Abstract
Background
Paraoxonase 1 (PON1) activity and von Willebrand factor (VWF) release are associated with lesion initiation in atherosclerosis. Diabetes can complicate coronary artery disease (CAD) due to the production of advanced glycation end products. This study evaluated PON1 activity and VWF levels in non-post-acute coronary syndrome, stable CAD (SCAD) patients without diabetes.
Material/Methods
Non-diabetic SCAD patients and patients experiencing acute stress periods were selected (n=130). Forty-seven cases with normal coronary angiography and 50 healthy individuals served as controls. The non-diabetic SCAD group was then stratified into single-vessel lesions, multiple-vessel lesions, and mild or severe luminal stenosis according to the number and the degree of luminal stenoses. Serum PON1 paraoxonase and arylesterase activities, and plasma VWF levels were measured, as well as serum total cholesterol, total triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A1. PON1 arylesterase activity was detected with an ordinary chemistry system using a novel phenylacetate derivative.
Results
Both PON1 paraoxonase and arylesterase were lower in the non-diabetic SCAD group, but VWF levels were higher (versus controls, all P<0.001). PON1 paraoxonase activity (OR=0.991), PON1 arylesterase activity (OR=0.981), and VWF (OR 2.854) influenced SCAD in multiple logistic regression. Decreased PON1 arylesterase activity and increased VWF levels were associated with severe atherosclerosis in non-diabetic SCAD patients. We also observed a slight negative correlation between VWF and PON1 paraoxonase/arylesterase.
Conclusions
PON1 and VWF are detectable markers that may predict the severity of stenoses, ideally facilitating a non-diabetic SCAD diagnosis before the sudden onset of life-threatening symptoms.
MeSH Keywords: Aryldialkylphosphatase, Bernard-Soulier Syndrome, Cardiology
Background
Research into the novel cardiovascular risk factors that influence the high prevalence of coronary artery heart disease (CAD) has attracted considerable global attention. However, developing markers for the risk assessment of CAD has been historically complicated by a diagnosis of diabetes mellitus (DM), due to the production of advanced glycation end products (RAGE) in these patients, which then activate receptors contributing to the development of atherosclerosis [1,2]. Although patients with DM are more susceptible to developing CAD [3], the production of RAGE does account for all the clinical characteristics of CAD and does nothing to explain the mechanisms leading to the development of CAD in non-diabetic patients. Chronic stable angina is the most common manifestation of CAD [4]. However, except for those who have later become stable following acute coronary syndrome (ACS), most patients with SCAD remain in a stable (undiagnosed) condition for decades prior to the sudden onset of an acute myocardial infarction (MI), heart failure (HF), or death. Therefore, it is imperative that novel markers for non-diabetic SCAD are identified to be used in risk assessment, which could prevent such negative outcomes related to the persistence of undetected disease.
An atherosclerosis lesion may initiate when endothelial impairment occurs and peroxidized low-density lipoproteins (ox-LDL) accumulate [5]. The major function of human paraoxonase 1 (PON1) is to reduce accumulation of ox-LDL [6,7]. PON1 is a 43 kDa enzyme that is produced by hepatocytes and is linked to high-density lipoprotein (HDL), principally HDL apolipoprotein A-I (ApoA1). Although the PON1 enzyme possesses at least 3 different activities, research into the origins of CAD mainly focuses on PON1 paraoxonase and arylesterase activity [8,9]. Nevertheless, the relationship between PON1 activity (paraoxonase and arylesterase activity) and non-diabetic SCAD remains poorly understood. Von Willebrand factor (VWF) may be a signal of endothelial impairment because it can be released from endothelial cells to facilitate platelet adhesion and aggregation at sites of injury [10,11]. VWF is released predominantly in response to a rise in cytosolic free calcium and cyclic adenosine monophosphate (cAMP) levels. cAMP levels are commonly regulated by stress hormones, such as epinephrine, and increased intracellular calcium (Ca2+) occurs in response to NO production [12]. However, far less is known about the levels of VWF in non-diabetic SCAD when acute stress factors are absent.
Since PON1 and VWF regulation shows a link between lesion initiation and the development of atherosclerosis, we hypothesized that PON1 and VWF may also be involved in the pathogenesis of non-diabetic SCAD. In this study, we set out to explore the PON1 activity and VWF levels in non-diabetic SCAD patients (post-ACS SCAD patients were excluded) and further evaluate the relation between these 2 factors. As the current methods for detection of PON1 arylesterase activity are unlikely to be used regularly in a clinical situation because they require ultraviolet detection, many standard clinical laboratories do not have the ability to incorporate UV detection into their chemistry systems [13]. We used a new method to measure PON1 arylesterase activity in a more traditional/ordinary chemistry system.
Material and Methods
Patients
We recruited 500 patients with chest pain from the Department of Cardiology in the Ninth People’s Hospital affiliated with Shanghai Jiaotong University School of Medicine from January 2012 to October 2013. The diagnosis of SCAD was made using ESC guidelines [14]. All the patients were initially diagnosed with SCAD and had no medical history of ACS. The inclusion and exclusion criteria was as follows: (a) the patients included in this study denied history of diabetes and had normal serum albumin, alanine transaminase, creatine, fasting plasma glucose (FPG), 2-h postprandial glucose (PPG), HOMA-IR (insulin resistance index), and glycosylated hemoglobin to exclude their influence on PON1 metabolism. (b) The patients had a normal bleeding time (BT), prothrombin time (PT), activated coagulation time (APTT), and platelet counts to exclude abnormal coagulation systems. (c) To exclude their regulation of PON1 expression, the patients included in this study denied having taken statins and fenofibrates in the last month. (d) Patients were included if their C-reactive protein (CRP) was lower than 3.0 mg/L after at least 19 h of chest pain to exclude VWF release in acute stress factors. (e) Patients were selected if their cardiac troponin I (TNI) and N-Terminal pro-brain natriuretic peptides (NT-proBNP) were within the normal range (TNI < 0.06 ng/ml, Beckman Access AccuTnI 10% CV; NT-proBNP <300pg/ml). (f) All the patients selected were to receive coronary angiography (CAG) within 20 to 48 h after the onset of chest pain.
Finally, 180 patients were selected from 500 patients, and 130 patients were confirmed for the non-diabetic SCAD group, while 47 patients were CAG (−) and had no objective clinical evidence of CAD and diabetes, which were put into the control group (CAG (−) control group). We excluded 323 cases from this study: 146 diabetic patients, 3 patients with HOMA-IR higher than normal, 81 patients with impaired glucose tolerance, 28 patients with abnormal creatine or alanine transaminase, 43 patients with acute coronary syndrome within 24 h after the onset, 13 patients with abnormal BT or platelet counts, 5 patients had received statins or fenofibrates within the previous month, and 4 patients had higher CRP levels 19 h after onset. Another 50 age- and sex-matched subjects without any known health problems were enrolled as healthy controls (the HC control group). HC individuals were selected from subjects for health examination. The inclusion and exclusion criteria were similar to that of the non-diabetic SCAD and CAG (−) group except for absence of CAG and chest pain. The CAG (−) and HC control groups were combined to form the non-diabetic SCAD (−) group. The non-diabetic SCAD group was then stratified into single-vessel or multiple-vessel lesions according to the number of stenosed coronary arteries and mild (≤ 50%) or severe (50%–90%) luminal stenosis, according to the degree of luminal stenosis.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Ninth People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine, approval number 2012104.
Blood chemical analysis
Plasma and serum samples were carefully collected from patients’ veins if they had remained stable with no clinical signs from 19 to 24 h after the onset of chest pain. Fasting venous blood samples were collected in citrated tubes for plasma samples. The serum and plasma samples were stored at −20°C and were assayed within 3 months.
Serum total cholesterol (TC), triglycerides (TG), low- and high-density lipoprotein cholesterol (LDL-C and HDL-C), and apolipoprotein A-I (ApoA1) were measured with standard laboratory techniques using the ADVIA 2400 Chemistry System (Siemens Medical Solution Diagnostics, Inc, NY, USA)
VWF antigen (VWF: Ag) was measured in plasma samples by enzyme-linked immunosorbent assay (ELISA) using polyclonal rabbit anti-human VWF antibodies and horseradish peroxidase (HRP)-conjugated anti-human VWF antibodies (DakoCytomation, Glostrop, Denmark) for capturing and tagging, respectively. The intra-assay coefficient of variation was 3.2% and the inter-assay coefficient of variation (CV) was 9.8%.
PON1 paraoxonase activity was measured according to the method of Gan et al. [13]. Briefly, 8 μl of serum was added to a 100-μl reaction mixture containing paraoxon substrate solution (1 mmol/L), glycine-NaOH (pH 10.0) buffer, and CaCl2 (2.0 mmol/L). The rate of p-nitrophenol product generation [15] was determined at 410 nm using the ADVIA 2400 Chemistry System.
PON1 arylesterase activity was measured using a modified method [13] with a new substrate (phenyl acetate derivative, donated by Jianhua Zhang, Department of Biochemistry, School of Medicine, Shanghai Jiaotong University) dissolved in dimethyl-sulfoxide. Three μl of serum was added to a 100-μl reaction mixture containing 1 mmol/L new substrate and Tris-HCl buffer (pH 8.0). The rate of product generation of the nitrophenol derivative [15] was determined using a wavelength of 410 nm (ADVIA 2400 Chemistry System), and the molar absorption coefficient was 14314 mol/L/cm. The detection evaluation performance is shown in Supplemental Table 1 and Figure 1.
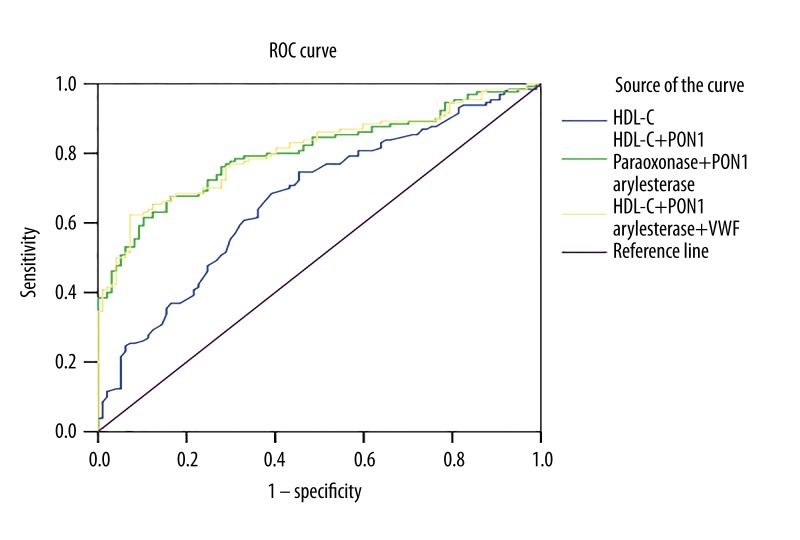
Figure 1.
Receiver-operator characteristic (ROC) curve for the prediction of non-diabetic SCAD. HDL-C, high-density lipoprotein cholesterol; PON1, paraoxonase 1; VWF, von Willebrand factor.
We detected PON1 paraoxonase and PON 1 arylesterase activity according to the guidelines for evaluation of precision performance of clinical chemistry devices (NCCLS-EP5A) [16]. Briefly, we analyzed a sample 20 times a day during a period of 20 continuous days, and the CV was less than 5%. We also detected the CV of the ELISA kit for measuring VWF level with a method similar to PON1 paraoxonase and PON 1 arylesterase activities, except we analyzed the same sample during a period of 10 continuous days.
Statistical analysis
A comparison of continuous variables among groups was performed using one-way ANOVA (if homogeneity of variables was assumed) and non-parametric test (if variables were not homogenous). The differences between groups were determined by t-test or Manny-Whitney U test. Categorical variables were estimated with the use of the χ2 test. Multivariate logistic regression was used to model different variables in the study groups, including the CAG (−) and HC control groups and the non-diabetic SCAD group. Multivariate logistic regression was also used to model independent factors associated with the number and degree of stenosed coronary arteries among the non-diabetic SCAD patients. Selected variables that had p values less than 0.2 in the bivariate analyses were considered for inclusion in the regression model. The discriminative ability to identify members of the non-diabetic SCAD group with risk markers of HDL-C, PON1 paraoxonase, PON1 arylesterase, and VWF levels was verified using the area under the receiver operating curve (ROC). Pearson correlation analysis was done between PON1 activity and VWF level. Data were analyzed using SPSS, version 17.0 (IBM Corp, Armonk, NY, USA). The statistical significance level was set at 0.05 for all tests.
Results
Validation of the new method of PON1 arylesterase activity measurement
We established a modified method for measuring PON1 arylesterase activity with a new substrate, which could be detected at a wavelength of 410 nm using the ADVIA 2400 Chemistry System. The intra- and inter-assay coefficients of variation were below 5%, while the linear range and analytic sensitivity was within the detectable range in patients (Supplemental Table 1). Correlation tests between our new method and the classical method were performed according to NCCLS-EP9A using patient serum; the square of the correlation coefficient reached 0.986 (Supplemental Figure 1).
Low PON1 activity and high VWF levels in the SCAD group
The basic relevant clinical factors of the participants are shown in Table 1. The patients in the non-diabetic SCAD and CAG (−) groups had a higher prevalence of cigarette smoking and a higher incidence of hypertension than the HC group. The concentrations of PON1 paraoxonase and PON1 arylesterase activity (PON1 paraoxonase: 287.9±95.0 U/L, PON1 arylesterase: 184.5±30.9 U/L) were significantly lower in the non-diabetic SCAD group than in the HC group (PON1 paraoxonase: 365.9±70.0 U/L, PON1 arylesterase: 210.3±16.0 U/L) and CAG (−) group (PON1 paraoxonase: 365.3±69.9 U/L, PON1 arylesterase: 206.1±29.3 U/L). The level of VWF in the non-diabetic SCAD group (113±47) was significantly higher than in the HC group (91±17) and the CAG (−) group (87±20). There were no significant differences between the HC group and the CAG (−) control for lipid parameters, PON1 activity, and VWF level. Comparison of the non-diabetic SCAD group with the 2 control groups together (non-diabetic SCAD (−) group) showed significant differences between the groups for body mass index (BMI) at 21.3±2.1 and 20.3±1.5, respectively (P<0.001), and HDL-C at 1.15±0.33 and 1.34±0.34, respectively.
Table 1.
Baseline characteristics of non-diabetic SCAD and control groups.
| Variables | HC group, n=50 | CAG (−) group, n=47 | Non-diabetic SCAD (−) group, n=97 | Non-diabetic SCAD group, n=130 | P (non-diabetic SCAD group vs. non-diabetic SCAD (−) group) |
|---|---|---|---|---|---|
| Gender n (%) | |||||
| Female | 25 (50) | 22 (47) | 47 (48) | 65 (50) | 0.893 |
| Male | 25 (50) | 25 (53) | 50 (52) | 65 (50) | |
| Age (years) | 64.7±14.0 | 65.6±11.0 | 65.1±12.6 | 67.0±11.0 | 0.235 |
| BMI (Kg/m2) | 20.2±1.6 | 20.4±1.4 | 20.3±1.5 | 21.3±2.1*,** | <0.001 |
| Blood type n (%) | |||||
| O | 7 (14) | 6 (13) | 13 (13) | 16 (12) | 0.842 |
| Non-O | 43 (86) | 41 (87) | 84 (87) | 114 (82) | |
| Hypertension n (%) | 0 | 20 (42)* | 20 (20) | 52 (40)* | 0.002 |
| Smoking n (%) | 16 (32) | 27 (43)* | 43 (44) | 60 (46)* | 0.789 |
| TC (mmol/L) | 4.37±0.78 | 4.55±0.99 | 4.46±0.899 | 4.54±1.28 | 0.546 |
| TG (mmol/L) | 1.29±0.59 | 1.32±0.68 | 1.30±0.63 | 1.45±0.81 | 0.154 |
| HDL-C (mmol/L) | 1.41±0.36 | 1.28±0.31 | 1.34±0.34 | 1.15±0.33*,** | <0.001 |
| LDL-C (mmol/L) | 2.56±0.60 | 2.76±0.78 | 2.66±0.70 | 2.81±1.08*,** | 0.204 |
| ApoA1 (mmol/L) | 1.04±0.19 | 1.11±0.22 | 1.07±0.20 | 1.05±0.21 | 0.422 |
| PON1 paraoxonase (U/L) | 365.9±70.0 | 365.3±69.9 | 365.6±69.6 | 287.9±95.0*,** | <0.001 |
| PON1 arylesterase (U/L) | 210.3±16.0 | 206.1±29.3 | 208.3±23.4 | 184.5±30.9*,** | <0.001 |
| VWF (%) | 91±17 | 87±20 | 89±19 | 113±47*,** | <0.001 |
P<0.05 vs. HC group;
P<0.05 vs. CAG (−) group.
HC group: healthy controls; CAG (−) group: CAG (−) and had no objective clinical evidence of CAD and diabetes; non diabetic SCAD group: patients were confirmed for the SCAD group without diabetes; non diabetic SCAD (−) group including healthy controls and CAG (−) group. Hypertension was diagnosed if systolic blood pressure exceeded 140 mmHg and/or diastolic blood pressure was above 90 mmHg.
SCAD – stable coronary artery disease; TC – total cholesterol; TG – triglycerides; HDL-C – high density lipoprotein-cholesterol; LDL-C – low density lipoprotein-cholesterol; ApoA1 – apolipoprotein A-I; PON1 – paraoxonase 1; VWF – Von Willebrand factor.
To explore the factors associated with SCAD, different independent variables that had p values less than 0.2 in bivariate analyses were considered in the logistic regression models. PON1 paraoxonase activity, PON1arylesterase activity, and VWF levels, as well as BMI and hypertension, showed significant associations with the outcome of SCAD and another 2 factors (TG and HDL-C) were excluded in the model (Table 2). Odds ratio of PON1 paraoxonase activity and PON1 arylesterase activity was 0.991 and 0.981(OR<1), respectively, and the odds ratio of VWF was 2.854 (OR>1).
Table 2.
Multivariate logistic regression analysis of independent factors associated with non-diabetic stable coronary artery disease.
| Variables | odds ratio | 95% CI | P |
|---|---|---|---|
| Hypertension | 2.854 | 1.33–6.121 | 0.007* |
| BMI (Kg/m2) | 1.670 | 1.333–2.093 | <0.001* |
| TG (mmol/L) | 1.153 | 0.688–1.931 | 0.590 |
| HDL-C (mmol/L) | 0.380 | 0.128–1.128 | 0.081 |
| PON1 paraoxonase (U/L) | 0.991 | 0.987–0.996 | <0.001* |
| PON1 arylesterase (U/L) | 0.981 | 0.968–0.994 | 0.004* |
| VWF (%) | 2.854 | 1.330–6.121 | 0.007* |
Cox & Snell R2=0.365;
P<0.05.
BMI – body mass index; TG – triglycerides; HDL-C – high density lipoprotein cholesterol; PON1 – paraoxonase 1; VWF – von Willebrand factor; 95% CI – 95% Confidence interval.
Improvement of SCAD prediction with association of PON1 paraoxonase activity, arylesterase activity, VWF level, and traditional coronary risk markers
We added HDL-C, HDL-C+ PON1 paraoxonase activity+PON1 arylesterase activity, HDL-C+PON1 paraoxonase activity+ PON1arylesterase activity+VWF for ROC curve analysis because HDL-C was a traditional risk marker for CAD. The area under the ROC curve (AUC) for the SCAD group (Figure 1 and Table 3) prediction reached 0.671, 63% specificity, and 65% sensitivity when only HDL-C was used. However, the area expanded when PON1 paraoxonase activity and PON1 arylesterase activity were imported (AUC=0.803, 84% specificity, and 68% sensitivity). When adding VWF to ROC, AUC further expanded to 0.808, 93% specificity, and 62% sensitivity.
Table 3.
Receiver-operator characteristic (ROC) curve for the prediction of non-diabetic SCAD.
| Variables | AUC | Specificity (%) | Sensitivity (%) | Cut-off value | P |
|---|---|---|---|---|---|
| HDL-C | 0.671 | 63% | 65% | 0.29 | |
| HDL-C +PON1 paraoxonase +PON1 arylesterase | 0.803 | 84% | 68% | 0.52 | 0.004 |
| HDL-C +PON1 paraoxonase +PON1 arylesterase +VWF | 0.808 | 93% | 62% | 0.55 | 0.003 |
SCAD – stable coronary artery disease; HDL-C – high density lipoprotein cholesterol; PON1 – paraoxonase 1; VWF – von Willebrand factor, AUC – area under curve.
Low PON1 arylesterase activity and high VWF level in severe stenosed coronary arteries
We further tested whether decreased PON1 paraoxonase activity, arylesterase activity, and increased VWF levels promoted coronary atherosclerosis in non-diabetic SCAD patients. Table 4 shows variables that had significant differences between groups. PON1 arylesterase activity and VWF level were factors that were lower in severely stenosed coronary arteries. Independent variables that had p values less than 0.2 were considered in the logistic regression of the number and degree of stenosed coronary arteries. In multivariate analysis for severely stenosed coronary arteries as a dependent variable, only PON1 arylesterase activity and VWF levels showed significant associations with the severity of atherosclerosis. Table 5 shows the odds ratio of PON1 arylesterase activity and VWF were OR=0.940 and OR=6.863, respectively. The model of severity of atherosclerosis could account for 38% of patients in the SCAD group (Cox & Snell R2=0.380).
Table 4.
Baseline characteristics of non-diabetic SCAD patients categorized by extent of stenosed coronary arteries.
| Variables | Number of stenosed coronary arteries | P | Degree of stenosed coronary arteries | P | ||
|---|---|---|---|---|---|---|
| Single-vessel lesions | Multiple-vessel lesions | Mild stenosis | Severe occlusions | |||
| n=50 | n=80 | n=44 | n=86 | |||
| Gender, n (%) | ||||||
| Female | 29 (58) | 36 (45) | 0.149 | 19 (43) | 46 (53) | 0.266 |
| Male | 21 (42) | 44 (55) | 25 (57) | 40 (47) | ||
| Age, (years) | 65.1±11.5 | 68.2±10.5 | 0.111 | 64.0±10.7 | 68.5±10.9 | 0.026* |
| BMI (Kg/m2) | 21.3±2.1 | 21.3±2.1 | 0.944 | 21.4±2.0 | 21.3±2.1 | 0.763 |
| Blood type, n (%) | ||||||
| Non-O | 44 (88) | 70 (88) | 0.933 | 37 (84) | 77 (89) | 0.371 |
| O | 6 (12) | 10 (12) | 7 (16) | 9 (11) | ||
| Hypertension, n (%) | 21 (42) | 31 (39) | 0.713 | 18 (41) | 34 (40) | 0.317 |
| Smoking, n (%) | 19 (38) | 41 (51) | 0.140 | 23 (52) | 37 (43) | 0.880 |
| TC (mmol/L) | 4.51±0.97 | 4.57±1.44 | 0.795 | 4.75±1.07 | 4.44±1.36 | 0.194 |
| TG (mmol/L) | 1.56±0.81 | 1.38±0.80 | 0.214 | 1.43±0.90 | 1.45±0.76 | 0.905 |
| HDL-C (mmol/L) | 1.20±0.32 | 1.12±0.36 | 0.181 | 1.19±0.36 | 1.13±0.31 | 0.331 |
| LDL-C (mmol/L) | 2.73±0.79 | 2.86±1.22 | 0.473 | 2.99±0.91 | 2.71±1.14 | 0.158 |
| ApoA1 (mmol/L) | 1.09±0.21 | 1.03±0.21 | 0.104 | 1.10±0.26 | 1.03±0.18 | 0.124 |
| PON1 paraoxonase (U/L) | 303.0±97.2 | 278.6±93.0 | 0.155 | 310.4±112.3 | 276.5±83.2 | 0.081 |
| PON1 arylesterase (U/L) | 186.7±33.0 | 183.2±29.6 | 0.544 | 209.5±27.7 | 171.8±23.9 | <0.001* |
| VWF (%) | 117±47 | 110±46 | 0.430 | 91±26 | 124±51 | <0.001* |
P<0.05.
SCAD – stable coronary artery disease; BMI – body mass index; TG – triglycerides; TC – total cholesterol; HDL-C – high density lipoprotein cholesterol; LDL-C – low density lipoprotein cholesterol; PON1 – paraoxonase 1; VWF – von Willebrand factor, AUC – area under curve.
Table 5.
Multivariate logistic regression analysis of independent factors associated with the number and degree of stenosed coronary arteries among non-diabetic SCAD patients.
| Characteristic | SCAD with single or multiple vessel lesions | SCAD with mild or severe lesions | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Age (years) | 1.036 | 0.998–1.075 | 0.064 | 1.006 | 0.957–1.057 | 0.811 |
| non-Smoking | 0.482 | 0.222–1.044 | 0.064 | / | / | / |
| Gender(male) | 1.739 | 0.783–3.860 | 0.174 | / | / | / |
| TC | / | / | / | 1.656 | 0.426–6.437 | 0.466 |
| ApoA1 | / | / | / | 0.552 | 0.040–7.643 | 0.658 |
| HDL-C | 0.736 | 0.142–3.821 | 0.716 | / | / | / |
| LDL-C | / | / | / | 0.501 | 0.110–2.281 | 0.371 |
| PON1 paraoxonase | 0.998 | 0.994–1.002 | 0.390 | 1.004 | 0.999–1.010 | 0.120 |
| PON1 arylesterase | / | / | / | 0.940 | 0.915–0.965 | <0.001* |
| VWF | / | / | / | 6.863 | 1.404–33.560 | 0.017* |
Cox & Snell R2=0.0084; Cox & Snell R2=0.380
P<0.05.
SCAD – stable coronary artery disease; TC – total cholesterol; HDL-C – high density lipoprotein cholesterol; LDL-C – low density lipoprotein cholesterol; PON1 – paraoxonase 1; VWF – von Willebrand factor; 95% CI – 95% confidence interval.
Correlation between VWF and PON1 activity
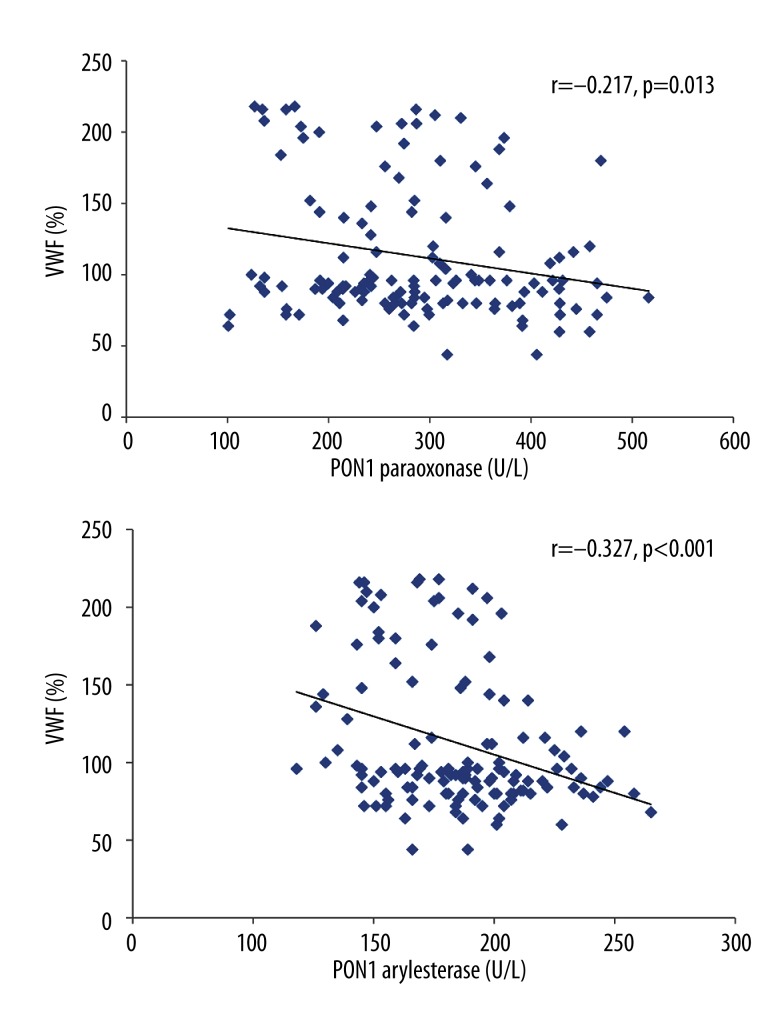
In the non-diabetic SCAD group, VWF was also slightly negatively correlated with PON1 paraoxonase (r=−0.217, P=0.013) and PON1 arylesterase activity (r=−0.327, P<0.001) (Figure 2).
Figure 2.
Pearson correlation analysis of the relationships between von Willebrand factor (VWF) and paraoxonase 1 (PON1) paraoxonase and arylesterase activities in patients with non-diabetic stable coronary artery disease (SCAD).
Discussion
This study demonstrated that non-diabetic SCAD patients have lower PON1 paraoxonase and PON1 arylesterase activity and higher VWF levels than control patients without SCAD. Specifically, lowered PON1 arylesterase activity and higher VWF levels were prevalent in the subgroup of patients with severely stenosed lesions. This was further confirmed by using a better logistic model for severely stenosed lesions, which indicated that PON1 arylesterase activity and VWF levels have a profound influence on the development of atherosclerosis in coronary arteries.
Other studies have shown that CAD patients have lower PON1 paraoxonase and arylesterase activity than patients serving as controls that become hospitalized with other unrelated diseases [17,18]. However, the distinction of PON1 paraoxonase and arylesterase activity as a measure to predict the occurrence of severely-stenosed lesions was somewhat unexpected. In our study, we confirmed that PON1 arylesterase activity was associated with severely stenosed lesions of coronary arteries. In clinical studies, individuals with severe atherosclerosis were more prone to experience ACS (acute coronary syndrome) or MI. A study by Tang et al. concluded that PON1 arylesterase activity could have incremental prognostic value and allows for the clinical reclassification of stable subjects at risk for developing major adverse cardiovascular events such as death, myocardial infarction, and stroke [19]; these findings support our conclusion.
Our study indicates that PON1 arylesterase activity is a valuable parameter for predicting severely stenosed coronary arteries in lesions in non-diabetic SCAD patients. Few ordinary chemical analytical systems can detect PON1 arylesterase activity because they lack the ability to detect and quantify UV wavelengths [13]. In our study, we explored a new phenyl acetate derivative for monitoring PON1 arylesterase activity, which could be detected at a wavelength of 410 nm and had an acceptable r2 value, in contrast to traditional methods.
Previous studies emphasized the importance of VWF in mediating platelet adhesion and thrombus formation, yet most of these studies focused on VWF in patients with acute coronary syndrome (ACS) [20,21]. However, it is still unclear whether VWF levels directly determine the rate and severity of arterial thrombus formation or whether they merely reflect an alteration in endothelial function. Increases in VWF can occur as a response to local endothelial damage and/or through acute inflammation. According to studies of acute bacterial inflammation, circulating concentrations of VWF were strongly correlated with circulating values of C-reactive protein (CRP). Therefore, we selected non-diabetic SCAD patients with CRP levels below 3.0 mg/L after 19 h, which was selected as our time frame, based on the half-life of CRP. Although Lee et al. previously observed that SCAD patients had higher VWF levels than healthy controls [22], to the best of our knowledge this is the first time that increased VWF levels have been associated with severe atherosclerosis in SCAD patients. We suggest that patients with severe atherosclerosis may have profound endothelial impairment, which is why VWF could be a good a marker for identifying severe atherosclerosis.
In recent years, improvement in the primary and secondary prevention of CAD has led to a considerable decrease in mortality [23]. However, severely stenosed lesions with more than 50% of the coronary arteries stenosed are in the secondary prevention stage, a situation that can unexpectedly and rapidly degrade, leading to complications, including MI and death. Therefore, SCAD patients need a rapid and effective method for risk stratification to allow for intervention before secondary prevention methods must be employed.
Our study strongly suggests that PON1 arylesterase activity and relative VWF levels could be excellent markers for SCAD risk stratification. In our study, this inverse relationship between PON1 paraoxonase/arylesterase activity and VWF levels was observed in all the patients, not just the non-diabetic SCAD group. Although the exact relationship between these 2 factors remains unknown, the following possibility should be considered. The regulation of VWF secretion in cultured endothelial cells is mainly dependent on cytosolic free calcium and cAMP. Calcium-raising agents can be inhibited by nitric oxide (NO) production [24]. HDL-associated PON1 activity leads to decreased ox-HDL and increased NO production through eNOS-activating pathways [25]. We anticipate that impaired HDL, with less protection of PON1 activity, inactivates the eNOS pathway, in turn inhibiting eNOS-dependent NO production, which then ultimately fails to inhibit VWF release. More HDL loses its function in patients with CAD, but less HDL-C is reduced [26]. Based on our results, we hypothesize that the relationship between VWF and HDL-C levels was decreased in the non-diabetic SCAD group, although the correlation between VWF and PON1 activity was more pronounced in the non-diabetic SCAD group. PON1 may have a role in inhibiting VWF release, but this requires more investigation.
This study has some limitations. The relatively small study population was necessary to exclude any of the complicating factors discussed above, but a larger study would add weight to the evidence presented here. This was a study in a Chinese population, so the results should be validated against other populations in case of differences due to racial background. Indeed, there have been studies that reveal the importance of single-nucleotide polymorphisms and genetic mutations on PON1 activity [7]; therefore, potential differences in activity should be considered in future studies and the polymorphisms measured. We found the new substrate used in the PON1 activity measurements to be beneficial but this will become more accepted when other investigators have also validated the method.
Conclusions
In summary, we have established a new method to detect PON1 arylesterase activity in our laboratory system, which should be readily implementable to clinical investigation. We have also successfully evaluated PON1 paraoxonase and arylesterase activity in non-diabetic SCAD patients, finding that low PON1 paraoxonase and arylesterase activity occurs along with high VWF levels in patients with non-diabetic stable coronary artery disease. PON1 arylesterase activity and VWF levels prove to be promising metrics for predicting the occurrence of severely stenosed coronary arteries in non-diabetic SCAD patients of Chinese origin.
Supplemental Table 1.
Detection evaluation of PON1 arylesterase activity.
| PON1 arylesterase activity | Intra-assay CV (%)* | Inter-assay CV (%)* | Linear range (U/L)** | Biologic limit of detection (U/L)*** |
|---|---|---|---|---|
| Classical method | 2.20% | 5.20% | 24.8–214.3 | 12.4 |
| Modified method | 0.90% | 2.40% | 24.5–344.5 | 12.3 |
Detection of intra- and inter-assay CV was performed according to NCCLS-EP5A and the maximum CV of control with different level was below 5%.
Detection of the linear range was performed according to NCCLS-EP6A and was found to be first-order linear within the range.
Detection of biologic limit of detection was performed according to NCCLS-EP17A.
The classical method of PON1 arylesterase activity is phenylacetate dissolved in methanol and Tris-HCl pH 8.0 reaction buffer. Correlation between these 2 methods was performed according to NCCLS-EP9A and the square of the correlation coefficient r2>0.95.
Footnotes
Source of support: Youth Research Foundation of Shanghai Municipal Commission of Health and Family Planning, China (Grant No. 2013118)
References
- 1.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 2.Colhoun HM, Betteridge DJ, Durrington P, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–85. doi: 10.2337/db11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care. 2011;34:1337–43. doi: 10.2337/dc10-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trujillo TC, Dobesh PP. Traditional management of chronic stable angina. Pharmacotherapy. 2007;27:1677–92. doi: 10.1592/phco.27.12.1677. [DOI] [PubMed] [Google Scholar]
- 5.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackness B, Quarck R, Verreth W, et al. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–50. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 7.Precourt LP, Amre D, Denis MC, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Ma Y, Fang Y, et al. Association between PON1 activity and coronary heart disease risk: a meta-analysis based on 43 studies. Mol Genet Metab. 2012;105:141–48. doi: 10.1016/j.ymgme.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 9.van Himbergen TM, van der Schouw YT, Voorbij HA, et al. Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis. 2008;199:408–14. doi: 10.1016/j.atherosclerosis.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Whincup PH, Danesh J, Walker M, et al. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002;23:1764–70. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- 11.Derhaschnig U, Jilma B. Assessment of platelets and the endothelium in patients presenting with acute coronary syndromes – is there a future? Thromb Haemost. 2009;102:1144–48. doi: 10.1160/TH09-07-0427. [DOI] [PubMed] [Google Scholar]
- 12.Zarei S, Frieden M, Kaufmann JE, Vischer UM. The regulation of endothelial VWF secretion by nitric oxide: is it physiological? J Thromb Haemost. 2006;4:263–65. doi: 10.1111/j.1538-7836.2005.01626.x. [DOI] [PubMed] [Google Scholar]
- 13.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–6. [PubMed] [Google Scholar]
- 14.Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet. 2008;1:147–52. doi: 10.1161/CIRCGENETICS.108.811638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards (NCCLS) NCCLS document EP5-A, Approved Guideline. Vol. 19. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999. Evaluation of Precision Performance of Clinical Chemistry Devices; pp. 20–36. [Google Scholar]
- 17.Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: is the gene or the protein more important? Free Radic Biol Med. 2004;37:1317–23. doi: 10.1016/j.freeradbiomed.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Najafi M, Gohari LH, Firoozrai M. Paraoxonase 1 gene promoter polymorphisms are associated with the extent of stenosis in coronary arteries. Thromb Res. 2009;123:503–10. doi: 10.1016/j.thromres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Tang WH, Hartiala J, Fan Y, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2012;32:2803–12. doi: 10.1161/ATVBAHA.112.253930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morange PE, Simon C, Alessi MC, et al. Endothelial cell markers and the risk of coronary heart disease: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. Circulation. 2004;109:1343–48. doi: 10.1161/01.CIR.0000120705.55512.EC. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 22.Lee KW, Lip GY, Tayebjee M, et al. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood. 2005;105:526–32. doi: 10.1182/blood-2004-03-1106. [DOI] [PubMed] [Google Scholar]
- 23.Young F, Capewell S, Ford ES, Critchley JA. Coronary mortality declines in the U.S. between 1980 and 2000 quantifying the contributions from primary and secondary prevention. Am J Prev Med. 2010;39:228–34. doi: 10.1016/j.amepre.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost. 2006;4:1186–93. doi: 10.1111/j.1538-7836.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- 25.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–74. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.
Detection evaluation of PON1 arylesterase activity.
| PON1 arylesterase activity | Intra-assay CV (%)* | Inter-assay CV (%)* | Linear range (U/L)** | Biologic limit of detection (U/L)*** |
|---|---|---|---|---|
| Classical method | 2.20% | 5.20% | 24.8–214.3 | 12.4 |
| Modified method | 0.90% | 2.40% | 24.5–344.5 | 12.3 |
Detection of intra- and inter-assay CV was performed according to NCCLS-EP5A and the maximum CV of control with different level was below 5%.
Detection of the linear range was performed according to NCCLS-EP6A and was found to be first-order linear within the range.
Detection of biologic limit of detection was performed according to NCCLS-EP17A.
The classical method of PON1 arylesterase activity is phenylacetate dissolved in methanol and Tris-HCl pH 8.0 reaction buffer. Correlation between these 2 methods was performed according to NCCLS-EP9A and the square of the correlation coefficient r2>0.95.