Abstract
We examined the long-term clinical and economic benefits of quadrivalent human papillomavirus (qHPV) vaccine as a secondary/adjunct prevention strategy in the prevention of recurrent high-grade intraepithelial neoplasia (HGAIN) in HIV-negative men who have sex with men (MSM) and are 27 years or older. We constructed a Markov model to evaluate the clinical effectiveness and cost-effectiveness of two strategies: (1) no qHPV vaccine after treatment for HGAIN versus (2) qHPV vaccine after treatment for HGAIN. Model parameters, including natural history of anal cancer, vaccine efficacy measured in terms of hazard ratio (HR) (risk of recurrent HGAIN), HGAIN treatment efficacy, utilities, and costs, were obtained from the literature. The outcomes were measured in terms of lifetime risk of anal cancer, lifetime cost, quality-adjusted life years, and incremental cost-effectiveness ratios (ICERs). Sensitivity analysis was conducted on all model parameters. We found that vaccinating HIV-negative MSM reduced the lifetime risk of anal cancer by 60.77% at an ICER of US$87,240 (95% CI, $22,301–$144,187) per quality-adjusted life-year. The results were highly sensitive to vaccine efficacy, transition of HGAIN to anal cancer, cost of treatment for HGAIN, vaccine degree of protection over time, and the vaccine duration of protection and less sensitive to HPV clearance, cost of qHPV, and the transitions from normal to low-grade anal intraepithelial neoplasia (LGAIN) and normal to HGAIN. With an HR of 0.3, the ICER was well below a $50,000 willingness-to-pay threshold; with an HR of 0.5, the ICER was still below a threshold of $100,000. The most critical disease-related factor influencing the cost-effectiveness was the progression of HGAIN to anal cancer. At an annual transition probability below 0.001, the ICER was below $50,000. Vaccinating HIV-negative MSM treated for HGAIN decreases the lifetime risk of anal cancer and is likely to be a cost-effective intervention.
Keywords: Human papillomavirus, Cost-effectiveness analysis, Quadrivalent human papillomavirus vaccine, Secondary/adjunct prevention, Anal neoplasia, High-grade intraepithelial neoplasia
1. INTRODUCTION
Recent evidence suggests that the overall age-adjusted incidence rates for anal cancer in the United States have been increasing over the past 30 years [1]. In the general population, the incidence rate has increased from 1.2/100,000 to 2.8/100,000 person-years. However, certain populations appear to be at higher risk, including human immunodeficiency virus (HIV)-negative men who have sex with men (MSM), who have a reported anal cancer incidence rate of 5.1 (95% confidence interval [CI], 0.0–11.5) per 100,000 person-years [2].
Persistent human papillomavirus (HPV) infection is highly associated with the development of anal cancer. It is believed that chronic infection with HPV leads to the neoplastic progression from infected tissue to invasive cancers. Like other HPV-related cancers, squamous cell carcinoma of the anus develops over a period of years in a stepwise progression. High-grade anal intraepithelial neoplasia (HGAIN) is believed to be a precursor to anal cancer [3]. The removal of HGAIN is thought to prevent progression to anal cancer in almost all patients [4]. However, the recurrence rate of HGAIN is almost 62% within the first year after treatment in HIV-negative MSM [5].
The quadrivalent human papillomavirus (qHPV) vaccine (Gardasil, Merck, Whitehouse Station, NJ, USA) has proved efficacious in the prevention of genital lesions and anogenital HPV infections related to HPV types 6, 11, 16, and 18 in boys and men, preventing almost 90% of incidences [6]. The vaccine was also determined to be cost-effective for the primary prevention of genital warts and anal cancer in MSM [7]. On the basis of these data and according to the guidance provided by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP), in December 2010, the Food and Drug Administration approved use of qHPV vaccine for preventing anal carcinoma and associated precancerous lesions attributed to HPV types 6, 11, 16, and 18 in boys and men ages 9 through 26 years. In 2011, the ACIP revised their guidelines to recommend use of the vaccine in males age 11 through 26 years [8].
Recently, Swedish et al [9] studied HIV-uninfected MSM with a history of HGAIN and found that vaccinating these men with qHPV vaccine was associated with an almost 50% reduction in the risk for recurrent or persistent HGAIN after treatment. During the 340.4 person-years of follow-up, Swedish et al [9] found that 13.6% of vaccinated patients and 30.7% of unvaccinated patients developed recurrent HGAIN. The authors concluded that the decreased risk of recurrent HGAIN appeared to endure for at least 3 years. Swedish et al hypothesized that the mechanism of decreased HGAIN risk after vaccination was associated with the prevention of further infection and integration of HPV infection (either new or residual HPV infection after treatment) through antibody production. These findings are consistent with a recently published study by Joura et al [10], who found that previous vaccination with qHPV vaccine among women who had surgical treatment for HPV-related disease significantly reduced the risk of subsequent cervical high-grade disease by 64.9%.
It will be several more years before the results from the AIDS Clinical Trials Group’s A5298 trial [11] evaluating the efficacy of qHPV vaccine for secondary prevention in HIV-positive MSM will be available; meanwhile, decision makers should know the potential long-term economic and clinical benefits of the vaccine as a post-treatment adjunct form of therapy. Here, we evaluate the potential clinical effectiveness and cost-effectiveness of extending qHPV vaccine to HIV-negative MSM, 27 years or older, treated for HGAIN.
2. METHODS
2.1 Model Overview
We created a state-transition model to simulate a cohort of HIV-uninfected MSM based on the natural history of HPV infection and anal carcinoma, along with treatment characteristics and vaccine properties. Annual transition occurs based on the probabilities of moving from one health state to another. The costs and utilities of spending time in each health state contribute to the population-level outcomes of quality-adjusted life years (QALYs) and lifetime costs; decreased annual risk of recurrent HGAIN contributed to the outcome of decreased lifetime risk of anal cancer.
The health states include: (1) baseline prevalence of HPV16/18, (2) histological characteristics (i.e., normal, low-grade anal intraepithelial neoplasia (LGAIN), HGAIN, and cancer), (3) HGAIN recurrence (first, second, and third recurrence), (4) survivorship, and (5) death. The model starts with a cohort of HIV-negative 27-year-old MSM distributed into health states —HPV16/18, LGAIN, or HGAIN— based on the baseline prevalence data from a meta-analysis [2]. Time horizon was divided into equal intervals of 1 year referred to as Markov cycles. A hypothetical cohort of 100,000 HIV-negative MSM was assumed to be acquiring and clearing HPV infection. Those with HPV infection could develop LGAIN or HGAIN. Those with LGAIN could progress to HGAIN, regress to normal, or remain in the same health state. The patients with HGAIN could regress to LGAIN or normal. The MSM with HGAIN who were treated with local excision or targeted ablative therapies were either vaccinated with 3-dose qHPV vaccine or not. Following treatment for HGAIN, patients either developed recurrent HGAIN or remained in the same health state. Untreated or recurrent HGAIN could lead to anal cancer. Those who developed anal cancer at any point were treated. In every cycle, the MSM were subjected to competing all-cause mortality risks. The model structure is presented in Supplemental Figure 1.
2.2 Clinical Data
Clinical variables, including natural history parameters, are presented in Table 1. The baseline prevalence of HPV16/18, LGAIN, and HGAIN and incidence of HPV types 16 and 18 in HIV-negative MSM were based on a meta-analysis [2]. The natural history parameters for disease progression and regression were based on a cost-effectiveness analysis by Czoski-Murray et al [12], who derived these estimates from two large prospective cohort studies [13, 14]. Data from Tong et al [15] were used to estimate HGAIN regression. The progression rates of HPV to LGAIN and HPV to HGAIN are unknown. Therefore, under the assumption that HPV is a precursor to precancerous lesions in all HIV-negative patients [16], we used the progression rates for normal MSM (HIV-negative MSM with no HPV infection) to LGAIN and normal MSM to HGAIN. The progression rates from normal to LGAIN and normal to HGAIN are similar to the progression rate from HPV infection to cervical intraepithelial neoplasia, a precursor to cervical cancer (another HPV-associated cancer that follows similar disease progression)[17]. We based the clearance rate for HPV16/18 in HIV-negative MSM on data from Machalek et al [2].
Table 1.
Modeling Parameters
| Parameters | HIV-Negative MSM | Distribution | Source |
|---|---|---|---|
| Baseline prevalence (range) | [2] | ||
| HPV16/18 | 0.174 (0.087 – 0.348) | Beta (5.46, 25.95) | |
| LGAIN | 0.106 (0.063 – 0.149) | Beta (36.65, 399.61) | |
| HGAIN | 0.152 (0.00 – 0.309) | Beta (3.00, 16.74) | |
| Incidence of HPV (range) | [2] | ||
| Type 16 | 0.118 (0.092 – 0.149) | Beta (57.96, 433.36) | |
| Type 18 | 0.061 (0.043 – 0.083) | Beta (33.49, 515.61) | |
| Progression (annual transition probability) | |||
| Normal to LGAIN | 0.072 (0.034 – 0.122) | Beta (9.47, 122.10) | [12] |
| LGAIN to HGAIN | 0.350 (0.129 – 0.599) | Beta (5.18, 9.63) | |
| Normal to HGAIN | 0.057 (0.024 – 0.103) | Beta (7.46, 123.85) | |
| HGAIN to anal cancer | 0.000239 (0.000119 – 0.000478) | [2] | |
| Regression (annual transition probability) | [2] | ||
| LGAIN to normal | 0.071 (0.002 – 0.236) | Beta (1.24, 16.26) | |
| HGAIN to normal | 0.301 (0.150 – 0.602) | Beta (4.46, 10.36) | |
| Clearance of HPV16/18 (range) | 0.445 (0.27 – 0.62) | Beta (13.34, 16.64) | [2] |
| Vaccine efficacy in prevention of HGAIN recurrence (vaccinated vs not vaccinated), HR (95% CI) | |||
| First year | 0.42 (0.22 – 0.82) | Beta (3.94, 5.45) | [9] |
| Second year | 0.50 (0.26 – 0.98) | Beta (3.20, 3.20) | |
| Third year | 0.52 (0.27 – 1.02) | Beta (3.02, 2.79) | |
| Fourth year onwards | 0.48 (0.24 – 0.72) | Beta (6.40, 8.84) | Assume |
| Recurrence of HGAIN in patients previously treated with ablation (range) | [5] | ||
| After first treatment | 0.62 (0.31 – 0.93) | Beta (5.22, 3.20) | |
| After second treatment | 0.48 (0.24 – 0.72) | Beta (7.52, 8.14) | |
| After third treatment | 0.57 (0.285 – 0.855) | Beta (6.04, 4.55) | |
| 5-y survival (range) | 0.656 (0.313 – 0.796) | Beta (9.09, 4.77) | [27] |
| Costs, adjusted to 2010 U.S. $ | |||
| Cost of anal cancer treatment | 38,011 (18,998 – 76,024) | Gamma (6.83, 5567.60) | [23] |
| Cost of HGAIN treatment | 3,983 (1,991 – 5,974) | Gamma(15.36, 259.20) | [22] |
| Cost of vaccine | 500 (360 – 700) | Gamma (33.23, 15.04) | Retail price |
| Utilities (range) | |||
| HGAIN | 0.98 (0.95 – 0.99) | Beta (183.49, 3.74) | [12] |
| Anal cancer | 0.58 (0.5 – 0.7) | Beta (53.69, 38.88) | [12] |
Abbreviations: HIV, human immunodeficiency virus; MSM, men having sex with men; HPV, human papillomavirus; LGAIN, low-grade anal intraepithelial neoplasia; HGAIN, high-grade anal intraepithelial neoplasia; IRC, infrared coagulation; QALY, quality-adjusted life-years.
2.3 Vaccine Efficacy
Vaccine efficacy, the reduction in the risk of developing recurrent HGAIN, was based on data from Swedish et al [9]. They found that qHPV vaccine was associated with a significantly decreased risk of recurrent HGAIN (hazard ratio [HR]=0.42; 95% CI, 0.22–0.82) at 1 year of treatment; at 2 years, the risk increased to 0.50 (95% CI, 0.26–0.98); and at 3 years, the risk increased to 0.521 (95% CI, 0.27–1.02).
2.4 Modeling Assumptions
We made the following conservative assumptions on the basis of the best available published evidence: (1) HPV is a precursor to LGAIN and HGAIN in HIV-negative MSM. (2) We assume the incidence of anal HPV infection is constant across all ages above 27 years. (3) All MSM with abnormal cytological tests will undergo high-resolution anoscopy and biopsy. (4) High-resolution anoscopy and biopsy determine the true histology of a patient’s lesion. (5) MSM diagnosed with HGAIN are treated with local excision or targeted ablative therapies and followed every 12 months to determine the possibility of HGAIN recurrence. (6) All MSM in the vaccination group receive qHPV vaccine in addition to treatment for HGAIN. (7) The probabilities of first, second, and third recurrences after treatment for HGAIN are based on Goldstone et al [5]. (8) After every event of HGAIN recurrence, those who are not compliant with the treatment follow the natural history of the cancer and progress from having HGAIN to anal cancer at a progression rate of 1 in 4196 patients per year [2]. For the base case analysis, we assumed 100% compliance with treatment for HGAIN, HGAIN recurrences, and anal cancer. (9) In the model, those who survive 5 years after anal cancer treatment assume the life expectancy based on U.S. life tables [18]. (10) The vaccine benefits only those who are vaccinated (i.e., indirect benefits were not incorporated). (11) For the base case analysis, we assumed that after the first 3 years of protection, qHPV vaccine is associated with a decrease in the lifetime risk of HGAIN, with an HR of 0.48 (95% CI, 0.24–0.72).
2.5 Utilities
We adjusted the life expectancy by incorporating utilities, measures of preference-based quality of life. The utility scores were based on a cost-effectiveness analysis by Czoski-Murray [12], which used the utility value of 0.98 (range, 0.95–0.99) for patients receiving treatment for HGAIN and 0.58 (range, 0.50–0.70) for patients receiving treatment for anal cancer.
2.6 Costs
Costs for anal cancer treatment were based Hu and Goldie [19], and costs for HGAIN treatment were based on Goldie et al [20]. Using consumer price indices, we converted these amounts to reflect costs in terms of 2010 U.S. dollars [21]. The cost of a 3-dose qHPV vaccine, including the administration cost, was assumed to be $500.
2.7 Analysis
The model calculated lifetime costs, effectiveness in terms of survival and QALYs, and cost-effectiveness of the following strategies: (1) no qHPV vaccine after treatment for HGAIN and (2) qHPV vaccine after treatment for HGAIN. The incremental cost-effectiveness ratio (ICER) was determined as the additional cost of including vaccination in the treatment regimen for HGAIN, divided by additional clinical benefits in terms of QALYs, compared with no vaccination. The analysis was conducted from the health-care perspective. Using the Panel on Cost-Effectiveness in Health and Medicine guidelines [22], we applied a discount rate of 3% to discount future costs and QALYs.
To determine robustness, we performed deterministic and probabilistic sensitivity analysis (PSA). One-way sensitivity analysis was conducted by varying vaccine efficacy and HGAIN to anal cancer progression. We also conducted two-way sensitivity analyses varying vaccine efficacy (base case vaccine efficacy, high vaccine efficacy [HR=0.25], and low vaccine efficacy [HR=0.75]) and HGAIN to anal cancer transition, age at the time of vaccination, degree of protection over time, duration of protection, HPV clearance, progression from normal to LGAIN and normal to HGAIN, compliance with recurrent HGAIN treatments, cost of HGAIN treatment, cost of qHPV vaccine, or cost of anal cancer treatment. For the analysis that included degree of vaccine protection over time, we assumed that the degree of protection remained constant throughout the duration of protection and then decreased to zero. Additionally, we examined scenarios in which the decline in vaccine efficacy followed a normal or exponential distribution.
The 95% CI of the ICER was estimated using 10,000 simulations in a PSA. The joint uncertainty in costs and effectiveness was explored using the simulations and was summarized in the form of a cost-effectiveness acceptability curve. The probability distributions for the PSA were selected using Briggs et al’s guidelines on decision modeling for health economic evaluation [23]. As the sample moments μ̄ and s2 were known, we used the functions α = μ̄2 (1 − μ̄)/s2 − μ̄ and β = α [(1 − μ)/μ], and then fitted a beta (α, β) distribution. The gamma distribution was parameterized as gamma (α, β) where α = μ̄2/s2 and β = s2/μ. These functions can be derived using the method of moments that equates the sample moments to the distribution moments [23]. We used TreeAge Pro 2013 (Williamstown, MA, USA) for modeling and analysis.
3. RESULTS
3.1 Model Validation
The face validity of our model was confirmed by comparing the predicted annual incidence and age-specific distribution of anal cancer in the cohort of 100,000 in the absence of treatment and vaccination to alternative data sources that were not used to populate the model. In the absence of treatment and vaccination, the absolute lifetime risk of anal cancer in HIV-negative MSM was 1.31%. The incidence of anal cancer in HIV-negative MSM as predicted by our model was comparable to the incidence rate reported in the literature [2, 24]. The incidence peaked between the age range of 51 and 53 years at a rate of 29 per 100,000. Our model predicted age-specific incidence was similar in distribution to that predicted by the Surveillance, Epidemiology, and End Results program [25]. Model-predicted age-specific incidences in the absence and presence of a vaccination program are presented in Supplemental Figure 2.
3.2 Base Case Analysis
In the base case analysis, vaccination increased QALYs by 0.0168 (QALYs for no vaccination after treatment and vaccination after treatment were 24.9617 and 24.9785, respectively) and increased total lifetime cost by $1,446 (total costs for no vaccination after treatment and vaccination after treatment were $4,652 and $6,118, respectively); therefore, the base case ICER was $87,240 per QALY (Table 2). If treatment and vaccination occurred at age 27 years, qHPV vaccine reduced the lifetime risk of anal cancer by 60.77%.
Table 2.
Base Case and Sensitivity Analyses
| Vaccine Efficacy (HR = 0.25) | Vaccine Efficacy (Base Case) | Vaccine Efficacy (HR = 0.75) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | ICER, $/QALY | Reduction in Lifetime Anal Cancer Risk, % | ICER, $/QALY | Reduction in Lifetime Anal Cancer Risk, % | ICER, $/QALY | Reduction in Lifetime Anal Cancer Risk, % |
| Treatment and vaccination age | ||||||
| 27 years (base case) | 30,867 | 86.12 | 87,240 | 60.77 | 170,915 | 29.43 |
| 30 years | 31,774 | 86.77 | 94,040 | 61.38 | 189,131 | 29.63 |
| 35 years | 32,970 | 87.62 | 106,977 | 62.22 | 226,872 | 30.16 |
| 40 years | 33,542 | 88.72 | 121,954 | 63.42 | 276,490 | 30.74 |
| 45 years | 33,096 | 89.76 | 138,521 | 64.88 | 341,385 | 31.71 |
| 50 years | 31,162 | 90.57 | 155,246 | 66.67 | 424,418 | 32.70 |
| 55 years | 27,436 | 91.53 | 169,035 | 68.64 | 524,079 | 33.90 |
| Duration of protection | ||||||
| Assumption 1. (base case) | ||||||
| Vaccine wanes in 10 years | 158,593 | 8.61 | 197,662 | 5.74 | 260,198 | 2.39 |
| Vaccine wanes in 20 years | 87,370 | 22.49 | 139,402 | 15.07 | 219,059 | 6.94 |
| Vaccine wanes in 30 years | 57,486 | 39.47 | 111,869 | 27.03 | 194,427 | 12.92 |
| Assumption 2. | ||||||
| Vaccine wanes in 10 years | 147,958 | 9.80 | 189,391 | 6.46 | 255,175 | 2.87 |
| Vaccine wanes in 20 years | 83,297 | 24.16 | 135,917 | 16.03 | 216,492 | 7.42 |
| Vaccine wanes in 30 years | 58,746 | 39.23 | 117,057 | 24.40 | 206,452 | 9.57 |
| Assumption 3. | ||||||
| Vaccine wanes in 10 years | 157,983 | 8.85 | 209,525 | 5.02 | 290,035 | 0.95 |
| Vaccine wanes in 20 years | 106,373 | 19.38 | 185,598 | 8.37 | 290,035 | 0.95 |
| Vaccine wanes in 30 years | 90,436 | 28.95 | 184,955 | 8.37 | 290,035 | 0.95 |
| HPV16/18 prevalence | ||||||
| Prevalence of 8.7% | 30,875 | 86.17 | 87,514 | 60.68 | 171,703 | 29.37 |
| Prevalence of 34.8% | 30,851 | 85.98 | 86,718 | 60.75 | 169,416 | 29.21 |
| HPV16/18 clearance | ||||||
| Clearance of 27% | 31,166 | 86.39 | 89,114 | 60.80 | 175,470 | 29.39 |
| Clearance of 62% | 30,597 | 85.76 | 84 276 | 60.47 | 161,950 | 29.17 |
| Normal to LGAIN progression | ||||||
| 0.034 | 30,721 | 86.02 | 85,722 | 60.69 | 166,898 | 29.29 |
| 0.122 | 31,016 | 86.30 | 88 389 | 60.87 | 173,831 | 29.35 |
| Normal to HGAIN progression | ||||||
| 0.024 | 30,727 | 86.02 | 86,002 | 60.48 | 167,706 | 29.30 |
| 0.103 | 31,008 | 86.17 | 88,051 | 60.85 | 172,844 | 31.49 |
| Compliance with recurrent HGAIN treatments | ||||||
| 25% | 23,147 | 85.14 | 62,017 | 60.14 | 134,485 | 29.01 |
| 50% | 26,789 | 85.19 | 75,289 | 60.71 | 154,988 | 29.29 |
| 75% | 29,204 | 85.85 | 82,619 | 60.77 | 165,004 | 29.19 |
| Anal cancer cost per case, U.S. $ | ||||||
| 18,998 | 31,885 | 86.12 | 88,259 | 60.77 | 171,667 | 29.43 |
| 76,024 | 28,832 | 86.12 | 85,216 | 60.77 | 169,413 | 29.43 |
| HGAIN cost per case, U.S. $ | ||||||
| 1,991 | 19,442 | 86.12 | 49,041 | 60.77 | 95,200 | 29.43 |
| 5,974 | 42,286 | 86.12 | 123,776 | 60.77 | 246,594 | 29.43 |
| qHPV cost per case, U.S. $ | ||||||
| 360 | 28,051 | 86.12 | 83,361 | 60.77 | 164,609 | 29.43 |
| 700 | 34,890 | 86.12 | 92,781 | 60.77 | 179,325 | 29.43 |
Abbreviations: HR, hazard ratio; ICER, incremental cost-effectiveness ratio; HPV, human papillomavirus; LGAIN, low-grade anal intraepithelial neoplasia; HGAIN, high-grade anal intraepithelial neoplasia.
Assumption 1. Constant degree of protection, assuming that the vaccine completely loses effectiveness after the given duration.
Assumption 2. Decline in degree of protection over time at a rate function that follows normal distribution (constant waning rate of 1/30 years).
Assumption 3. Decline in degree of protection over time at a rate function that follows an exponential distribution (mean = 30 years, standard deviation = 5 years).
3.3 Sensitivity Analysis
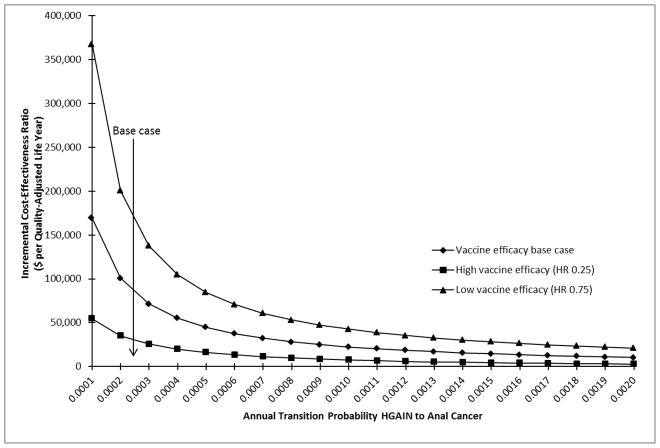
The 95% CI as determined from the 10,000 simulations was $22,301 and $144,187 per QALY. The ICER was highly sensitive to vaccine efficacy (Fig. 1) and the transition from HGAIN to anal cancer (Fig. 2). Vaccine efficacies of HR=0.2, 0.5, and 0.8 corresponded to ICERs of $15,229, $95,761, and $191,183 per QALY; decreases in lifetime risk of anal cancer of 91.16%, 59.53%, and 25.58%; and absolute lifetime risks of anal cancer of 0.038%, 0.174%, and 0.32%, respectively (data not shown). Annual transition probabilities of 0.001, 0.01, and 0.02 for the progression from HGAIN to anal cancer corresponded to ICERs of $169,431, $22,450, and $10,488 per QALY in the base case analysis; the ICERs under the assumptions of high vaccine efficacy for this transition were $54,622, $7,523, and $2,650 per QALY; and under the assumption of low vaccine efficacy the ICERs were $367,753, $42,616, and $21,034 per QALY, respectively. The outcomes were also sensitive to the treatment and vaccination age, degree of protection, duration of protection, compliance with recurrent HGAIN treatments, and HGAIN cost per case (Table 2). Results were less sensitive to HPV prevalence, HPV clearance, cost of qHPV, and the transitions from normal to LGAIN and normal to HGAIN. In the PSA, we determined that the probability of cost-effectiveness of qHPV vaccine was 22% at a willingness-to-pay threshold of $50,000 and 74% at a threshold of $100,000 (Fig. 3).
Figure 1. One-Way Sensitivity Analysis on Vaccine Efficacy.
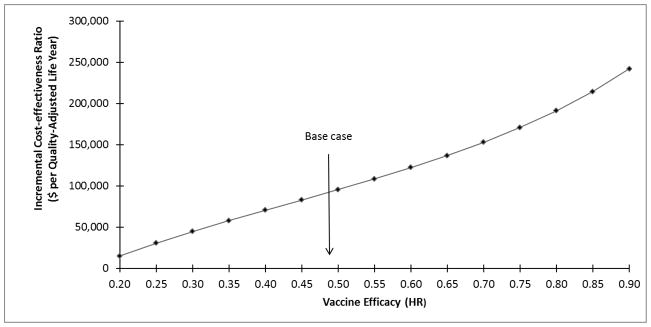
One-way sensitivity analysis shows the impact of vaccine efficacy on the incremental cost-effectiveness ratio (ICER). The vaccine effectiveness is expressed in terms of hazard ratio (HR; i.e., the chance of HGAIN recurrence with vaccination divided by the chance of recurrence without vaccination). The base case efficacy is indicated by an arrow labeled “base case”. As the vaccine efficacy decreases, the ICER increases.
Figure 2. One-way Sensitivity Analysis on Annual Transition Probability of High-Grade Squamous Intraepithelial Lesion to Anal Cancer.
One-way sensitivity analysis shows the impact of varying the annual transition probability of high-grade squamous intraepithelial lesion to anal cancer on the incremental cost-effectiveness ratio (ICER). The base case annual transition probability is indicated by an arrow labeled “base case”. With the increases in disease transition probability, the ICER decreases.
Figure 3. Cost-effectiveness Acceptability Curves.
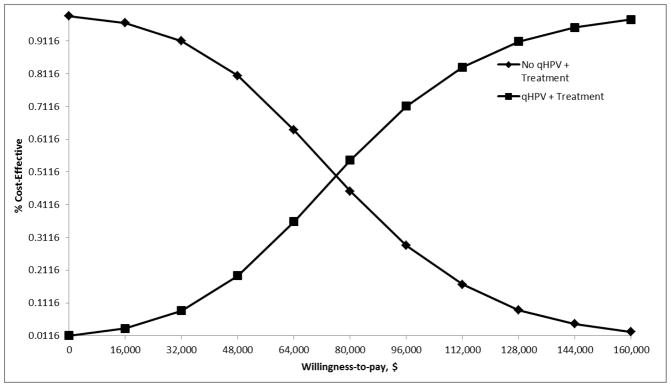
The cost-effectiveness acceptability curves show the effect of joint density of all model parameters on the probability of cost-effectiveness. With the increases in the willingness to pay threshold, the probability that the vaccine is cost-effective increases.
4. DISCUSSION
We found that post-treatment adjunct vaccination in HIV-negative older MSM can reduce the lifetime risk of anal cancer and is likely to be a cost-effective intervention. High vaccine efficacy and faster disease progression were associated with an increase in cost-effectiveness and a decrease in the lifetime risk of anal cancer. In 2011, the ACIP approved the use of qHPV vaccine in males up to age 26 years, but our results indicate that older men may benefit from vaccination as well.
Swedish et al [9] ascertained that the qHPV vaccine was efficacious in preventing HGAIN recurrence for at least the first 3 years after vaccination, although effectiveness declined slightly each year. An earlier study by Villa et al [26] determined that the qHPV vaccine was effective through 5 years for primary disease prevention, and other primary prevention studies demonstrated that the vaccine was efficacious for 6–8 years [27, 28]. Furthermore, previous economic evaluation studies assumed a duration of primary disease prevention of 30 years for the vaccine [7, 29]. As the long-term efficacy of qHPV in the prevention of HGAIN recurrence is not known, the duration and degree of protection were varied in our sensitivity analysis. The ICER was below a willingness-to-pay threshold of $100,000 only when high vaccine efficacy (HR=0.25) and a duration of protection of at least 20 years were assumed.
Our study is not without limitations. First, we captured only direct clinical and economic benefits of the vaccine. The indirect benefits, such as herd-immunity and cross-protective effects, were not included. Inclusion of these properties would improve the cost-effectiveness of qHPV vaccine. In addition, the vaccine efficacy data were based on a retrospective study; confirmation by a randomized clinical trial is required. Furthermore, we made several conservative assumptions regarding disease transition. We also did not consider the probability of acquiring HIV infection. As shown by Chin-Hong [30], infection with 2 or more HPV types was associated with HIV seroconversion, with an HR of 3.5 (95% CI, 1.2–10.6; P=0.002).
This analysis will need to be revisited as more data become available on the natural history of HPV in anal cancer patients, clearance of HPV, qHPV vaccine efficacy, and duration of qHPV vaccine protection. In particular, the long-term benefits of the vaccine in HIV-positive patients will need to be determined as these patients may benefit differently than the HIV-negative patients.
Moreover, the results of a randomized clinical trial evaluating the safety, efficacy, and immunogenicity of a nonavalent HPV vaccine will be available soon [31]. This vaccine could be more efficacious and cost-effective than currently available vaccines [32, 33]. There is also a need to evaluate secondary prevention properties of the nonavalent vaccine.
The clinical effectiveness of the qHPV vaccine in preventing anal cancer after HGAIN treatment will be highly dependent on the screening test characteristics (i.e., sensitivity and specificity) of anal cytology for the detection of HGAIN as identified by high-resolution anoscopy-guided biopsy. For example, Berry et al [34] found that the sensitivity of abnormal cytology to detect cases of HGAIN in HIV-negative MSM was only 55%. Nevertheless, screening HIV-negative MSM with anal cytology every 2–3 years was determined to be cost-effective [20]. The current lack of U.S. clinical guidelines on screening anal cytology will make it difficult to comprehensively identify cases of HGAIN among MSM.
To our knowledge, our study is the first to explore the clinical and economic value of qHPV vaccine as adjunct therapy after HGAIN treatment in the high-risk population of HIV-negative MSM. Currently, no established guidelines exist for vaccinating men older than 26 years. However, given the potential benefits and economic value of qHPV vaccine as an adjunct prevention strategy, consideration should be given to expanding the guidelines to include this target group.
Supplementary Material
Research highlights.
We examined the benefits of qHPV vaccine for secondary prevention of anal cancer.
We evaluated the cost-effectiveness of qHPV vaccine as part of HGAIN treatment.
Vaccination reduced the lifetime risk of anal cancer by 60.77%.
Vaccination costs US$87,240 per quality-adjusted life year gained.
The results were sensitive to vaccine efficacy and transition of HGAIN to cancer.
Acknowledgments
The authors wish to thank Jagpreet Chhatwal, Ph.D., and Van Thi-Ha Nghiem, M.S.P.H., for analytical suggestions and Melissa Burkett, Luanne Jorewicz, Dawn Chalaire, and Jennifer Gatilao for editorial contributions that enhanced the quality of the manuscript, all from The University of Texas MD Anderson Cancer Center.
Funding: US National Cancer Institute (R01 CA163103), and partially supported by The Janice Davis Gordon Postdoctoral Fellowship in Colorectal Cancer Prevention and the National Institute of Health through MD Andersons’s Cancer Center Support Grant CA016672.
Footnotes
Declaration of interests: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson RA, Levine AM, Bernstein L, Smith DD, Lai LL. Changing patterns of anal canal carcinoma in the United States. J Clin Oncol. 2013;31:1569–75. doi: 10.1200/JCO.2012.45.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 3.Gervaz P, Hahnloser D, Wolff BG, Anderson SA, Cunningham J, Beart RW, Jr, et al. Molecular biology of squamous cell carcinoma of the anus: a comparison of HIV-positive and HIV-negative patients. J Gastrointest Surg. 2004;8:1024–30. doi: 10.1016/j.gassur.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Goldstone SE, Kawalek AZ, Huyett JW. Infrared coagulator: a useful tool for treating anal squamous intraepithelial lesions. Dis Colon Rectum. 2005;48:1042–54. doi: 10.1007/s10350-004-0889-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum. 2011;54:1284–92. doi: 10.1097/DCR.0b013e318227833e. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10:845–52. doi: 10.1016/S1473-3099(10)70219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males. Advisory Committee on Immunization Practices (ACIP), 2011. 2011;60:1705–8. [PubMed] [Google Scholar]
- 9.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–8. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 10.Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross C, Ming Y, Pawel P, Michelle C, Elizabeth C, Jennifer W-C, et al. Baseline data of a phase 3 trial of the quadrivalent HPV vaccine in HIV+ males and females: ACTG 5298. Conference on Retrovirus and Opportunistic Infections (CROI); Boston, Massachusetts. 2014. [Google Scholar]
- 12.Czoski-Murray C, Karnon J, Jones R, Smith K, Kinghorn G. Cost-effectiveness of screening high-risk HIV-positive men who have sex with men (MSM) and HIV-positive women for anal cancer. Health Technol Assess. 2010;14:iii–iv. ix–x, 1–101. doi: 10.3310/hta14530. [DOI] [PubMed] [Google Scholar]
- 13.Critchlow CW, Surawicz CM, Holmes KK, Kuypers J, Daling JR, Hawes SE, et al. Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. AIDS. 1995;9:1255–62. doi: 10.1097/00002030-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Palefsky JM, Holly EA, Hogeboom CJ, Ralston ML, DaCosta MM, Botts R, et al. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:314–9. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Tong WW, Jin F, McHugh LC, Maher T, Sinclair B, Grulich AE, et al. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS. 2013;27:2233–43. doi: 10.1097/QAD.0b013e3283633111. [DOI] [PubMed] [Google Scholar]
- 16.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–83. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 17.Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151:1158–71. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 18.Murphy Sherry L, Xu Jiaquan, Kochanek KD. National Vital Statistics Reports. Hyattsville, MD: U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System; 2013. pp. 1–118. [Google Scholar]
- 19.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500, e1–7. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men. Am J Med. 2000;108:634–41. doi: 10.1016/s0002-9343(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 21.Statistical Abstract of the United States. Consumer price indexes (CPI-U) by major groups: 1990 to 2010. Washington, DC: Bureau of the Census; 2012. [Google Scholar]
- 22.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–7. [PubMed] [Google Scholar]
- 23.Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 24.Koblin BA, Hessol NA, Zauber AG, Taylor PE, Buchbinder SP, Katz MH, et al. Increased incidence of cancer among homosexual men, New York City and San Francisco, 1978–1990. Am J Epidemiol. 1996;144:916–23. doi: 10.1093/oxfordjournals.aje.a008861. [DOI] [PubMed] [Google Scholar]
- 25.US National Cancer Institute. Surveillance, epidemiology, end results (SEER) cancer statistics review. 1992–2010. [Google Scholar]
- 26.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowhani-Rahbar A, Mao C, Hughes JP, Alvarez FB, Bryan JT, Hawes SE, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27:5612–9. doi: 10.1016/j.vaccine.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 29.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–67. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Chin-Hong PV, Husnik M, Cranston RD, Colfax G, Buchbinder S, Da Costa M, et al. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS. 2009;23:1135–42. doi: 10.1097/QAD.0b013e32832b4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute of Health. Broad Spectrum HPV (Human Papillomavirus) Vaccine Study in 16 to 26 Year-Old Women (V503-001 AM2) [Google Scholar]
- 32.Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer. 2013;134:2264–8. doi: 10.1002/ijc.28541. [DOI] [PubMed] [Google Scholar]
- 33.Van de Velde N, Boily MC, Drolet M, Franco EL, Mayrand MH, Kliewer EV, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 34.Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum. 2009;52:239–47. doi: 10.1007/DCR.0b013e31819793d9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.