Abstract
Introduction
Lung function is inversely associated with coronary heart disease (CHD) and cardiovascular disease (CVD). We evaluated the prospective association of reduced lung function by spirometry and CHD or CVD events in older community-dwelling adults.
Methods
We studied 1,548 participants (mean age 73.6±9.2 years, 42% males) from the Rancho Bernardo Study using age, sex, and risk-factor adjusted Cox regression to assess pulmonary function (FEV1, FVC, and FEV1/FVC ratio) as a predictor of CHD and CVD events followed for up to 22 years.
Results
Of CVD risk factors, older age, male sex, current/past smoking, physical exercise (<3x a week), and prevalent CVD predicted an increased risk of CHD and CVD. Higher FEV1 and FVC were each associated with a decreased risk of CHD [HR 0.80 (0.73-0.88) for both FEV1 and FVC, per SD, p<.01] and CVD [HR 0.82 (0.74-0.91) for both FEV1 and FVC, per SD, p<.01]. Those in the lowest quartiles of FEV1 and FVC had hazard ratios of 1.68 (1.33-2.13) and 1.55 (1.21-2.00) respectively for CHD and 1.74 (1.34-2.25) and 1.49 (1.13-1.96) respectively for CVD (all p<.01, relative to those in the highest quartile). Similar findings were observed for CHD and CVD mortality. Sex- and age-stratified analyses showed the strongest associations for CHD and CVD events in women and in the oldest participants.
Conclusions
FEV1 and FVC are inversely associated with risk of future CHD and CVD events in older community-dwelling adults and may add to CVD risk stratification in the elderly.
Keywords: Pulmonary Function Test, Coronary Heart Disease, Cardiovascular Disease, Epidemiology
Introduction
In spite of the current evidence-based approaches to cardiovascular disease (CVD) reduction, CVD and coronary heart disease (CHD) remain the leading cause of mortality in many industrialized countries. Previous epidemiological studies have shown reduced lung function is a significant predictor of CHD and CVD mortality1-3, as well as with all-cause mortality4-6. In one study of non-smokers, poor lung function was shown to be a better predictor of CVD and total mortality than established cardiovascular risk factors such as serum cholesterol7.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death and affects 65 million worldwide, and the primary cause of COPD is tobacco smoke8. Its predictive capacity for cardiovascular disease incidence and mortality has been well documented in the past9-11. Because spirometry is the usual prognostic tool for diagnosing COPD and its severity, it serves as a common risk factor in evaluating the association of lung function with CHD and CVD. As early as 1983, the Framingham Study identified FVC as a prognostic indicator for CVD, in which different FVC indexes could vary the risk of CVD death by a three- to four-fold range1. Reduced FEV1 has also been shown to predict cardiovascular morbidity and mortality in other studies3.
Our study evaluates the association of reduced lung function and CVD or CHD events within the Rancho Bernardo prospective study of CVD, a well-established cohort of older predominantly Caucasian adults. No study has been published on the association of lung function and CVD in the Rancho Bernardo study cohort, and there are limited data on the long-term prognostic significance of lung function in population-based cohorts of older adults accounting for prevalent CVD.
Methods
Between 1972 and 1974, 6629 adults representing 82% of adult residents in Rancho Bernardo, a suburban Southern California community, participated in the baseline examination of the Rancho Bernardo Heart and Chronic Disease Study (RBS)12. Nonrespondents tended to have more CVD and history of smoking, but less hyperlipidemia and family history of CVD.13 Residents were followed up in 1984-87 when 2479 participants attended a follow-up visit, and again in 1988-91 when new data on cardiovascular risk factors and pulmonary function (1988-91) were obtained. We studied 1548 participants from the Rancho Bernardo community-based cohort (mean age 73.6±9.2 years, 42% males, primarily Caucasian) to assess pulmonary risk factors associated with CHD and CVD events. We also included demographic and diverse health-related information including body mass index, blood pressure, cholesterol, diabetes, physical exercise, smoking, and pulmonary function tests. Pulmonary function tests were performed using a water-sealed spirometer (Warren E. Collins, Eagle models, Braintree, MA) by a specially trained graduate student (Catherine Frette) who adhered to the 1987 American Thoracic Society guidelines14 and performed from three to six tests to satisfy the ATS standards of acceptability and reproducibility. RBS participants were followed for a maximum of 22 years after spirometry. All participants gave written informed consent; the study was approved by the institutional review board of the University of California, San Diego.
Chronic obstructive pulmonary disease (COPD) risk groups were categorized as none (FEV1/FVC> 70%), mild (FEV1/FVC <70%, FEV1≥80%), and moderate/severe (FEV1/FVC <70%, FEV1<80%) according to the GOLD (Global Initiative for Chronic Obstructive Pulmonary Disease) criteria. Moderate and severe categories were combined due to the number of participants within these groups.
Prevalent CVD was defined as physician-diagnosed myocardial infarction, coronary artery revascularization, congestive heart failure, stroke or transient ischemic attack, carotid surgery, peripheral arterial surgery, or physician-diagnosed intermittent claudication. Total (or incident) CHD and CVD refer to time of non-fatal or fatal CHD or CVD, whichever occurred first. Non-fatal CHD was defined as heart attack, coronary bypass, or angioplasty, while CVD additionally included stroke, TIA, and peripheral artery revascularization. Classification of incident CHD and CVD events were based on history, physician diagnosis, and/or ECG criteria with vital status confirmed by death certificates with underlying cause of death coded by a certified nosologist using ICD-9. Validation of self-reported heart attack (by chest pain, enzyme elevation, and ECG) was achieved for 72% of a subset for whom hospital records could be obtained.
All analyses were done using SAS version 9.1.3 (SAS institute, Cary, NC). Analysis of variance (ANOVA) was used to compare means between different COPD risk groups for continuous variables, and chi-squared test of proportions was used to compare proportions between the risk groups for categorical variables. We also calculated CHD and CVD mortality per 1000 person years associated with quartile of FVC, FEV1, and ratio of FEV1/FVC. Cox proportional hazards regression was used to determine the risk of total CHD or CVD from the standard CVD risk factors: age, sex, systolic blood pressure, diastolic blood pressure, body mass index, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein (LDL-C), diabetes, smoking, exercise, COPD, and prevalent CVD, both adjusted and unadjusted for covariates. Additionally, we used Cox regression to assess the relationship of pulmonary function tests [forced expiratory volume in 1 minute (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio] and total CHD and CVD. Standardized hazard ratios (HR) are presented to compare the HR's of each measure per standard deviation increment. We then categorized FEV1 and FVC values into quartiles and compared each the 1st, 2nd, and 3rd quartiles with the 4th quartile using Cox regression for risk of CHD and CVD mortality. Finally, we performed gender and age stratified analyses for CHD and CVD outcomes. All tests of pulmonary function variables were adjusted for standard CVD risk factors.
Results
In our study, there were significant differences in risk factors according to COPD severity. With increasing COPD group severity, the following trends were observed: older age, lower FEV1, lower FVC, higher rate of CHD/CVD death, higher proportion of CVD death, higher proportion of prevalent CHD/CVD, higher proportion of current and past smokers, and lower proportion of those who reported that they exercised regularly (Table 1).
Table 1.
Means and proportions for individuals with different severities of COPD
| Total (n=1548) 100% | No COPD (n=1165) 75.3% | Mild COPD (n=252) 16.3% | Moderate and Severe COPD (n=131) 8.5% | P value | |
|---|---|---|---|---|---|
|
Means ± Standard Deviation | |||||
| Age (yr) | 73.6±9.2 | 72.8±9.3 | 75.4±8.6 | 77.1±8.2 | <.0001*** |
| SBP (mmHg) | 135.7±20.3 | 135.1±20.4 | 136.9±20.7 | 138.7±18.4 | 0.0303* |
| DBP (mmHg) | 75.8±9.2 | 75.7±9.2 | 75.7±9.4 | 75.9±9.1 | 0.8788 |
| BMI (kg/m^2) | 25.1±3.7 | 25.3±3.8 | 24.2±3.3 | 24.6±4.0 | 0.0002*** |
| Cholesterol (mg/dL) | 222.1±39.9 | 224.6±40.0 | 213.0±37.6 | 218.1±40.4 | 0.0006*** |
| HDL (mg/dL) | 62.7±18.7 | 62.6±18.6 | 62.6±19.4 | 63.7±18.2 | 0.5835 |
| LDL (mg/dL) | 136.4±36.7 | 138.6±37.3 | 128.0±33.4 | 131.9±35.0 | 0.0003*** |
| FEV1 (L) | 2.3±0.8 | 2.4±0.7 | 2.3±0.7 | 1.2±0.5 | <.0001*** |
| FVC (L) | 3.1±0.9 | 3.1±0.9 | 3.5±1.0 | 2.4±0.8 | 0.0002*** |
| FEV1/FVC ratio *100 | 74.2±10.4 | 78.7±5.0 | 64.5±4.6 | 52.8±13.7 | <.0001*** |
| Follow-up CHD Incidence (yr) | 10.5±5.8 | 11.0±5.7 | 9.7±5.6 | 7.9±5.4 | <.0001*** |
| Follow-up CVD Incidence (yr) | 10.1±5.8 | 10.6±5.8 | 9.3±5.6 | 7.6±5.3 | <.0001*** |
| Follow-up Mortality, CHD or CVD (yr) | 12.2±5.9 | 12.8±5.7 | 11.3±5.8 | 8.6±5.7 | <.0001*** |
|
Proportions [%(n)] | |||||
| Male | 41.5 (642) | 37.8 (440) | 56.4 (142) | 45.8 (131) | <.0001*** |
| Diabetes | 12.5 (193) | 12.9 (150) | 10.3 (26) | 13.0 (17) | 0.5283 |
| Current Smokers | 9.1(141) | 6.8 (79) | 15.5 (39) | 17.6 (23) | <.0001*** |
| Past Smokers | 47.8 (740) | 44.3 (516) | 56.8 (143) | 61.8 (81) | |
| Ex 3x a week | 34.0 (527) | 36.4 (424) | 31.8 (80) | 17.6 (23) | <.0001*** |
| CHD Death | 11.2 (173) | 10.3 (120) | 13.1 (33) | 15.3 (20) | 0.1324 |
| CVD Death | 26.7 (413) | 24.1 (281) | 32.5 (82) | 38.2 (50) | 0.0002*** |
| Prevalent CHD | 18.4 (284) | 16.7 (195) | 21.0 (53) | 27.5 (36) | 0.0052** |
| Prevalent CVD | 24.2 (375) | 22.4 (261) | 25.8 (65) | 37.4 (49) | 0.0006*** |
| Total CHD | 19.8 (250) | 19.5 (189) | 22.1 (44) | 17.9 (17) | 0.6227 |
| Total CVD | 39.8 (467) | 38.3 (346) | 45.5 (85) | 43.9 (36) | 0.1388 |
p<.05
p<.01
p<.001
Of the standard risk factors, age, male sex, current/past smoking, <3x a week physical exercise, and prevalent CVD were significantly associated with total CHD and CVD, in addition to higher LDL-C and having COPD for only total CHD (Table 2). When controlled for standard risk factors, higher FEV1 and FVC were significantly associated with decreased risk of both CHD [HR 0.80 (0.73-0.88) for FEV1 and FVC, per SD, p<.01] and CVD [HR 0.82 (0.74-0.91) for FEV1 and FVC, per SD, p<.01] (Table 3). Of note, since FEV1 and FVC are often adjusted for height, adjustment for height instead of BMI resulted in virtually identical results (data not shown). Additionally, the effects appear stronger in women and in older persons after adjustments (Table 3). The upper cutpoints for the quartiles of FEV1 were 1.80 liters, 2.25 liters, and 2.78 liters, and the upper cutpoints for the quartiles of FVC were 2.41 liters, 2.98 liters, and 3.70 liters. Those in the lowest quartiles of FEV1 and FVC had hazard ratios of 1.68 (1.33-2.13) and 1.55 (1.21-2.00) respectively for CHD and hazards ratios of 1.74 (1.34-2.25) and 1.49 (1.13-1.96) for CVD (all p<.01, relative to those in the highest quartile) (Table 4). The FEV1/FVC ratio was inversely associated with CHD and CVD events in unadjusted analyses, but not after adjustment for standard risk factors and prevalent CVD.
Table 2.
Standard Risk Factors: Standardized Hazards Ratio Survival Analysis
| Total CHD HR | CHD Death HR | Total CVD HR | CVD Death HR | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Age | 1.74** | 1.71** | 1.79** | 1.78** | 1.64** | 1.59** | 1.54** | 1.55** |
| Gender (Male vs. Female) | 1.12* | 1.16* | 1.17 | 1.32** | 1.14* | 1.18* | 1.16* | 1.33** |
| Systolic Blood Pressure | 1.14** | 0.95 | 1.14** | 0.98 | 1.12** | 0.96 | 1.06** | 0.96 |
| Diastolic Blood Pressure | 0.91** | 0.98 | 0.90** | 0.94 | 0.89** | 0.95 | 0.89** | 0.93 |
| Body Mass Index | 0.97 | 0.97 | 0.95 | 0.95 | 0.98 | 0.99 | 0.97 | 0.98 |
| HDL Cholesterol | 0.97 | 0.97 | 0.98 | 0.99 | 0.97 | 1.02 | 1.00 | 1.03 |
| LDL Cholesterol | 0.94* | 0.92** | 0.96 | 0.95 | 0.96 | 0.95 | 0.97 | 0.97 |
| Diabetes (Yes vs. No) | 1.24* | 1.06 | 1.24* | 1.05 | 1.24* | 1.06 | 1.15 | 1.01 |
| Smoking (Current/Past vs. Never) | 1.06 | 1.14** | 1.08 | 1.16** | 1.11* | 1.17** | 1.12* | 1.17** |
| Exercise 3x a week (Yes vs. No) | 0.60** | 0.71** | 0.64** | 0.72** | 0.60** | 0.69** | 0.64** | 0.69** |
| COPD (Yes vs. No) | 1.31** | 1.11* | 1.40** | 1.16** | 1.31** | 1.09 | 1.31** | 1.12* |
| Prevalent CVD | 1.51** | 1.27** | 1.27** | 1.05 | 1.91** | 1.60** | 1.21* | 1.05 |
p<.05
p<.01, Hazards ratios are expressed per standard deviation.
Table 3.
Pulmonary Function Estimates: Standardized Hazards Ratio Survival Analysis
| Adjusted vs. Unadjusted | Total CHD HR (95% CI) | CHD Death HR (95% CI) | Total CVD HR (95% CI) | CVD Death HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| FEV1 (Actual) | 0.73** (0.69-0.78) | 0.80** (0.73-0.88) | 0.74** (0.69-0.79) | 0.77** (0.70-0.85) | 0.75** (0.70-0.81) | 0.82** (0.74-0.91) | 0.80** (0.75-0.86) | 0.81** (0.73-0.90) |
| FVC (Actual) | 0.80** (0.75-0.84) | 0.80** (0.73-0.88) | 0.81** (0.77-0.86) | 0.79** (0.72-0.87) | 0.82** (0.77-0.87) | 0.82** (0.74-0.91) | 0.86** (0.81-0.92) | 0.82** (0.74-0.91) |
| FEV1/FVC ratio | 0.83** (0.79-0.88) | 0.96 (0.90-1.03) | 0.81** (0.77-0.86) | 0.93* (0.88-0.99) | 0.83** (0.78-0.89) | 0.97 (0.90-1.04) | 0.85** (0.79-0.90) | 0.95 (0.89-1.03) |
| Adjusted, Stratified by Gender | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Male | Female | Male | Female | Male | Female | Male | Female |
| FEV1 (Actual) | 0.82** (0.71-0.93) | 0.81** (0.71-0.94) | 0.82** (0.71-0.94) | 0.73** (0.63-0.83) | 0.84* (0.73-0.97) | 0.82* (0.71-0.96) | 0.88 (0.75-1.03) | 0.74** (0.64-0.86) |
| FVC (Actual) | 0.82** (0.73-0.93) | 0.81** (0.70-0.93) | 0.85* (0.76-0.96) | 0.73** (0.64-0.84) | 0.84** (0.73-0.96) | 0.82* (0.70-0.96) | 0.90 (0.78-1.03) | 0.74** (0.64-0.86) |
| FEV1/FVC ratio | 0.94 (0.84-1.05) | 0.96 (0.89-1.04) | 0.91 (0.82-1.02) | 0.93 (0.86-1.01) | 0.95 (0.85-1.07) | 0.96 (0.87-1.05) | 0.95 (0.84-1.08) | 0.94 (0.86-1.03) |
| Adjusted, Stratified by Median Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Age ≤74.2 | Age>74.2 | Age ≤74.2 | Age>74.2 | Age ≤74.2 | Age>74.2 | Age ≤74.2 | Age>74.2 |
| FEV1 (Actual) | 0.83** (0.73-0.95) | 0.62** (0.54-0.70) | 0.82** (0.72-0.93) | 0.59** (0.52-0.67) | 0.86* (0.75-0.99) | 0.63** (0.54-0.72) | 0.85* (0.75-0.97) | 0.64** (0.55-0.75) |
| FVC (Actual) | 0.85* (0.75-0.97) | 0.61** (0.54-0.70) | 0.86* (0.76-0.97) | 0.60** (0.53-0.68) | 0.89 (0.78-1.01) | 0.61** (0.53-0.70) | 0.89 (0.78-1.00) | 0.64** (0.55-0.74) |
| FEV1/FVC ratio | 0.73 (0.83-1.04) | 0.94 (0.87-1.02) | 0.90* (0.81-1.00) | 0.92* (0.85-1.00) | 0.93 (0.82-1.05) | 0.95 (0.87-1.04) | 0.92 (0.82-1.03) | 0.95 (0.86-1.05) |
Variables adjusted for standard risk factors: age, systolic blood pressure, diastolic blood pressure, body mass index, HDL, LDL, sex, diabetes, smoking, exercise, and prevalent CVD
p<.05
p<.01, Hazards ratios are expressed per standard deviation.
Table 4.
Adjusted Hazards Ratio on risk of CHD/CVD Outcomes
| Total CHD HR (95% CI) | CHD Death HR (95% CI) | Total CVD HR (95% CI) | CVD Death HR (95% CI) | |
|---|---|---|---|---|
| FEV1 | ||||
| FEV1 Q3 vs. Q4 | 1.26* (1.06-1.51) | 1.23* (1.03-1.47) | 1.27* (1.05-1.54) | 1.18 (0.97-1.44) |
| FEV1 Q2 vs. Q4 | 1.20 (0.98-1.46) | 1.25* (1.02-1.52) | 1.15 (0.92-1.43) | 1.19 (0.95-1.48) |
| FEV1 Q1 vs. Q4 | 1.68** (1.33-2.13) | 1.83** (1.45-2.31) | 1.74** (1.34-2.25) | 1.75** (1.35-2.26) |
| FVC | ||||
| FVC Q3 vs. Q4 | 1.19 (1.00-1.43) | 1.16 (0.97-1.38) | 1.11 (0.92-1.35) | 1.13 (0.93-1.38) |
| FVC Q2 vs. Q4 | 1.21 (0.97-1.51) | 1.20 (0.96-1.50) | 1.06 (0.83-1.37) | 1.11 (0.87-1.43) |
| FVC Q1 vs. Q4 | 1.55** (1.21-2.00) | 1.69** (1.32-2.16) | 1.49** (1.13-1.96) | 1.68** (1.28-2.21) |
Variables adjusted for standard CVD risk factors: age, systolic blood pressure, diastolic blood pressure, body mass index, HDL, LDL, sex, diabetes, smoking, exercise, and prevalent CVD
p<.05
p<.01
We attained similar results when isolating the CHD and CVD mortality outcomes specifically. Age (HR 1.78 CHD/ 1.55 CVD, per SD, p<.01) and sex (HR 1.32 CHD/ 1.33 CVD, male vs. female, p<.01) had the strongest positive associations, while exercise 3x a week (0.72 CHD/0.69 CVD, p<.01) had the strongest negative association. A history of COPD was significantly associated with CHD but not CVD death. When controlled for standard risk factors, higher FEV1 and FVC were significantly associated with decreased risk of both CHD and CVD death. FEV1/FVC ratio was only mildly associated with CHD death. Those in the lowest quartiles of FEV1 and FVC had the highest hazards ratios for CHD and CVD death (all p<.01, relative to those in the highest quartile) (Table 4).
Secondary analyses were also conducted with the prevalent CVD cases omitted from the cross-sectional analyses (new sample 1,173). Adjusted hazards ratios for FEV1 and FVC were 0.80 (0.72-0.89) and 0.81 (0.73-0.90) respectively for CHD death and 0.83 (0.74-0.94) and 0.84 (0.75-0.94) respectively for CVD death, all p<.01. FEV1/FVC ratio was not statistically significant for either endpoint. In quartile analysis, those in the lowest quartiles of FEV1 and FVC had hazard ratios of 1.54 (1.17-2.01) and 1.64 (1.24-2.18) respectively for CHD death and hazards ratios of 1.54 (1.14-2.07) and 1.63 (1.19-2.22) for CVD Death (all p<.01, compared to those in the highest quartile).
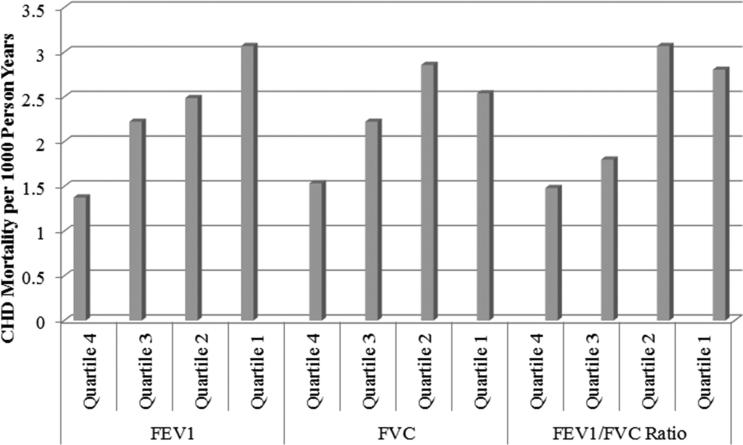
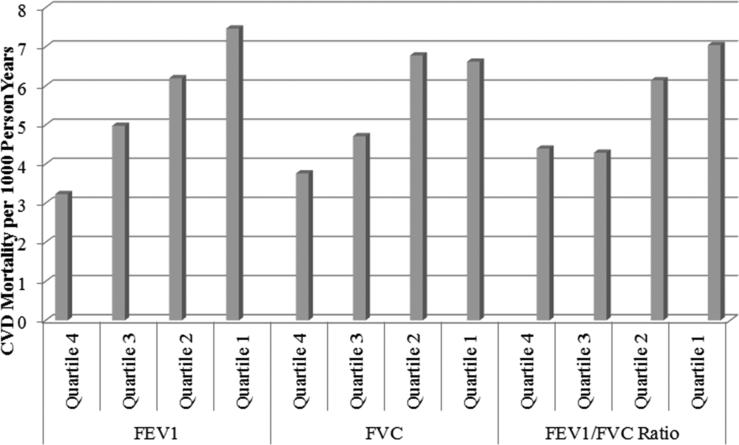
From highest to lowest quartiles of FVC and FEV1, rates of CHD mortality (per 1000 person years) ranged from 1.53 to 2.54 for FVC and 1.38 to 3.07 for FEV1 and rates of CVD mortality from 3.76 to 6.62 for FVC and 3.23 to 7.46 for FEV1 (Figures 1 and 2). For the FEV1/FVC ratio from highest to lowest quartiles, rates of CHD mortality varied from 1.48 to 2.80 and CVD mortality from 4.39 to 7.04, respectively.
Figure 1.
Quartile Comparison of Pulmonary Function Indicators with CHD Mortality
Figure 2.
Quartile Comparison of Pulmonary Function Indicators with CVD Mortality
Discussion
Our study is the first to evaluate the long-term prognostic significance of lung spirometry in a large and relatively healthy community-dwelling cohort of older adults followed for up to 22 years. We report here the potential clinical utility of these measures examining the association between classic CVD risk factors and the severity of COPD. We demonstrated the strong and similar prognostic value of FEV1 and FVC for total CHD and CVD and for mortality from CHD and CVD. Specifically, increased COPD severity, defined by spirometry, was significantly correlated with many CVD risk factors including age, SBP (systolic blood pressure), male sex, lack of exercise, and current or past smoking and was significantly associated with increased CVD mortality.
Although several CVD risk factors such as cholesterol and BMI showed significant differences among COPD severity groups, there were no clear trends. While past studies have suggested that cholesterol is a risk factor for CVD but not COPD15, BMI has been previously established as an independent prognostic factor in all-cause mortality among COPD individuals16-19.
After adjustment, many of the same COPD severity factors (older age, male sex, current/past smoking, <3x a week physical exercise) were significantly associated with incidence/death of CHD and CVD. Prevalent CVD was a predictor of future CHD or CVD cases, but not mortality. The prevalence of COPD itself was significantly associated with higher risk of CHD incidence and death. Both FEV1 and FVC were strongly and inversely associated with the risk of CHD and CVD outcomes after adjusting for standard risk factors; however, the FEV1/FVC ratio commonly used in diagnosis of lung disease, while predictive of outcomes in unadjusted analyses, did not remain significantly associated with outcomes after adjustment for risk factors and CVD. It is possible that reduced variability in the FEV1/ FVC ratio in our largely healthy cohort (75% without COPD) and adjustment for risk factors that could confound the relation of the FEV1/FVC ratio with outcomes may explain this.
FVC has been suggested to add predictive power to CHD mortality over traditional CHD risk factors20; the current study confirms that higher FVC is associated with decreased risk of CHD and CVD death after controlling for standard cardiovascular risk factors. In the Framingham study, Kannel et al. reported that risk of congestive heart failure was significantly increased among those in the lower quartiles of FVC; we similarly observed increased CHD and CVD death among those with lower FVC in our cohort. FEV1 is a well-studied risk factor for CVD1,3,19-26; our study confirms these relationships and found that the negative association was particularly prominent in the highest risk quartile (Q1), which increased the risks of CVD death and CHD death nearly two-fold compared to the lowest risk quartile (Q4). The magnitude of FEV1 and FVC in predicting these outcomes was not impacted heavily by those without baseline CVD, as indicated in our secondary analysis which yielded similar numbers after excluding individuals with prevalent CVD. Importantly, Lundback and colleagues in the Obstructive Lung Disease in Northern Sweden (OLIN) Study have shown the strong prognostic significance of COPD; the survival after 20 years was only 19% in those with severe and very severe COPD, and disease severity and concomitant ischemic heart disease or heart failure at entry was significantly predictive of death.27
We additionally performed analyses stratified by sex and age, and reported that these associations were strongest among women and older individuals in our study. Despite an overall lower risk of CVD death in women in our study, the strength of the inverse correlation of pulmonary function with cardiovascular outcomes was slightly greater in women which may have implications for preventative measures in pulmonary disease treatment. The Framingham study1 reflects our results showing that women had stronger associations than men of low vital capacity index with CVD incidence. The stronger associations among the oldest individuals support the particular utility of using spirometry to assess CVD risk as a primary prevention tool in the elderly.
There are several strengths and limitations to our study. Our measures of spirometry used three to six tests with the ATS standard of acceptability and reproducibility, although was limited by the single visit where lung function measures were obtained. Thus, we were unable to examine the prognostic significance of worsening of COPD as could be possible from serial measures of pulmonary function.27 Additionally, only a limited number of participants met the classifications of moderate and severe COPD, so we had limited statistical power to examine the impact of severe COPD separately. Furthermore, the sample of upper middle class, predominately older Caucasian participants, nearly all of whom had health insurance, should not be generalized to more diverse populations. On the other hand, baseline data were collected at a time when individuals of this community were less informed about healthy lifestyle habits or not taking effective medications for cholesterol and blood pressure, making the associations less confounded compared to more recent years, an advantage compared to more contemporary cohorts.
In conclusion, FEV1 and FVC are inversely associated with risk of future CHD and CVD outcomes in older community-dwelling adults, largely independent of classic cardiovascular risk factors, and may be useful measures for risk stratification in older adults.
Highlights.
We studied 1548 older adults from the Rancho Bernardo prospective study.
Participants had measures of pulmonary function and up to 22 year follow-up.
Higher FEV1 and FVC were associated with a decreased risk of incident CHD and CVD.
Lung function measures may be useful in assessing CHD or CVD risk in older persons.
Acknowledgements
The research for this Rancho Bernardo Study was supported by grants from NIH [National Institutes of Health/National Institute on Aging grants AG07181 and AG028507] and the National Institute of Diabetes and Digestive and Kidney Diseases grant DK31801. The authors thank the participants of the Rancho Bernardo Study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest for any of the authors.
References
- 1.Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: The Framingham study. American Heart Journal. 1983;105(2):311–315. doi: 10.1016/0002-8703(83)90532-x. doi:10.1016/0002-8703(83)90532-X. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder EB, Welch VL, Couper D, et al. Lung function and Incident Coronary Heart Disease The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158(12):1171–1181. doi: 10.1093/aje/kwg276. doi:10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Wu L, Man SFP. The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. doi:10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 4.Higgins MW, Keller JB. Predictors of Mortality in the Adult Population of Tecumseh. Archives of Environmental Health: An International Journal. 1970;21(3):418–424. doi: 10.1080/00039896.1970.10667260. doi:10.1080/00039896.1970.10667260. [DOI] [PubMed] [Google Scholar]
- 5.Hole DJ, Watt GCM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. doi:10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tockman MS, Comstock GW. Respiratory risk factors and mortality: longitudinal studies in Washington County, Maryland. Am Rev Respir Dis. 1989;140(3 Pt 2):S56–63. doi: 10.1164/ajrccm/140.3_Pt_2.S56. doi:10.1164/ajrccm/140.3_Pt_2.S56. [DOI] [PubMed] [Google Scholar]
- 7.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Spirometric findings and mortality in never-smokers. Journal of Clinical Epidemiology. 1990;43(9):867–873. doi: 10.1016/0895-4356(90)90070-6. doi:10.1016/0895-4356(90)90070-6. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Chronic obstructive pulmonary disease (COPD) fact sheet. World Health Organization; [December 19, 2012]. website. www.who.int/mediacentre/factsheets/fs315/en/index.html. Published November 2012. [Google Scholar]
- 9.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001*. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. doi:10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 10.Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. doi:10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 11.Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart. 2012;98(14):1055–1062. doi: 10.1136/heartjnl-2012-301759. doi:10.1136/heartjnl-2012-301759. [DOI] [PubMed] [Google Scholar]
- 12.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–12. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 13.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108:367–72. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 14.Frette C, Barrett-Connor E, Clausen JL. Effect of active and passive smoking on ventilatory function in elderly men and women. Am J Epidemiol. 1996;143(8):757–765. doi: 10.1093/oxfordjournals.aje.a008813. [DOI] [PubMed] [Google Scholar]
- 15.Lee HM, Lee J, Lee K, Luo Y, Sin DD, Wong ND. Relation between COPD severity and global cardiovascular risk in US adults. Chest. 2012;142(5):1118–1125. doi: 10.1378/chest.11-2421. doi:10.1378/chest.11-2421. [DOI] [PubMed] [Google Scholar]
- 16.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. doi:10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body Weight in Chronic Obstructive Pulmonary Disease: The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. American Review of Respiratory Disease. 1989;139(6):1435–1438. doi: 10.1164/ajrccm/139.6.1435. doi:10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 18.Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):961–966. doi: 10.1164/ajrccm.153.3.8630580. doi:10.1164/ajrccm.153.3.8630580. [DOI] [PubMed] [Google Scholar]
- 19.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017. doi:10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J. 2010;36(5):1002–1006. doi: 10.1183/09031936.00042410. doi:10.1183/09031936.00042410. [DOI] [PubMed] [Google Scholar]
- 21.Persson C, Bengtsson C, Lapidus L, Rybo E, Thiringer G, Wedel H. Peak Expiratory Flow and Risk of Cardiovascular Disease and Death a 12-Year Follow-up of Participants in the Population Study of Women in Gothenburg, Sweden. Am J Epidemiol. 1986;124(6):942–948. doi: 10.1093/oxfordjournals.aje.a114483. [DOI] [PubMed] [Google Scholar]
- 22.Cook DG, Shaper AG. Breathlessness, lung function and the risk of heart attack. Eur Heart J. 1988;9(11):1215–1222. doi: 10.1093/oxfordjournals.eurheartj.a062432. [DOI] [PubMed] [Google Scholar]
- 23.Ebi-Kryston KL. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall study. Journal of Clinical Epidemiology. 1988;41(3):251–260. doi: 10.1016/0895-4356(88)90129-1. doi:10.1016/0895-4356(88)90129-1. [DOI] [PubMed] [Google Scholar]
- 24.Knuiman MW, James AL, Divitini ML, Ryan G, Bartholomew HC, Musk AW. Lung Function, Respiratory Symptoms, and Mortality: Results from the Busselton Health Study. Annals of Epidemiology. 1999;9(5):297–306. doi: 10.1016/s1047-2797(98)00066-0. doi:10.1016/S1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Ockene JK, Townsend M, Browner W, Meilahn E, Wentworth DN. The epidemiology of pulmonary function and COPD mortality in the multiple risk factor intervention trial. Am Rev Respir Dis. 1989;140(3 Pt 2):S76–81. doi: 10.1164/ajrccm/140.3_Pt_2.S76. doi:10.1164/ajrccm/140.3_Pt_2.S76. [DOI] [PubMed] [Google Scholar]
- 26.Keys A, Aravanis C, Blackburn H, et al. Lung function as a risk factor for coronary heart disease. Am J Public Health. 1972;62(11):1506–1511. doi: 10.2105/ajph.62.11.1506. doi: 10.1089/dna.2011.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundback B, Eriksson B, Lindberg A, Ekerljung L, Muellerova H, Larsson LG, Ronmark E. A 20-year follow-up of a population-based COPD cohort-report from the obstructive lung disease in Northern Sweden studies. J of COPD. 2009;6:263–71. doi: 10.1080/15412550903061483. [DOI] [PubMed] [Google Scholar]