombinant IL-33 or infected with N. brasiliensis to stimulate ILC2 responses show lasting improvements in metabolic homeostasis while on HFD [4,13,72]. Do these findings extend to chronically parasitized humans? Can ILC2 targeting improve organismal homeostasis without causing type 2-associated pathologies, such as fibrosis? These are exciting possibilities, and the study of homeostatic type 2 responses has also provided important insights into the basic biology and evolution of type 2 immunity. This will likely remain an active area of research in the coming years.
Highlights.
Innate type 2 immune pathways are protective in metabolic dysregulation, atherosclerosis, and tissue damage.
Group 2 innate lymphoid cells initiate and maintain homeostatic type 2 responses.
Group 2 innate lymphoid cells are activated by disruptions in tissue homeostasis.
Innate type 2 immunity may not have evolved primarily to counteract helminth infection.
Acknowledgments
The authors thank members of the Locksley lab for discussions that helped shape the ideas presented in this review. The lab is supported by NIH grants (AI026918, AI030663, AI119944), the Diabetes Endocrinology Research Center grant (DK063720), the Howard Hughes Medical Institute and the Sandler Asthma Basic Research Center at the University of California San Francisco. J.vM. is an HHMI Fellow of the Damon Runyon Cancer Research Foundation (DRG-2162-13).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Walker JA, Barlow JL, McKenzie ANJ. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. Excellent review of the recent discovery and characterization of innate lymphoid lineages. [DOI] [PubMed] [Google Scholar]
- 2*.Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie ANJ, Takei F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. Demonstrates a role for ILC2-derived IL-13 in the migration of dendritic cells to the lymph node and subsequent activation of adaptive immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csóka B, Koscsó B, Töro G, Kókai E, Virág L, Németh ZH, Pacher P, Bai P, Haskó G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee C-H. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–271. doi: 10.1172/JCI64941. Demonstrates a direct metabolic role for IL-13 via inhibition of glucose production in hepatocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Molofsky AB, Nussbaum JC, Liang H-E, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. First paper to show how ILC2s can regulate fat homeostasis by maintaining AAMs and eosinophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. First study to directly examine the role of IL-13 in atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foks AC, van Puijvelde GHM, Bot I, ter Borg MND, Habets KLL, Johnson JL, Yagita H, van Berkel TJC, Kuiper J. Interruption of the OX40-OX40 ligand pathway in LDL receptor-deficient mice causes regression of atherosclerosis. J Immunol Baltim Md 1950. 2013;191:4573–4580. doi: 10.4049/jimmunol.1200708. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Gao S, Xu W, Zhao S, Zhou J, Wang N, Yuan Z. Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice. J Mol Cell Cardiol. 2014;72C:20–27. doi: 10.1016/j.yjmcc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Hadri K, Mahmood DFD, Couchie D, Jguirim-Souissi I, Genze F, Diderot V, Syrovets T, Lunov O, Simmet T, Rouis M. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1445–1452. doi: 10.1161/ATVBAHA.112.249334. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, Lu Y, Zhou G, Liao X, Kapil P, Anand P, Mahabeleshwar GH, Stamler JS, Jain MK. Myeloid Krüppel-like factor 4 deficiency augments atherogenesis in ApoE−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32:2836–2838. doi: 10.1161/ATVBAHA.112.300471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder CJ, Hartvigsen K, Chang M-K, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. Demonstrates the important wound-healing role of type II signaling during helminth infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh YPS, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. First report of impaired muscle regeneration in the absence of IL4/13 and eosinophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeAngelis RA, Markiewski MM, Kourtzelis I, Rafail S, Syriga M, Sandor A, Maurya MR, Gupta S, Subramaniam S, Lambris JD. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol Baltim Md 1950. 2012;188:641–648. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol. 2013;133:2461–2470. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE. Traumatic brain injury induces macrophage subsets in the brain. Eur J Immunol. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol Baltim Md 1950. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 31.Shono J, Sakaguchi S, Suzuki T, Do M-KQ, Mizunoya W, Nakamura M, Sato Y, Furuse M, Yamada K, Ikeuchi Y, et al. Preliminary time-course study of antiinflammatory macrophage infiltration in crush-injured skeletal muscle. Anim Sci J Nihon Chikusan Gakkaih. 2013;84:744–750. doi: 10.1111/asj.12105. [DOI] [PubMed] [Google Scholar]
- 32*.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol Baltim Md 1950. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. Uses conditional depletion of macrophages to demonstrate their protective role during wound healing in the skin. [DOI] [PubMed] [Google Scholar]
- 33.Varin A, Gordon S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nei Y, Obata-Ninomiya K, Tsutsui H, Ishiwata K, Miyasaka M, Matsumoto K, Nakae S, Kanuka H, Inase N, Karasuyama H. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A. 2013;110:18620–18625. doi: 10.1073/pnas.1311668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. First report of constitutive IL-5 production in ILC2s and their role in maintaining systemic eosinophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie ANJ, Krummel MF, Liang H-E, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. Genetic dissection of upstream cytokine pathways required for ILC2 activation during innate type II inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY, Ramji DP. IL-33 reduces macrophage foam cell formation. J Immunol Baltim Md 1950. 2010;185:1222–1229. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau J-L, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 41*.Liang H-E, Reinhardt RL, Bando JK, Sullivan BM, Ho I-C, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. Together with Ref #43, maps the segregation of IL-4 and IL-13 production and function in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan BM, Liang H-E, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CDC, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie ANJ. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e1–4. doi: 10.1016/j.jaci.2011.09.041. Together with Ref #41, maps the segregation of IL-4 and IL-13 production and function in vivo. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KSL, Gao B, Lee C-H, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PloS One. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. Identifies cysteinyl leukotriene D4 as a novel activating signal for ILC2s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie ANJ, Spits H, Klenerman P, Ogg G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–740. doi: 10.1038/mi.2013.92. Identifies TL1A as a novel activating signal for ILC2s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Turner J-E, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld J-C, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. Describes the importance of autocrine IL-9 signaling in ILC2 function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Licona-Limón P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limón I, Ishigame H, Hao L, Herbert DR, Flavell RA. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39:744–757. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cayrol C, Girard J-P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. Together with Ref #70, identifies a novel role for IL-4/13 and eosinophils in beiging of fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. First paper to describe cross-talk between the ILC2-activating cytokine TSLP and neuronal signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong T, Yeung J, Hildebrand KJ, Junker AK, Turvey SE. Human primary immunodeficiencies causing defects in innate immunity. Curr Opin Allergy Clin Immunol. 2013;13:607–613. doi: 10.1097/ACI.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 57.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 61.Flohr C, Quinnell RJ, Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2009;39:20–32. doi: 10.1111/j.1365-2222.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 62.Riner DK, Ferragine CE, Maynard SK, Davies SJ. Regulation of innate responses during pre-patent schistosome infection provides an immune environment permissive for parasite development. PLoS Pathog. 2013;9:e1003708. doi: 10.1371/journal.ppat.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol Baltim Md 1950. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubuc TQ, Traylor-Knowles N, Martindale MQ. Initiating a regenerative response, cellular and molecular features of wound healing in the cnidarian Nematostella vectensis. BMC Biol. 2014;12:24. doi: 10.1186/1741-7007-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, Bañuelos K, Romo-Fewell O, Aroian RV, Traver D. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood. 2010;116:3944–3954. doi: 10.1182/blood-2010-03-267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L, Pan P, Fang W, Shao J, Xiang L. Essential role of IL-4 and IL-4Rα interaction in adaptive immunity of zebrafish: insight into the origin of Th2-like regulatory mechanism in ancient vertebrates. J Immunol Baltim Md 1950. 2012;188:5571–5584. doi: 10.4049/jimmunol.1102259. [DOI] [PubMed] [Google Scholar]
- 67.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dijkstra JM. TH2 and Treg candidate genes in elephant shark. Nature. 2014;511:E7–9. doi: 10.1038/nature13446. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70**.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. Together with Ref #54, identifies a novel role for IL-4/13 and eosinophils in beiging of fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khaled WT, Read EKC, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, Sprigg N, McKenzie ANJ, Watson CJ. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell. Development. 2007;134:2739–2750. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- 72.Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]