SUMMARY
In the vertebrate retina, glutamate is traditionally thought to be released only by photoreceptors and bipolar cells to transmit visual signals radially along parallel ON and OFF channels. Lateral interactions in the inner retina are mediated by amacrine cells, which are thought to be inhibitory neurons. Here, we report calcium-dependent glutamate release from vGluT3-expressing amacrine cells (GACs) in the mouse retina. GACs provide an excitatory glutamatergic input to ON-OFF and ON direction-selective ganglion cells and a subpopulation of W3 ganglion cells, but not to starburst amacrine cells. GACs receive excitatory inputs from both ON and OFF channels, generate ON-OFF light responses with a medium-center, wide-surround receptive field structure, and directly regulate ganglion cell activity. The results reveal a functional glutamatergic circuit that mediates non-canonical excitatory interactions in the retina and likely plays a role in generating ON-OFF responses, crossover excitation, and lateral excitation.
INTRODUCTION
Glutamate is known to be released in the retina by photoreceptors (PRs) and bipolar cells (BCs) at ribbon synapses in the outer and inner plexiform layers (OPL and IPL), respectively, to mediate radial (vertical) signal processing along ON and OFF visual pathways. Amacrine cells (ACs), which mediate lateral interactions between the vertical visual channels release either GABA or glycine (Masland, 2012; Wassle et al., 2009). The recent discovery of vesicular glutamate transporter 3 (vGluT3) in a subpopulation of ACs in rat, mouse, and macaque monkey retinae (Fremeau et al., 2002; Gong et al., 2006; Grimes et al., 2011; Haverkamp and Wassle, 2004; Johnson et al., 2004; Stella et al., 2008) raises an intriguing possibility that glutamate may also be released by an AC subtype at conventional (non-ribbon) synapses to mediate excitatory interactions in an unconventional way.
GACs comprise a small subset (~1%) of ACs in the mammalian retina. They exhibit immunoreactivity for vGluT3 and glutamate, as well as glycine and glycine transporter GlyT1 (but not vesicular inhibitory amino acid transporter) (Haverkamp and Wassle, 2004; Johnson et al., 2004). They have a medium dendritic field and ramify diffusely between the ON and OFF cholinergic bands (Grimes et al., 2011; Haverkamp and Wassle, 2004; Johnson et al., 2004), allowing them to interact potentially with both the ON and OFF channels. The possibility that GACs receive and release glutamate in both ON and OFF sublaminae of the IPL is fascinating, because it suggests a novel excitatory circuit that is capable of combining ON and OFF excitation and/or mediating lateral excitation between the ON and OFF channels. However, it is unknown whether GACs actually release glutamate as a transmitter, since vGluT3 is also thought to play a role in creating an intracellular store to buffer cytoplasmic glutamate or to facilitate the vesicular transport of another transmitter (El Mestikawy et al., 2011; Johnson et al., 2004). It is also unclear whether GACs receive excitatory inputs from both ON and OFF BCs (Grimes et al., 2011). In order to gain an insight into the physiological role and synaptic circuitry of the vGluT3 system in the retina, several basic questions must be answered. (1) Do GACs actually release glutamate and use it as a classic neurotransmitter? (2) What are the postsynaptic targets of GACs? (3) What are the response polarity and receptive field properties of GACs? (4) What are the functional effects of GACs on their postsynaptic targets?
The present study addressed above questions using a combination of optogenetics, single and dual patch-clamp recordings, visual stimulation, and two-photon imaging in transgenic mice that had Cre-dependent expression of channel rhodopsin 2 (ChR2) in GACs. We report the functional release of glutamate by GACs, as well as the receptive field properties, postsynaptic targets, and potential postsynaptic actions of GACs.
RESULTS
GACs provide excitatory inputs to specific postsynaptic cell types
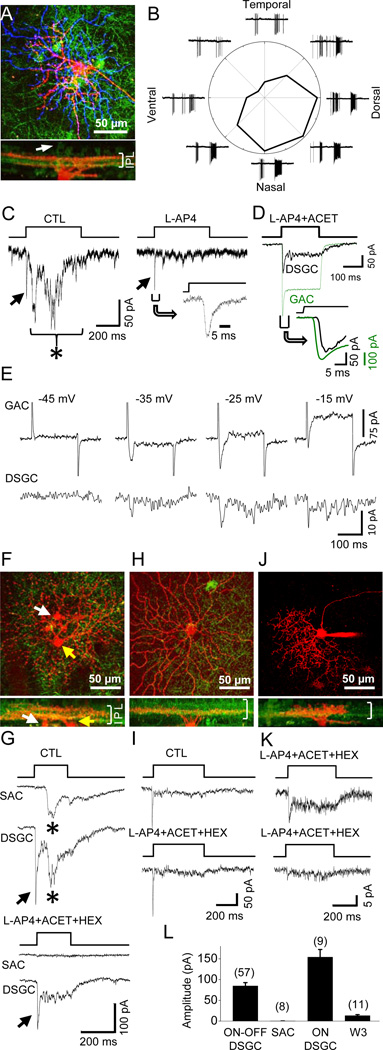
To determine whether GACs make excitatory synapses onto specific postsynaptic neurons, we optically activated ChR2-expressing GACs in wholemount retinae of vGluT3-Cre/ChR2-YFP mice with a flash of intense full-field blue light (referred to as blue light henceforth), while recording from cells in the ganglion cell layer (GCL) under voltage clamp at the chloride equilibrium potential ECl (−70 mV). We found that ON-OFF DSGCs, identified based on dendritic morphology and/or response properties to moving light bars (Fig. 1A, 1B), responded to blue light activation of GACs with a fast inward current (Fig. 1C, arrow), followed, with a delay of 30–50 ms, by a barrage of excitatory synaptic currents (Figure 1C, left, asterisk). The delayed synaptic currents were blocked by the group III metabotropic glutamate receptor agonist L-AP4 (20 µM, which blocks synaptic transmission from PRs to ON BCs (Slaughter and Miller, 1981) (Figure 1C, right), indicating that they were visually driven inputs from ON bipolar cells (the visually driven response, especially the OFF response, was not present in every cell, due to photo bleaching of PRs by repeated blue light flashes). However, the fast inward current response was resistant to L-AP4 (Figure 1C, right, arrow), or L-AP4 + ACET (10 µM, a KA receptor antagonist (Pinheiro et al., 2013) that blocks synaptic transmission from PRs to OFF BCs in mouse (Borghuis et al., 2014)) + hexamethonium (HEX, 300 µM, a nicotinic blocker) (Figure 1G), suggesting it was evoked by an excitatory neurotransmitter released from GACs. The fast response was activated within 4–8 ms of the onset of the blue light (Figure 1C, inset), much earlier than the activation of BC-mediated visual responses (40–60 ms after light onset). Compared to the blue light-evoked ChR2 current recorded from GACs in the presence of L-AP4 + ACET (Figure 1D, green trace, peak amplitude at −70 mV: 232 ± 22 pA, n =13), the onset of the fast synaptic current in the ON-OFF DSGC was delayed by <2 ms (n=5) (Figure 1D inset), consistent with a monosynaptic response to an excitatory transmitter released by GACs. Similar responses were found from 57 out of 66 morphologically/physiologically confirmed On-OFF DSGCs.
Figure 1. Excitatory synaptic transmission from GACs to specific postsynaptic targets.
(A) Upper: collapsed two-photon images of ON (red) and OFF dendrites (blue) of an ON-OFF DSGC after whole-cell recording in a vGluT3-Cre/ChR2-YFP (green) retina. Lower: z-projection image of the same cell. Arrow: an example of ChR2-YFP-expressing GAC (green). (B) Loose-patch responses of the cell in (A) to a light bar (500 µm × 100 µm) moving at 500 µm/s in 8 directions. (C) Voltage-clamp responses (at −70 mV) of the cell in (A) to blue light flashes, showing a fast GAC-activated postsynaptic current (left, arrow), followed by a delayed BC-mediated visual response (left, asterisk). Inset: expanded view of the fast, L-AP4-resistant current. (D) Overlay of the ChR2-current in a GAC (green, at −70 mV) and the fast postsynaptic current in an ON-OFF DSGC (black, −70 mV) in response to a blue light flash. Inset: expanded view, showing a ~2-ms onset delay between the ChR2 current and the postsynaptic response. (E) Dual recording from a pair of GAC and ON-OFF DSGC in the presence of L-AP4, ACET and HEX, showing voltage-gated currents in GAC in response to depolarizing steps from −70 mV to −45, −35, −25, and −15 mV (upper traces, with the Na+ channel blocker QX 314 in pipette solution) and inward postsynaptic currents in the ON-OFF DSGC at −70 mV (lower traces). (F, G) Simultaneous recording (at −70 mV) from a pair of ON SAC (F, white arrow) and ON-OFF DSGC (F, yellow arrow), showing delayed, L-AP4-ACET-HEX-sensitive currents in both ON SAC and ON-OFF DSGCa (G, asterisks), and a fast, L-AP4-ACET-HEX-resistant current only in the ON-OFF DSGC, but not the ON SAC (G, arrow). The reduction in the fast response amplitude after drug application, seen in this particular case, was likely due to response rundown after a long recording period, but not to pharmacological blockade (see text related to Figure 2). (H, I) An ON DSGC in response to blue light flashes under voltage clamp at −70 mV, showing a fast synaptic response that is resistant to L-AP4 + ACET + HEX. (J) A putative W3 cell (upper, shown only in red channel), with narrow, diffuse dendritic ramification in the middle of IPL (lower, both red and green channels shown). (K) Responses (at −70 mV) of two putative W3 cells from two different retinae to blue light flashes, showing a small peak response and a sustained component. (L) Summary of the peak current response amplitudes (at −70 mV) of different cell types to blue light flashes in the presence of L-AP4 + ACET + HEX. Numbers in parentheses, cells tested. Error bars, SEM. Drug concentrations (in µM): ACET, 10; HEX, 300; and L-AP4, 20. See also Figure S1.
As previously reported (Grimes et al., 2011), we did not detect any ChR2-YFP expression in BCs or PRs in this mouse line (Figure S1), but some cells in the GCL are ChR2-YFP-positive. To exclude the possibility of a blue light-evoked glutamate input from axon collaterals of ChR2-YFP-expressing GCs or from BCs that express a low (microscopically undetectable) level of ChR2-GFP, we made dual patch-clamp recordings from pairs of GACs and ON-OFF DSGCs in the whole-mount retina (Figure 1E, Figure S1) in the presence of L-AP4, ACET, and HEX. Depolarizing a GAC with voltage steps activated Ca2+ currents in the GAC (Figure 1E, upper traces) and, correspondingly, inward excitatory postsynaptic currents in an overlapping ON-OFF DSGC at −70 mV (Figure 1E, lower traces). Similar responses were obtained from three successfully recorded pairs of GACs and ON-OFF DSGCs (Figure S1), demonstrating the presence of functional excitatory synapses between individual GACs and ON-OFF DSGCs (see Discussion).
In addition to ON-OFF DSGCs, we also found similar postsynaptic responses to optical activation of GACs from ON DSGCs (Figure 1H). These responses also persisted in the presence of L-AP4 + ACET + HEX (Figure 1I, n=9), suggesting a direct excitatory input from GACs to ON DSGCs. In contrast, we did not detect any response from SACs (7 ON SACs and 1 OFF SAC) to optical activation of GACs in the presence of L-AP4 + ACET + HEX (Figure 1F, 1G). Because ON SAC dendrites closely co-fasciculate with those of ON and ON-OFF DSGCs, this result suggests that GACs make selective excitatory synaptic contacts with specific postsynaptic targets.
We also tested the response of small-soma GCs with dendritic morphologies resembling those of W3 cells (Kim et al., 2010; Zhang et al., 2012) to optical activation of GACs (Figure 1J). Although W3 cells ramify diffusely and overlap with GAC processes in the band between the ON and OFF cholinergic strata in the IPL (Figure 1J) (Kim et al., 2010), we detected only small inward currents (mean peak amplitude at −70 mV: 13.4 ± 8.3 pA, n=11) in 11 out of 20 putative W3 cells tested in the presence of L-AP4 + ACET + HEX (Figure 1K), with the remaining 9 cells showing no detectable response. We also found that optical activation of GACs evoked fast inward currents in a small number of other types of bistratified and monostratified GCs (including OFF GCs) in the presence of L-AP4 (data not shown), but more extensive studies are required to identify and characterize these cells.
Ca2+-dependent excitatory glutamatergic transmission from GACs to ON-OFF and ON DSGCs
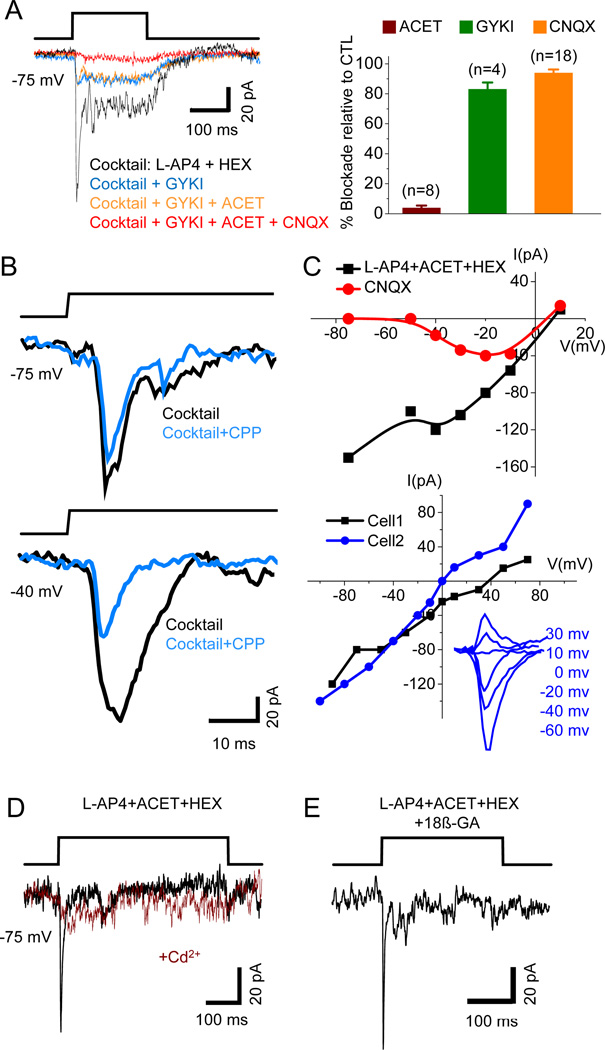
The blue light-evoked responses in ON-OFF DSGCs (at −70 mV in the presence of L-AP4 and HEX) were largely blocked by the AMPA receptor antagonist GYKI52466 (20 µM, 83.0 ± 4.5% blockade, n=4), or the AMPA/KA receptor antagonist CNQX (40 µM, 93.9 ± 2.4% blockade, n=18), but was little affected by the KA receptor-selective antagonist ACET (10 µM, 5.4 ± 1.9% blockade, n=10) (Figure 2A). L-AP4 or HEX alone did not have a significant effect on the early fast response to blue light (0.4 ± 5.5% blockade, n=5, or 1.8 ± 13.7% blockade, n=7, respectively). Application of the muscarinic receptor blocker, atropine (ATRO, 2 µM) also did not have any effect (0.7 ± 4.4% blockade, n=3). At more depolarized membrane potentials (e.g., −40 mV), a CPP (20 µM)-sensitive NMDA component was also detected (Figure 2B), suggesting that both AMPA and NMDA receptors mediate the glutamatergic synaptic transmission from GACs to ON-OFF DSGCs. The current-voltage (I–V) relation of the peak response showed a J-shaped region, presumably due to voltage-dependent Mg2+ block of NMDA currents (Figure 2C, upper, black curve); and, in the presence of CNQX (40 µM), a nonlinear NMDA component was clearly resolvable (Figure 2C, upper, red curve). In the presence of CPP, L-AP4, HEX, SR95531 (50 µM), and strychnine (1 µM), the I–V relation was nearly linear at negative potentials, but many ON-OFF DSGCs showed a more positive reversal potential than 0 mV (~ECation) or an inward rectification at positive membrane potentials (Figure 2C, lower, Cell 1), possibly due to inadequate voltage clamp at more depolarized potentials. Together, the above results demonstrate functional glutamatergic synaptic transmission from GACs to ON-OFF DSGCs, mediated by postsynaptic AMPA/NMDA receptors.
Figure 2. Properties of the glutamatergic transmission from GACs to ON-OFF DSGCs.
(A) Left, effects of GYKI52466 (GYKI), ACET and CNQX on the response of an ON-OFF DSGC to blue light flashes recorded at −75 mV in the presence of a control cocktail of L-AP4 and HEX. Right, statistical summary of the effects. Numbers in parentheses, cells tested. Error bars, SEM. (B) Voltage-dependent block of the NMDA component of blue light-evoked responses by CPP in an ON-OFF DSGC. (C) Upper: I–V relations of blue light-evoked responses in an ON-OFF DSGC in the presence of L-AP4, ACET, and HEX before (black) and after (red) the addition of CNQX. Lower: I–V relations of AMPA receptor-mediated responses of two other ON-OFF DSGCs to blue light flashes in the presence of CPP, L-AP4, HEX, ACET, SR95531, and strychnine. (D) CdCl2 blocked the response of an ON-OFF DSGC to blue light stimulation in the presence of L-AP4, ACET, and HEX. (E) A blue light-evoked response in an ON-OFF DSGC remained intact after 25-min incubation in 18β-GA and L-AP4, ACET, and HEX. Drug concentrations (in µM): ACET, 10; CdCl2, 300; CNQX, 40; CPP, 20; GYKI, 20; HEX, 300; L-AP4, 20; SR95531, 50; strychnine, 1; 18β-GA, 25. See also Figure S2.
Because of the relatively low frequency of encountering ON DSGCs in our sample, we only tested a few essential pharmacological agents to confirm the glutamatergic nature of the excitatory transmission from GACs to ON DSGCs. The response of ON DSGCs to optical activation of GACs was resistant to L-AP4 + ACET + HEX (n=5, Figure 1H, 1I), but completely blocked by CNQX (n=4) at −70 mV, and partially blocked by CPP (29.8 ± 8.7% blockade of the peak current, n=3) at −40 mV, indicating fast excitatory glutamatergic synaptic transmission mediated by both non-NMDA and NMDA receptors.
Because ChR2-YFP is also expressed in some cells in the GCL (Figure S1), we only recorded from GCs that did not show any YPF fluorescence. Indeed, ChR2 current was never detected in our recorded GCs because the blue light-evoked response was consistently blocked by glutamate receptor antagonists (Figure 2A). To confirm that blue-light-evoked responses in DSGCs were mediated by Ca2+-dependent vesicular transmitter release, but not by direct gap-junction coupling between the recorded DSGCs and ChR2-YFP-expressing cells, we showed that the Ca2+ channel blocker, CdCl2 (300 µM), blocked the responses of DSGCs (4 ON-OFF DSGCs, 1 ON DSGC) to blue light stimulation in the presence of L-AP4, ACET and HEX (Figure 2D). Incubating the retina with the gap junction blocker 18β-GA (25 µM) for 15–45 min also did not block the response of ON-OFF DSGCs to blue-light activation of GACs (Figure 2E, n=6), arguing against a gap-junction-mediated current. In contrast, 15-min incubation of 25 µM 18β-GA effectively blocked gap-junction-coupled spikelets in superior-preferring ON-OFF DSGCs (n=4, Figure S2) as previously reported (Trenholm et al., 2013; Xu et al., 2013).
GACs generate ON-OFF light responses, with a medium-center, wide-surround receptive field structure
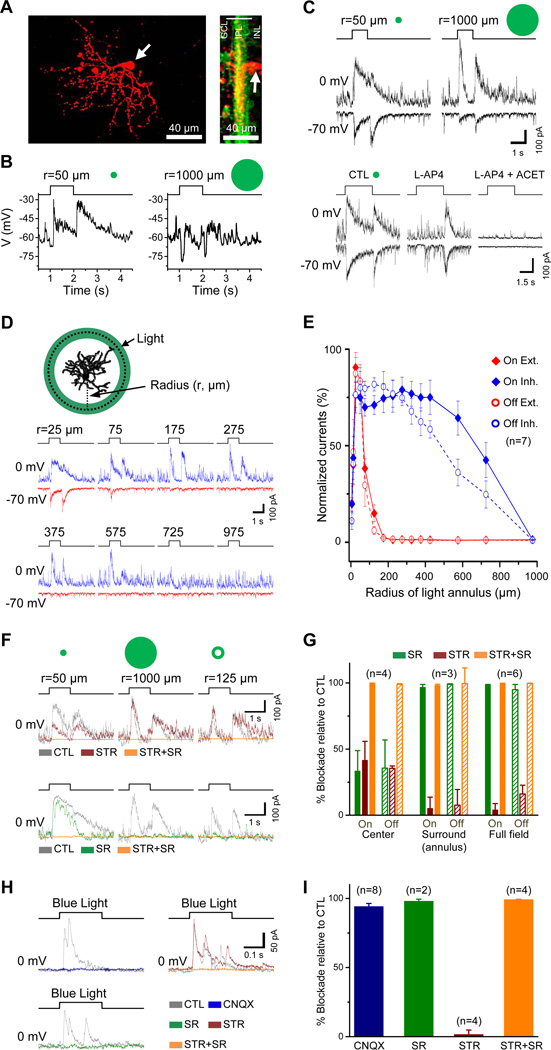
Consistent with the dendritic stratification of GACs in both sublamina a and b (Figure 3A), whole-cell current-clamp recording from GACs in the light-adapted whole-mount retina (see Experimental Procedures) showed a fast depolarization in response to both the onset and offset of a 50-µm-radius light spot at the receptive center (Figure 3B, left, n=5). However, when stimulated with a large spot (1000 µm in radius), the same GACs responded with a hyperpolarization, rather than a depolarization, at both light onset and offset (Figure 3B right, n=5), suggesting that GACs are strongly inhibited by the receptive field surround. Voltage-clamp recordings further demonstrated that GACs received excitatory and inhibitory synaptic inputs at both light onset and offset (Figure 3C). When the light spot size increased from 50 to 1000 µm in radius, the excitatory inputs became greatly reduced, while the inhibitory inputs became stronger and more transient (Figure 3C), suggesting a concerted presynaptic feedback suppression of the BC excitatory output and a postsynaptic feedforward inhibition of the GACs by the surround. L-AP4 blocked both the excitatory and inhibitory inputs at the center light spot onset, but not at the offset. Subsequent application of ACET on top of L-AP4 eliminated the excitatory and inhibitory inputs at the light offset (Figure 3C), suggesting that GACs received excitatory and inhibitory inputs from both the ON and OFF channels. When stimulated with a light ring of expanding radius (Figure 3D), GACs showed a medium excitatory receptive-field center of ~100 µm in radius and a wide inhibitory surround of up to 1000 µm in radius (Figure 3E). While the spatial profile (response amplitude vs. light annulus radius) of the excitatory input was similar between ON and OFF responses, the spatial profile of the inhibitory input was slightly broader for ON than for OFF responses (Figure 3E), suggesting a relatively stronger wide-field inhibition in the ON input than the OFF.
Figure 3. Receptive-field properties of GACs.
(A) Whole-mount (left) and cross-sectional (right) views of a GAC (red) after whole-cell recording in a vGluT3Cre/ChR2-YFP (green) mouse retina. White arrows, GAC soma. (B) Responses of a GAC to 1-s-long flashes of light spots under current clamp, showing depolarizing ON-OFF responses to a small spot (50 µm in radius, left) and hyperpolarizing ON-OFF responses to a large spot (1000 µm in radius, right). (C) Upper: responses of another GAC under voltage clamp to 1-s light flashes of two different sizes (50 and 1000 µm in radius, centered on the soma), showing inward ON-OFF EPSC (at −70 mV) and outward ON-OFF IPSC (at 0 mV). Lower: responses of the same GAC to 3-s flashes of a 50-µm-radius light spot, showing selective blockade of ON responses by L-AP4 and of both ON and OFF responses by L-AP4 and ACET. (D) Responses of a GAC to 1-s flashes of light annuli (50 µm in thickness), showing EPSCs (red, −70 mV) and IPSCs (blue, 0 mV) evoked by light annuli of various radii. (E) Normalized peak EPSCs and IPSCs as a function of annulus radius (n=7 GACs). Thickness of the annuli was 50 µm, except the smallest two annuli, which were 10 and 25 µm, respectively. (F) Effects of STR and SR (applied in two different sequences) on IPSCs evoked by a center light spot (50 µm in radius, left), a full-field light spot (1000 µm in radius, center), and a surround light annulus (125 µm in radius, right). (G) Summary of effects of SR and STR on ON-OFF IPSCs evoked by center, surround, and full-field light spot shown in (F). (H) Effects of CNQX, SR, and STR on inhibitory currents (at 0 mV) evoked by blue light stimulation of ChR2-expressing GACs in the presence of L-AP4, ACET, HEX, and atropine. (I) Summary of effects in (H), indicating that blue light evoked glutamate release from GACs onto unidentified amacrine cells, which, in turn, were induced to release GABA onto GACs. Numbers in parentheses, cells tested. Error bars, SEM. Drug concentrations (µM): ACET, 10; atropine, 2; CNQX, 40; HEX, 300; L-AP4, 20; SR, 50; STR, 2. See also Figure S3.
The inhibitory input from the surround was predominantly mediated by GABAA receptors, which was blocked completely by SR95531 (50 µM, n=3, Figure 3F,G). However, the inhibitory input at the center could be blocked completely only by a combination of SR95531 (25 µM) and strychnine (1 µM) (Figure 3F, n=4), suggesting that the center inhibitory input to the GAC was mediated by both glycinergic and GABAergic amacrine cells. When a light bar moved across the receptive field in different directions, GACs gave a depolarizing response to both the leading and the trailing edge of the light bar, but the somatic depolarization showed no detectable directional selectivity (n=5, Figure S3).
Voltage-clamp recording from GACs (at 0 mV) in the presence of L-AP4 + ACET + HEX showed that blue-light evoked outward inhibitory currents in GACs themselves, which could be completely blocked by either SR95531 (50 µM) or CNQX (40 µM), but not by strychnine (2 µM) (Figure 3H, I). Since BCs were already inhibited by L-AP4 and ACET, this result suggests that GACs provided an excitatory input to other amacrine cells which, in turn, inhibited GACs via GABAergic synapses. Thus, GACs also make glutamatergic synapses onto certain amacrine cells. Because CNQX blocked all blue light-evoked outward current responses of GACs at 0 mV (Figure 3H), this result also suggests that GACs do not make direct inhibitory synapses onto each other even if they should release an inhibitory transmitter (e.g., glycine) in addition to glutamate. Due to the presence of a large ChR2 current in GACs (at −70 mV) during blue light stimulation (Figure 1D), we did not test the possibility that neighboring GACs directly excite each other via glutamatergic synapses.
GACs directly influence the spike activity of DSGCs
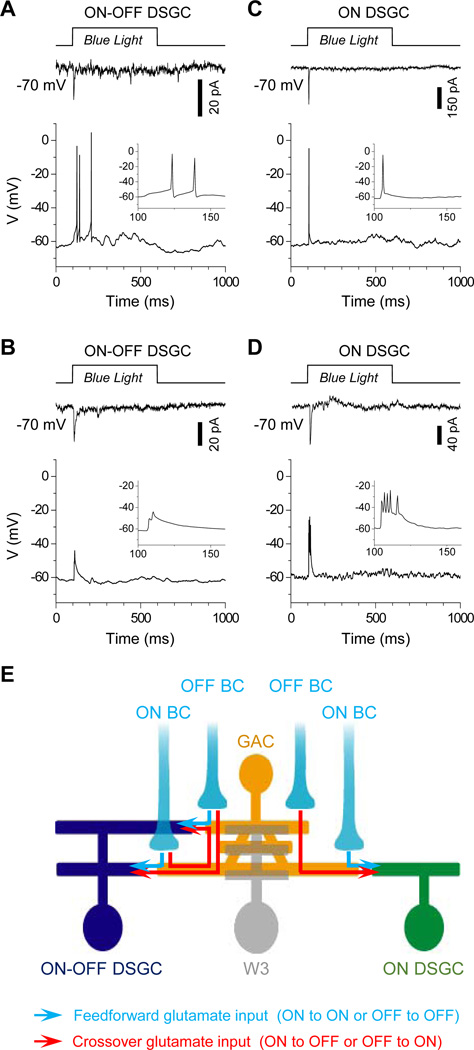
To determine the impact of GACs on DSGC function, we recorded the membrane potential of ON-OFF and ON DSGCs under whole-cell current clamp (with a K+-based pipette solution, see Experimental Procedures), while activating GACs with blue light. With the light-driven BC output blocked with L-AP4 and ACET (and nicotinic transmission blocked by HEX as a precaution), optical activation of GACs was sufficient to evoke transient spikes from 4 out of the 9 ON-OFF and 2 out of the 3 ON DSGCs tested (Figure 4A, 4C). The remaining 5 ON-OFF DSGCs and 1 ON DSGC gave either a sub-threshold depolarization or small spikes (Figure 4B, 4D) that resembled the dendritic spikes previously reported (Oesch et al., 2005). To confirm that the blue light-evoked responses in GACs were within the physiological range, we compared the voltage response of GACs to full-field blue light with that to visual stimulation (center white light spot, without L-AP4 and ACET). The amplitude of the voltage responses was comparable between these two stimulation conditions (n=7, Figure S4), indicating that GACs provide a physiologically relevant excitatory drive to DSGCs.
Figure 4. GACs directly influence DSGC spike patterns.
Responses of ON-OFF (A, B) and ON DSGCs (C, D) under voltage-(−70 mV, top) and current-clamp (0 pA, bottom) to blue light stimulation in the presence of L-AP4, ACET and HEX, showing examples of transient somatic spikes (A, C) and small (dendritic-like) spikes (B, D). Insets: blown-up view of the voltage responses. (E) Model of the GAC circuit, showing GACs receiving glutamatergic inputs from ON and OFF BCs and sending glutamatergic outputs to ON-OFF and ON DSGCs to mediate feedforward or lateral excitation (blue arrows) and crossover excitations (red arrows). GACs also provide a small excitatory input to a subset of W3 cells (gray). Not shown in the scheme are the strong pre- and postsynaptic inhibition of GACs from the surround and GAC outputs to other unidentified ACs and GCs. See also Figure S4.
Finally, we also investigated whether GACs provide a direct glycinergic drive to ON-OFF DSGCs. In response to blue light in the presence of LAP4 + ACET + CNQX + CPP + HEX, 9 out of the 11 ON-OFF DSGCs tested at 0 mV showed no detectable outward current, suggesting a lack of direct glycinergic or GABAergic synaptic input (Figure S4). The remaining 2 of the 11 cells displayed small (<15pA), strychnine-sensitive outward currents (Figure S4), but it is unclear whether these small currents were evoked by GACs or potentially by ChR2-expressing ACs in the GCL. Overall, these results indicate a lack of significant (if any) glycinergic synaptic transmission from GACs to DSGCs, consistent with our paired recordings from GACs and ON-OFF DSGCs, which failed to detect any outward current responses from DSGCs at 0 mV (n=3). However, it remains to be determined whether GACs release glycine onto other cell types.
DISCUSSION
A non-canonical glutamatergic circuit in the inner retina
The present study demonstrated functional glutamate release from GACs. We identified ON and ON-OFF DSGCs as postsynaptic targets of GACs. Because our dual patch clamp recordings found only small synaptic currents (Figure 1E, Figure S1D, S1E), it seems that each GAC makes only a small number of glutamatergic synapses onto an ON-OFF DSGC and that a DSGC integrates inputs from a number of GACs to generate a sizable whole-cell response. Notably, even though most W3 dendrites ramify in the same diffuse band in the IPL as do GAC dendrites (Figure 1J), only a subpopulation (or subtype) of W3 GCs responded to GAC activation, and their responses to full-field blue light activation were much smaller than those of ON-OFF and ON DSGCs (Figure 1K). In addition to DSGCs, a few other bistratified and monostratified (including OFF monostratified) GCs also gave excitatory postsynaptic responses (data not shown) to optical activation of GACs in the presence of L-AP4, though most other GC types recorded were not responsive to GAC activation. As indicated by the data shown in Figure 3H, some ACs may also receive glutamate input from GACs. However, GACs apparently avoid making glutamatergic synapses onto SACs, even though SACs and DSGCs co-fasciculate closely. Thus, GACs are expected to influence multiple, but specific, postsynaptic cell types.
The receptive field structure and potential functional role of GACs
We showed that GACs receive excitatory inputs from both ON and OFF BCs and generate ON-OFF depolarization to small center flashes. They also receive strong surround inhibition and generate ON-OFF hyperpolarization to large/full-field flashes. The ON-OFF center response implies that GACs can integrate excitatory inputs from separate ON and OFF channels into an ON-OFF glutamatergic output at individual output synapses. This glutamatergic synaptic organization contrasts the current dogma that ON and OFF channels are segregated in the IPL, and that ON and OFF channels typically inhibit each other through diffuse, narrow-field glycinergic amacrine cells (crossover inhibition) (Werblin, 2010), but do not excite each other through excitatory synapses. We propose that GACs provide a mechanism for “crossover excitation”, in which ON channel excites the OFF channel and vice versa (Fig. 4E, red arrows). This circuit can potentially enhance the ON-OFF response of bi-stratified or diffusely stratified cells, such as ON-OFF DSGCs, by coordinating/synergizing the responses between the ON and OFF dendrites, thus providing an additional level of processing between ON and OFF channels. There is currently no evidence that GACs interfere with directional computation of ON-OFF DSGCs, since the glutamatergic input from GACs is relatively small compared to the input from BCs and is likely counter-balanced by a strong asymmetric GABAergic input from SACs during null direction movement, in analogy to the situation in which the cholinergic input to a DSGC is rendered ineffective during null direction movement (Lee et al., 2010). The somatic responses of GACs suggest that these cells are not direction-selective, though we cannot rule out the possibility of directional glutamate release at GAC dendrites or directional connectivity with DSGC dendrites.
Notably, even some monostratified cells, such as ON DSGCs, also receive ON-OFF glutamate signals from GACs. Although most monostratified GCs are so far not known to generate ON-OFF center responses, ON DSGCs (including those that do not have ectopic OFF dendritic branches) in the mouse retina have been reported to receive L-AP4-resistant OFF excitation (Ackert et al., 2009; Farajian et al., 2011; He et al., 2005; Sun et al., 2006). The functional role of this OFF excitatory input is currently unclear, but it is known that the OFF center responses in ON GCs (and vice versa) are often masked by GABAergic inhibition (Farajian et al., 2011), which may be attributable, in part, to the receptive field properties of GACs (Figure 3). It has been recently reported that many OFF GCs give ON responses under certain adaptation conditions (Munch and Tikidji-Hamburyan, 2014). These anomalous responses, while difficult to explain by the canonical glutamatergic circuits, seem compatible with the GAC circuit. Finally, GACs may also play a role in lateral excitation, especially in predicting local lateral movement (Berry et al., 1999) and in synchronizing responses among GCs over a medium spatial range (Brivanlou et al., 1998; Mastronarde, 1983; Meister et al., 1995; Schnitzer and Meister, 2003).
EXPERIMENTAL PROCEDURES
vGluT3-Cre/ChR2-YFP mice (4–10 weeks old) were generated by crossbreeding vGlut3-Cre mice (Grimes et al., 2011) with ChR2-YFP mice (strain B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J, Jackson Laboratory, Bar Harbor, ME). All animal procedures were approved by Yale University IACUC. Patch-clamp recordings were made in the wholemount retina in oxygenated ACSF (composition in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 0.5 L-glutamine, 26 NaHCO3, and 20 glucose, pH7.4) at 32–34°C. Pipette solution contained (in mM) for loose patch: ACSF; for voltage clamp: 105 CsMeSO4, 0.5 CaCl2, 10 HEPES, 5 EGTA, 5 Na2-phosphocreatine, 2 ATP-Mg, 0.5 GTP-2Na, 2 ascorbic acid, 8 QX314-Cl, pH 7.2, with 20–30 CsOH; and for current clamp: 105 potassium gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 10 HEPES, 5 Na2-phosphocreatine, 2 ATP-2Na, 0.5 GTP-2Na, 2 ascorbic acid, pH 7.2 with 5 NaOH and 15 KOH. Recorded cells were filled with Alexa Fluor 594 and imaged under a two-photon microscope ChR2 was activated by full-field flashes of blue light (λpeak, 460–470 nm; intensity at retina: 2–5.5 ×1010 photons µm−2 s−1). GACs were identified under brief (2–10 s each time, 2–5 times in total) epi-fluorescence illumination (1010 photons µm−2s−1 at retina, 465 ± 15 nm in wavelength) and recorded under white light (trans-illumination, 105 photons µm−2s−1 at retina). Stable light responses were obtained from GACs after 1–5 min of dark adaptation following the establishment of a patch clamp configuration. Visual stimulus patterns were generated on a black-and-white LCD (contrast: 100:1; maximum image intensity at retina: ~2×105 photons µm−2s−1) and projected to the retina via the microscope condenser lens. Drugs were applied at the same concentrations as first described in the text. Results were expressed as mean ± SEM. Statistical significance was determined at the level of α=0.05 by paired Student’s t-test (Figure S4B). See additional information in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH R01EY17353 (ZJZ), NIH T32 EY22312 (ZJZ and MY), and Research to Prevent Blindness (RPB) Inc. Unrestricted Grant to Yale Eye Center, and by the Marvin L. Sears Endowed Professorship (ZJZ). We thank Dr. Shigang He for scientific discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackert JM, Farajian R, Volgyi B, Bloomfield SA. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J Physiol. 2009;587:4481–4495. doi: 10.1113/jphysiol.2009.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, 2nd, Brivanlou IH, Jordan TA, Meister M. Anticipation of moving stimuli by the retina. Nature. 1999;398:334–338. doi: 10.1038/18678. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Looger LL, Tomita S, Demb JB. Kainate Receptors Mediate Signaling in Both Transient and Sustained OFF Bipolar Cell Pathways in Mouse Retina. J Neurosci. 2014;34:6128–6139. doi: 10.1523/JNEUROSCI.4941-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20:527–539. doi: 10.1016/s0896-6273(00)80992-7. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Farajian R, Pan F, Akopian A, Volgyi B, Bloomfield SA. Masked excitatory crosstalk between the ON and OFF visual pathways in the mammalian retina. J Physiol. 2011;589:4473–4489. doi: 10.1113/jphysiol.2011.213371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Jellali A, Mutterer J, Sahel JA, Rendon A, Picaud S. Distribution of vesicular glutamate transporters in rat and human retina. Brain Res. 2006;1082:73–85. doi: 10.1016/j.brainres.2006.01.111. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Seal RP, Oesch N, Edwards RH, Diamond JS. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis Neurosci. 2011;28:381–392. doi: 10.1017/S0952523811000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468:251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- He S, Sun W, Deng Q. ON Direction-Selective Ganglion Cells in the Mouse Retina. Invest Ophthalmol Vis Sci. 2005;46:2335. [Google Scholar]
- Johnson J, Sherry DM, Liu X, Fremeau RT, Jr, Seal RP, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477:386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA Cotransmission in Detecting Image Motion and Motion Direction. Neuron. 2010;68:1159–1172. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983;49:303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Munch TA, Tikidji-Hamburyan A. The output of the retina qualitatively changes at different light levels. ARVO Meeting Abstracts. 2014;55:5008. [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–750. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crepel V, Perrais D, Mulle C. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cerebral cortex. 2013;23:323–331. doi: 10.1093/cercor/bhs022. [DOI] [PubMed] [Google Scholar]
- Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003;37:499–511. doi: 10.1016/s0896-6273(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Li S, Sabatini A, Vila A, Brecha NC. Comparison of the ontogeny of the vesicular glutamate transporter 3 (VGLUT3) with VGLUT1 and VGLUT2 in the rat retina. Brain Res. 2008;1215:20–29. doi: 10.1016/j.brainres.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Deng Q, Levick WR, He S. ON direction-selective ganglion cells in the mouse retina. J Physiol. 2006;576:197–202. doi: 10.1113/jphysiol.2006.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Awatramani GB. Dynamic tuning of electrical and chemical synaptic transmission in a network of motion coding retinal neurons. J Neurosci. 2013;33:14927–14938. doi: 10.1523/JNEUROSCI.0808-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zeng Q, Shi X, He S. Changing coupling pattern of The ON-OFF direction-selective ganglion cells in early postnatal mouse retina. Neuroscience. 2013;250:798–808. doi: 10.1016/j.neuroscience.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci U S A. 2012;109:E2391–E2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.