Abstract
Persistent pain following breast cancer surgery is well-documented. However, it is not well characterized in terms of the anatomic site effected (i.e., breast, arm). In two separate growth mixture modeling analyses, we identified subgroups of women (n=398) with distinct breast pain and arm pain trajectories. Based on the fact that these latent classes differed by anatomic site, types if tissue affected, and neural innervation patterns suggests the need for separate evaluations of these distinct persistent pain conditions. Purposes of this companion study were to identify demographic and clinical characteristics that differed between the two arm pain classes and determine if differences existed over time in sensitivity in the upper inner arm and axillary lymph node dissection (ALND) sites, pain qualities, pain interference, and hand and arm function; as well as to compare findings with persistent breast pain. Higher occurrence rates for depression and lymphedema were found in the Moderate Arm pain class. Regardless of pain group membership, sensory loss was observed in the upper inner arm and ALND site. Arm pain was described similarly to neuropathic pain and interfered with daily functioning. Persistent arm pain was associated with sustained impairments in shoulder mobility.
Perspective: For persistent breast and arm pain, changes in sensation following breast cancer surgery were notable. Persistent arm pain was associated with sustained interference with daily functioning and upper body mobility impairments. Long-term management of persistent pain following breast cancer surgery is warranted to improve the quality of survivorship for these women.
Keywords: arm pain, breast cancer surgery, pain qualities, pain interference, range of motion, grip strength, sensory changes, persistent pain, chronic pain
INTRODUCTION
The occurrence of persistent pain following breast cancer surgery is a well-documented pain condition. This pain, which can occur in the breast and/or upper extremity, affects 25% to 60% of patients.24 However, considerable variation in the precise definition of this pain condition has limited the identification of definitive risk factors.1 Several studies evaluated for changes in sensation and upper extremity mobility following breast cancer surgery. In general, sustained impairments in mobility and a high prevalence of sensory loss were found, particularly following axillary lymph node dissection (ALND).5,7.8,12–15,23 However, changes in sensations and mobility associated with persistent arm pain were not evaluated. In fact, little is known about the qualities of persistent arm pain; as well as its association with changes in sensation, daily functioning, muscle strength, and shoulder mobility.
In an effort to better characterize this complex and persistent pain condition, our research team evaluated worst pain trajectories in the breast and arm/shoulder (referred to as “arm pain” in the remainder of this paper) as distinct persistent pain conditions in a sample of women (n=398) followed prospectively for six months after breast cancer surgery.17–18 These separate assessments of arm versus breast pain were designed purposefully to be comparable so that differences in persistent pain between these two distinct anatomic sites (e.g. different tissue types, different innervation patterns) could be evaluated.
Using growth mixture modeling (GMM), distinct subgroups of women who reported similar worst pain trajectories were identified. For the breast pain analysis, four distinct subgroups (32% No Pain; 43% Mild Pain; 13% Moderate Pain; 12% Severe Pain) were identified.17 For the arm pain analysis, only three distinct subgroups (42% No Pain; 23% Mild Pain; 35% Moderate Pain) were identified.18 Of note, only 20% of these patients were classified in the No Pain groups for both breast and arm pain. Given the differences in tissue types and neural innervation patterns in breast tissue and in the area of the sentinel lymph node biopsy or ALND (i.e., axilla), as well as the differential classification of patients based on separate GMM analyses of persistent breast and arm pain, we hypothesized that these two pain conditions would have distinct phenotypic characteristics.
In fact, in the initial publications that included the No Pain Groups,17,18 a number of demographic and clinical characteristics were significantly associated with both breast and arm pain group membership (e.g., age, education, functional status, comborbidity scores, occurrence of preoperative breast pain). However, several clinical characteristics differentiated between the breast and arm pain classes. For example, preoperative numbness and hardness in the breast, comorbid hypertension and rheumatoid arthritis, receipt of radiation therapy, and re-excision/mastectomy within six months after surgery differentiated among the breast pain classes.17 In contrast, receipt of neoadjuvant chemotherapy, stage of disease, number of positive lymph nodes, number of drains placed, type of surgery, occurrence of postoperative complications, and the proportion of patients who received physical therapy differentiated among the arm pain classes.18 Taken together, these findings suggest that breast and arm pain are distinct persistent pain conditions following breast cancer surgery.
This initial phenotyping suggested that a more detailed evaluation of these two persistent pain conditions was warranted. Therefore, in parallel with our study of persistent breast pain [16], the purposes of this study were to identify demographic and clinical characteristics that differed between the two arm pain classes (i.e., Mild, Moderate) and to determine if any differences existed over time between the two persistent arm pain classes in sensations in the upper inner arm and node dissection site; pain qualities; pain interference; and hand and arm function (i.e., grip strength, shoulder mobility). In addition these findings will be compared with findings from the persistent breast pain study.17
MATERIALS AND METHODS
Patients and Settings
The methods are described in detail elsewhere18 and are abbreviated for the purposes of the current study in our companion paper of persistent breast pain.16 The reader is referred to this companion paper for a more comprehensive description of the material and methods. In brief, adult women who were scheduled for unilateral breast cancer surgery, without distant metastasis; were able to complete the questionnaires; and gave written informed consent were eligible to participate.
Subjective Measures
The demographic questionnaire obtained information on age, marital status, education, ethnicity, employment status, living situation, and financial status. The Karnofsky Performance Status (KPS) scale was used to evaluate functional status.11 The Self-Administered Comorbidity Questionnaire (SCQ) was used to measure comorbidity.2,20
The occurrence of pain in the arm/shoulder was assessed using the Arm/Shoulder Symptoms Questionnaire (ASQ). In addition, patients were asked to rate the intensity of their average and worst pain, in the past week, using a numeric rating scale (NRS) that ranged from 0 (no pain) to 10 (worst imaginable pain). Patients rated the level of interference caused by arm/shoulder pain with sixteen activities using a 0 (does not interfere) to 10 (completely interferes) NRS. Patients completed the ASQ at 1, 2, 3, 4, 5, and 6 months after surgery. At the Month 1 assessment, patients were asked to rate the intensity of their postoperative pain in the first 24 to 48 hours after surgery.
Pain qualities were evaluated using the valid and reliable Pain Qualities Assessment Scale (PQAS).9,25 Sixteen items evaluated the magnitude of the different pain quality descriptors. Three subscale scores were calculated (i.e., Paroxysmal, Surface, Deep).25 Patients completed the PQAS associated with arm pain at 1, 2, 3, 4, 5, and 6 months after surgery.
Objective Measures
Sensitivity in the upper inner arm and ALND site along the length of the scar was tested at 4 to 8 sites depending on the length of the scar, using a 5.07 gram monofilament and compared to the corresponding area on the unaffected side. For each site tested, patients reported whether it was “much less sensitive than the opposite side”, “same as the opposite side”, or “much more sensitive than the opposite side”. The percentages for the total number of sites classified as “much less,” “same,” and “much more” were calculated. Sensitivity in the upper inner arm and ALND incision sites were evaluated at 1, 2, 3, 4, 5, and 6 months following surgery (i.e., six assessments).
Grip strength in kilograms (kg) was measured using a Jamar hydraulic hand dynamometer (Patterson Medical, Bolingbrook, IL) according to the procedures described by Spijkerman and colleagues.21 Shoulder mobility was assessed using goniometric measurements of range of motion (ROM). While the patient was lying supine, ROM was measured, in degrees, for 4 positions (i.e., flexion, abduction, internal rotation, external rotation). Grip strength and shoulder mobility were measured prior to surgery and 1, 2, 3, 4, 5, and 6 months following surgery (i.e., seven assessments).
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. After providing written informed consent prior to surgery, eligible patients completed the enrollment questionnaires. Then, the research nurse performed the baseline objective measures.
The research nurse met with the patient either in their home or in the Clinical Research Center at 1, 2, 3, 4, 5, and 6 months after surgery. During each of the study visits, the women completed the study questionnaires and had the objective measures done by the research nurse. Over the course of the study, medical records were reviewed for disease and treatment information. Inter-rater reliability among the research nurses for each of the objective measures, evaluated every six months, exceeded 0.80.
Characterization of the persistent arm pain phenotypes
A description of the GMM analysis that was used to characterize the persistent arm pain phenotypes was reported previously.18 In brief, at each assessment, patients were asked, “Are you experiencing pain in your shoulder, arm, or hand on the side of the surgery?”. If the patient reported pain, she was asked to rate her worst pain during the past week using a 0 (no pain) to 10 (worst pain) NRS. Prior to conducting the GMM analysis, patients who reported no pain in their arm, shoulder, or hand for all six assessments (i.e., 1, 2, 3, 4, 5, and 6 months) were identified (n=164; 42%) and were not included in the GMM analysis. For the remaining 230 women, the six ratings of worst arm pain were used in the GMM analysis to assign each patient into a latent class. The GMM analysis was done using Mplus 6.1.[19]. Because patients in the No Arm Pain class did not complete the other pain measures (i.e., BPI, PQAS), these data could not be modeled. Therefore, only data from the two pain classes (i.e., Mild, Moderate) were evaluated.
Statistical Analyses
Data were analyzed using SPSS version 21.0. Descriptive statistics and frequency distributions were calculated for patients’ demographic and clinical characteristics. Independent Student t-tests, as well as Mann-Whitney U, Chi-square, or Fisher Exact tests were performed to evaluate for differences in demographic and clinical characteristics between the two arm pain classes.
Linear mixed effects models fit by restricted maximum likelihood estimation (REML) were evaluated to determine whether any differences existed over time between the latent classes in: sensitivity in the upper inner arm and ALND (for those women who had ALND) sites; PQAS subscale scores; individual pain interference items; grip strength; and shoulder mobility. The tests of Group x Time interactions determined whether changes in any of these outcomes were significantly different between the arm pain classes. In addition, group effects (differences between the classes) and time effects (changes over time across the classes) were evaluated using mixed-model tests of main effects. Time effects were described based on evaluation of estimated marginal means and respective plots, and supplemented by post-hoc pairwise comparisons between the monthly timepoints. A P-value of <0.05 was considered statistically significant.
RESULTS
Differences in Demographic and Clinical Characteristics between the Arm Pain Classes
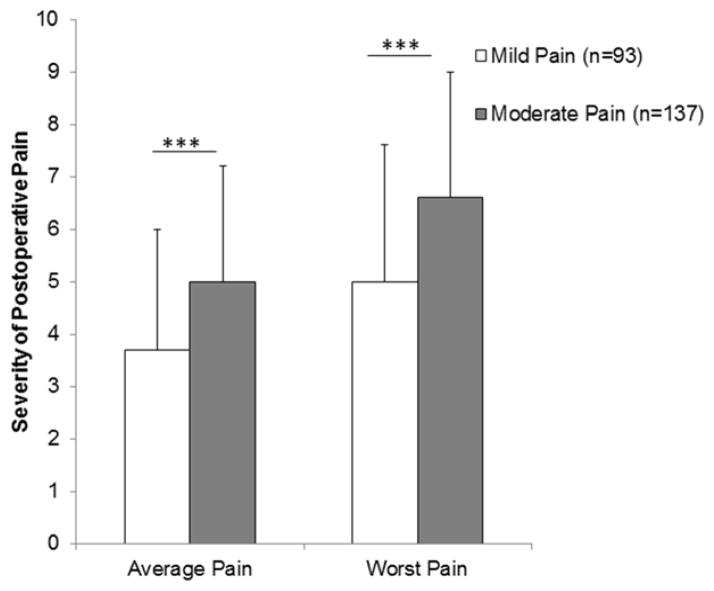
A comprehensive description of the differences in demographic and clinical characteristics among the three arm pain classes (i.e., No, Mild, Moderate Pain) is provided elsewhere.18 Differences in select demographic and clinical characteristics between the two arm pain classes are displayed in Table 1. Women in the Moderate Pain class had significantly fewer years of education, lower KPS scores, and higher comorbidity scores than women in the Mild Pain class. An overall difference in the distribution of the ethnic groups was found between the two arm pain classes (P = .014). Post hoc tests revealed that a significantly higher proportion of Black women was in the Moderate Pain class than in the Mild Pain class (P = .02). The proportion of White women was significantly higher in the Mild Pain class than in the Moderate Pain class (P = .006). Income was significantly higher in the Mild Pain class than the Moderate Pain class (P = .006). Finally, a significantly higher proportion of women in the Moderate Pain class reported comorbid depression, preoperative swelling in the affected breast, and developed lymphedema than women in the Mild Pain class. As shown in Figure 1, women in the Moderate Pain class reported significantly higher average and worst postoperative pain ratings than women in the Mild pain class.
Table 1.
Differences in Demographic and Clinical Characteristics Between the Arm/Shoulder Pain Classes
| Characteristic | Mild Pain (1) 93 (40.4%) |
Moderate Pain (2) 137 (59.6%) |
Statistics |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | ||
|
| |||
| Age (years) | 52.7 (9.7) | 52.9 (11.3) | t=−0.80, P=.423 |
|
| |||
| Education (years) | 16.3 (2.7) | 15.3 (2.7) | t=6.76, P<.001 |
|
| |||
| Karnofsky Performance Status score | 93.1 (10.0) | 89.3 (12.4) | t=2.46, P=.015 |
|
| |||
| Self-Administered Comorbidity Scale score | 3.8 (2.4) | 5.0 (3.1) | t=−3.24, P=.001 |
|
| |||
| Body mass index (kg/m2) | 26.3 (6.2) | 28.1 (6.9) | t=−1.92, P=.056 |
|
| |||
| % (N) | % (N) | ||
|
| |||
| Ethnicity | |||
| White | 68.8 (64) | 50.0 (68) | |
| Black | 7.5 (7) | 19.1 (26) | χ2=10.61, P=.014 |
| Asian/Pacific Islander | 14.0 (13) | 14.0 (19) | |
| Hispanic/Mixed Ethnic Background/Other | 9.7 (9) | 16.9 (23) | |
|
| |||
| Lives alone | |||
| Yes | 19.4 (18) | 24.6 (33) | FE, P=.419 |
| No | 80.6 (75) | 75.4 (101) | |
|
| |||
| Marital status | |||
| Married/partnered | 35.5 (33) | 43.0 (58) | FE, P=.274 |
| Single, separated, widowed, divorced | 64.5 (60) | 57.0 (77) | |
|
| |||
| Currently working for pay | |||
| Yes | 53.3 (49) | 43.1 (59) | FE, P=.139 |
| No | 46.7 (43) | 56.9 (78) | |
|
| |||
| Total annual household income | |||
| <$10,000 to $29,999 | 18.1 (15) | 29.9 (32) | |
| $30,000 to $99,999 | 34.9 (29) | 42.1 (45) | χ2=7.88, P=.019 |
| ≥$100,000 | 47.0 (39) | 28.0 (30) | |
|
| |||
| Occurrence of comorbid conditions | |||
| Depression | 14.0 (13) | 27.0 (37) | FE, P=.022 |
|
| |||
| Swelling in breast pre-operatively | |||
| Yes | 5.4 (5) | 13.9 (19) | FE, P=.048 |
| No | 94.6 (88) | 86.1 (118) | |
|
| |||
| Type of surgery | |||
| Breast conserving | 79.6 (74) | 74.5 (102) | FE, P=.429 |
| Mastectomy | 20.4 (19) | 25.5 (35) | |
|
| |||
| Sentinel lymph node biopsy | |||
| Yes | 86.0 (80) | 83.9 (115) | FE, P=.407 |
| No | 14.0 (13) | 16.1 (22) | |
|
| |||
| Axillary lymph node dissection | |||
| Yes | 47.3 (44) | 51.1 (70) | FE, P=.593 |
| No | 52.7 (49) | 48.9 (67) | |
|
| |||
| Received hormonal therapy during the 6 months | |||
| Yes | 45.2 (42) | 38.0 (52) | FE, P=.279 |
| No | 54.8 (51) | 62.0 (85) | |
|
| |||
| Received radiation therapy during the 6 months | |||
| Yes | 54.8 (51) | 54.7 (75) | FE, P=1.000 |
| No | 45.2 (42) | 45.3 (62) | |
|
| |||
| Received adjuvant chemotherapy during the 6 months | |||
| Yes | 38.7 (36) | 38.0 (52) | FE, P=1.000 |
| No | 61.3 (57) | 62.0 (85) | |
|
| |||
| Occurrence of lymphedema | 15.1 (14) | 29.4 (40) | FE, P=.017 |
Abbreviations: FE = Fisher’s Exact; kg/m2 = kilograms per meter squared; SD = standard deviation
Figure 1.
Differences in average and worst postoperative pain ratings (on 11-point numeric rating scale) for 24 to 48 hours following breast cancer surgery between the pain classes. Values are plotted as means and standard deviations. ***P < .001.
Changes in Sensations in the Upper Inner Arm and the ALND Site over Time between the Arm Pain Classes
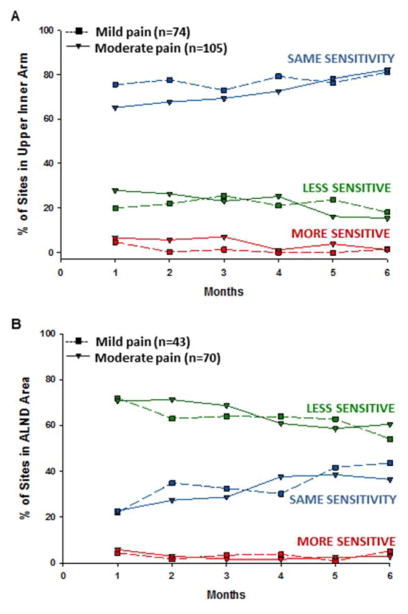
Across the two arm pain classes, 15% to 30% of the sites tested in the upper inner arm and 50% to 75% of the sites tested in ALND site were much less sensitive than the unaffected side. As shown in Figures 2A and 2B, for both sites regardless of arm pain class, less than 6% of sites tested were more sensitive than the affected side.
Figure 2.
Plots of the estimated marginal means over time between the arm pain classes for the mixed effects model for the percentage of sites in the upper inner arm (A) and axillary lymph node dissection (ALND) sites reported as less sensitive (green), the same (blue), and more sensitive (red) than the unaffected side. Statistically significant findings for upper inner arm: Percentage less sensitive – time effect: P = .04; Percentage the same – time effect: P = .006; Percentage more sensitive – group effect: P = .01 and time effect: P = .993.
For the upper inner arm, compared to the Mild Pain class, a higher percentage of sites in the affected upper inner arm of women in the Moderate Pain class were classified as more sensitive (P < .05). In addition, significant time effects were found for the percentage of sites classified as less, same, and more sensitive (all P < .05). Regardless of arm pain class, the percentage of sites in the upper inner arm that were classified as less sensitive and more sensitive decreased significantly over time, while the percentage of sites classified as the same increased significantly over time (Figure 2A). For the ALND site, no statistically significant group, time, or Group x Time effects were found (Figure 2B).
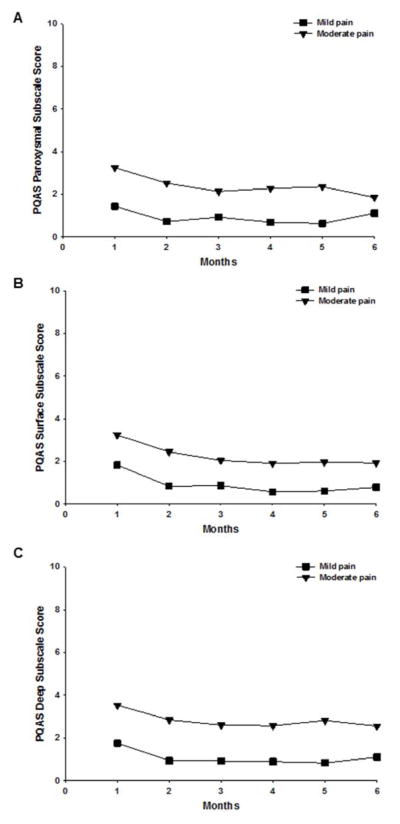
Changes in Pain Qualities over Time between the Arm Pain Classes
Figure 3 illustrates changes over time between the arm pain classes in the PQAS Paroxysmal, Surface, and Deep subscale scores. No significant Group x Time interactions were found for any of the PQAS subscales. Significant group effects were found for all of the PQAS subscale scores (all P < .001), with the Moderate Arm Pain class reporting higher scores than the Mild Pain class. In addition, significant time effects were observed for all of these subscales (P < .001), such that regardless of arm pain class, these scores decreased over time relative to Month 1.
Figure 3.
Plots of the estimated marginal means over time between the arm pain classes for the mixed effects models for the Pain Qualities Assessment Scale (PQAS) Paroxysmal (A); Surface (B); and Deep (C) subscale scores between the arm pain classes. Statistically significant findings: Group and time effects for Paroxysmal, Surface, and Deep, all P < .001.
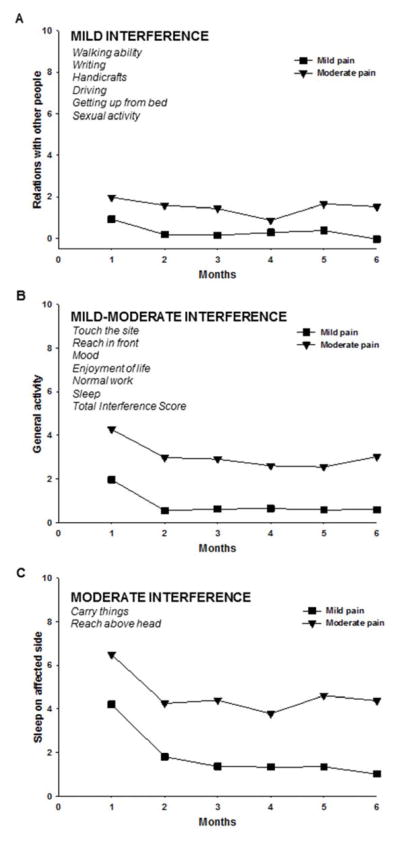
Changes in Pain Interference over Time between the Arm Pain Classes
Figure 4 illustrates changes over time between the arm pain classes in pain interference scores. Each of the panels displays an exemplar plot for the pain interference items that demonstrated: mild (i.e., ≤ 2, A); mild-moderate (i.e., 2.1 – 3.9, B); or moderate (i.e., ≥ 4, C) interference scores based on the average score across time for the Moderate Pain classes. Mild interference was observed for: relations with other people (exemplar); ability to walk, write, do handicrafts, drive, and get up from bed; as well as sexual activity. Mild-moderate interference was observed for: general activity (exemplar); ability to touch the operative site and reach in front; mood, and enjoyment of life. Moderate interference was observed for: ability to sleep on the affected side (exemplar), carry things, and reach above the head.
Figure 4.
Plots of the estimated marginal means over time, between the arm pain classes, for the mixed effects models for pain interference scores. Each of the panels displays an exemplar plot for the pain interference items that demonstrated: (A) mild interference (≤ 2); (B) mild-moderate interference (2.1 – 3.9); (C) moderate interference (≥ 4), based on the average pain interference score across time for the Moderate Pain class.
None of the pain interference items demonstrated significant Group x Time interactions. However, all of the pain interference items demonstrated a significant group effect (all P < .001). Women in the Moderate Pain class reported higher interference scores than women in the Mild Pain class. In addition, all of the pain interference items demonstrated a significant time effect (all P < .05), such that, regardless of pain class, interference scores decreased over time relative to Month 1.
Changes in Grip Strength and Shoulder Mobility over Time between the Arm Pain Classes
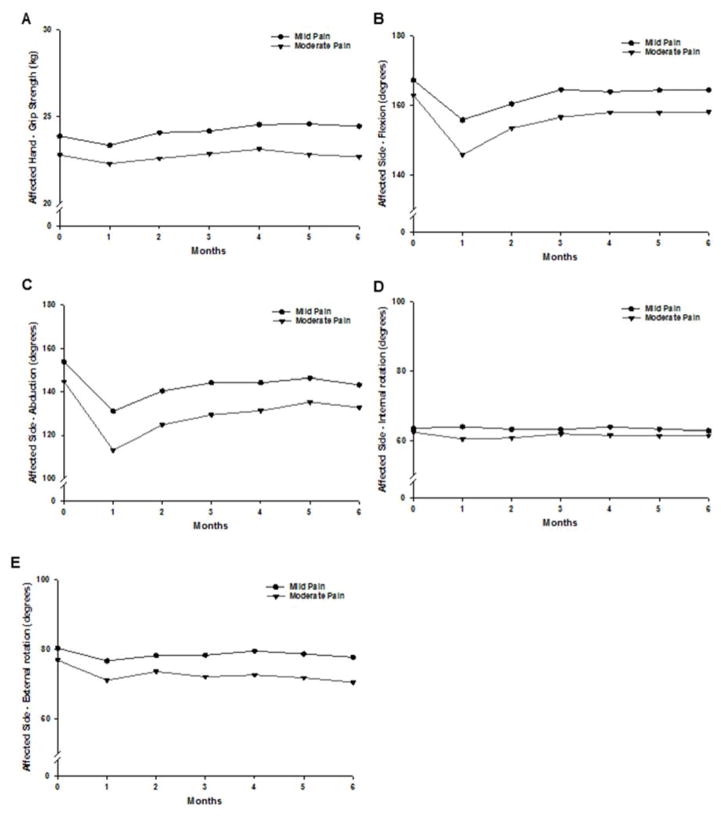
Figure 5 illustrates changes over time in grip strength and shoulder mobility (i.e., flexion, abduction, internal rotation, external rotation) between the two arm pain classes. For all four measures, no significant Group x Time interactions were observed. For grip strength, only a significant time effect (P < .001) was found, where on average, grip strength increased after Month 1. For flexion, abduction, and external rotation, significant group (all P < .001) and time (all P < .001) effects were observed. Women in the Moderate Pain class had decreased flexion, abduction, and external rotation measurements compared to women in the Mild Pain class. As depicted in Figures 5B and 5C, flexion and abduction appear to follow a quadratic pattern. Measurements decreased from Month 0 to Month 1, increased from Month 1 to Month 3, then plateaued, remaining lower than Month 0. As shown in Figure 5D, external rotation decreased over time relative to Month 0. No significant group, time or Group x Time effects were observed for internal rotation.
Figure 5.
Plots of the estimated marginal means over time between the arm pain classes for the mixed effects model for grip strength (A), flexion (B), abduction (C), internal rotation (D), and external rotation (E). Statistically significant findings: Grip strength - time effect: P < .001; Flexion - group effect: P < .001 and time effect: P < .001; Abduction - group effect: P < .001 and time effect: P < .001; External rotation - group effect: P < .001 and time effect: P < .001.
DISCUSSION
This study provides a detailed phenotypic characterization of persistent arm pain following breast cancer surgery. In addition, this analysis extends our previous work16–18 and confirms our hypothesis that breast and arm pain following breast cancer surgery are distinct persistent pain conditions. Throughout the discussion, similarities and differences between the breast and arm pain sites, particularly between the two more severe pain classes (i.e., Moderate Arm Pain, Severe Breast Pain) are highlighted.
In terms of demographic and clinical characteristics, women in the Moderate Arm Pain class were more likely to be Black, had less education, lower annual incomes, lower functional status scores, higher comorbidity scores, a higher number of postoperative complications, and higher occurrence rates for depression. Of note, findings related to years of education, annual income, as well as functional status and comorbidity scores, were similar for the Severe Breast Pain class. However, additional characteristics associated with membership in the Severe Breast Pain class, but not in the Moderate Arm Pain class included higher body mass index and higher occurrence rates of hypertension and rheumatoid arthritis.
In terms of preoperative breast symptoms and postoperative pain, a higher percentage of women in the Moderate Arm Pain class reported preoperative swelling in the affected breast and higher average and worst postoperative pain intensity scores. While findings regarding preoperative breast swelling and postoperative pain intensity scores were similar for the Severe Breast Pain class,16 a higher percentage of women in this latent class reported numbness in the affected breast prior to surgery. Of note, the occurrence of lymphedema differed between the arm, but not the breast pain classes. This finding warrants additional investigation because findings regarding the association lymphedema and extremity pain are inconsistent.4,6,10 The phenotypic differences between the persistent breast and arm pain conditions warrant confirmation in an independent sample.
Changes in sensations in the upper inner arm or ALND incision site did not differ between the persistent arm pain classes. In the upper inner arm, the majority of the sites had the same sensitivity as the unaffected arm. However, 15% to 30% of these sites were much less sensitive than the unaffected arm. Consistent with sites in the breast scar area, ALND sites were most frequently classified as “much less” sensitive than the unaffected side. While sensory loss or numbness after surgery, particularly following ALND, was reported previously,5,7,8,12,14,15,23 it is surprising that despite persistent pain, only a small percentage of sites in both the upper inner arm and ALND area were classified as hypersensitive (<6% for both areas tested). Collectively, these findings suggest that sensory loss, particularly at the sites of the surgical incisions, is highly prevalent and persistent. As noted in our companion paper,16 the co-occurrence of sensory loss and persistent arm pain may reflect an underlying neuropathic mechanism. Alternatively, ongoing pain and touch-evoked sensations may differ mechanistically.
In terms of the qualities of arm pain, compared to women in the Mild Pain class, women in the Moderate Pain class reported higher scores on all of the PQAS subscales (i.e., Paroxysmal, Deep, Surface) that persisted over the six months of the study. Subscale scores for the Mild Pain class stabilized by 2 months, whereas subscale scores for the Moderate Pain class stabilized by 3 months after surgery. These between-group differences in the time to plateau suggest differential postoperative healing periods. The finding that postoperative complications were most prevalent in the Moderate Pain class18 supports this hypothesis. Of note, in contrast to breast pain, patients did not report on the qualities of arm pain prior to surgery (Month 0). Therefore, we do not know if women experienced arm or shoulder pain prior to surgery.
Consistent with findings from the sensory examination, the pattern of PQAS scores reported by the Moderate Arm Pain class was consistent with those observed in a neuropathic pain condition (i.e., carpal tunnel syndrome).25 Of note, this finding contrasts with the pattern of PQAS subscale scores reported by the Severe Breast Pain class that was more consistent with the pattern of scores observed in patients with non-neuropathic pain conditions (i.e., low back pain, osteoarthritis).25 This distinction between the pattern of PQAS scores for the breast and arm pain classes highlights the importance of separate evaluations of breast and arm pain as distinct persistent pain conditions.
With regards to pain interference, a significant group effect was found for each of the items in the expected direction (Moderate Pain > Mild Pain class). A time effect was observed for all of the interference items, such that scores decreased from one to two months following surgery. This decrease over time can be attributed to normal postoperative healing. Unlike breast pain, which showed variations in the trajectories of pain interference by latent class membership, all of the pain interference items for arm pain demonstrated the same trajectory and only main effects of group and time. Pain interference was fairly mild for the majority of items. Scores for patients in the Moderate Pain class were in the moderate range (>4) for only three physical function items, which involved the use of the arm or shoulder (i.e., sleep on affected side, ability to carry things, reaching above the head). In contrast, women in the Severe Breast Pain class reported higher interference scores overall, and moderate pain interference for more items, including psychological items, such as mood and enjoyment of life. These differences may be due to the nature of the class construction (“Severe” breast pain versus “Moderate” arm pain), with the Severe Breast Pain class reflective of a more extreme phenotype. However, it is surprising that, compared to the Mild Arm Pain class, higher pain interference scores for other physical function items that involve the use of the arm, shoulder or hand, were not reported by patients in the Moderate Arm Pain class.
In terms of grip strength and shoulder mobility, compared to women in the Mild Pain class, women in the Moderate Pain class had persistently lower measures of flexion, abduction, and external rotation. Group effects for grip strength (P = .06) and internal rotation (P = .05) approached, but did not reach statistical significance. Interestingly, consistent with persistent breast pain, these between-groups differences (with the exception of internal rotation) occurred prior to surgery. Additional research is warranted to determine if arm pain occurs prior to surgery and how this pain affects women’s arm and shoulder mobility.
Time effects were observed for grip strength, flexion, abduction, and external rotation. In both the Mild and Moderate Pain classes, an overall increase in grip strength was observed, while range of motion initially decreased over time. In particular, both statistically significant and clinically meaningful impairments in flexion and abduction were observed from prior to through one month following surgery. This reduction in mobility at the one month assessment for both arm pain classes can be attributed to the effects of surgery. Although no statistically significant interaction was found, the rate of change (i.e., steepness of line) from enrollment to Month 1 appears greater for women in the Moderate Pain class. The higher proportion of women with postoperative complications in the Moderate Pain class18 may explain this finding. Although improvements were observed after one month, flexion, abduction, and external rotation did not return to preoperative levels six months after surgery, which highlights the need for ongoing clinical evaluation and rehabilitation. Of note, no significant differences were found in the proportion of women in the Mild and Moderate Pain classes who underwent physical therapy on a monthly basis (Supplemental Figure 1).
A number of studies evaluated arm morbidity following breast cancer surgery.5,7,8,13–15 Many of these studies focused on morbidity after ALND5,8,14 or evaluated ALND as a risk factor for more severe morbidity.13,15 In general, sustained mobility impairments and more severe impairments in strength and mobility were found in patients who had a complete ALND. Interestingly, although a similar proportion of patients in the Mild and Moderate Pain classes had an ALND (47% and 51%, respectively), statistically significant differences in the degree of impairment in flexion, abduction, and external rotation, were found. Previous studies did not evaluate associations between pain and upper extremity morbidity. Therefore, our findings suggest that the severity of persistent arm pain has an independent role in modulating functional impairments after breast cancer surgery. Persistent arm pain may result in an adaptive response, such that patients restrict arm and shoulder movement in order to promote healing. However, this response may result in long term impairments in arm and shoulder mobility. Efforts to manage this pain may reduce these functional impairments.
Medium effect sizes were observed for the differences between the Mild and Moderate Pain classes with respect to flexion (d=0.55), abduction (d=0.59), and external rotation (d=0.61). The observed differences between the classes did not reach published standards for clinically meaningful differences of 20°.13 However, a 20° change represents severe physical impairments.26 The more subtle changes that persist over time may represent clinically meaningful decrements in these patients.
Limitations of this study need to be acknowledged. A larger sample size might have allowed for detection of more subtle differences between the classes, including interaction effects. In addition, generalizability may be limited as a result of this relatively homogeneous sample of women. Patients were asked to recall the severity of postoperative pain at the Month 1 assessment. In future studies, postoperative pain should be assessed prospectively. In addition, future studies that evaluate persistent arm pain should determine whether pain exists preoperatively, and if so, how pain changes following surgery. Finally, approximately 8% of the current sample belonged to both the Moderate Arm Pain class and the Severe Breast Pain class. This subset of women may be at particularly high risk for more negative outcomes, such as greater interference with function and more significant functional impairments. Therefore, further study of this subgroup of women, including an evaluation of risk factors and a detailed description of their pain experience, is warranted.
In summary, consistent with persistent breast pain,16 the overall differences in pain qualities, interference, and function between the arm pain classes were largely differences of severity, suggestive of a similar underlying mechanism. As such, similar interventions that differ only by dose or intensity may be effective for both arm pain subgroups. Similarities between persistent breast and arm pain, in a number of demographic and clinical characteristics, were observed. One important similarity was the association between postoperative pain intensity and membership in the Moderate Arm Pain and Severe Breast Pain classes. This finding highlights the need for adequate acute pain management. In addition, it may reflect inter-individual variability in pain sensitivity3 or responses to analgesic medications.22 However, arm pain was described more similarly to neuropathic pain and exhibited less variability in patterns of change across all measures. These differences suggest that breast and arm pain are two distinct persistent pain conditions. Mechanistic insights may be gleaned from parallel efforts to identify similarities and differences in molecular markers associated with latent class membership for both persistent breast and arm pain. Importantly, for both persistent breast and arm pain, changes in sensation were notable. In addition, persistent arm pain was associated with sustained interference with daily functioning and upper body mobility impairments. As such, long term clinical evaluation and management of both types of pain in women following breast cancer surgery are warranted to improve the quality of survivorship for these women.
Supplementary Material
Supplemental Figure 1. Percentages of women in the Mild and Moderate Pain classes who underwent physical therapy during the previous month (e.g., Month 1 = any physical therapy from prior to through six months following surgery).
Acknowledgments
This study was funded by grants from the National Cancer Institute (NCI; CA107091 and CA118658). Dr. Bradley Aouizerat was funded through the National Institutes of Health (NIH) Roadmap for Medical Research Grant (KL2 RR624130). Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor and has a K05 award from the NCI (CA168960). Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
Disclosures:
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison RN. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46:30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment - A nationwide study of prevalence and associated factors. Breast. 2010;19:506–515. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Hamner JB, Fleming MD. Lymphedema therapy reduces the volume of edema and pain in patients with breast cancer. Ann Surg Oncol. 2007;14:1904–1908. doi: 10.1245/s10434-006-9332-1. [DOI] [PubMed] [Google Scholar]
- 7.Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, Schmitz KH. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118:2237–2249. doi: 10.1002/cncr.27467. [DOI] [PubMed] [Google Scholar]
- 8.Ivens D, Hoe AL, Podd TJ, Hamilton CR, Taylor I, Royle GT. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7:823–832. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Jeong HJ, Sim YJ, Hwang KH, Kim GC. Causes of shoulder pain in women with breast cancer-related lymphedema: a pilot study. Yonsei Med J. 2011;52:661–667. doi: 10.3349/ymj.2011.52.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 12.Karydas I, Fentiman IS, Habib F, Hayward JL. Sensory changes after treatment of operable breast cancer. Breast Cancer Res Treat. 1986;8:55–59. doi: 10.1007/BF01805925. [DOI] [PubMed] [Google Scholar]
- 13.Kootstra JJ, Dijkstra PU, Rietman H, de Vries J, Baas P, Geertzen JH, Hoekstra HJ, Hoekstra-Weebers JE. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat. 2013;139:125–134. doi: 10.1007/s10549-013-2509-y. [DOI] [PubMed] [Google Scholar]
- 14.Kuehn T, Klauss W, Darsow M, Regele S, Flock F, Maiterth C, Dahlbender R, Wendt I, Kreienberg R. Long-term morbidity following axillary dissection in breast cancer patients--clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat. 2000;64:275–286. doi: 10.1023/a:1026564723698. [DOI] [PubMed] [Google Scholar]
- 15.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242–4248. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Langford DJ, Paul SM, West C, Levine JD, Hamolsky D, Elboim C, Cooper B, Abrams G, Aouizerat BE, Miaskowski C. Persistent breast pain following breast cancer surgery is associated with persistent sensory changes, interference, and functional impairments. J Pain. doi: 10.1016/j.jpain.2014.08.014. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, Abrams G, Hamolsky D, Dunn L, Dodd M, Neuhaus J, Baggott C, Dhruva A, Schmidt B, Cataldo J, Merriman J, Aouizerat BE. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miaskowski C, Paul SM, Cooper B, West C, Levine JD, Elboim C, Hamolsky D, Abrams G, Luce J, Dhruva A, Langford DJ, Merriman JD, Kober K, Baggott C, Aouizerat BE. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18:242–253. doi: 10.1016/j.ejon.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthen L, Muthen B. Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen; 2010. [Google Scholar]
- 20.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 21.Spijkerman DC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991;23:203–206. [PubMed] [Google Scholar]
- 22.Stamer UM, Stuber F. The pharmacogenetics of analgesia. Expert Opin Pharmacother. 2007;8:2235–2245. doi: 10.1517/14656566.8.14.2235. [DOI] [PubMed] [Google Scholar]
- 23.Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: an investigation of women’s experiences. Pain. 1995;61:61–68. doi: 10.1016/0304-3959(94)00162-8. [DOI] [PubMed] [Google Scholar]
- 24.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 25.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. The dimensions of pain quality: Factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24:550–555. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- 26.Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90:76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Percentages of women in the Mild and Moderate Pain classes who underwent physical therapy during the previous month (e.g., Month 1 = any physical therapy from prior to through six months following surgery).