Abstract
Cancer metastasis is a multi-step process in which tumor cells gain the ability to invade beyond the primary tumor and colonize distant sites. The mechanisms regulating the metastatic process confer changes to cell adhesion receptors including the integrin family of receptors. Our group previously discovered that the α6 integrin (ITGA6/CD49f) is post translationally modified by urokinase plasminogen activator (uPA) and its receptor, urokinase plasminogen activator receptor (uPAR), to form the variant ITGA6p. This variant of ITGA6 is a cleaved form of the receptor that lacks the ligand-binding domain. Although it is established that the uPA/uPAR axis drives ITGA6 cleavage, the mechanisms regulating cleavage have not been defined. Intracellular integrin dependent “inside-out” signaling is a major regulator of integrin function and the uPA/uPAR axis. We hypothesized that intracellular signaling molecules play a role in formation of ITGA6p to promote cell migration during cancer metastasis. In order to test our hypothesis, DU145 and PC3B1 prostate cancer and MDA-MB-231 breast cancer cell lines were treated with small interfering RNA targeting actin and the intracellular signaling regulators focal adhesion kinase (FAK), integrin linked kinase (ILK), and paxillin. The results demonstrated that inhibition of actin, FAK, and ILK expression resulted in significantly increased uPAR expression and ITGA6p production. Inhibition of actin increased ITGA6p, although inhibition of paxillin did not affect ITGA6p formation. Taken together, these results suggest that FAK and ILK dependent “inside-out” signaling, and actin dynamics regulate extracellular production of ITGA6p and the aggressive phenotype.
1. Introduction
In cancer, metastatic lesions are responsible for 90% of cancer related mortalities, not the primary tumor [1]. Prostate cancer patients diagnosed with confined disease have a 5-year patient survival rate of 100%, while breast cancer patients with confined disease have a 5-year patient survival rate of 98% [2]. However, for prostate and breast cancer patients diagnosed with metastatic disease the 5-year survival rate drastically decreases to 28% and 24% respectively [2]. Therefore it is imperative to develop targeted therapies to prevent, delay, or inhibit the invasion and migration of cancer cells. Migrating cancer cells rely on cell surface receptors and the mechanisms that control proper function of these molecules. The cell adhesion receptors that bind extracellular matrix, such as the integrins, are often post-translationally modified to promote migration and invasion during metastasis [3,4]. During prostate cancer progression, the laminin-binding integrins are expressed while all other integrin family members are not [5–8]. Integrin alpha 6 (ITGA6/CD49f) is expressed in 70% of advanced prostate carcinomas and in prostate cancer derived micro-metastases [5,6,9]. Previous studies by our group have identified a structural variant of ITGA6 called ITGA6p, that lacks the ligand binding extracellular domain and is formed following cleavage of ITGA6 by urokinase-type plasminogen activator (uPA) [10,11]. In addition to the necessary role of uPA in cleaving ITGA6, recent work by our group has shown that macrophages can stimulate uPA/uPAR production in tumor cells and increase ITGA6 cleavage. These data suggested that tumor activated macrophages promote prometastatic integrin-dependent pericellular proteolysis and the metastatic phenotype [12]. Furthermore, ITGA6 cleavage has been shown to contribute to cell invasion and migration on laminin, and inhibition of ITGA6 cleavage was shown to substantially delay the onset of bone metastasis and promote curative-type bone metastasis lesions in xenograft mouse models [13–15]. In addition to the role of extracellular regulators in ITGA6p production, our group has shown that cleavage of ITGA6 was dependent on actin [16]. The integrin-actin complex is essential for “inside-out” integrin signaling [17–20] and intracellular signaling molecules such as focal adhesion kinase (FAK) and integrin linked kinase (ILK) and structural complex molecules such as paxillin, vinculin and talin all play pivotal roles in cancer progression and with integrin in the formation of focal adhesions [21–28]. We hypothesized that key intracellular signaling molecules involved with cell migration and invasion promote cleavage of ITGA6 and modulate the invasive phenotype. The goal of this study was to identify whether the integrin-actin axis and FAK, ILK and focal adhesion adaptor molecules regulate ITGA6p production in aggressive prostate and breast cancer tumor cells.
2. Materials and Methods
2.1. Antibodies and reagents
The anti-ITGA6 rabbit polyclonal (pAb) antibody AA6A was generated against the intracellular COOH-terminal domain of ITGA6 and purified by Bethyl Laboratories Inc (Montgomery, TX). The AA6A pAb is specific for the last 16 amino acids of human ITGA6 sequence and the cytoplasmic domain of α3 integrin (ITGA3) [13]. The anti-ITGA6 rabbit pAb, AA6NT antibody was generated using a recombinant fragment of the N-terminal β-barrel domain as previously described [14]. The J1B5 rat anti-ITGA6 monoclonal antibody (mAb), was generated in the laboratory of Dr. Caroline Damsky (University of California, San Francisco, CA) [29]. The anti-ITGA3 rabbit pAb (1920), was, purchased from Chemicon (Temecula, CA). The mAb anti-uPAR antibodies included mouse antibodies 3936 (Sekisui Diagnostics, Lexington, MA) and 62022 (R&D Systems, Minneapolis, MN). The anti-β-actin antibodies included the rabbit mAb 13E5 (Cell Signaling, Danvers, MA) and rabbit pAb AAN01 (Cytoskeleton, Denver, CO). The anti-ILK mouse mAb (611802) and the anti-paxillin Ab were purchased from BD Biosciences (San Jose, CA). The anti-FAK rabbit pAb (06-543) and anti-α-Tubulin mouse mAb DM1A were purchased from Millipore (Bedford, MA). Secondary conjugated Alexa-488 antibodies, donkey anti-rat and goat anti-mouse were purchased from Life Technologies (Carlsbad, CA) and secondary anti-rabbit and anti-mouse horseradish peroxidase antibodies used were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The ILK inhibitor QLT0267 was a generous gift from Dr. Shoukat Dedhar (University of British Columbia, Vancouver, Canada).
2.2. Cell lines and culture conditions
All cells lines were maintained in Iscove’s Modified Dulbecco’s medium (Life Technologies) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and 1% penicillin/streptomycin (Life Technologies) at 37°C in a humidified atmosphere of 95% air and 5% CO2. The DU145 prostate carcinoma and the breast carcinoma cell line MDA-MB-231 were obtained from American Type Culture Collection (Manassas, VA). PC3B1 cells were generated as an aggressive subclone of PC3 cells isolated from bone metastatic lesions produced in a xenograft model as previously described [14].
2.3. Immunoprecipitations and SDS-PAGE/Immunoblotting
Cells were rinsed two times with HEPES buffer (5% HEPES; 7.59% NaCl; 0.4% KCl, 0.16% MgCl2, 0.15% CaCl2) and lysed using cold RIPA buffer (50 mM Tris; 150 mM NaCl; 1% Triton X-100; 0.1% SDS; 1% Na-Deoxycolate; pH 7.4), 1 mM PMSF, 1 μg/ml Leupeptin and 1 μg/ml Aprotinin. Lysates were sonicated 8 times at one-second intervals and then were pre-cleared with 40 μl Protein-G sepharose beads (GE Healthcare, Piscataway, NJ) for one hour at 4°C with agitation. The immunoprecipitation reactions contained 250 μg of protein, 30 μl of Protein-G sepharose beads and 3 μL of the J1B5 antibody. The final volume was adjusted to 500 μl using RIPA buffer and the reactions were incubated on a rotational inverter for 18 hours at 4°C. The protein complexes were then analyzed by SDS-PAGE and immunoblot was performed using chemiluminescence (ECL Western Blotting Detection System, GE Healthcare).
2.4. Flow cytometry
Cells were harvested using cold PBS containing 5mM EDTA, washed in PBS and resuspended in 200 μL of 1x PBS with specific antibodies (1:200 dilution) and incubated on ice for 30 min. Cells were then washed in PBS and incubated with either an Alexa-488 anti-rat secondary antibody (1:500) or an Alexa-488 anti-mouse secondary antibody (1:500) in PBS for 30 minutes on ice. Cells were analyzed using a BD FACScan and FlowJo (version 7.2.2).
2.5. Small Interfering RNA
Small interfering RNA (siRNA) targeting ITGA6, actin, ILK, FAK, paxillin or a non-targeting control (siControl) were purchased from Dharmacon (GE Healthcare) The transfections were performed per instructions provided by the manufacturer and at the optimized concentration of 30 nM using DharmaFECT 4 reagent. The transfected cells were then incubated at 37°C with 5% CO2 for 48, 72, or 96 h. The cells were transfected a second time for incubations lasting longer than 48 h.
2.6. Real-time PCR
RNA was quantified using Eppendorf’s BioPhotometer 6131. Equal amounts (1250 ng) of RNA were prepared for real-time PCR (RT-PCR) and Applied Biosystems TaqMan One Step RT-PCR Master Mix Reagent Kit was used. Probes for ILK and GAPDH from Applied Biosystems were used. Cycle parameters for RT-PCR experiment using Applied Biosystems ABI Prism 7000 sequence Detection System were as followed; one cycle of 50°C for two minutes to convert RNA to DNA using reverse-transcriptase, one cycle of 95°C for 10 minutes to denature reverse-transcriptase, 40 cycles of (95°C for 15 seconds and 60°C for one minute) quantified amplification. Analysis of data was performed using the Relative expression software tool (REST 2009). Independent experiments were performed twice.
3. Results
3.1. Inhibiting actin expression increased ITGA6p formation
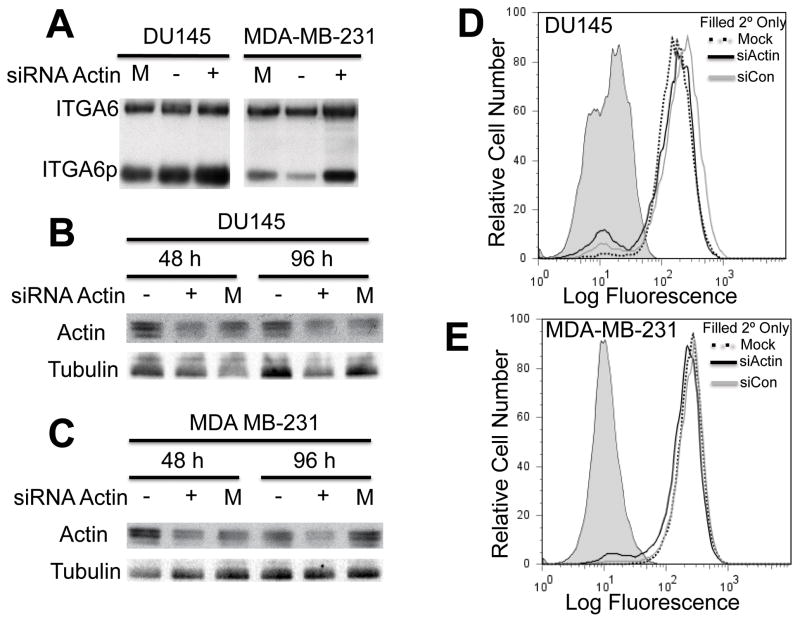
DU145 cells and MDA-MB-231 cells were transiently transfected with siRNA targeting actin or a non-targeting control for 48 and 96 h. The cells were harvested using RIPA lysis buffer and J1B5 was used to immunoprecipitate all forms of ITGA6. The results in Fig 1A show that ITGA6p formation increased in DU145 and MDA-MB-231 cells following actin depletion for 96 h. Actin silencing occurred at 48 and 96 h after siRNA treatment in both cell lines (Fig 1B and 1C). Flow cytometric analysis indicated that total cell surface expression of ITGA6 did not change in either cell line (Fig 1D and 1E). These results show that although depleting actin from cells for 96 h increased ITGA6p formation, it did not affect total cell surface levels of the integrin.
Fig. 1.
(A) DU145 and MDA-MB-231 cells were treated with siRNA targeting actin (siActin (+)), a non-targeting siRNA (siCon (−)) or mock treatment (M) for 48 or 96 h. (A) ITGA6 was immunoprecipitated from DU145 and MDA-MB-231 cells and immunobot analysis was used to detect ITGA6 and ITGA6p following a 96 h siRNA treatment. DU145 (B) and MDA-MB-231 (C) cells were harvested following 48 or 96 h of siRNA treatment and immunoblot analysis was performed to detect actin expression. Tubulin was used as a loading control. Flow cytometric analysis of cell surface ITGA6 in (D) DU145 and (E) MDA-MB-231 cells after 96 h of siRNA treatment. Secondary only is the grey filled peak, siActin is the black solid line, siCon is the grey line, and mock is the black dotted line. Independent experiments were performed three times.
3.2. Inhibition of FAK and ILK increased ITGA6 cleavage
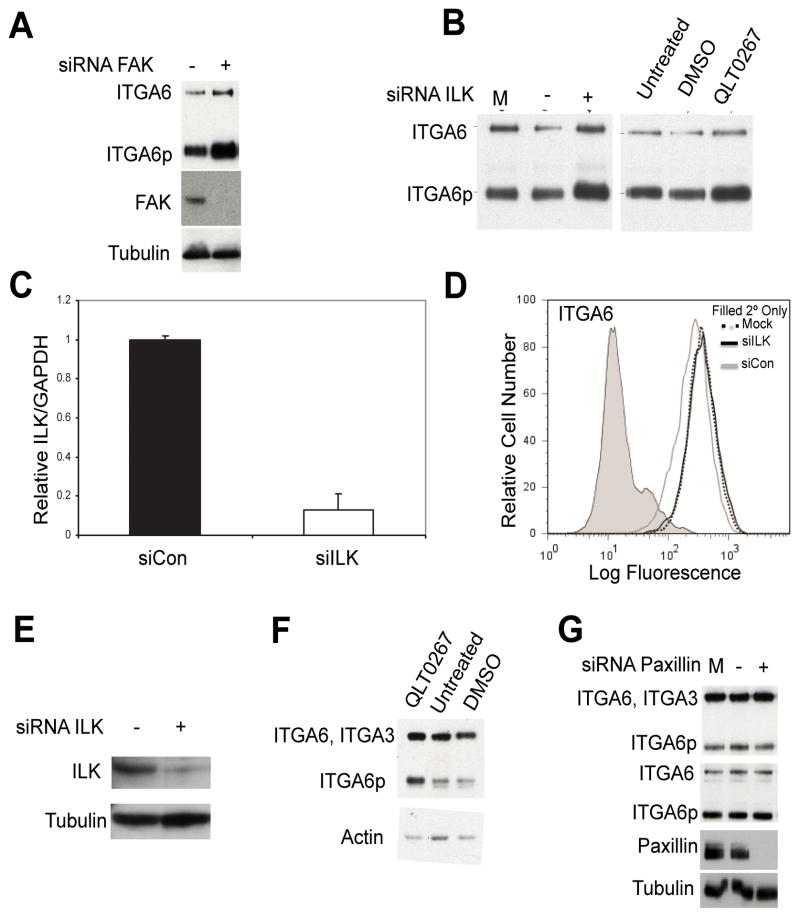
DU145 cells were transiently transfected with siRNA targeting FAK, LK, paxillin or siControl for 48, 72 or 96 h. Cells were harvested using RIPA lysis buffer and ITGA6 was retrieved by immunoprecipitation using the mAb J1B5. ITGA6 was then separated on a non-reducing 10% SDS-PAGE gel and both forms of ITGA6 (ITGA6 and ITGA6p) were detected using the pAb AA6A. The results in Figure 2A and 2B show that ITGA6p increased after silencing FAK or ILK expression, respectively. ILK knockdown was confirmed in DU145 cells via real-time PCR and immunoblot (Fig 2C & 2E). Flow cytometry analysis of PC3B1 cells treated with siRNA targeting ILK showed no change in cell surface levels of ITGA6 (Fig 2D). The ILK kinase inhibitor QLT0267 also increased protein levels of ITGA6p (Fig 2B & 2F). Decreased paxillin expression at 72 h post-siRNA treatment did not affect ITGA6 or ITGA6p levels in DU145 cells (Fig 2G).
Fig. 2.
(A) DU145 cells were transfected for 72 h with siRNA targeting FAK (+) or non-targeting control (siCON (−)) and blotted for ITGA6, FAK, or tubulin. Cells were harvested and ITGA6 was immunoprecipitated. Immunoblot analysis indicates expression of ITGA6 and ITGA6p (top blot) and FAK with tubulin as a loading control (lowest blots). (B) DU145 cells were transfected for 48 h with siRNA targeting ILK (siILK), siCON or mock treated (M). Cells were harvested and ITGA6 was immunoprecipitated and detected using immunoblot analysis (left blot). DU145 cells were treated with the ILK inhibitor QLT0267, DMSO, or left untreated. ITGA6 and ITGA6p expression were analyzed using immunoblot analysis (right blot). (C) Messenger RNA levels determined by real-time PCR following 72 h siILK or siCon. Flow cytometry analysis was performed on PC3B1 cells after 96 h siILK (black line), siCon (grey line), mock transfection (black dotted) and secondary only (grey filled) controls. (D) Cell surface expression of ITGA6 in PC3B1 cells is reported. (E) DU145 cells were trabsfected for 72 h with siILK (+) or siCon (−). Whole cell lysates were harvested and immunoblot analysis of ILK and tubulin was performed. (F) DU145 cells were harvested for WB after 48 hours treatment with ILK inhibitor (QLT0267), DMSO, or untreated and blotted for ITGA6 and actin. (G) DU145 cells were transfected with siRNA targeting paxillin (+), siCON (−), or mock treated (M) for 72 h. Cells were harvested and ITGA6 was detected using immunoblot analysis of whole cell lysate with the AA6A antibody (top blot) or following immunoprecipitation of ITGA6 (middle blot). Immunoblot analysis of paxillin was performed using whole cell lysates with tubulin as a loading control (lowest blots). Independent experiments were performed three times.
3.3. Depletion of FAK and actin increased uPAR in DU145 cells
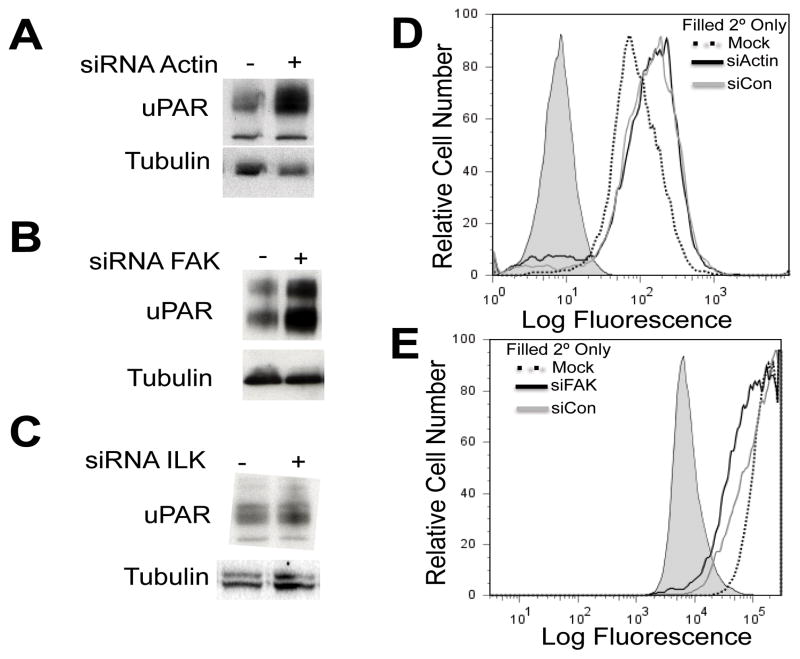
We next determined if increased ITGA6 cleavage following siRNA treatment for FAK, ILK, or actin coincided with an increase in uPAR expression. The results in Fig 3A and 3B show that silencing of actin or FAK expression increased uPAR protein levels in DU145 cells. In contrast, siRNA depletion of ILK for 96 h did not affect uPAR protein levels in DU145 cells (Fig 3C). DU145 cells treated with siRNAs targeting, actin, FAK or siControl for 72 or 96 hours were harvested for flow cytometric analysis of cell surface uPAR. The results show that cell surface uPAR expression was unchanged following siRNA treatment of DU145 cells (Fig 3D & 3E).
Fig. 3.
(A) DU145 cells were transfected with siRNA targeting (A) actin for 96 h (B) FAK for 72 h and (C) ILK for 72 h. Immunoblot analysis using whole cell lysates was used to detect uPAR expression for each experiment. DU145 cells were transfected with siRNA targeting (D) actin for 96 h and (E) FAK for 72 h and cell surface expression of uPAR was detected by flow cytometry using the mAB 3936. Independent experiments were performed three times.
4. Discussion
The lethality of cancer lies with the ability to spread to distant sites and impair normal vital organ function. Continued investigation of the changes that occur to a cancer cell that bestow the ability to invade, migrate and metastasize are constantly underway [30–32]. Previous studies by our group have identified a cleaved form of ITGA6, called ITGA6p, which has been shown to contribute to cell invasion and migration [13]. Inhibition of ITGA6 cleavage substantially delayed the onset of bone metastasis and promoted curative-type bone metastasis lesions in xenograft mouse models [14,15]. Due to the high prevalence and pro-metastatic phenotype of ITGA6p, we further investigated selected molecular candidates as modifiers of ITGA6p production.
Six molecular candidates (actin, ILK, FAK, paxillin, vinculin and talin) were investigated since their involvement in the integrin-actin signaling and structural complex has been established [17–20]. All have pivotal roles with integrin in the formation of focal adhesions [21–23]. The results indicated that suppression of actin, FAK and ILK expression using a siRNA approach led to an increase of ITGA6 cleavage (Figs 1 & 2). Loss of the actin cytoskeleton and functionally related signaling molecules resulted in the observed increase in ITGA6 cleavage.
While the production of ITGA6p was increased by suppression of ILK or FAK expression, it was unaffected by suppression of paxillin (Fig 2), talin or vinculin (data not shown). A potent ILK kinase inhibitor, QLT0267, [33] also increased production of ITGA6p (Fig 2B & 2F). In a breast cancer study, QLT0267 also altered F-actin distribution [34]. Taken together, these results suggested that perturbing enzymatic signaling associated with actin dynamics was a determinant of ITGA6p production. The involvement of actin dynamics as a determining factor is consistent with the established concept of “inside-out” regulation of integrin function [35,36].
The rationale for the current study included the knowledge that integrin cleavage was dependent upon the uPA/uPAR axis and the reports that uPAR and integrins interact [37–40]. Recently, published work indicates that ITGA6 cleavage is closely associated with uPAR and uPA levels [12]. These observations of increased ITGA6 cleavage prompted us to determine if uPAR levels were increasing as well. Loss of actin, FAK and ILK expression resulted in an increase in ITGA6 cleavage in DU145 cells (Fig 1A, Fig 2A & 2B) and under these conditions, DU145 cells showed a significant increase in uPAR expression (Fig 3A & 3B). We note with interest that while DU145 cells showed an increase in the total amount of uPAR protein, flow cytometry analysis of uPAR levels resulted in no change of uPAR levels at the cell surface (Fig 3D). This suggests that a dynamic component of uPAR may be involved rather than simply static levels of the receptor as a determinant of ITGA6p production.
Since the defining feature of ITGA6p is the loss of the laminin ligand binding domain, the results suggested that an actin dependent trigger exists which would increase the loss of the laminin binding function of ITGA6. Previous work in model organisms has shown an actin dependent coordination of laminin and fibronectin adhesion. For example, in normal development of the follicular epithelium during Drosophila oocyte maturation, changes from a columnar to squamous morphology is accompanied by a switch from laminin binding integrin to RGD binding integrin. During these changes, integrin adhesive structures remain at actin fiber ends [41]. It is possible that human tumors, during metastasis and collective migration, retain the ability to modify laminin adhesion similar to developing embryos by utilizing actin dynamics.
Finally, we note that the responsiveness of tumor cells to alterations in actin function to modulate integrin dependent laminin adhesion (via integrin cleavage) may be most relevant to tumors that retain collective migration properties and E-cadherin expression, consistent with collective migration and tubulogenesis models of metastasis [42,43]. DU145 cells express E-cadherin whereas PC3 and MDA-MB-231 cells have undergone epithelial to mesenchymal transition (EMT) [44,45]. Previous work in vitro suggested an increase in ITGA6p occurs during increased cell-cell interactions stimulated during the in vitro differentiation of skin keratinocytes or induced by overexpression of the androgen receptor in vitro [10,46]. Future studies will be directed at understanding the potential role of actin dynamics in regulating the production of ITGA6p via the UPA/uPAR axis in collective migration.
Highlights.
Actin inhibition increased ITGA6 cleavage in prostate and breast cancer cell lines.
Inhibition of FAK and ILK increased ITGA6p formation in prostate cancer cell lines.
Depletion of FAK and actin increased uPAR in prostate cancer DU145 cells.
Acknowledgments
The work was supported in part by NIH grants 1RO1 CA159406, CA23074 and the TACMASS core service of the University of Arizona Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. Cancer Statistics Review, 1975–2011 - SEER Statistics. Natl. Cancer Institute; Bethesda, MD: http//seer.cancer.gov/csr/1975_2011/, Based Novemb. 2013 SEER Data Submission, Posted to SEER Web Site, April 2014. (2014). http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 3.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 4.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. [accessed August 30, 2014];Cancer Metastasis Rev. 1995 14:219–28. doi: 10.1007/BF00690293. http://www.ncbi.nlm.nih.gov/pubmed/8548870. [DOI] [PubMed] [Google Scholar]
- 6.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. [accessed August 30, 2014];Am J Pathol. 1995 146:1498–507. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1870922&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 7.Fornaro M, Tallini G, Bofetiado CJ, Bosari S, Languino LR. Down-regulation of beta 1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. [accessed August 30, 2014];Am J Pathol. 1996 149:765–73. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1865133&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 8.Perlino E, Lovecchio M, Vacca RA, Fornaro M, Moro L, Ditonno P, et al. Regulation of mRNA and protein levels of beta1 integrin variants in human prostate carcinoma. [accessed August 30, 2014];Am J Pathol. 2000 157:1727–34. doi: 10.1016/s0002-9440(10)64809-2. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1885729&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. [accessed August 30, 2014];Cancer Res. 1999 59:241–8. http://www.ncbi.nlm.nih.gov/pubmed/9892213. [PubMed] [Google Scholar]
- 10.Davis TL, Rabinovitz I, Futscher BW, Schnölzer M, Burger F, Liu Y, et al. Identification of a novel structural variant of the alpha 6 integrin. J Biol Chem. 2001;276:26099–106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetriou MC, Pennington ME, Nagle RB, Cress AE. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp Cell Res. 2004;294:550–8. doi: 10.1016/j.yexcr.2003.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sroka IC, Sandoval CP, Chopra H, Gard JMC, Pawar SC, Cress AE. Macrophage-dependent cleavage of the laminin receptor α6β1 in prostate cancer. Mol Cancer Res. 2011;9:1319–28. doi: 10.1158/1541-7786.MCR-11-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawar SC, Demetriou MC, Nagle RB, Bowden GT, Cress AE. Integrin alpha6 cleavage: a novel modification to modulate cell migration. Exp Cell Res. 2007;313:1080–9. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ports MO, Nagle RB, Pond GD, Cress AE. Extracellular engagement of alpha6 integrin inhibited urokinase-type plasminogen activator-mediated cleavage and delayed human prostate bone metastasis. Cancer Res. 2009;69:5007–14. doi: 10.1158/0008-5472.CAN-09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landowski TH, Gard J, Pond E, Pond GD, Nagle RB, Geffre CP, et al. Targeting integrin α6 stimulates curative-type bone metastasis lesions in a xenograft model. Mol Cancer Ther. 2014;13:1558–66. doi: 10.1158/1535-7163.MCT-13-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis TL, Buerger F, Cress AE. Differential regulation of a novel variant of the alpha(6) integrin, alpha(6p) [accessed July 24, 2014];Cell Growth Differ. 2002 13:107–13. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2824497&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 17.Brakebusch C, Fässler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesner S, Legate KR, Fässler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–99. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Vicente-Manzanares M, Potvin-Trottier L, Wiseman PW, Horwitz AR. The integrin-ligand interaction regulates adhesion and migration through a molecular clutch. PLoS One. 2012;7:e40202. doi: 10.1371/journal.pone.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 23.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–56. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 24.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–72. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 25.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165:1087–95. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz C, Holz DR, Oeggerli M, Schneider S, Gonzales IM, Kiefer JM, et al. Amplification and overexpression of vinculin are associated with increased tumour cell proliferation and progression in advanced prostate cancer. J Pathol. 2011;223:543–52. doi: 10.1002/path.2828. [DOI] [PubMed] [Google Scholar]
- 28.Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2011;22:327–41. doi: 10.1091/mbc.E10-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. [accessed August 26, 2014];Development. 1994 120:3657–66. doi: 10.1242/dev.120.12.3657. http://www.ncbi.nlm.nih.gov/pubmed/7529679. [DOI] [PubMed] [Google Scholar]
- 30.Banyard J, Zetter BR. The role of cell motility in prostate cancer. [accessed August 30, 2014];Cancer Metastasis Rev. n.d 17:449–58. doi: 10.1023/a:1006150007710. http://www.ncbi.nlm.nih.gov/pubmed/10453290. [DOI] [PubMed] [Google Scholar]
- 31.Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem. 2004;91:41–6. doi: 10.1002/jcb.10665. [DOI] [PubMed] [Google Scholar]
- 32.Banyard J, Chung I, Migliozzi M, Phan DT, Wilson AM, Zetter BR, et al. Identification of genes regulating migration and invasion using a new model of metastatic prostate cancer. BMC Cancer. 2014;14:387. doi: 10.1186/1471-2407-14-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troussard AA, McDonald PC, Wederell ED, Mawji NM, Filipenko NR, Gelmon KA, et al. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006;66:393–403. doi: 10.1158/0008-5472.CAN-05-2304. [DOI] [PubMed] [Google Scholar]
- 34.Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, et al. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outc. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faull RJ, Ginsberg MH. Inside-out signaling through integrins. [accessed July 29, 2014];J Am Soc Nephrol. 1996 7:1091–7. doi: 10.1681/ASN.V781091. http://www.ncbi.nlm.nih.gov/pubmed/8866399. [DOI] [PubMed] [Google Scholar]
- 36.Kolanus W, Seed B. Integrins and inside-out signal transduction: converging signals from PKC and PIP3. [accessed July 29, 2014];Curr Opin Cell Biol. 1997 9:725–31. doi: 10.1016/s0955-0674(97)80127-5. http://www.ncbi.nlm.nih.gov/pubmed/9330877. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. [accessed August 06, 2014];J Cell Biol. 1999 147:89–104. doi: 10.1083/jcb.147.1.89. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2164973&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. [accessed August 06, 2014];Mol Biol Cell. 2001 12:2975–86. doi: 10.1091/mbc.12.10.2975. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=60149&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, et al. Regulation of integrin function by the urokinase receptor. [accessed August 06, 2014];Science. 1996 273:1551–5. doi: 10.1126/science.273.5281.1551. http://www.ncbi.nlm.nih.gov/pubmed/8703217. [DOI] [PubMed] [Google Scholar]
- 40.Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. [accessed August 14, 2014];Curr Pharm Des. 2003 9:1565–74. doi: 10.2174/1381612033454658. http://www.ncbi.nlm.nih.gov/pubmed/12871068. [DOI] [PubMed] [Google Scholar]
- 41.Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J Cell Sci. 2009;122:4363–74. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 43.Nagle RB, Cress AE. Metastasis Update: Human Prostate Carcinoma Invasion via Tubulogenesis. Prostate Cancer. 2011;2011:249290. doi: 10.1155/2011/249290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furbert-Harris PM, Parish-Gause D, Hunter KA, Vaughn TR, Howland C, Okomo-Awich J, et al. Activated eosinophils upregulate the metastasis suppressor molecule E-cadherin on prostate tumor cells. [accessed August 06, 2014];Cell Mol Biol (Noisy-Le-Grand) 2003 49:1009–16. http://www.ncbi.nlm.nih.gov/pubmed/14682382. [PubMed] [Google Scholar]
- 45.Lombaerts M, van Wezel T, Philippo K, Dierssen JWF, Zimmerman RME, Oosting J, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–71. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. [accessed September 11, 2014];Cell Growth Differ. 2002 13:1–11. http://www.ncbi.nlm.nih.gov/pubmed/11801526. [PubMed] [Google Scholar]