Abstract
The infections with herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) have been prevalent since the ancient Greek times. To this day, they still affect a staggering number of over a half billion individuals worldwide. HSV-2 infections cause painful genital herpes, encephalitis, and death in newborns. HSV-1 infections are more prevalent than HSV-2 infections and cause potentially blinding ocular herpes, oro-facial herpes and encephalitis. While genital herpes in mainly caused by HSV-2 infections, in recent years, there is an increase in the proportion of genital herpes caused by HSV-1 infections in young adults, which reach 50% in some western societies. While prophylactic and therapeutic HSV vaccines remain urgently needed for centuries their development has been notoriously difficult. During the most recent National Institute of Health (NIH) workshop titled "Next Generation Herpes Simplex Virus Vaccines: The Challenges and Opportunities", basic researchers, funding agencies, and pharmaceutical representatives gathered: (i) to assess the status of herpes vaccine research; and (ii) to identify the gaps and propose alternative approaches in developing a safe and efficient herpes vaccine. One “common denominator” among previously failed clinical herpes vaccine trials is that they either used a whole virus or whole viral proteins, which contain both pathogenic “symptomatic” and protective “asymptomatic” antigens/epitopes. In this report, we continue to advocate that using an “asymptomatic” epitope-based vaccine strategy that selectively incorporates protective epitopes which: (i) are exclusively recognized, in vitro, by effector memory CD4+ and CD8+ TEM cells from “naturally” protected seropositive asymptomatic individuals; and (ii) protect, in vivo, human leukocyte antigen (HLA) transgenic animal models from ocular and genital herpes infections and diseases, could be the answer to many of the scientific challenges facing HSV vaccine development. We review the role of animal models in herpes vaccine development and discuss its current status, challenges, and prospects.
Keywords: Herpes simplex virus, clinical trials, vaccines, immunotherapeutic, symptomatic, asymptomatic, epitopes
INTRODUCTION
In the current era of effective drug therapies, many of the maladies that struck down our ancestors have been eliminated. However, herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) infections, which have been prevalent since the ancient Greek times, still affect a staggering number of the world's population to this day [1]. Over a half billion individuals, between fourteen and forty-nine years of age, around the world are clinically affected by HSV-2 [1]. The sub-Saharan African populations are most dramatically afflicted, with up to 50% of women and 40% of men in some regions suffering from genital herpes (NHANES-2005–2010). HSV-1 & HSV-2 infections cause a wide range of diseases throughout human life [1–9] (Fig. 1). Globally, HSV-1 is much more prevalent than HSV-2 (CDC), causing significant morbidity especially among young adults in western societies, where up to 63% are sero-positive. Genital herpes is one of the most common sexually transmitted infections, with a higher prevalence in women than men. Recent immuno-epidemiological evidence suggest that: (i) there is an increasing proportion of genital herpes cases associated with HSV-1 and (ii) the majority of infected individuals exhibit frequent and brief shedding episodes that are most often asymptomatic [10] and Fig. 2A. This non-apparent shedding likely contributes to high HSV transmission rates [10]. In the United States, there are 500,000 cases/year of oral herpes caused by HSV-1, 300,000 cases/year of genital herpes caused by HSV-1 and/or HSV-2, 20,000 cases/year of ocular herpes caused by HSV-1 and 1500 cases/year of herpes encephalitis [11, 12]. HSV-2, but not HSV-1, appears to be linked with a two- to three-fold increase of risk of HIV-1 acquisition [1]. In addition to causing painful blisters, HSV-2 can cause encephalitis and death in newborns from vertical transmission [1].
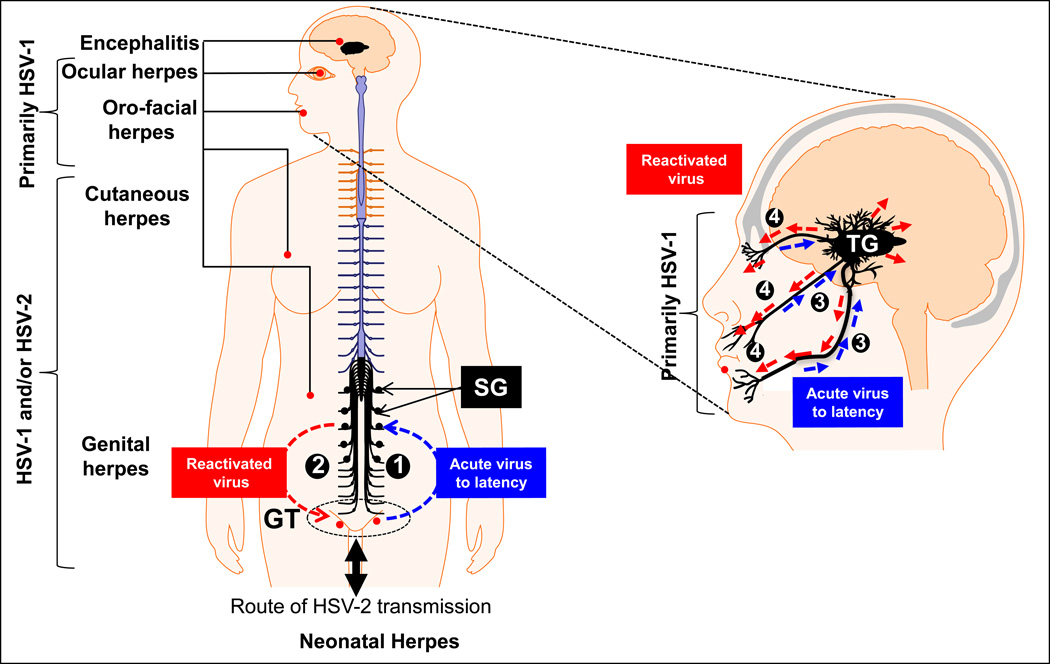
Figure 1.
The natural history of genital (left) and oro-facial (right) herpes infection. HSV-1 and HSV-2 are transmitted by close interpersonal contact (such as during intravaginal/oral sex, during birth or eye contact), and preferentially infects muco-cutaneous epithelium around the genital tract (GT), around the lips (cold sores), nose and eyes. Right : While most of genital herpes is caused by HSV-2 reports of HSV-1 genital infection are increasing. HSV-2 infections is a major public health problem. (1) The virus replicates in the TG and then travels along nerves to the sacral ganglia (SG) that control the GT, where it establishes a latent infection. (2) Recurrent genital herpes is the most prevalent sexually transmitted disease. Left : (1) Ocular herpes is mainly caused by HSV-1, which infects the cornea and then establishes latency in sensory neurons of the trigeminal ganglia (TG). (2) Sporadic spontaneous reactivation of HSV-1 from latently infected neurons leads to viral shedding in saliva and tears which can cause symptomatic recurrent Herpes Stromal Keratitis (HSK), a blinding corneal disease.
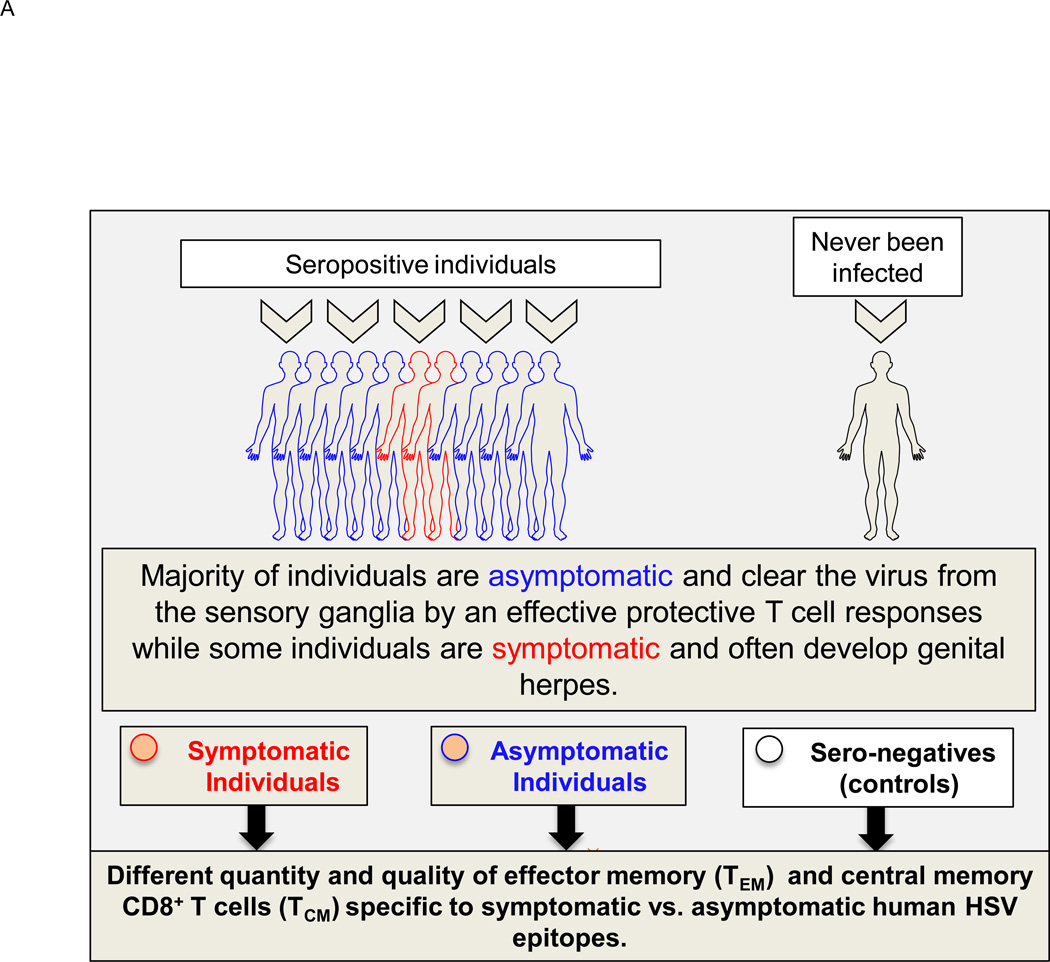
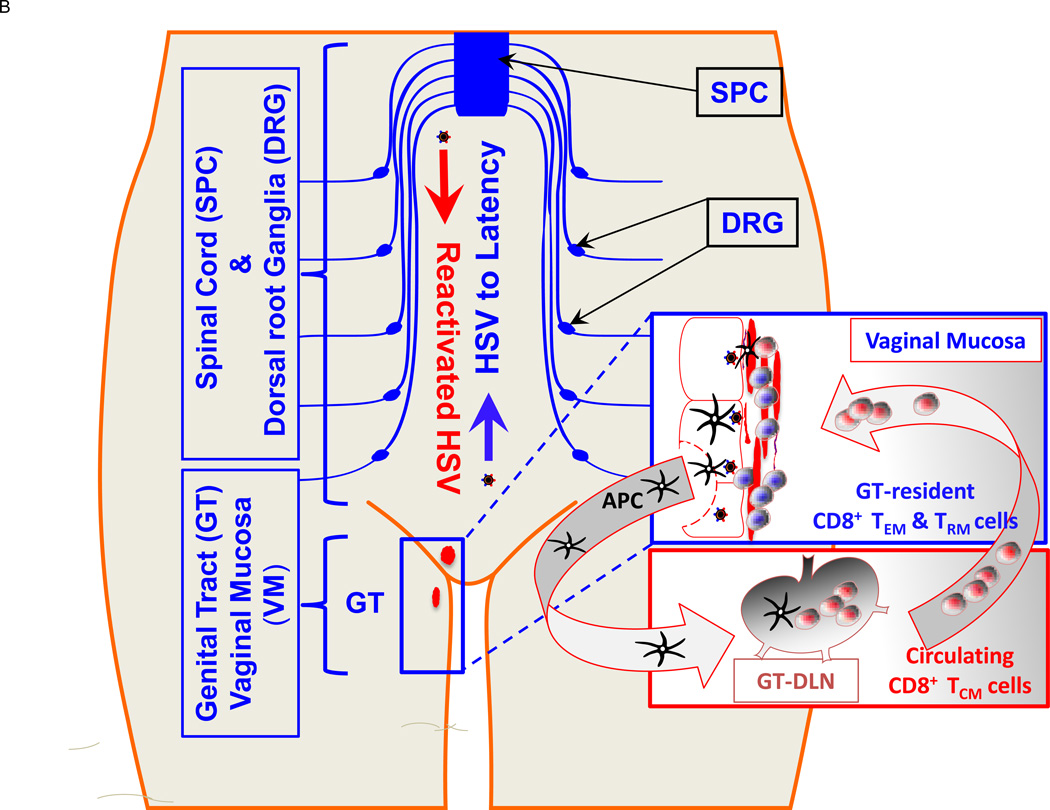
Figure 2. Symptomatic and asymptomatic genital herpes infection in humans.
(A) Following intravaginal infection with HSV-1 or HSV-2; stimulation with pathogenic “symptomatic” and protective “asymptomatic” T cell epitopes, expressed by the HSV, contributes to development of various subsets of HSV-specific memory CD8+ T cells: either GT and DRG-resident effector memory CD8+ T cells (TEM) or lymphoid resident central memory CD8+ T cells (TCM) (Fig. 2B). (B) Protective role of various subsets of HSV-specific memory CD8+ T cells. HSV reactivates from DRG and SPC re-infect of the GT. CD8+ TCM cells in GT-DLN traffic through the bloodstream into the vaginal mucosa of GT to clear the virus. In contrast, CD8+ TEM cells reside in the vaginal mucosal tissues of GT and DRG and are more rapidly mobilized upon re-infection. (See text for details).
The development of effective antiviral medications has had little discernible impact on herpes epidemiology [13, 14]. Meanwhile, the development of effective vaccines against herpes viruses has been notoriously difficult, largely because HSV-1 & HSV-2 have complex life cycles, and the majority of infections remain clinically dormant and silent (i.e. latent) in the body for long periods of time (Fig. 1). Of note, it is surprising that only one vaccine strategy (i.e. vaccination with glycoproteins B and D (gB and gD)) has been tested during the last 18 years in human trials to prevent genital herpes [15–17]. No other vaccine strategy and vaccine trial against ocular or oro-facial herpes have succeed to reach phase II or phase II for over a decade and half now. This by itself attests to the scientific and logistical difficulties facing HSV vaccine development. The latest failures of clinical herpes vaccines involving the employment of HSV-2 gD have brought on additional challenges in securing financial support from pharmaceutical companies for vaccine development and clinical trials [15–17].
We strongly believe that the appropriate response to the recent “failure” of clinical HSV vaccine trials using whole recombinant HSV-2 gB and gD is to intensify our efforts and to not be discouraged as there is much work to do to increase our understanding of human herpes humoral and cellular protective immunity in order to develop and test novel vaccine approaches in reliable animal models, such as HLA transgenic mice, rabbits and guinea pigs.
A “common denominator” among previously failed clinical herpes vaccine trials is that they either used a whole virus or whole glycoproteins, which contain both protective “asymptomatic” antigens/epitopes and pathogenic “symptomatic” antigens/epitopes (Fig. 2 and Fig. 3). We therefore continue to advocate our “asymptomatic” herpes approach through basic immuno-virology (reviewed in [9]). This new approach is based on understanding the immune mechanisms by which seropositive asymptomatic individuals are “naturally” protected from recurrent herpes disease throughout their lives. The T cell-based immune system in the mucosa lining of the genital tract that prevents HSV-2 acquisition, and a better mucosal vaccine approach to boost effector memory T cell responses, we feel, will be instrumental to development of an effective HSV vaccine [1, 18].
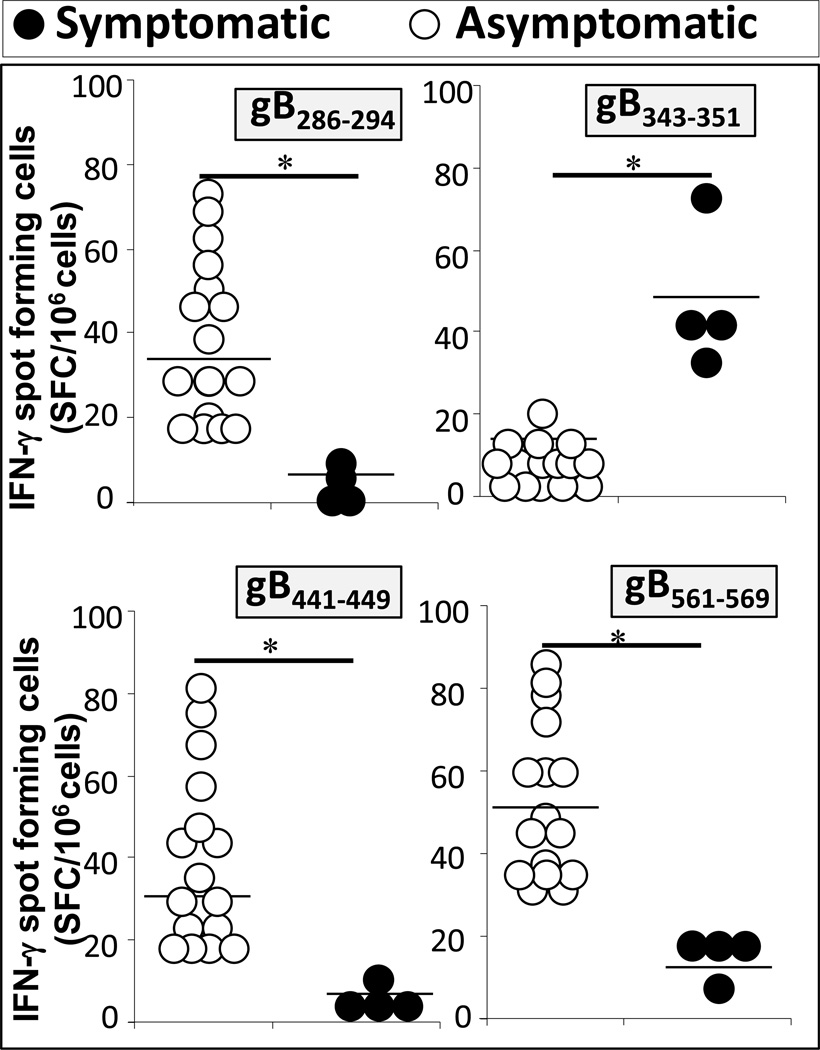
Figure 3. CD8+ T-cells from HSV-seropositive symptomatic and asymptomatic individuals recognize different gB epitopes.
CD8+ T-cells isolated from HLA-A*0201-matched symptomatic (n = 4) and asymptomatic patients (n = 16) were stimulated with autologous DC that were pulsed with 10 µg/ml of the indicated gB peptide for 5 days. The number of IFN-γ-producing CD8+ T-cells specific to each gB epitope was determined by IFN-γ-ELISpot assay in duplicate.
In the most recent workshop on "Next Generation Herpes Simplex Virus Vaccines: The Challenges and Opportunities" convened by the National Institute of Allergy and Infectious Diseases (NIAID) in Washington, DC, (October 22–23rd 2012), the future of the HSV vaccine was discussed among basic researchers, clinicians, funding agencies, and pharmaceutical representatives [11, 12] (See the list of participants in Appendix A). The objectives were: (i) to assess the current status of herpes vaccine research, (ii) to identify the gaps in our knowledge, and (iii) to propose our best approach in developing the next generation of herpes vaccines.
Although much remains unknown about the protective immune effector of ocular, genital, and oro-facial herpes (Fig. 1 and Fig. 2), improved knowledge of HSV immuno-epidemiology, pathogenesis and host immunity should help guide new vaccine strategies for disease prevention and control.
1. GOALS FOR NEXT GENERATION HERPES VACCINES
The first set of questions in a clinical trial often concerns the indications for a successful HSV vaccine – the endpoint, and the goal of vaccination – to reduce herpes disease, shedding, and transmission.
α. Prophylactic and therapeutic vaccines
As with vaccines against any infectious pathogen, the goals that must be achieved with a prophylactic herpes vaccine are often different from those of a therapeutic herpes vaccine. Prophylactic vaccination, given to non-infected individuals, aims to prevent future initial infection. Therapeutic vaccination, given to already infected individuals, aims to reduce both viral shedding and/or ameliorates/eliminates recurrent diseases. One simple and straightforward approach to evaluate the efficacy of a candidate prophylactic vaccine is to look for sero-conversion (i.e. the presence of serum IgG or saliva, tears, or vaginal secretion IgG/IgA specific to herpes proteins). In contrast, therapeutic vaccination would be much more difficult, since the goal of reducing shedding and disease to statistically significant levels may be difficult to attain. While HSV-2 has traditionally been the main target for genital herpes vaccination, expanding coverage to HSV-1 has also become important due to its high prevalence in younger populations, particularly in western societies [10]. Recent immuno-epidemiological evidence points to an increasing proportion of genital herpes cases associated with HSV-1 [10]. Because of structural and sequential overlapping between the proteins and epitopes of HSV-1 and HSV-2, an ideal vaccine against HSV-2 will also protect against HSV-1.
β. Therapeutic vaccine to prevent shedding vs. a therapeutic vaccine to cure the disease: endpoints and public health benefits
When considering the goals of therapeutic herpes vaccine development, several potential obstacles in measurement of endpoints become apparent. Namely, how much reduction in shedding is required to reduce transmission? While the default target is currently around 30% to 40% reduction in shedding, there appears to be no epidemiological data to support this assumption. Additionally, monitoring transmission rates with statistically significant results would require a much larger cohort of volunteers and hence a more expensive and difficult clinical trial study to analyze, compared to measuring sero-conversion on an individual basis. In an attempt to help monitor transmission rates, the Corey group recently proposed the use of several surrogate markers of protection reported in their recent antiviral drug clinical trial [19]. These surrogate markers of protection, in addition to the current approach of only measuring shed virus, may prove to be useful in determining vaccination trials endpoints. Nevertheless, a therapeutic vaccine that would control recurrent herpes disease (in contrast to one that aims to control HSV transmission) would still be relatively more achievable and the results more testable, since it would require a much smaller sample size and a much smaller cohort of volunteers. This is especially true if the candidate therapeutic vaccine is to be tested in a population from the sub-Saharan region where the prevalence of genital herpes disease remains high (e.g. Kenya where 20 % of women are affected by genital herpes) [20]. In other words, a positive effect of a therapeutic vaccine candidate would be much more easily identified in such a populations and the study would have required fewer individuals.
A therapeutic herpes vaccine aimed solely at the reduction of the disease symptoms --without reducing viral shedding -- would be less beneficial for overall public health, resulting in an acceleration of herpes epidemic over time. Recent immuno-epidemiological evidence indicates that asymptomatic, frequent and brief shedding episodes likely contribute to high HSV transmission rates [10] and Fig. 2. Therapeutically vaccinated individuals with no apparent herpes symptoms would continue to shed and spread the virus. Therefore, a therapeutic vaccine against herpes symptoms would be a vaccine that masks the disease rather than eradicating the roots of the disease. Nevertheless, an endemic population may still benefit from a therapeutic vaccine that reduces shedding and disease even if the virus continues to circulate among that population (i.e. herd immunization). Therefore, a therapeutic vaccine against herpes disease that would reduce symptoms is viable in endemic populations but it is less desirable in general populations. Although it would be practically difficult to assess whether an immunotherapeutic vaccine did indeed prevent shedding of the virus, since it would require a larger sample size of some 5,000 to 10,000 sero-positive individuals, a therapeutic vaccine capable of preventing virus reactivation from latency and viral shedding in the body fluids (e.g. vaginal secretions, tears and saliva) should nevertheless be the target of the next immunotherapeutic vaccine trials for these reasons.
χ. Does sero-conversion correlate with shedding and vice versa?
A challenge for herpes vaccinologists is to determine whether or not the degree of sero-conversion (quantitative HSV-specific IgG/IgA antibodies) will correlate with the amount of viral shedding. Alternatively, does the amount of shedding proportionally correlate with the titer of developed HSV-specific antibodies? Can a vaccinated individual shed the virus without sero-converting? Is it possible that a seropositive individual will not shed the virus? The guinea pig model of genital herpes shed some light to these questions [21].
δ. Neonatal herpes vaccine
Many women receiving obstetric care in the United States are infected with HSV-2, with their newborns particularly susceptible to neonatal infection, permanent brain damage, birth defects and death [22, 23]. Neonatal herpetic infection is defined as the infection within 28 days of birth that results from transmission of the virus from the mother to the newborn [24]. The risk of vertical transmission (i.e. passage of virus from mother to newborn) is highest among women who have newly acquired HSV-2 in the genital tract (i.e. women naturally infected during the third trimester of pregnancy) and who were otherwise seronegative [25, 26]. This risk of transmission is increased 300 fold if vaginal shedding occurs at the time of delivery [27]. The overwhelming majority (~85%) of infections are perinatally transmitted to newborns in the birth canal [25, 26, 28]. The outcome of mother-to-infant HSV-2 vertical transmission and neonatal infection is determined by the interplay of virus and maternal/placental immunity [29, 30]. Because vertical transmission of HSV leads to considerable morbidity and mortality (with HSV-1 causing more than 51% of neonatal herpes), the use of a preventive vaccine is highly desirable. In a large cohort of over 40,000 pregnant women, Richard Whitley and co-workers recently showed that most cases of neonatal herpes resulted from primary infection during pregnancy [23, 31, 32]. This study highlights the importance of preventing neonatal herpes, and brings up more questions: Which quality and what quantity of maternal antibodies are necessary to prevent placental transmission of the virus to the newborn? How long must the transmitted antibodies remain in the newborn in sufficient concentration to prevent infection and disease? What is the role of maternal T cells in preventing vertical transmission?
2. ROLE OF ANIMAL MODELS IN HERPES SIMPLEX VACCINE DEVELOPMENT
Reliable animal models of herpes infection and disease are the key to the development of an effective therapeutic vaccine against HSV-1 and HSV-2. In an ideal animal model: (i) the infection would be initiated via a mucocutaneous route similar to that in which humans are commonly infected, i.e., inoculation of the ocular, oro-facial or genital mucosal epithelium; (ii) a small proportion of the animals would develop clinical disease similar in both pathology and severity to those seen in the minority of symptomatic humans, (iii) a large proportion of the animals, would develop immune responses after infection that would protect the animal from the disease, similar to those seen in the majority of asymptomatic humans (Fig. 2A). In these “asymptomatic” animals the immune response can be scrutinized to determine which aspects are important for protection, including innate, humoral, and/or cellular immune responses. The “symptomatic” animals, which cannot spontaneously control the pathogen, provide a situation for the investigation of potential protective immune responses through immunization. Immune responses could then be elicited through parenteral mucosal immunizations and the quality and quantity of those responses would be analyzed before and after inoculation of the pathogen and the immunogen. If immunized animals are protected from infection and/or disease, and the unimmunized animals are not, then the immune responses can be further scrutinized to determine the important aspects for protection. Although there is no perfect animal model that emulates HSV infections and disease in human, both rabbits (for ocular HSV-1) and guinea pigs (for genital HSV-2) are being used for the development of therapeutic herpes vaccines and each model has its own strengths and weaknesses.
During the past decades, several vaccine candidates have shown promising protective results in animal models (mostly mice) but they, unfortunately, have not been effective in clinical trials [33, 34]. Of note, the recent recombinant gD vaccine that failed in clinical trials was successful in animal models [21, 33–35]. This indicates that we must re-evaluate our animal model pre-clinical models to advance herpes vaccines candidates to clinical trials [36]. The development of appropriate and reliable animal models is an important step in the development of vaccines and immunotherapies against any human diseases. As we recently reviewed in [37], one challenge in herpes simplex vaccine development relates to the reliability of currently used animal models and the derived pre-clinical results needed to advance the vaccine into phase I clinical trial. Some clinical herpes immunologists are currently hesitant about using the mouse model in pre-clinical development of therapeutic vaccines because mice do not adequately mimic spontaneous viral shedding or recurrent symptomatic diseases, as occurs in human. Alternatives to mouse models are rabbits and guinea pigs in which reactivation arises spontaneously with clinical features relevant to human disease. Current consensus among many herpes vaccinologists is that pre-clinical animal models can provide relevant information to the design of the next clinical vaccine approach if the endpoint of the pre-clinical vaccine trial in animal models (both immunogenicity and protective efficacy) is set to be similar to the clinical trial. In order to gain useful data, however, current animal models must be improved to simulate the disease process and progression that occurs in humans.
α. Mouse models of herpes infection and disease
Because of the obvious ethical and practical considerations in assessing candidate herpes vaccines directly in humans, finding a species that would be the most appropriate animal model remains critical. For most immunologists, mouse models appear to be the preferred due to the availability of: (i) unlimited inbred and transgenic strains; (ii) specific immune molecule knockout strains; and (iii) the well-characterized immunological probes to study the immune response to specific therapies. In that perspective, the majority of preclinical studies are evaluating HSV vaccine efficiencies on mouse models. Unfortunately, in contrast to humans, recurrent HSV shedding and recurrent herpetic disease do not occur in mice because spontaneous reactivation of HSV is either extremely rare or does not occur in mice [38]. Therefore, although mouse studies have provided ample crucial information regarding immune response against primary HSV-1 and HSV-2 infections (reviewed in [37]), the efficacy of human epitope-based therapeutic vaccines against recurrent shedding and disease cannot be assessed in mice. Several clinical trials have been carried out based on mice data, but almost none have led to clinical success. HSV infections in the murine model preclude its use to study horizontal or vertical transmission or to evaluate strategies designed to prevent reactivation. The lack of an accurate animal model that translates human immune responses and diseases, certainly limits the proper preclinical assessment.
One advantage of mice is the availability of human leukocyte antigen (HLA) transgenic mice that can develop immune responses to human HLA-restricted CD8+ T cell epitopes. In fact, we have recently used HLA transgenic mice to study the immunogenicity and protective efficacy of several HLA-A*020-restricted epitopes against primary HSV-1 [39, 40]. We now have an HLA transgenic rabbit model (referred as HLA Tg rabbits), in which one major component of the rabbit immune system is replaced by the identical component taken from its human counterpart (i.e. HLA-A*0201 class I molecules). For this study we selected a strain in which the rabbit HLA class I expression was compromised and high levels of HLA-A*0201 molecules were expressed (See Fig. 1 and [41]). The HLA Tg rabbit model is capable of mounting T cell responses specific to human CD8+ T cell epitopes and developing spontaneous HSV-1 reactivation. This will allow us for the first time to investigate whether immunization with human CD8+ T-cell epitopes can decrease HSV-1 spontaneous reactivation, and ultimately reduce or eliminate recurrent HSV-induced ocular disease in an appropriate animal model. There are several proposed ways in which such improvement can be made. We continue to advocate that using the HLA transgenic animal models is the ultimate answer to some of challenges facing pre-clinical testing of herpes vaccine. Among these animal models are: (1) humanized HLA transgenic guinea pigs for genital herpes, (2) using humanized HLA transgenic rabbits for ocular herpes.
Mice often provide good infection models as they are readily available in outbred and inbred lines, are relatively inexpensive, and have many immunological reagents available. Mice can be infected with HSV via several routes including footpad, flank, ocular, intravaginal, and each route has its own unique properties. HSV infection at any of these sites initiates localized viral replication, which leads to the development of lesions, establishment of latent infection within sensory neurons and persistence of latent viral DNA within local sensory ganglia and in some cases, central nervous disease. However, the reactivation of HSV from latent infection in mice is extremely rare in vivo making the mouse not a reliable model to assess therapeutic vaccines. While spontaneous reactivation of the virus from sensory ganglia and shedding of the virus in tears or in genital tract do not seem to occur in mice (as opposed to rabbits, guinea pigs and humans), reactivation of HSV from latent infection is readily observed in vitro when ganglia are explanted in culture [42, 43]. Therefore, the potential effect of vaccination against HSV recurrences in mice can be inferred from the levels of latent viral DNA within neurons [44], as these measurements correlate with the number of ex vivo recurrence rates in mice [45–47]. HSV reactivation in mice can be induced to a limited extent by ultraviolet irradiation [48] or elevated temperature [49] or hormone [50]. Furthermore, mice develop a wide range of innate, humoral and cellular immune responses against HSV that are readily measured with the many commercially available immunological reagents. Evidence suggests that both humoral and cellular immune responses can protect mice from HSV infection [37]. In most cases, passive transfer of HSV-specific antibody can prevent encephalitis but not reduce mucosal replication [47, 51, 52]. Both CD4+ and CD8+ T-cell responses appear to play a role in protection from infection or reduction in levels of viral replication, latent viral loads and neuropathy, although CD4+ T-cells appear to be the most important in protecting mice against corneal infection [47, 52] and intravaginal infection [53].
The mouse genital infection model is derived from Parr et al. [54] where female mice are treated with medroxyprogesterone to thin the genital epithelium lining and make the mice uniformly susceptible to HSV-2 vaginal infection. These mouse models have been useful for vaccine studies where one can quantify: (i) the levels of acute viral replication; and (ii) the levels of latent viral loads within the ganglia of sensory neurons that enervate the site of infection. Through these analyses, immunization can reduce viral shedding, latent viral loads, and encephalitis in a dose-dependent manner [55].
β. The guinea pig model
Guinea pig is currently the gold standard for studying genital HSV-2 infection and disease by many researchers because HSV-2 infection in guinea pigs shares many features similar to humans. Intravaginal inoculation of HSV-2 into guinea pigs leads to acute replication and disease at the site of infection, establishment of a latent viral reservoir within the enervating sensory neurons, and periodic reactivation leading to viral shedding and even recurring disease as detected by visible lesions [47, 56]. The development of both acute and recurrent disease provides the opportunity to test both the prophylactic and the therapeutic effects of potential vaccines in guinea pig model. Infected animals develop immune responses that consist of both humoral and cellular immunity [37]. Due to the paucity of immunological reagents for the guinea pig, the full range of immune effector mechanisms is not known, but humoral immunity seems to be sufficient for protection in the guinea pig genital herpes model because passive immunization [47, 57] and subunit vaccines are protective [33, 47, 58].
χ. Generation of human leukocyte antigens transgenic guinea pigs
Preclinical genital herpes vaccine experiments in animal models that use human leukocyte antigen- (HLA-) restricted human T cell epitopes are unfortunately limited to prophylactic vaccines in HLA transgenic mice because there is currently no animal model of recurrent genital herpes that can mount specific T cell responses to HLA-restricted human epitopes (Fig. 3). Like other mice, our HLA transgenic mice do not have recurrent herpes. Guinea pigs have been used to study infection and immunity against genital herpes and share many common genetic diseases with humans. Genital HSV infection in the guinea pigs generated genital herpes disease symptoms that are similar to humans and hence this model will be extremely useful for pre-clinical development of a therapeutic vaccine [58–65]. However, there are still potential challenges that need to be addressed: such as that the genital tract in guinea pigs may differ from that of a human’s. Progress in genital herpes vaccine has been hindered because the tools necessary to undertake a complete immunological analysis of the guinea pig’s cellular immune response against HSV-1 and HSV-2 have been lacking. Although a guinea pig is presently considered the gold standard model for pre-clinical studies of recurrent genital herpes, there are no HLA transgenic guinea pigs available. Development of “humanized” HLA transgenic guinea pig models has long been awaited, due to the technical challenges including: (a) longer gestation times (60–75 days) relative to that of mice (20–30 days); (b) smaller average litter sizes (4 vs. ≥7); (c) limited numbers of litters/ year (5 vs. 10); (d) difficulties in obtaining fertilizable eggs; (e) restraints due to the unavailability of embryonic stem cells, thus no transgenic guinea pigs have been generated; and (f) limited availability of monoclonal antibodies to guinea pigs cell surface antigens, cytokines and cell lines. However, in recent years, (i) physical and chemical delivery methods of naked plasmid DNA directly by spermatogonium, before the sperm can be detected, has provided a powerful tool and opportunity to generate guinea pigs; (ii) many guinea pig’s specific reagents have been characterized in more detail, and are available either commercially or directly from the labs that produced them. We are currently applying, in collaboration with Francina Langa-Vives from Pasteur Institute (Paris), a new transgenic technology to generate an HLA transgenic guinea pig model, over-expressing the human HLA-A*0201 and HLA-DR molecules. This “humanized” animal model would serve as a valuable research model to: (i) speed up pre-clinical development of successful T-cell based vaccines against many infectious pathogens and diseases including genital herpes disease; (ii) duplicate T cell immunity in humans; and (iii) help development of assays that would predict protective immunity against many infectious diseases. The aim of this proposal is to develop HLA-A*0201 and HLA-DR transgenic guinea pigs, characterize the expression pattern of the human HLA-A*0201 and HLA-DR and study its effect on the generation of CD4+ and CD8+ T cell responses against genital herpes human epitopes, recently discovered in our laboratory (Fig. 3).
δ. HLA transgenic rabbit model of ocular HSV-1 and latency for studying the therapeutic efficacy of HLA-restricted CD8+-T cell epitopes-based vaccines in suppressing HSV-1 spontaneous reactivation
HSV-1 infects the cornea and then establishes latency in sensory neurons of the trigeminal ganglia (TG). Sporadic spontaneous reactivation of HSV-1 from neuronal latency causes viral shedding in tears leading to a spreading of the virus to other individuals, and can also cause recurrent Herpes Stromal Keratitis (HSK), a blinding ocular disease. Ocular herpes is one of the more frequent causes of unilateral blindness in the United States with over 450,000 adults having a history of recurrent ocular herpes. Herpes infection is ubiquitous, with an estimated one third of the population worldwide suffering from recurrent infections, which cause thousands to become blind every year. A major gap in our current knowledge remains: “How can a vaccine prevent or significantly reduce virus shedding in tears and HSV-induced ocular disease due to spontaneous reactivation of latent virus in the TG?” HSV-specific CD8+ T-cells producing IFN-γ and granzyme B (GrB) appear to decrease in vitro induced HSV-1 reactivation in explanted mouse TG in a major histocompatibility complex- (MHC-) dependent manner [66–69]. Unfortunately, the in vivo spontaneous HSV-1 shedding and the subsequent recurrent eye disease are extremely rare in mice [38], so the relevance of these findings to in vivo HSV-1 spontaneous reactivation remains to be determined. Spontaneous HSV-1 reactivation occurs in rabbits [70–72], and we now have a “humanized” HLA transgenic rabbit model of ocular HSV-1 that mounts “human-like” CD8+ T-cell immune responses (HLA Tg rabbits). We recently found that therapeutic immunization of latently infected HLA Tg rabbits with 3 human CD8+ T-cell epitopes from HSV-1 gD decreased spontaneous reactivation 4-fold (BenMohamed submitted). This will now allow us, for the first time, to test the hypothesis that appropriate T-cell responses to HSV-1 human epitopes recognized by “asymptomatic T-cells can decrease spontaneous virus shedding in eyes and HSV-induced ocular disease (Fig. 1 and Fig. 3). To our knowledge, this novel HLA Tg rabbit model is the only animal model with spontaneous HSV-1 reactivation that can develop “humanized” CD8+ T cell responses to human HSV-1 epitopes. For better pre-clinical assessment of ocular herpes vaccines in the HLA Tg rabbit model we set up the endpoints to be similar to the endpoints expected in a clinical trial. Our data indicate that therapeutic vaccination of latently infected HLA-transgenic rabbits with asymptomatic lipopeptide vaccines bearing immunodominant human CD8+ T cell epitopes selected from HSV-1 induced strong CD8+ T cell-dependent protective immunity that significantly reduce spontaneous reactivation (shedding of HSV-1 in tears and HSV-induced recurrent eye disease (Submitted).
Similarities between rabbit and human eyes
From a practical standpoint, the sizes of a rabbit’s cornea, conjunctiva and TG are significantly larger than those of mice and offer plentiful amount of tissues for in vitro characterization of T cell responses. In addition, compared to mice, the surface of the rabbit and human eye are relatively immunologically isolated from systemic immune responses [73, 74]. This may be because capillaries are only present in the outer 1 mm of the cornea, effectively isolating the central cornea in humans and rabbits (12–14 and 14–15 mm diameter corneas), while in mice (2 mm diameter cornea) circulating antibody and immune effector cells can rapidly diffuse from the peripheral capillaries into the central cornea (Figs. 1, 2, and 3). This may explain why a serum neutralizing antibody efficiently protects the mouse, but not the rabbit or human cornea, against ocular HSV-1. HSV-1 induced recurrent disease (i.e. HSK) is similar in HLA Tg rabbits (Fig. 2) and humans but differ in mice [73, 74]. In addition, rabbit conjunctiva associated lymphoid tissue (CALT) closely resembles that of humans CALT [73, 74] while the mouse differs [73]. Microanatomy and immuno-histological studies indicate that rabbit conjunctival mucosa is comparable to that of humans and has a typical follicular ultra-structure with an abundance of “conjunctival lymphoid follicles” (CLF), whereas no lymphoid tissue was identified in mice [73, 75–78].
Recently there are more readily available of immunological reagents to study rabbit immune responses. Although the state of the art in rabbit immunology still lags behind those of the mouse and human, several monoclonal and polyclonal antibodies specific to rabbit immune cell CD markers, cytokines and growth factors are now available commercially. In the past eight years we have dedicated a lot of effort in determining which reagents are useful for studying the rabbit immune system. We now have a tested panel of antibodies specific to the rabbit immune cells, allowing for the unprecedented opportunity to assess the induction of rabbit CD8+ T cells and their deployment in TG to decrease HSV-1 spontaneous reactivation, and ultimately reduce or eliminate recurrent eye disease. Anti-rabbit CD8+ T cell mAbs and human tetramers have already allowed us to analyze HSV-specific CD8+ T cell infiltrates in TG and conjunctiva of acutely infected HLA Tg rabbits [8]. Altogether, the striking similarities between HLA Tg rabbit and human in terms of HSV-1 infection and immunity suggests that the HLA Tg rabbit is a preferred model to study the role of CD8+ T cells in controlling spontaneous HSV-1 reactivation and recurrent HSK.
3. CHALLENGES IN HERPES IMMUNOLOGY: IDENTIFYING LIKELY IMMUNE CORRELATES OF “NATURAL” AND “ARTIFICIAL” VACCINE-INDUCED PROTECTION
Another challenge in herpes vaccine development is to determine the immune correlates of protection (e.g. role of antibody vs. cellular immunity and role of mucosal and innate immunity). Immune correlate of protection can emerge from natural history or from clinical trials. It is crucial to determine from a phase II trial the immune arms that correlate with protection in order for potential preventive and therapeutic vaccines to advance to phase III trials. This obviously involves studying the human immunology of HSV infection and disease. Bearing in mind that nature may be working towards better protection against herpes than humans do, we strongly believe that a good starting point for the development of an effective herpes vaccine would be to identify the protective immune mechanisms from “naturally” protected asymptomatic seropositive patients (Fig. 2A). Thus, our novel “asymptomatic herpes vaccine concept involves including “protective” “asymptomatic” epitopes in a herpes vaccine, and excluding the “symptomatic” epitopes that might be “pathogenic” and harmful (Fig. 3).
Mapping of human “asymptomatic” and “symptomatic” T-cell epitopes would: (i) provide a better understanding of the immune responses that correlate with protection and; (ii) help to develop effective immunotherapeutic vaccine strategies against ocular, genital, and oro-facial herpes. If those with a history of severe recurrent disease (i.e., symptomatic people) have a tendency to develop T cells that recognize a subset of epitopes (i.e., symptomatic epitopes) that differ from those recognized by T cells from asymptomatic people (i.e., asymptomatic epitopes), it would be logical to exclude those sets of symptomatic epitopes from vaccines on the grounds that they may enhance rather than diminish the recurrent disease (Fig. 3).
Posovad et al., has reported T cells specific to HSV-2 IE protein antigen in seropositive asymptomatic individuals, the majority of which are CD4+ T cells [79, 80]. Immune seronegative individuals share a specific phenotype whereby the local immune response may be key for better recognition of the IE proteins, which in turn may be a potential target for vaccine development [79, 80]. Comparatively, CD8+ responses appear to be more beneficial for protection than CD4+ T cell response. It has also been reported that a high level of IL-15 (an anti-viral) correlates with protection against HSV-2 in humans [81, 82]. As expected, patients with severe disease, as indicated by multiple outbreaks of genital herpes, had weaker or no responses to these same proteins [83]. Rosenthal and co-workers recently reported a role of HSV-2 virions host shutoff (VHS) on innate immune sensing pathways in human vaginal epithelial cells [84]. They also found that VHS of HSV-2 is forty times more potent than VHS of HSV-1 CD8+ T cell suppression. Overall, their findings strongly suggest that HSV-2 VHS plays roles in selectively inhibiting TLR3, RIG-I/Mda-5, and TLR2-mediated antiviral pathways for sensing dsRNA and effectively suppresses IFN-β antiviral responses in human vaginal epithelial cells [84].
Adding to herpes vaccine challenges, Knipe and collaborators isolated American and African strains of herpes and showed sequence variations that have led to concerns that a generic vaccine cannot produce sufficient coverage among all strains [47]. Similar comparison studies in sequence variations may be worth extending to other HSV strains. Sub-Saharan Africa is by far the most affected by the herpes epidemic with up to 80% woman and 50 % men afflicted with genital herpes; Asian populations have the lowest prevalence (NHANES-2005–2010). Hence, comparing the sequence variation of HSV-1 and HSV-2 isolated from these two continents may reveal information in specific viral genes that make the African strain more prone to reactivate and likely to cause significant disease than the Asian strains. Nevertheless, this simplistic viral concept does not take into account other variations in the immune system between those extreme situations such as the presence of various susceptible vs. resistant HLA alleles, as we recently reported [1].
Our recent finding of “asymptomatic and “symptomatic” epitopes on gB and gD, as well as on VP11/12 and VP13/14, does not exclude the involvement of other herpes antigens/epitopes in shaping T-cell-mediated protective or immunopathological responses (Fig. 3). We are currently performing genome-based bioinformatics searches to identify new HSV-1 and HSV-2 epitopes presented by major HLA class I supertypes HLA-A*0201 (covering over 50% of the human population). A total of 20 HSV-1 and HSV-1 peptide T cell epitopes have been identified from the 84+ HSV open reading frames (ORF). Novel candidate immunodominant HSV CD8+ epitopes recognized by polyfunctional CD8+ T cells during control of infection were identified showing that the HSV-epitope/Ag repertoire for human CD8+ T cells is much broader than previously suspected (BenMohamed, unpublished data). Human HSV-specific CD8+ T cells appear to persist in the genital muco-cutaneous tissues, particularly in the epidermal-dermal junction [85]. It is now warranted to determine the phenotype of mucosal-resident “asymptomatic” CD8+ T cells that correlates with “natural protection” (i.e. TEM, TCM and TRM phenotype) (Fig. 2B). Laser capture and array studies show that mucosal resident CD8+ T cells produce RANTES, TNF-α, perforin, granzyme B, and IFN-γ, demonstrating T cell activity in genital tract (GT) mucosal sites [85]. While CD8+ T cells appear to be critical for local control of those GT mucosal sites, their relevance in HSV acquisition is still unclear. With respect to the innate immunity, the dendritic cells are also critical in imprinting the homing of T cells to GT mucosal sites, the eye, and sensory dorsal root ganglia, creating a balanced, poly-functional CD8+ T memory cell population.
4. PARAMETERS FOR THE CLINICAL EVALUATION OF THE EFFICACITY OF HERPES VACCINE CANDIDATES
Among the questions to be asked during the clinical evaluation of a herpes vaccine are: What are the immunogenicity and the ultimate clinical targets to attain for a candidate vaccine to be called protective? What study design can be used for preventative and therapeutic vaccines? What are the best study populations? Other challenges concern the technical aspects of evaluating the immunological and virological results of the clinical vaccine study itself. These challenges include the lack of standardization of the immunological, virological and protective assays among research laboratories and pharmaceutical companies. This lack of standardization leads to difficulties in deciding which formulation among the currently developed vaccine candidates should be used, Hence, it is of priority to establish a peer-reviewed consensus or employ independent laboratories that would use standardized techniques to make objective recommendations.
Clinical responses (protection against disease vs. virus shedding) must be measured in phase III studies. Young adults of both genders are the target population for prophylactic vaccines with the clinical endpoint being reduction in severity of disease. As mentioned above, while the rate of sero-conversion may be monitored, whether or not the vaccine has an effect on the transmission rate could be difficult to assess, as reduction in shedding may not correlate with reduction in transmission. An ideal therapeutic vaccine should prevent shedding and/or recurrent disease as clinical endpoints. The ideal target of a therapeutic herpes vaccine would be the population living in endemic areas (e.g. sub-Saharan countries) suffering from frequent recurrent genital herpes (e.g. more than once a month) with the endpoint of reducing these recurrences. As mentioned above, Kenya, with 20% of its population affected by genital herpes, may benefit from a therapeutic vaccine. Nonetheless, there are still concerns that variations in HLA alleles [1] and variations of sequences between viruses can affect results when the same vaccine is tested in a different population, rendering the vaccine less efficacious in areas outside of the region in which the original vaccine trial is conducted.
5. CHALLENGES FROM THE VIEWPOINT OF THE PHARMACEUTICAL INDUSTRY
From the industry prospective, pharmaceutical companies have inevitably begun to raise doubts about herpes vaccines due to recent setbacks in vaccine trials [15–17]. The recent failures of clinical trials, using a recombinant glycoprotein D (gD), have brought in additional challenges in securing funding for herpes vaccine research including financial endorsement from pharmaceutical companies for expensive clinical trials. This brought additional challenges related to the regulatory, licensing, and marketing processes. Many pharmaceutical companies are now assessing their vaccine portfolio much more aggressively with requests for additional epidemiological studies and, in some cases even for positive results from a phase I clinical trial, before further investment. Unlike larger pharmaceutical companies, small start-up companies with limited resources that are pursuing a new herpes vaccine often need to evaluate the process of “de-risking the program” more aggressively. For better pre-clinical assessment of candidate vaccines in animal models, the targeted endpoints must correlate to the endpoints expected in clinical trials.
The most recent clinical herpes vaccine (Herpevac Trial) contained envelope HSV-2 gD used with the goal that it would stimulate a protective antibody-mediated response and prevent the virus from establishing an infection. However, in this randomized, double blind clinical trial involving 8,323 women, the vaccine failed to reach its primary endpoint, which is reduction in occurrence of genital herpes disease from either HSV-1 or HSV-2. While there was modest reduction in HSV-1 genital disease, there was no reduction in genital disease caused by HSV-2. The results, recently published in The New England Journal of Medicine [15], continue to be puzzling given the promising results of the same vaccine in previous, smaller clinical trials [16, 17]. Early tests of the gD vaccine suggested that it protected more than 70% of the women against HSV-2 while demonstrating minimal response in men [16, 17]. Belshe attributes the disparity of protection between men and women to differences in genital tract (GT) anatomy – for example, the greater mucosal surface in female GT compared to the male allows secretion of greater amounts of protective IgG and IgA protective antibodies. However, it is likely that the higher level of Th1 responses, which are consistently detected in women compared to men, may account for these gender differences. There also appears to be differences in the parameters of the vaccine trials. Early studies tested the vaccine in uninfected partners from discordant couples (whose partners are positive for HSV-2). This larger trial sponsored by the London-based pharmaceutical firm GlaxoSmithKline (GSK) and the US NIAID, enrolled uninfected women regardless of the status of their partners. This, according to Belshe, may also account for the different results [15]. It is likely that the uninfected partner, in the discordant couples, has developed a yet-to-be-determined natural partial resistance to infection and/or disease. If so, that partial resistance could have contributed the “effectiveness” of the vaccine in the earlier trial [16, 17].
What can we learn from the Herpevac study? We believe further collaboration between basic research laboratories, such as obtaining samples from the GlaxoSmithKline (GSK) Herpevac vaccine trial to better characterize the protective vs. non-protective (or maybe even pathogenic) immune responses, would also be instrumental in proposing alternative vaccine strategies to advance the development of the next generation of herpes vaccine.
While some pharmaceutical companies are more reluctant in investing in the development of herpes vaccine, others maintain their foothold in the field. Sanofi-Pasteur, the vaccines division of Paris-based drug maker Sanofi, is working on licensing a vaccine candidate from Knipe’s laboratory that is based on a whole live virus (HSV-2 dl5–29) genetically rendered incapable of replication [11]. Last year, Amgen, a biotechnology firm based in Thousand Oaks, California, purchased a small company named BioVex of Woburn, Massachusetts, that is testing a different vaccine strategy that uses live, weakened HSV-2. Both vaccines are still in the early stages of testing, and, if successful, may take years to obtain FDA approval. A subunit vaccine containing secreted gD2, and truncated ICP4, which was identified as a CD8+ T-cell antigen through a high-throughput proteomic screening method showed efficacy against infection and recurrent disease in the guinea pig model [83], and is currently being tested with Matrix M-2 new adjuvant in a Phase I/IIa trial as a therapeutic vaccine in men and women (ages 18 to 50 years) with documented genital HSV-2 genital infection (NCT01667341). Among the questions that remain to be answered are: How can we minimize the risk of failure in choosing a herpes vaccine candidate moving from pre-clinical studies to phase I, II and III for clinical trials? Which animal model should we use in pre-clinical studies?
The recent findings that different sets of HSV epitopes are recognized by T cells from symptomatic versus asymptomatic individuals might lead to a fundamental immunologic advance in vaccine development against herpes infection and/or diseases (Fig. 2 and Fig. 3). An efficient immunotherapeutic herpes vaccine would include only the protective (asymptomatic) T-cell epitopes and exclude the pathogenic (symptomatic) epitopes. The lack of an appropriate animal model with a humanized immune response (HLA-Tg) and spontaneous HSV reactivation from latency has stalled the preclinical development of an immunotherapeutic vaccine against the virus. To our knowledge, the recently developed HLA Tg rabbit model is the only animal model with spontaneous HSV-1 reactivation that can respond to humanized T-cell epitopes, and thus can be used for human vaccine development. Finally, newly introduced needle-free mucosal (i.e., topical ocular and intravaginal) lipopeptide vaccines provide an unprecedented strategy against ocular and genital herpes.
There is much work to do to increase our understanding of human herpes humoral and cellular protective immunity and to test novel vaccine approaches in reliable animal models, such as HLA transgenic mice, rabbits and guinea pigs. A good starting point for the development of an effective herpes vaccine would be to identify the protective immune mechanisms from “naturally” protected asymptomatic seropositive patients. Thus, our novel “asymptomatic herpes vaccine concept involves including “protective” “asymptomatic epitopes in a herpes vaccine, and excluding the symptomatic epitopes that might be “pathogenic” and harmful (Fig. 2 and Fig. 3).
6. CONCLUSIONS
In the current era with effective anti-viral therapies, many of the maladies that struck down our ancestors have for the most part been eliminated. However, a staggering number of the world's population still lives with many infectious pathogens including, HSV-1 & HSV-2 that cause a wide range of diseases throughout their life. Over a half billion individuals, between fourteen and forty-nine years of age, around the world are infected with HSV-2 alone. Despite the urgent need, the development of effective vaccines against herpes viruses has been notoriously difficult, largely because HSV-1 & HSV-2 have complex life cycles, and the majority of infections remain latent in sensory neurons, away from the immune system control, for the lifetime.
The latest failure of clinical herpes vaccines involving the employment of the envelope recombinant gD has brought on additional challenges in securing financial support from pharmaceutical companies. Despite these setbacks, we continue to advocate our “asymptomatic” epitope-based herpes approach through basic immuno-virology (Fig. 2 and Fig. 3). This new approach is based on understanding and harnessing the immune mechanisms by which seropositive asymptomatic individuals are “naturally” protected from recurrent herpes disease throughout their life. Fundamentally, it is means to elicit a T cell-based responses in the mucosa lining the genital tract to prevent HSV-2 acquisition, and the development of a better mucosal therapeutic vaccine approach to boost effector memory T cell (TEM cells) responses (Fig. 2B).
Most investigators prefer to use mice as the animal model because there is many well characterized probes commercially available to study the mouse immune response and the inbred transgenic knockout mouse strains are readily available. However, there are two concerns regarding mouse models compared to other models: (i) HSV-1 spontaneous reactivation is either extremely rare or does not occur in mice [38] in contrast to rabbits where spontaneous reactivation occurs at levels similar to that of humans; and (ii) although induction of a systemic immune response (e.g. neutralizing antibody) or a passive transfer of CD8+ T cells can protect the mouse, it does protect humans against ocular herpes disease [2–4, 37, 39, 86]. Such differences make herpes vaccine candidates, developed based on mouse pre-clinical studies, difficult to extrapolate to humans. Thus, although mouse studies have provided much useful and important information regarding ocular HSV-1 infection and immunity and despite the tremendous knowledge about mouse immunology in general, the mouse is not an ideal model for the pre-clinical study of protective immunity against herpes.
There has been a tendency to ignore differences in herpes infection and immunity between mice and humans. This leads to a risk of overlooking aspects of human herpes infection and immunity that do not occur, or cannot be modeled, in mice. Persistent effort on developing herpes therapeutic vaccines using mice as the preclinical model may therefore not be the best approach. Currently, rabbits and guinea pigs are alternative to mice as animal models for pre-clinical testing of ocular and genital herpes therapeutic vaccine candidates, respectively. We hope that the newly introduced HLA transgenic rabbit model (and the HLA transgenic guinea pigs currently being developed in our lab) with spontaneous reactivation and recurrent herpes disease, in addition to their ability to mount “human-like” T-cell responses to HLA-restricted CD8+ T-cell epitopes, will help elucidate some of the questions raised during the preclinical phase of herpes vaccine development. These two animal models (i.e. HLA Tg rabbits and HLA Tg guinea pigs) will help to address the following two major questions: (i) Can spontaneous herpes shedding and recurrent ocular or genital disease be reduced by induction of a vigorous “protective” HSV-specific CD8+ T cells in the sensory ganglia specifically induced by “asymptomatic” epitopes? and (ii) Conversely, can recurrent herpetic disease and spontaneous shedding be exacerbated by “pathogenic” CD8+ T-cells induced by “symptomatic” epitopes?
From the industry prospective, pharmaceutical companies have inevitably began to raise doubts in herpes vaccines due to recent setbacks in vaccine trials that have hindered further progress. We strongly believe that the appropriate response to the recent “failure” of clinical HSV vaccine trials using one or two HSV glycoproteins with adjuvant is to continue evaluate alternative approaches. There is much work to do to improve our understanding of human herpes humoral and cellular immunology and to test novel vaccine approaches in reliable animal models, described previously, in order to improve the preclinical demonstration of safety, immunogenicity and protective efficacy profile of future clinical vaccine candidates. Although much remains unknown about the immune effector(s) that protect against herpes infection and disease, improved knowledge of HSV immuno-epidemiology, pathogenesis and host immunity should help guide new vaccine strategies for herpes disease prevention and control.
The lessons learned from past vaccine clinical trials must serve as a stimulus for new strategies, study designs, and endpoint determinations. Further collaboration between basic research laboratories, such as obtaining samples from the GlaxoSmithKline (GSK) vaccine trial to better characterize the protective (asymptomatic) vs. non-protective (pathogenic or symptomatic) immune responses, would also be instrumental to making strides.
Among other challenges concerning the technical aspects of herpes vaccine development include the lack of standardization of the immunological and protective assays among research laboratories and pharmaceutical companies, which leads to difficulties in deciding which currently developed vaccine candidate is of high priority. A peer-reviewed consensus on standardization of laboratory techniques would therefore be necessary to make recommendations objectively.
ACKNOWLEDGEMENTS
This work is supported by Public Health Service research grants NIH-EY14900 and NIH-EY019896 to LBM, by The Discovery Eye Foundation, by The Henry L. Guenther Foundation, and by an unrestricted Research to Prevent Blindness Challenge grant.
APPENDIX A LIST OF WORKSHOP PARTICIPANTS
Elizabeth Adams, Jeff Alexander, Kenneth Bagley, Lynn B. Barclay, Christopher Beisel, Abbie Bellamy, Robert Belshe, Lbachir BenMohamed, David Bernstein, Nigel Bourne, Heather Bradley, Nathalie Broutet, Jill Brown, David Burns, Cathy Cai, Danilo Casimiro, Mark Challberg, Cara J. Chrisman, Jeffrey I. Cohen, Lawrence Corey, Hagit David, Carolyn Deal, Simon Delagrave, Walla Dempsey, Betty Dodet, Lesia Dropulic, Emily Erbelding, Marian Ewell, Ali Fattom, Anthony Fauci, Neil Finlayson, Doran Fink, Jessica Flechtner, Timothy Fouts, Harvey Friedman, Ulrich Fruth, Gary Fujii, Anne Gershon, Jonathan Glock, Jennifer L. Gordon, Heather Greenstone, Dennis L. Guberski, Susan Guerry, Marc Gurwith, Barbara Hahn, William Halford, Thomas C. Heineman, Seth Hetherington, Thomas Hiltke, David M. Knipe, David M. Koelle, Philip R. Krause, Philip LaRussa, Catherine Laughlin, Peter Leone, Daniel Li, Douglas Lowy, Lauri Markowitz, Gregg N. Milligan, Tulin Morcol, Luwy Musey, Anthony B. Nesburn, Deborah Palliser, Dorothy L. Patton, Carlos V. Paya, Christine Posavad, Elizabeth Rogers, Kenneth L. Rosenthal, Susan L. Rosenthal, Robert P. Ryall, George R. Siber, Jonathan Smith, Deborah Spector, Sean M. Sullivan, Shuang Tang, Anna Wald, Kening Wang, D. Heather Watts, Lisa L. Wei, Richard Whitley, Peter Wolff, and Kejian Yang.
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists
DISCLAIMER
The authors alone are responsible for the views expressed in this review article, and they do not necessarily represent the decisions, policy or views of the institutions, which with they are affiliated.
REFERENCES
- 1.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F DS, et al. Associations of HLA-A, HLA-B and HLA-C Alleles Frequency with Prevalence of Herpes Simplex Virus Infections and Diseases Across Global Populations: Implication for the Development of an Universal CD8+ T-Cell Epitope-Based Vaccine Human Immunoligt. 2014 Feb 5;5(5) doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol. 2013 Nov 15;191(10):5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chentoufi AA, Dervillez X, Rubbo PA, Kuo T, Zhang X, Nagot N, et al. Current trends in negative immuno-synergy between two sexually transmitted infectious viruses: HIV-1 and HSV-1/2. Curr Trends Immunol. 2012;13:51–68. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol. 2012 Nov 1;189(9):4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, et al. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by proteome-wide antibody profiling. J Virol. 2012 Apr;86(8):4328–4339. doi: 10.1128/JVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, et al. Future of an "Asymptomatic" T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virol. 2012 Apr 1;7(4):371–378. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, et al. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol. 2012 Jun;25(3):204–215. doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, et al. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010 Mar 1;184(5):2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofstetter AM, Rosenthal SL, Stanberry LR. Current thinking on genital herpes. Curr Opin Infect Dis. 2014 Feb;27(1):75–83. doi: 10.1097/QCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 11.Knipe DM, Corey L, Cohen JI, Deal CD. Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on "Next Generation Herpes Simplex Virus Vaccines". Vaccine. 2014 Mar 20;32(14):1561–1562. doi: 10.1016/j.vaccine.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi S, Friedman HM. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol. 2014 Mar 11;6C:6–12. doi: 10.1016/j.coviro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Schiffer JT, Swan D, Al Sallaq R, Magaret A, Johnston C, Mark KE, et al. Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract. Elife. 2013;2:e00288. doi: 10.7554/eLife.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer JT, Corey L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med. 2013 Mar;19(3):280–290. doi: 10.1038/nm.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002 Nov 21;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 17.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999 Nov 4;341(19):1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Srivastava R, Lopes PP, Wang C, Pham TT, Cochrane J, et al. Asymptomatic memory CD8 T cells: From development and regulation to consideration for human vaccines and immunotherapeutics. Hum Vaccin Immunother. 2014 Feb 5;10(4) doi: 10.4161/hv.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010 Feb 4;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyiro JU, Sanders EJ, Ngetsa C, Wale S, Awuondo K, Bukusi E, et al. Seroprevalence, predictors and estimated incidence of maternal and neonatal Herpes Simplex Virus type 2 infection in semi-urban women in Kilifi, Kenya. BMC Infect Dis. 2011;11:155. doi: 10.1186/1471-2334-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein DI, Ashley RL, Stanberry LR, Myers MG. Detection of asymptomatic initial herpes simplex virus (HSV) infections in animals immunized with subunit HSV glycoprotein vaccines. J Clin Microbiol. 1990 Jan;28(1):11–15. doi: 10.1128/jcm.28.1.11-15.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handel S, Klingler EJ, Washburn K, Blank S, Schillinger JA. Population-based surveillance for neonatal herpes in New York City, April 2006-September 2010. Sex Transm Dis. 2011 Aug;38(8):705–711. doi: 10.1097/OLQ.0b013e31821b178f. [DOI] [PubMed] [Google Scholar]
- 23.Thompson C, Whitley R. Neonatal herpes simplex virus infections: where are we now? Adv Exp Med Biol. 2011;697:221–230. doi: 10.1007/978-1-4419-7185-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009 Oct 1;361(14):1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol. 2014 May;88(9):4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011 Apr 13;305(14):1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of Vaccine-induced Primary and Memory CD8+ T-cell Responses by Soluble PD-1. J Immunother. 2011 Apr;34(3):297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]
- 28.Brown ZA, Gardella C, Wald A, Morrow RA, Corey L. Genital herpes complicating pregnancy. Obstet Gynecol. 2005 Oct;106(4):845–856. doi: 10.1097/01.AOG.0000180779.35572.3a. [DOI] [PubMed] [Google Scholar]
- 29.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011 Dec 1;121(12):4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009 Jun;7(5):559–568. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009 Sep;83(3):207–213. doi: 10.1016/j.antiviral.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitley R, Davis EA, Suppapanya N. Incidence of neonatal herpes simplex virus infections in a managed-care population. Sex Transm Dis. 2007 Sep;34(9):704–708. doi: 10.1097/01.olq.0000258432.33412.e2. [DOI] [PubMed] [Google Scholar]
- 33.Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 34.Myers MG, Bernstein DI, Harrison CJ, Stanberry LR. Herpes simplex virus glycoprotein treatment of recurrent genital herpes reduces cervicovaginal virus shedding in guinea pigs. Antiviral Res. 1988 Nov;10(1–3):83–88. doi: 10.1016/0166-3542(88)90016-2. [DOI] [PubMed] [Google Scholar]
- 35.Stanberry LR, Harrison CJ, Bernstein DI, Burke RL, Shukla R, Ott G, et al. Herpes simplex virus glycoprotein immunotherapy of recurrent genital herpes: factors influencing efficacy. Antiviral Res. 1989 May-Jun;11(4):203–214. doi: 10.1016/0166-3542(89)90005-3. [DOI] [PubMed] [Google Scholar]
- 36.Johnston C, Koelle DM, Wald A. Current status and prospects for development of an HSV vaccine. Vaccine. 2014 Mar 20;32(14):1553–1560. doi: 10.1016/j.vaccine.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011 Aug 11;29(35):5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhardt BM, Halford WP. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J. 2005 Aug 18;2:67. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, et al. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009 Mar;2(2):129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008 Jan 1;180(1):426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 41.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006 Dec 1;177(11):8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 42.van Velzen M, Laman JD, Kleinjan A, Poot A, Osterhaus AD, Verjans GM. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol. 2009 Aug 15;183(4):2456–2461. doi: 10.4049/jimmunol.0900890. [DOI] [PubMed] [Google Scholar]
- 43.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2008 Mar 1;372(1):56–63. doi: 10.1016/j.virol.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007 Aug;81(15):8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawtell NM. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997 Jul;71(7):5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011 May 15;203(10):1434–1441. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laycock KA, Lee SF, Brady RH, Pepose JS. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest Ophthalmol Vis Sci. 1991 Sep;32(10):2741–2746. [PubMed] [Google Scholar]
- 49.Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992 Apr;66(4):2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker M, Noisakran S, Gebhardt BM, Kriesel JD, Carr DJ. The relationship between interleukin-6 and herpes simplex virus type 1: implications for behavior and immunopathology. Brain Behav Immun. 1999 Sep;13(3):201–211. doi: 10.1006/brbi.1999.0572. [DOI] [PubMed] [Google Scholar]
- 51.Eis-Hubinger AM, Schmidt DS, Schneweis KE. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J Gen Virol. 1993 Mar;74(Pt 3):379–385. doi: 10.1099/0022-1317-74-3-379. [DOI] [PubMed] [Google Scholar]
- 52.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006 Jan 5;344(1):230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Morrison LA. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology. 2008 Jun 20;376(1):205–210. doi: 10.1016/j.virol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994 Mar;70(3):369–380. [PubMed] [Google Scholar]
- 55.Da Costa XJ, Morrison LA, Knipe DM. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology. 2001 Sep 30;288(2):256–263. doi: 10.1006/viro.2001.1094. [DOI] [PubMed] [Google Scholar]
- 56.Krause PR, Stanberry LR, Bourne N, Connelly B, Kurawadwala JF, Patel A, et al. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J Exp Med. 1995 Jan 1;181(1):297–306. doi: 10.1084/jem.181.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourne N, Pyles RB, Bernstein DI, Stanberry LR. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol. 2002 Nov;83(Pt 11):2797–2801. doi: 10.1099/0022-1317-83-11-2797. [DOI] [PubMed] [Google Scholar]
- 58.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003 Feb 15;187(4):542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 59.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, et al. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol. 2014 Feb;88(4):2000–2010. doi: 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernstein DI, Earwood JD, Bravo FJ, Cohen GH, Eisenberg RJ, Clark JR, et al. Effects of herpes simplex virus type 2 glycoprotein vaccines and CLDC adjuvant on genital herpes infection in the guinea pig. Vaccine. 2011 Mar 3;29(11):2071–2078. doi: 10.1016/j.vaccine.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, et al. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine. 2008 Jul 29;26(32):4034–4040. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillpotts RJ, Welch MJ, Ridgeway PH, Walkland AC, Melling J. A test for the relative potency of herpes simplex virus vaccines based upon the female guinea-pig model of HSV 2 genital infection. J Biol Stand. 1988 Apr;16(2):109–118. doi: 10.1016/0092-1157(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 63.Berman PW, Vogt PE, Gregory T, Lasky LA, Kern ER. Efficacy of recombinant glycoprotein D subunit vaccines on the development of primary, recurrent, and latent genital infections with herpes simplex virus type 2 in guinea pigs. J Infect Dis. 1988 May;157(5):897–902. doi: 10.1093/infdis/157.5.897. [DOI] [PubMed] [Google Scholar]
- 64.Thornton B, Baskerville A, Bailey NE, Melling JM, Hambleton P. Herpes simplex virus genital infection of the female guinea pig as a model for the evaluation of an experimental vaccine. Vaccine. 1984 Jun;2(2):141–148. doi: 10.1016/0264-410x(84)90006-9. [DOI] [PubMed] [Google Scholar]
- 65.Scriba M. Protection of guinea pigs against primary and recurrent genital herpes infections by immunization with live heterologous or homologous Herpes simplex virus: implications for a herpes virus vaccine. Med Microbiol Immunol (Berl) 1978 Nov 17;166(1–4):63–69. doi: 10.1007/BF02121135. [DOI] [PubMed] [Google Scholar]
- 66.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001 Nov;75(22):11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36(1–3):119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 68.Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004 May;25(5):230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008 Oct 10;322(5899):268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis. 2006 Sep 15;194(Suppl 1):S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 71.Nesburn AB, Elliott JH, Leibowitz HM. Spontaneous reactivation of experimental herpes simplex keratitis in rabbits. Arch Ophthalmol. 1967 Oct;78(4):523–529. doi: 10.1001/archopht.1967.00980030525021. [DOI] [PubMed] [Google Scholar]
- 72.Perng GC, Ghiasi H, Slanina SM, Nesburn AB, Wechsler SL. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J Virol. 1996 Feb;70(2):976–984. doi: 10.1128/jvi.70.2.976-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knop N, Knop E. Ultrastructural anatomy of CALT follicles in the rabbit reveals characteristics of M-cells, germinal centres and high endothelial venules. J Anat. 2005 Oct;207(4):409–426. doi: 10.1111/j.1469-7580.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005 Mar;206(3):271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, et al. Functional Foxp3+ CD4+ CD25(Bright+) "natural" regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol. 2007 Jul;81(14):7647–7661. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1163–1170. [PubMed] [Google Scholar]
- 77.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol. 2005 Dec;79(24):15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005 Jan 4;23(7):873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 79.Posavad CM, Magaret AS, Zhao L, Mueller DE, Wald A, Corey L. Development of an interferon-gamma ELISPOT assay to detect human T cell responses to HSV-2. Vaccine. 2011 Sep 16;29(40):7058–7066. doi: 10.1016/j.vaccine.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Posavad CM, Remington M, Mueller DE, Zhao L, Magaret AS, Wald A, et al. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol. 2010 Mar 15;184(6):3250–3259. doi: 10.4049/jimmunol.0900722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013 Dec;14(12):1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 82.Thatte A, DeWitte-Orr SJ, Lichty B, Mossman KL, Ashkar AA. A critical role for IL-15 in TLR-mediated innate antiviral immunity against genital HSV-2 infection. Immunol Cell Biol. 2011 Aug;89(6):663–669. doi: 10.1038/icb.2011.7. [DOI] [PubMed] [Google Scholar]
- 83.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol. 2013 Apr;87(7):3930–3942. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao XD, Rosenthal KL. Herpes simplex virus type 2 virion host shutoff protein suppresses innate dsRNA antiviral pathways in human vaginal epithelial cells. J Gen Virol. 2011 Sep;92(Pt 9):1981–1993. doi: 10.1099/vir.0.030296-0. [DOI] [PubMed] [Google Scholar]
- 85.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. 2013 May 23;497(7450):494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chentoufi AA, BenMohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]