Abstract
Objective
To evaluate the association of technological capacity with prostate cancer quality of care. Technological capacity was conceptualized as a market’s ability to provide prostate cancer treatment with new technology, including robotic prostatectomy and intensity-modulated radiotherapy (IMRT).
Methods
In this retrospective cohort study, we used data from the Surveillance Epidemiology and End Results (SEER) – Medicare linked database from 2004–2009 to identify men with newly diagnosed prostate cancer (n=46,274). We measured technological capacity as the number of providers performing robotic prostatectomy or IMRT per population in a healthcare market. We used multilevel logistic regression to assess the association of technological capacity with receiving quality care according to a set of nationally endorsed quality measures, while adjusting for patient and market characteristics.
Results
Overall, our findings were mixed with only subtle differences in quality of care when comparing high-tech to low-tech markets. High robotic prostatectomy capacity was associated with better adherence to some quality measures, such as avoiding unnecessary bone scans (79.8% vs. 73.0%, p=0.003) and having follow-up with urologists (67.7% vs. 62.6%, p=0.023). However, for most measures, neither high robotic prostatectomy nor high IMRT capacity were associated with significant increases in adherence rates. In fact, for one measure (treatment by a high-volume provider), high IMRT capacity was associated with lower performance (23.4% vs. 28.5%, p<0.001).
Conclusion
Our findings suggest that new technology is not clearly associated with higher quality of care. To improve quality, more specific efforts will be needed.
Keywords: prostate cancer, technology, quality of care, prostatectomy, IMRT
Introduction
New technology has transformed prostate cancer treatment over the last decade. For example, the proportion of radical prostatectomy procedures performed with robotic assistance increased from less than ten percent in 2004 to 67% in 2010.1 Similarly, intensity-modulated radiotherapy (IMRT) has largely supplanted more traditional treatment with three dimensional radiotherapy, with a more than tenfold increase in the use of IMRT from 2001 to 2007.2 Although these new technologies come at a significant investment cost of $2–3 million, they offer the promise of increased effectiveness and decreased toxicity. For example, IMRT delivers higher treatment doses with similar or less side effects.3–5
However, the dissemination of these new technologies may have additional indirect effects on the quality of care for men with prostate cancer. It is widely perceived that physicians at the forefront of technological innovation deliver the highest quality care.6–9 This perception is likely due to the fact that these physicians practice at specialized, high-volume hospitals,10,11 which also tend to be associated with quality. For instance, patients undergoing prostatectomy at high volume hospitals have shorter length of stay, fewer postoperative urinary complications, and lower in-hospital mortality.12,13 These associations suggest that physicians at specialized technologically advanced centers are more compliant with important processes of care. However, as technology disseminates more widely, the link between technology and quality may not persist. While new technology may increase a provider’s focus on prostate cancer and thus heighten awareness of guidelines, the pressure to recoup high upfront investment costs may also lead to overuse of well-reimbursed services (e.g. imaging) and decreased use of less lucrative care (e.g. follow-up visits).
For these reasons, we evaluated the extent to which technological capacity – conceptualized as a market’s ability to provide prostate cancer treatment with new technology – is associated with prostate cancer quality of care. Understanding this association provides valuable information for patients, who are interested in where they can obtain the highest quality care. Policymakers will appreciate a better understanding of the downstream effects of technology dissemination as they consider regulation of other new technologies.
Methods
Study population
We used Surveillance, Epidemiology, and End Results (SEER) – Medicare data for the years 2004 through 2009 to identify patients with newly diagnosed localized prostate cancer. We only included patients 66 years of age and older to allow for accurate measurement of baseline comorbidity status during the year preceding the diagnosis. Further, only patients in the fee-for-service program eligible for Parts A and B of Medicare for at least 12 months before and after prostate cancer diagnosis were included. Finally, we limited our study population to patients treated with radiotherapy or prostatectomy, because the quality measures only apply to these patients.14,15 Using these criteria, our study population consisted of 46,274 patients who were followed through December 31, 2010.
Measuring technological capacity
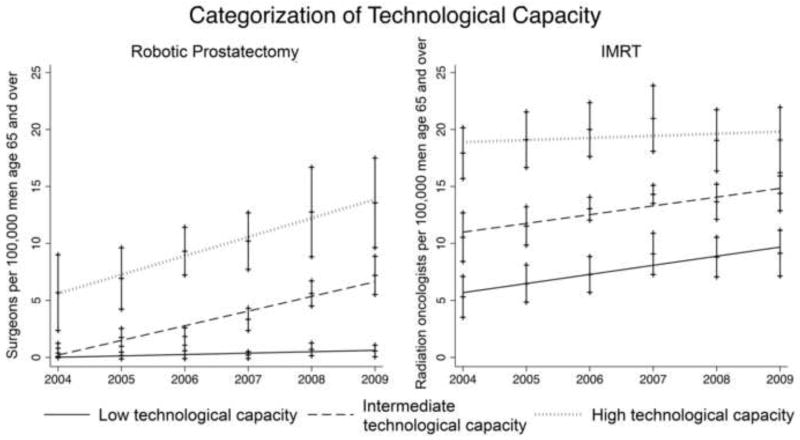
As previously described, we measured technological capacity at the level of the healthcare market.16,17 Healthcare markets were defined as Hospital Referral Regions (HRRs), which represent geographical areas in which Medicare beneficiaries typically receive their tertiary care.18 Patients and physicians (urologists and radiation oncologists) were assigned to their respective HRR based on their ZIP code. We then defined technological capacity as the number of urologists or radiation oncologists per population offering treatment with robotic prostatectomy or IMRT, respectively. Urologists and radiation oncologists offering treatment with robotic prostatectomy or IMRT were identified based on the Medicare claims submitted by these physicians. Technological capacity was calculated separately for each HRR and year. Finally, HRRs were categorized as low, medium, or high technological capacity based on tertiles of the continuous technological capacity measure (Figure 1).16,17 To validate the measure of robotic capacity, we assessed the correlation between our claims-based measure and data on the number of robotic surgeons retrieved from historical Intuitive Surgical websites (the sole manufacturer of the DaVinci Robot) and found a high level of agreement (r=0.81).16,17
Figure 1.
Healthcare markets (defined by Hospital Referral Regions) were categorized into those with low, intermediate, or high technological capacity, based on the number of providers offering robotic prostatectomy or intensity-modulated radiotherapy (IMRT) per population. Technological capacity increased significantly over the study period from 2004 to 2009 (p<0.001 for time-trends). Vertical bars represent 95% confidence intervals.
Measuring quality of care
As previously described,19 we used a set of nationally endorsed prostate cancer quality of care measures to provide a comprehensive view of prostate cancer care (see column headers in Table 1). These measures were initially developed by RAND in 2000,20,21 and several of them were subsequently endorsed by the National Quality Forum (NQF) and the Physician Consortium for Performance Improvement (PCPI).14,15 They encompass structure and outcome measures as originally defined by Donabedian.22 According to Donabedian, structure measures assess the setting in which care takes place, while process measures evaluate whether what is known to be “good” medical care has been 22 Specifically, applied. we assessed (1) the proportion of patients seen by both a urologist and a radiation oncologist between diagnosis and start of treatment (RAND process measure), (2) the proportion of patients with low-risk cancer avoiding receipt of a non-indicated bone scan (PCPI process measure endorsed by NQF), (3) the proportion of patients with high-risk cancer receiving adjuvant androgen deprivation therapy while undergoing radiotherapy (PCPI process measure endorsed by NQF), (4) the proportion of patients treated by a high volume provider (RAND structure measure), and (5) the proportion of patients having at least two follow-up visits with a treating radiation oncologist or urologist (RAND process measure).19
Table 1.
Adherence to quality measures stratified by patient and market characteristics. Quality measures: (1) proportion of patients seen by both a urologist and a radiation oncologist between diagnosis and start of treatment (“pretx counseling”), (2) proportion of patients with low-risk cancer avoiding receipt of a non-indicated bone scan (“bone scan”), (3) proportion of patients with high-risk cancer receiving adjuvant androgen deprivation therapy while undergoing radiotherapy (“adjuvant ADT”), (4) proportion of patients treated by a high volume provider (“high-volume provider”), (5a) proportion of patients having at least two follow-up visits with a treating radiation oncologist (“f/u with radonc”), (5b) proportion of patients having at least two follow-up visits with a treating urologist (“f/u with urologist”). Abbreviations: nr: not reported because of low numbers (<100) in the denominator; na: not applicable.
| Characteristics | N | % of patients receiving care adherent to quality measure | |||||
|---|---|---|---|---|---|---|---|
| (1) pretx counseling | (2) bone scan | (3) adjuvant ADT | (4) high-volume provider | (5a) f/u with radonc | (5b) f/u with urologist | ||
| Overall | 46,274 | 53.2 | 67.5 | 75.5 | 32.1 | 55.0 | 64.0 |
| Patient characteristics | |||||||
| Age, years | |||||||
| 66–69 | 15,842 | 42.7* | 69.6* | 71.1* | 32.1 | 57.8* | 62.9 |
| 70–74 | 16,838 | 53.9* | 67.1* | 74.1* | 32.5 | 56.2* | 65.6 |
| 75–79 | 9,916 | 64.0* | 66.1* | 77.3* | 31.7 | 53.6* | 66.5 |
| 80–84 | 3,140 | 66.8* | 57.0* | 80.3* | 31.6 | 48.2* | nr |
| 85+ | 538 | 62.3* | nr | 83.5* | 30.6 | 45.0* | nr |
| Race/ethnicity | |||||||
| White | 38,428 | 53.6 | 68.2** | 75.3 | 32.4** | 55.2 | 63.4 |
| Black | 4,374 | 52.1 | 63.6** | 74.6 | 29.9** | 55.8 | 68.7 |
| Hispanic | 848 | 49.1 | 62.7** | 84.2 | 24.9** | 51.6 | 69.6 |
| Asian | 1,226 | 50.5 | 68.8** | 77.1 | 33.9** | 53.1 | 67.4 |
| Other/unknown | 1,398 | 51.6 | 60.5** | 75.9 | 34.3** | 50.0 | 64.6 |
| Comorbidity | |||||||
| 0 | 30,711 | 51.5* | 68.8* | 75.0 | 32.6* | 55.2 | 63.2 |
| 1 | 10,369 | 55.6* | 65.1* | 75.7 | 31.3* | 56.1 | 66.2 |
| 2 | 3,246 | 57.5* | 64.8* | 76.4 | 31.1* | 52.6 | 68.4 |
| 3+ | 1,948 | 60.2* | 62.7* | 78.5 | 29.6* | 51.3 | 64.3 |
| D’Amico risk | |||||||
| Low | 12,585 | 54.9* | 67.5 | na | 35.1* | 59.2* | 63.4 |
| Intermediate | 16,484 | 51.6* | na | na | 33.2* | 54.7* | 62.9 |
| High | 12,266 | 57.2* | na | 75.5 | 28.4* | 50.8* | 64.6 |
| Year of diagnosis | |||||||
| 2004 | 7,812 | 52.1 | 67.5 | 77.4 | 33.0 | 56.3 | 67.9 |
| 2005 | 7,519 | 53.3 | 67.4 | 72.4 | 28.6 | 55.0 | 65.6 |
| 2006 | 8,148 | 53.5 | 66.2 | 76.1 | 32.6 | 55.8 | 63.0 |
| 2007 | 8,398 | 52.8 | 66.1 | 76.5 | 35.4 | 54.4 | 59.2 |
| 2008 | 7,466 | 52.1 | 69.0 | 74.8 | 33.0 | 54.6 | 64.0 |
| 2009 | 6,931 | 55.7 | 70.1 | 75.8 | 29.4 | 52.8 | 65.1 |
| Socioeconomic status25 | |||||||
| Low | 13,377 | 50.4* | 64.2* | 75.6 | 27.3* | 53.5* | 66.2 |
| Medium | 15,331 | 54.8* | 68.8* | 76.1 | 31.7* | 54.4* | 63.5 |
| High | 16,477 | 54.1* | 68.9* | 75.0 | 36.2* | 56.7* | 62.9 |
| Residing in urban area | |||||||
| non-urban | 3,831 | 53.9 | 62.7* | 76.4 | 18.8* | 46.5* | 60.9 |
| urban | 42,434 | 53.2 | 68.0* | 75.4 | 33.3* | 55.8* | 64.3 |
| Market characteristics | |||||||
| Urologists per 100,000 | |||||||
| Low (≤ 53) | 14,893 | 53.3 | 70.2* | 76.6 | 29.6* | 53.7* | 63.0 |
| Intermediate | 13,994 | 53.0 | 68.1* | 75.1 | 31.0* | 54.0* | 65.2 |
| High (≥ 87) | 17,378 | 53.3 | 64.8* | 74.9 | 35.1* | 57.0* | 63.7 |
| Radiation oncologists per 100,000 | |||||||
| Low (≤ 22) | 15,228 | 53.4 | 68.2 | 76.1 | 33.2 | 53.8* | 62.3* |
| Intermediate | 14,640 | 54.9 | 66.9 | 75.3 | 29.9 | 53.7* | 63.4* |
| High (≥ 37) | 16,397 | 51.6 | 67.5 | 75.2 | 33.0 | 57.4* | 65.8* |
| Hospital beds per 100,000 | |||||||
| Low (≤ 4,735) | 14,638 | 53.9* | 70.9* | 76.6 | 31.1 | 52.8* | 63.4 |
| Intermediate | 15,566 | 56.4* | 66.0* | 75.7 | 35.7 | 56.6* | 62.5 |
| High (≥ 6,854) | 16,061 | 49.5* | 66.0* | 74.3 | 29.6 | 55.5* | 65.6 |
| Medicare Managed Care penetration | |||||||
| Low (≤ 5.1%) | 15,319 | 57.6* | 67.7* | 76.3 | 25.9* | 53.9 | 62.9 |
| Intermediate | 15,469 | 54.9* | 62.2* | 76.3 | 40.3* | 57.9 | 65.0 |
| High (≥ 17.9%) | 15,477 | 47.2* | 73.4* | 74.0 | 30.0* | 52.8 | 64.1 |
| Technological capacity for robotic prostatectomy | |||||||
| Low | 11,103 | 53.7* | 64.9 | 80.8* | 29.0* | 52.9 | 62.5 |
| Intermediate | 18,097 | 55.8* | 70.1 | 72.2* | 33.9* | 57.2 | 62.9 |
| High | 17,074 | 50.2* | 66.3 | 75.4* | 32.2* | 53.9 | 65.6 |
| Technological capacity for IMRT | |||||||
| Low | 13,665 | 46.4* | 69.9* | 76.4 | 34.9* | 53.8* | 63.5 |
| Intermediate | 17,656 | 54.0* | 69.4* | 71.7 | 29.6* | 53.3* | 63.0 |
| High | 14,953 | 58.5* | 63.1* | 79.3 | 32.6* | 58.0* | 65.9 |
p≤0.001, Mantel-Haenszel chi square test;
p≤0.001, chi square test
Statistical analyses
We calculated the proportion of patients receiving care that adhered to the quality measures according to patient characteristics (age in years, race, comorbidity,23 D’Amico risk group,24 year of diagnosis, socioeconomic status25, and urban residence) and market characteristics (number of urologists, number of radiation oncologists, and number of hospital beds per 100,000 men aged 65 and older; Medicare managed care penetration). Market characteristics were obtained from the Health Resources and Services Administration’s Area Resource File. We assessed the statistical significance of these bivariate associations using Chi-Square tests.
For each of the quality measures described above, we fit multilevel logistic regression models to evaluate the association of technological capacity with adherence. For these models, our primary binary outcome was whether or not a patient received care according to the quality measure. The models allowed us to account for the nested structure of our data (i.e., patients nested within HRRs) by introducing an HRR-level random effect. We adjusted these models for patient and market covariates as well as for provider volume. The measures of robotic prostatectomy and IMRT capacity were introduced as separate continuous variables. We also included an interaction term between these two variables, but this was only statistically significant in the model assessing the quality measure “treatment by a high -volume calculate the adjusted probability of to provider.” Lastly, we used these models to calculate the adjusted probability of receiving care compliant with the quality measure for markets with low versus high technological capacity.
Because of concerns that we may not have captured all providers offering treatment with robotic prostatectomy or IMRT in HRRs that are partially outside of the SEER catchment areas, we performed sensitivity analyses excluding these markets (n=21). In addition, we performed sensitivity analyses not including provider volume as a covariate to assure that this covariate did not mask any effect of technological capacity. Results from these sensitivity analyses were not materially different from those of the main analyses, so only our main findings are presented.
We performed all analyses using Stata version 12SE and SAS version 9.3. All tests were 2-tailed and we set the probability of a Type 1 error at 0.05 or less. The University of Michigan Medical School Institutional Review Board exempted this study from review.
Results
Technological capacity increased significantly over the study period, both for robotic prostatectomy and IMRT (p<0.001 for time-trends, Figure 1). Table 1 summarizes the bivariate associations of patient and market characteristics with adherence to the quality measures. While many associations reached statistical significance, only few seem clinically relevant. For example, the association of socioeconomic status with pretreatment counseling was statistically significant, but adherence among patients of high socioeconomic status was only slightly higher than adherence among patients of low socioeconomic status (54% versus 50%). In contrast, the association of age with the adjuvant androgen deprivation therapy and pretreatment counseling measures was much more clinically relevant, with adherence being 12% to 20% higher for older patients. In bivariate analyses, high robotic prostatectomy capacity was associated with worse adherence to the pretreatment counseling and adjuvant ADT measures, but better adherence to the provider volume measure. High IMRT capacity was associated with worse adherence to the bone scan and provider volume measures, but better adherence to the pretreatment counseling and follow-up with radiation oncology measures (Table 1).
In multivariable analyses, associations between technological capacity and adherence to the quality measures remained mixed. High robotic prostatectomy capacity was associated with better adherence to some of the quality measures, such as avoiding unnecessary bone scans (79.8% vs. 73.0%, p=0.003) and having follow-up with urologists (67.7% vs. 62.6%, p=0.023, Figure 2). However, for most measures, high robotic prostatectomy capacity was not associated with significant increases in adherence rates (Figure 2). Similarly, high IMRT capacity was not associated with better adherence to the quality measures (Figure 3). In fact, for one measure (treatment by a high-volume provider), high IMRT capacity was associated with lower performance (23.4% vs. 28.5%, p<0.001, Figure 3).
Figure 2.
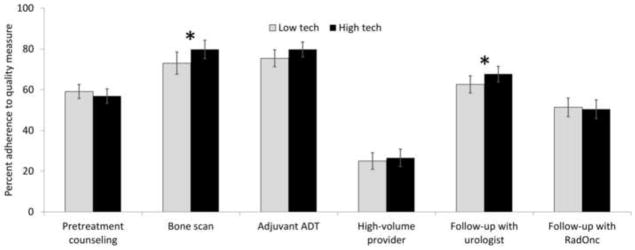
Adherence rates to a set of nationally endorsed quality measures comparing markets with low to those with high technological capacity for robotic prostatectomy (“low tech” vs. “high tech”). Adherence rates were calculated based on multivariable multilevel logistic regression models, adjusting for patient and market characteristics. * indicates p<0.05. ADT: androgen deprivation therapy; RadOnc; radiation oncologist.
Figure 3.
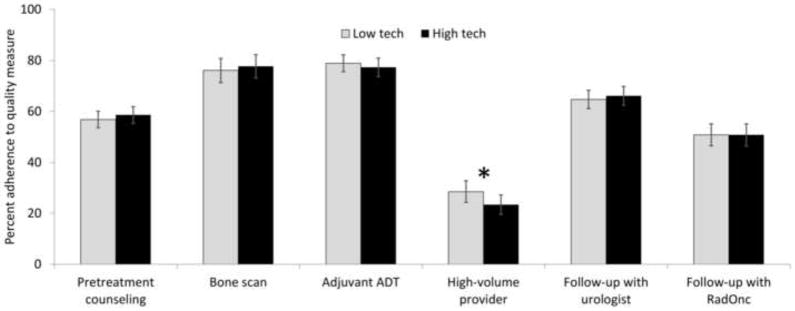
Adherence rates to a set of nationally endorsed quality measures comparing markets with low to those with high technological capacity for intensity-modulated radiotherapy (“low tech” vs. “high tech”). Adherence rates were calculated based on multivariable multilevel logistic regression models, adjusting for patient and market characteristics. * indicates p<0.05. ADT: androgen deprivation therapy; RadOnc; radiation oncologist.
Discussion
We found no consistent associations between technological capacity and adherence to nationally endorsed prostate cancer quality of care measures. While higher capacity for robotic prostatectomy was associated with better adherence to two measures (avoidance of unnecessary bone scans and recommended follow-up with the treating urologist), higher technological capacity for IMRT was associated with worse adherence to another measure (treatment by a high-volume provider). In addition, the better adherence to the two measures in markets with high robotic prostatectomy capacity was fairly small and likely not clinically meaningful.
There are several potential reasons for these findings. First, it is possible that new technology affects the details of what happens in the operating room or radiotherapy facility, as opposed to more broadly impacting the processes of care evaluated in this study. Second, the general perception that new technology is associated with higher quality of care may be due to the fact that this technology is typically first acquired by high-volume centers, which often are perceived as centers of excellence.10,11 However, the link between advanced technology and quality may disappear after it disseminates more widely. Third, new technology may increase a provider’s focus on prostate cancer, but increased awareness of guidelines may not directly translate into adherence to them. In fact, even after increasing knowledge of guidelines, additional multilevel interventions are often needed to increase measurable adherence to them, including patient education, improving access to specific care (e.g., access to a radiation oncologist for counseling), and decreasing facility level barriers to recommended care (e.g., opening up a sufficient number of follow-up visit slots in providers’ clinic schedules).26
Our current study is the first to evaluate the association between the dissemination of a new surgical technology and quality of care. Regarding other healthcare technologies, a recent systematic review concluded that adoption of healthcare information technology was associated with improvements in guideline concurrent care. However, this association was limited to four benchmark institutions. Data from a broader set of institutions did not support this association,27 which is in line with our findings. In another study which used rise in healthcare spending as a proxy for technology dissemination among patients with acute myocardial infarction, higher spending was associated with worse performance on a quality index and worse survival.28 Taken together, these studies imply that dissemination of new technology does not necessarily lead to improvements in the quality of care patients receive.
There are several limitations of our current study that are worth mentioning. First, we had to limit our analyses to patients diagnosed between 2004 and 2009, because several of the quality measures require adequate prostate cancer risk assessment, which is not possible within SEER prior to 2004. However, by 2004, dissemination of robotic prostatectomy and IMRT was well under way.2,16 Therefore, we cannot exclude an association of early technology adoption with high adherence to quality of care measures. Second, our measure of technological capacity may not have captured all providers offering treatment with new technology in HRRs that are partially outside of the SEER areas. Therefore, we performed sensitivity analyses excluding these HRRs (n=21), which confirmed our main findings. Third, given the nature of claims data and the relatively short follow-up, we were unable to assess outcomes of prostate cancer treatment such as quality of life and survival. Finally, the process of care measures examined in this study do not capture the full breadth of prostate cancer care patients receive. Thus, it is possible that some aspects of prostate cancer care were superior in markets with higher technological capacity in ways that could not be assessed in this study. However, the measures used in our study were developed based on expert consensus and several of them have been nationally endorsed by the PCPI and NQF.14,15 In fact, several of them have already been incorporated into systematic performance measurement by the Centers for Medicare and Medicaid Services.29
These limitations notwithstanding, our findings suggest that new technology is not clearly associated with better adherence to recommended processes of care. Further, these data provide valuable information for patients, who are naturally interested in where they can obtain the highest quality care. Policymakers will appreciate a better understanding of the downstream effects of technology dissemination as they consider regulation of other new technologies. While technology may increase a provider’s focus on prostate cancer, this does not appear to have a meaningful impact on established quality of care measures. Therefore, we believe that more specific efforts will be needed to improve quality of care in the future. For example, regional collaboratives of urologists were able to make important strides towards better care for prostate cancer patients by implementing a systematic system of provider education, quality measurement, and data feedback.30
Acknowledgments
Grant support: FRS is supported in part by grant T32 DK07782 from the NIH/NIDDK and by Postdoctoral Fellowship PF-12-118-01-CPPB from the American Cancer Society. BLJ is supported in part by grant KL2 TR000146 from the NIH. BKH is supported in part by Research Scholar Grant RSGI-13-323-01-CPHPS from the American Cancer Society.
Footnotes
Disclosures: Dr. Skolarus is a paid consultant for ArborMetrix, Inc.
Disclaimer: The views expressed in this article do not reflect the views of the federal government.
References
- 1.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–2092. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs BL, Zhang Y, Skolarus TA, et al. Growth Of High-Cost Intensity-Modulated Radiotherapy For Prostate Cancer Raises Concerns About Overuse. Health Affairs. 2012;31:750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:643–657. doi: 10.1016/j.clon.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative Effectiveness of External-Beam Radiation Approaches for Prostate Cancer. Eur Urol. 2014;65:162–168. doi: 10.1016/j.eururo.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheets NC, Goldin GH, Meyer A-M, et al. Intensity-Modulated Radiation Therapy, Proton Therapy, or Conformal Radiation Therapy and Morbidity and Disease Control in Localized Prostate Cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Robotics Institute. [accessed October 15, 2013];Florida Hospital Celebration Health. 2013 Available at: http://www.globalroboticsinstitute.com/sites/default/files/campus_map/celebration_map.pdf.
- 7.Varian Medical Systems. [accessed October 15, 2013];Eclipse: Benefits. 2013 Available at: http://www.varian.com/us/oncology/radiation_oncology/eclipse/benefits.html.
- 8.Chernew ME, Jacobson PD, Hofer TP, et al. Barriers to constraining health care cost growth. Health Aff (Millwood) 2004;23:122–128. doi: 10.1377/hlthaff.23.6.122. [DOI] [PubMed] [Google Scholar]
- 9.Luft HS, Robinson JC, Garnick DW, et al. The role of specialized clinical services in competition among hospitals. Inquiry. 1986;23:83–94. [PubMed] [Google Scholar]
- 10.Moses H, Thier SO, Matheson DHM. Why Have Academic Medical Centers Survived? JAMA. 2005;293:1495–1500. doi: 10.1001/jama.293.12.1495. [DOI] [PubMed] [Google Scholar]
- 11.Miller DC, Taub DA, Dunn RL, et al. Laparoscopy for renal cell carcinoma: diffusion versus regionalization? J Urol. 2006;176:1102–1106. doi: 10.1016/j.juro.2006.04.101. discussion 1106–1107. [DOI] [PubMed] [Google Scholar]
- 12.Yao SL, Lu-Yao G. Population-based study of relationships between hospital volume of prostatectomies, patient outcomes, and length of hospital stay. J Natl Cancer Inst. 1999;91:1950–1956. doi: 10.1093/jnci/91.22.1950. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 14.Thompson IM, Clauser S. [accessed September 13, 2011];Prostate Cancer Physician Performance Measurement Set. 2007 Available at: http://www.ama-assn.org/apps/listserv/x-check/qmeasure.cgi?submit=PCPI.
- 15.National Quality Forum (NQF) [accessed August 31, 2011];NQF-endorsed standards. 2011 Available at: http://www.qualityforum.org/Measures_List.aspx?#k=prostate.
- 16.Schroeck FR, Kaufman SR, Jacobs BL, et al. Technology diffusion and diagnostic testing for prostate cancer. J Urol. 2013;190:1715–1720. doi: 10.1016/j.juro.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeck FR, Kaufman SR, Jacobs BL, et al. The Impact of Technology Diffusion on Treatment for Prostate Cancer. Med Care. 2013;51:1076–84. doi: 10.1097/MLR.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Dartmouth Institute for Health Policy & Clinical Practice. [accessed April 7, 2012];Glossary - Dartmouth Atlas of Health Care. 2012 Available at: http://www.dartmouthatlas.org/tools/glossary.aspx.
- 19.Schroeck FR, Kaufman SR, Jacobs BL, et al. Regional Variation in Quality of Prostate Cancer Care. J Urol. 2014;191:957–963. doi: 10.1016/j.juro.2013.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwin M, Steinberg M, Malin J, et al. [accessed October 7, 2013];Prostate Cancer Patient Outcomes and Choice of Providers: Development of an Infrastructure for Quality Assessment. 2000 Available at: http://www.rand.org/pubs/monograph_reports/MR1227.html.
- 21.Spencer BA, Steinberg M, Malin J, et al. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol. 2003;21:1928–1936. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 22.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(Suppl):166–206. [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 26.Zapka J, Taplin SH, Ganz P, et al. Multilevel Factors Affecting Quality: Examples From the Cancer Care Continuum. J Natl Cancer Inst Monogr. 2012;2012:11–19. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhry B, Wang J, Wu S, et al. Systematic Review: Impact of Health Information Technology on Quality, Efficiency, and Costs of Medical Care. Ann Intern Med. 2006;144:742–752. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 28.Skinner JS, Staiger DO, Fisher ES. Is technological change in medicine always worth it? The case of acute myocardial infarction. Health Aff (Millwood) 2006;25:w34–47. doi: 10.1377/hlthaff.25.w34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services: Physician Quality Reporting System. Measure Specifications Manual for Claims and Registry Reporting of Individual Measures. 2011 Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/downloads/2011_PhysQualRptg_MeasureSpecificationsManual_033111.pdf.
- 30.Miller DC, Murtagh DS, Suh RS, et al. Regional Collaboration to Improve Radiographic Staging Practices Among Men With Early Stage Prostate Cancer. J Urol. 2011;186:844–849. doi: 10.1016/j.juro.2011.04.078. [DOI] [PubMed] [Google Scholar]