Abstract
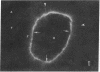
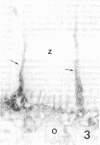
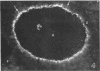
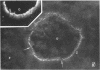
It was the aim of this study to test the hypothesis that the interaction of antibodies with antigens expressed on the plasma membrane of cells surrounded by a basement membrane or a basement membrane-like structure results in in situ formation of immune deposits. Ovary was chosen for the experiments because we found that a well-characterized protein, angiotensin-converting enzyme (ACE), is expressed in a diffuse pattern on the plasma membrane of mature oocytes. To investigate the events following the in vivo interaction of oolemma-ACE with its antibody, rabbits were injected with goat anti-rabbit ACE gamma-globulin or with Fab fragments of goat anti-rabbit ACE IgG in an ear vein for a maximum of 4 d; they were followed for up to 20 d thereafter. Ovary tissue was studied by immunofluorescence, and immunoelectron, light, and transmission electron microscopy. The results of this study document two new findings: First, that ACE is expressed on the oolemma of rabbit oocytes. Second, that the in vivo interaction of divalent antibodies to this cell surface antigen induces formation of granular immune deposits in the adjacent zona pellucida through a mechanism of "patching" and "shedding" of immune complexes, similar to that occurring in in vitro systems characterized by interaction of plasma membrane receptors with soluble ligands. This mechanism might have importance in the pathogenesis of Heymann glomerulonephritis and of other immunological diseases involving antigens expressed on the plasma membrane of cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accinni L., Albini B., Andres G., Dixon F. J. Deposition of immune complexes in ovarian follicles of mice with lupus-like syndrome. Am J Pathol. 1980 Jun;99(3):589–602. [PMC free article] [PubMed] [Google Scholar]
- Albini B., Ossi E., Neuland C., Noble B., Andres G. Deposition of immune complexes in the ovarian follicle of rabbits with experimental chronic serum sickness. I. Immunopathology. Lab Invest. 1979 Nov;41(5):446–454. [PubMed] [Google Scholar]
- Andres G. A., Accinni L., Beiser S. M., Christian C. L., Cinotti G. A., Erlanger B. F., Hsu K. C., Seegal B. C. Localization of fluorescein-labeled antinucleoside antibodies in glomeruli of patients with active systemic lupus erythematosus nephritis. J Clin Invest. 1970 Nov;49(11):2106–2118. doi: 10.1172/JCI106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann K. J., Tangelder M. M., Lange W. P., Tadema T. M., Koene R. A. Membranous glomerulonephritis in the mouse. Kidney Int. 1983 Sep;24(3):303–312. doi: 10.1038/ki.1983.159. [DOI] [PubMed] [Google Scholar]
- BLANDAU R. J. Ovulation in the living albino rat. Fertil Steril. 1955 Sep-Oct;6(5):391–404. doi: 10.1016/s0015-0282(16)32100-8. [DOI] [PubMed] [Google Scholar]
- Barabas A. Z., Lannigan R. Induction of an autologous immune-complex glomerulonephritis in the rat by intravenous injection of heterologous anti-rat kidney tubular antibody. I. Production of chronic progressive immune-complex glomerulonephritis. Br J Exp Pathol. 1974 Feb;55(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Barba L. M., Caldwell P. R., Downie G. H., Camussi G., Brentjens J. R., Andres G. Lung injury mediated by antibodies to endothelium. I. In the rabbit a repeated interaction of heterologous anti-angiotensin-converting enzyme antibodies with alveolar endothelium results in resistance to immune injury through antigenic modulation. J Exp Med. 1983 Dec 1;158(6):2141–2158. doi: 10.1084/jem.158.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigazzi P. E., Kosuda L. L., Hsu K. C., Andres G. A. Immune complex orchitis in vasectomized rabbits. J Exp Med. 1976 Feb 1;143(2):382–404. doi: 10.1084/jem.143.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Unanue E. R. B lymphocyte biology studied with anti-Ig antibodies. Immunol Rev. 1980;52:3–28. doi: 10.1111/j.1600-065x.1980.tb00328.x. [DOI] [PubMed] [Google Scholar]
- COCHRANE C. G., WEIGLE W. O. The cutaneous reaction to soluble antigen-antibody complexes; a comparison with the Arthus phenomenon. J Exp Med. 1958 Nov 1;108(5):591–604. doi: 10.1084/jem.108.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Wigger H. J., Fernandez L. T., D'Alisa R. M., Tse-Eng D., Butler V. P., Jr, Gigli I. Lung injury induced by antibody fragments to angiotensin-converting enzyme. Am J Pathol. 1981 Oct;105(1):54–63. [PMC free article] [PubMed] [Google Scholar]
- Clagett J. A., Wilson C. B., Weigle W. O. Interstitial immune complex thyroiditis in mice: the role of autoantibody to thyroglobulin. J Exp Med. 1974 Dec 1;140(6):1439–1456. doi: 10.1084/jem.140.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtoy P. J., Picton D. H., Farquhar M. G. Resolution and limitations of the immunoperoxidase procedure in the localization of extracellular matrix antigens. J Histochem Cytochem. 1983 Jul;31(7):945–951. doi: 10.1177/31.7.6304184. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Salant D. J. In situ immune complex formation and glomerular injury. Kidney Int. 1980 Jan;17(1):1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J. R., Sisson S. P., Vernier R. L. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest. 1979 Aug;41(2):168–173. [PubMed] [Google Scholar]
- Hoyer J. R. Tubulointerstitial immune complex nephritis in rats immunized with Tamm-Horsfall protein. Kidney Int. 1980 Mar;17(3):284–292. doi: 10.1038/ki.1980.34. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J., Milgrom F. M., McCluskey R. T. Studies of the antigens involved in an immunologic renal tubular lesion in rabbits. Am J Pathol. 1977 Jul;88(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Klassen J., Milgrom F. Autoimmune concomitants of renal allografts. Transplant Proc. 1969 Mar;1(1):605–608. [PubMed] [Google Scholar]
- Kåresen R., Godal T. Induction of thyroiditis in guinea-pigs by intravenous injection of rabbit anti-guinea-pig thyroglobulin serum. II. Studies with fluorescent antibody technique. Immunology. 1969 Dec;17(6):863–874. [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C. Solubility of proteins in the presence of polysaccharides. Fed Proc. 1966 May-Jun;25(3):1127–1127. [PubMed] [Google Scholar]
- Mannik M., Agodoa L. Y., David K. A. Rearrangement of immune complexes in glomeruli leads to persistence and development of electron-dense deposits. J Exp Med. 1983 May 1;157(5):1516–1528. doi: 10.1084/jem.157.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Neale T. J., Couser W. G., Salant D. J., Lowenstein L. M., Wilson C. B. Specific uptake of Heymann's nephritic kidney eluate by rat kidney: studies in vivo and in isolated perfused kidneys. Lab Invest. 1982 Apr;46(4):450–453. [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Capping and the lymphocyte: models for membrane reorganization. J Immunol. 1977 Nov;119(5):1549–1551. [PubMed] [Google Scholar]
- Soffer R. L., Reza R., Caldwell P. R. Angiotensin-converting enzyme from rabbit pulmonary particles. Proc Natl Acad Sci U S A. 1974 May;71(5):1720–1724. doi: 10.1073/pnas.71.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki T., Klassen J., Andres G. A., Milgrom F., McCluskey R. T. Passive transfer of Heymann nephritis with serum. Kidney Int. 1973 Feb;3(2):66–73. doi: 10.1038/ki.1973.13. [DOI] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- Wood B. T., Thompson S. H., Goldstein G. Fluorescent antibody staining. 3. Preparation of fluorescein-isothiocyanate-labeled antibodies. J Immunol. 1965 Aug;95(2):225–229. [PubMed] [Google Scholar]
- Zamboni L., Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983 Nov;228(2):173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]