Abstract
Objective
Virtual reality (VR)-based cue reactivity has been successfully used for the assessment of drug craving. Going beyond assessment of cue reactivity, a novel VR-based treatment approach for smoking cessation was developed and tested for feasibility.
Method
In a randomized experiment, 10-week treatment feasibility trial, 46 nicotine-dependent adults, completed the10-week program. Virtual reality skills training (VRST) combined with nicotine replacement therapy (NRT) was compared to NRT alone. Participants were assessed for smoking behavior and coping skills during, at end of treatment, and at posttreatment follow-up.
Results
Smoking rates and craving for nicotine were significantly lower for the VRST group compared to NRT-only group at the end of treatment. Self-confidence and coping skills were also significantly higher for the VRST group, and number of cigarettes smoked was significantly lower, compared to the control group at follow-up.
Conclusions
Feasibility of VRST was supported in the current study.
Keywords: addictions, behavior therapy, cognitive–behavioral, individual intervention, quantitative, randomized experiment, virtual reality, nicotine dependence, coping skills, craving
Introduction
The social and economic costs of cigarette smoking are well documented (Centers for Disease Control and Prevention [CDC], 2008). As a major health concern, cigarette smoking has occasioned considerable investments and efforts aimed at cessation treatments; however, success rates have remained stubbornly low after decades of research. Clearly, improvements in the effectiveness of current cessation programs are needed to more fully address this major public health challenge.
Cognitive–behavioral therapy (CBT) to teach smokers problem-solving skills to resist the temptation to smoke in high-risk situations has been used alone and in combination with nicotine replacement therapy (NRT; Garcia-Vera, 2004). CBT is particularly well suited for use in combination with the nicotine patch, as some studies have reported that cue-induced cravings can be resisted when cognitive and behavioral coping skills are mobilized during the craving episode (Araujo, Olivera, Pedroso, & Castro, 2009; Curry & Marlatt, 1985; Ferguson & Shiffman, 2009; Shiffman, 1984). Others have found the combination of NRT and CBT in general to be effective (Garcia-Vera, 2004). One of the strengths of CBT is the ability of the client and practitioner to confront high-risk situations and practice coping skills in a safe environment. However, the utility of this approach may be limited by the relatively artificial clinic setting. The client must imagine risky scenarios, and role-play in a setting that often lacks the proper smoking-related environmental context and, therefore, weakens the transfer of learning to real-world situations. Ideally, CBT would be brought into real-world settings (e.g., bar, party, and courtyard) that allow the client to learn and practice coping skills in meaningful and realistic scenarios. This approach may improve the effectiveness of CBT as a smoking cessation treatment, lead to better success rates, and help minimize the impact of smoking-related health costs.
Virtual reality (VR), a technological medium that effectively immerses individuals into realistic complex cue environments, appears an ideal tool to help improve the effectiveness of CBT training. The VR system uses a head-mounted display with a tracking system which responds to user movements by changing the scene being displayed in real time as if one was looking around the environment. Directional stereo audio, graphics, vibration platforms, and scent cues all add to the immersive experience for the participant. VR provides an opportunity for treatment-seeking persons to safely develop and practice meaningful coping skills while immersed within high-risk VR cue situations. While VR has not used in substance abuse treatment, the ability of the technology to present complex cue environments has been previously demonstrated effective in eliciting craving in smokers in laboratory studies (Bordnick et al., 2004; Bordnick, Graap, Copp, Brooks, & Ferrer, 2005; Traylor, Bordnick, & Carter, 2008).
These complex cue environments are appropriate for skill development and practice which will enhance smoking cue exposure techniques. The opportunity to practice skills in realistic, risky environments should engender realistic emotional responses which will provide teachable moments for specific skills. Engaging in coping skills training in VR may provide smokers with the additional support they need to more effectively resist cue-induced cravings and therefore increase their abilities to remain abstinent.
The advantage of combining CBT training with VR is well supported by learning theory. For example, State-dependent learning suggests that information learned in one state of mind and body is more accessible for retrieval when a person is in the same state of mind and body. Although clients can acquire some level of skill in the clinic setting, ultimately, according to Kanfer and Schefft (1988), ‘‘the center stage of therapeutic change programs is not the therapist’s office but the world in which the client lives’’ (pp. 234–235).
In addition, because VR offers an in-therapy experience that more closely resembles the real world than the clinic, it handily fulfills several of the goals of systematic behavioral assignments: (a) Facilitate generalization of newly learned behaviors from the safe therapeutic setting to the client’s natural setting; (b) Guide client efforts in making specific observations or obtaining information needed for the therapeutic process; (c) Permit access to private behaviors that cannot be readily observed or treated in a therapist’s office; and (d) Clarify the nature of behaviors that are vaguely defined at first and cannot be readily observed or simulated in the therapeutic session (Kanfer & Phillips, 1966).
This study tested the feasibility of using a set of smoking-related VR environments for virtual reality skills training (VRST) in a smoking cessation program. A two-group design was used, with one group receiving nicotine replacement therapy only (NRTO) plus (standard of care intervention) and the other group receiving NRT plus VRST. The primary goal of the study was to evaluate VRST for feasibility as a treatment adjunct to CBT skills training. The secondary goal of the study was to make basic comparisons in treatment effectiveness between the two groups.
Method
Participants
Eighty-six nicotine-dependent treatment-seeking cigarette smokers, referred by area professionals or recruited through television and newspaper advertisements, initially qualified for the study. The following inclusion criteria were used: having a Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV; American Psychiatric Association, 2000) diagnosis of nicotine dependence, being in self-reported good physical health and between 18 and 70 years, having a sixth grade or higher English reading level. Exclusion criteria included DSM-IV (American Psychiatric Association, 2000) diagnosis of severe mental illness or other substance dependence (nicotine not included), current use of psychotropic medications, pregnancy, history of seizures or seizure disorders, use of illicit drugs in the past 30 days, or other significant health problems that would preclude participation. Participants who were excluded were provided referrals to local smoking cessation programs.
Design and Procedures
This study was approved by the Institutional Review Board (IRB) of a large university in the Southeastern United States. The role of the IRB, along with study information, study rationale, risks, and potential benefits were explained to participants at the intake screening. After obtaining written informed consent, a member of the research team administered questionnaires, rating scales, and breath carbon monoxide (CO) measures. Participants who qualified were randomly assigned to one of the two 10-week treatment groups: Group 1—NRTO (n = 42), who received only nicotine patch or Group 2—VRST + NRT (n = 44), who received the same nicotine patch schedule plus 10 weeks of VRST. Within 1 week of the screening session, participants were scheduled for their first treatment session.
NRT medication dosing
Participants in both groups were provided Nicoderm CQ™ (GlaxosmithKline, Philadelphia, PA) patches by a research nurse at weekly clinic visits. The dosing schedule was the standard Nicoderm CQ 21 mg 10-week step-down regimen: 21 mg patch (Weeks 1–6), 14 mg patch (Weeks 7 and 8), and 7 mg patch (Weeks 9 and 10). At each weekly visit, participants were assessed by a research nurse for adverse events or difficulties with the use of the Nicoderm CQ patches and for compliance. At Week 1, all participants were provided instructions provided by the manufacturer on how to apply the patch and use it properly over the course of the treatment program.
Treatment conditions. Group 1—NRTO
Participants in the NRTO group attended 10 weekly clinic visits to pick up NRT patches and to complete assessment instruments. NRTO sessions were designed to deliver standard over-the-counter smoking cessation treatment as instructed in product directions and to serve as a standard-of-care intervention.
Group 2—VRST + NRT
Participants in the VRST group attended 1 hr sessions weekly for 10 weeks. During each visit, participants completed weekly assessment measures and engaged in a VRST treatment session. VRST sessions were manual based, individualized (e.g., preferred brand of cigarettes used in VR sessions), and conducted by a graduate-level trained therapist. The VRST intervention combined CBT techniques and VR-augmented cue exposure skills training conducted in VR. VRST session content was chosen based on the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Cognitive–Behavioral Coping Skills manual (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 1995) with modifications as required to adapt to VR therapy and smoking-specific items. A VRST treatment manual was developed at the start of the study and combined CBT (relapse prevention treatment) with VR cue exposure. During a typical VR session, while the participant was immersed in the environment, the therapist used the VR scenario to assess and teach coping skills. For example, the therapist would use the virtual party (with smokers) to help the participant identify high-risk triggers and social situations that elicit a strong desire to smoke. After triggers were identified, the therapist used the virtual party to teach skills and strategies (e.g., managing craving, planning an exit strategy) to cope effectively with the situation.
VRST provided exposure to real-world high-risk relapse scenarios described below. Exposure and skills practice (e.g., CBT) in the VR scenarios was a main component of treatment and was used in Sessions 1–9 to reinforce coping skills. The VRST therapist guided participants in the VR scenarios and controlled all interactions and stimuli through a real-time computer interface. Interactions and scenario difficulty allowed for specific skills to be practiced or repeated if necessary. A key to VRST is that once a participant became comfortable with a scenario and used positive coping skills, scenario difficulty was increased. This process allowed the therapist to overtrain the participant to effectively cope with extremely challenging smoking situations.
VRST scenarios
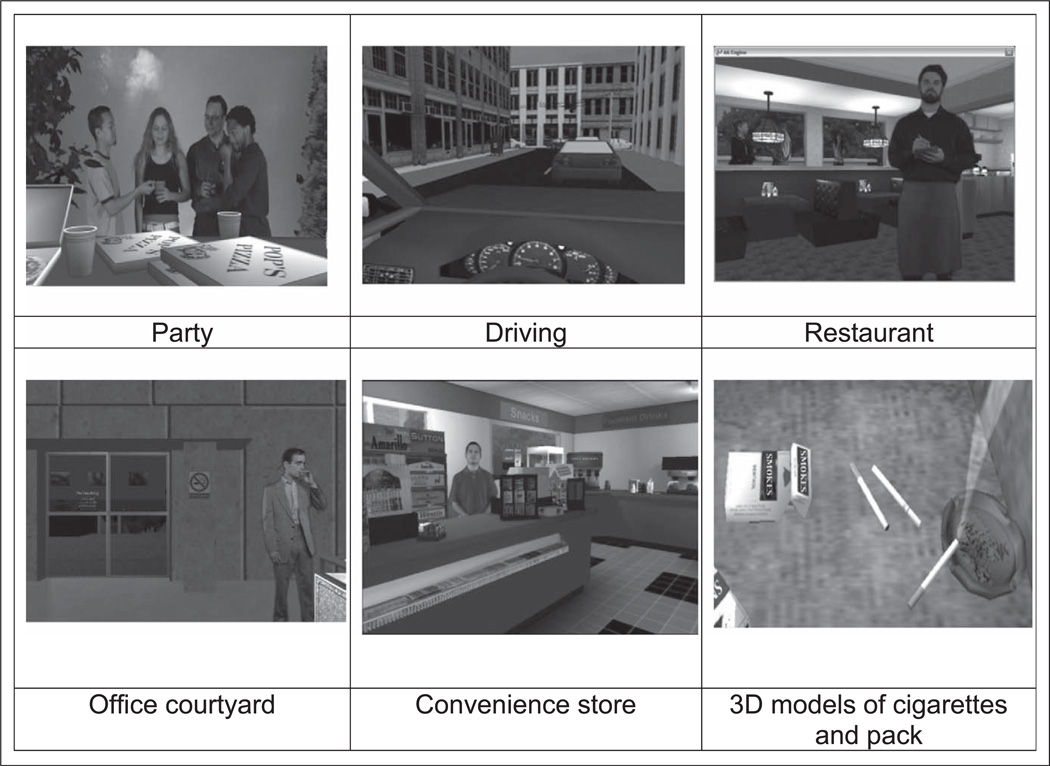
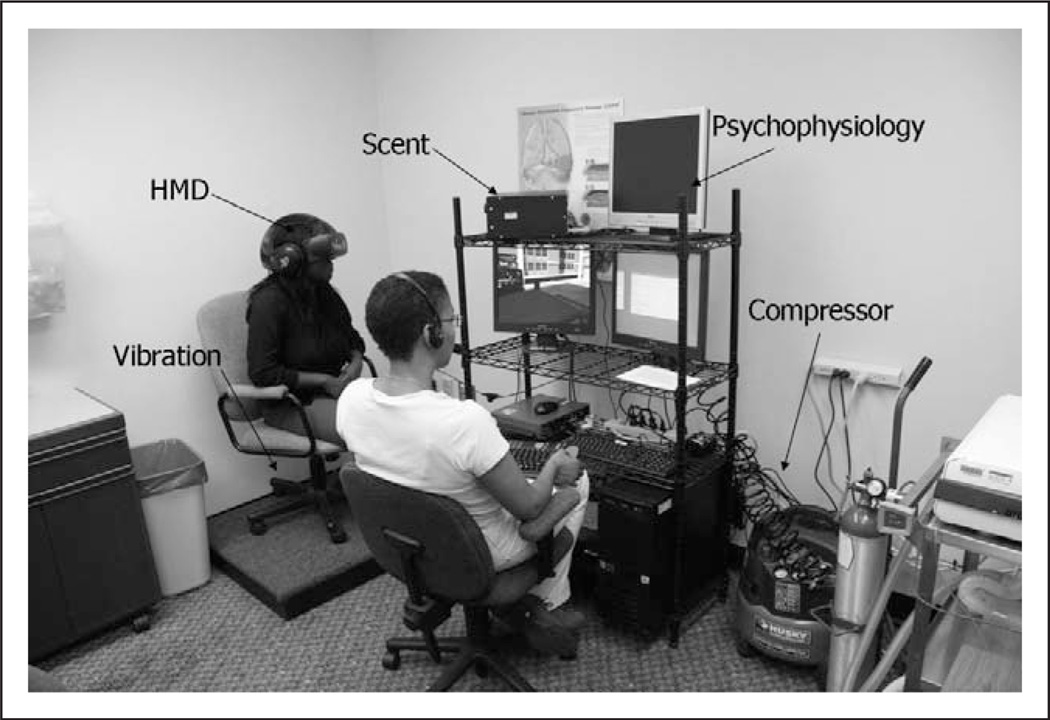
All VR scenarios used advanced three-dimensional (3D) computer graphics; 3D models with photo-realistic textures; integration of people for social interactions; spatial surround sound audio cues; and scents, including cigarette smoke, beer, pizza, and coffee, to produce interactive, immersive smoking scenarios that resemble real-world environments and social interactions. Screenshots of the VR scenarios are presented in Figure 1, and the clinical VR treatment setting is shown in Figure 2.
Figure 1.
Screen shots of virtual reality (VR) scenarios.
Figure 2.
Virtual reality clinical equipment setup.
Party
This setting consisted of a loft-based party with an out-door patio where people were smoking and drinking alcoholic and nonalcoholic beverages. Tasks included developing and practicing skills involving interactions with other party guests, including offers to join them in smoking.
Driving
This scenario consisted of a car interior and parking garage with various smoking cues including a cigarette package and cigarettes. Traffic sounds contributed to the realism of the environment and vibration was used to simulate movement. Participants walked around a parking garage to their car, then drove in traffic, listened to radio messages, and heard other drivers honking horns to increase stress. The participant’s brand of cigarettes was placed on the dashboard in a prominent position to increase cue salience.
Restaurant
This scenario consisted of a restaurant where people were eating and drinking. People smoking cigarettes outside the restaurant were visible from the inside of the restaurant. In the restaurant, a waiter interacted with participants by taking orders for food and drinks.
Office building and courtyard
This virtual environment consisted of an entrance to an urban high-rise office building and a courtyard where people were sitting on park benches or standing near commercial ashtrays, smoking, and drinking coffee. Participants walked around the courtyard, passed the smokers, and entered the office building, encountering a newsstand selling cigarettes.
Convenience store
This scenario included the inside and outside of a convenience store. Outside, the participant was surrounded by cigarette advertising, smokers, ashtray cans, and alcohol advertising. Cigarette brand representations that matched users’ preferred cigarette types were selected to appear in the scenario. Participants navigated from the outside pump area and entered the store where the cashier interacted with them.
Airport smoking lounge and gate
This setting consisted of an airport smoking lounge and gate area where the participant observed people smoking, drinking coffee, and waiting for flights.
Treatment fidelity and therapist training
The VR therapist was trained to deliver the manual-based VRST intervention. To ensure treatment fidelity, the therapist completed session checklists for all visits to verify that all content was addressed and presented according to the VRST manual. Random sessions from both groups were videotaped and reviewed for protocol adherence.
Measures
Nicotine dependence and use. Structured Clinical Interview for the DSM-IV (SCID I/P)
Participants were diagnosed as nicotine dependent using the SCID(First, Spitzer, Gibbon, & Williams, 1996). The SCID was also used to exclude participants with other mental illness or substance dependence diagnoses.
Smoking history
A self-report of smoking behavior was used to gather information regarding years smoking, number of quit attempts, and current and past use levels during screening.
The Fagerstrom test for nicotine dependence (FTND)
The FTND (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) was used to measure level of nicotine dependence during screening.
Time line follow back/daily use level
A modified time line follow back (Sobell & Sobell, 1995) was used to assess the number of cigarettes smoked per day at intake, weekly sessions, and follow-ups.
Coping skills. Smoking Confidence Questionnaire (SCQ; Condiotte & Lichtenstein, 1981)
A modified SCQ was used to assess confidence and self-efficacy of participant’s ability to resist smoking. The SCQ consists of 44 high-risk scenarios under which participants rate their ability to resist smoking. The SCQ has been used extensively to assess coping skills in behavioral research studies. In addition, the SCQ is formulated based on the Marlatt’s relapse prevention model (Condiotte & Lichtenstein, 1981; Schmitz, Spiga, Rhoades, Fuentes, & Grabowski, 1999; Stevens & Hollis, 1989), so it was highly applicable to assess coping skills in this study. The SCQ was used at baseline and follow-up (1, 2, 3, and 6 months) posttreatment to monitor confidence in the use of coping skills after participants were exposed to cues in their daily lives outside treatment.
Smoking abstinence self-efficacy (SASE)
The SASE (Diclemente & Prochaska, 1985) is a 20-item scale used to assess coping skills and confidence level of smokers to resist smoking. The SASE lists 20 challenging situations, and smokers rate how confident they are that they would be able to resist smoking in each situation on a scale from 1 (not at all tempted) to 5 (extremely tempted). This instrument was administered each week during treatment.
Craving. Brief Questionnaire of Smoking Urges (QSU-Brief)
The QSU-Brief (Cox, Tiffany, & Christen, 2001) is an 11-item craving scale derived from the long version of the QSU (Tiffany & Drobes, 1991). The QSU-Brief was administered at baseline and end of treatment to assess craving and urges.
Cigarette Craving Visual Analogue scale (CCVAS)
The CCVAS was used to measure cigarette craving at baseline and during weekly sessions. Participants were asked to rate how they felt at this moment by selecting a position along a line anchored on the left by not at all and on the right by extremely. The CCVAS consists of 6 items: I am thinking about how I can get cigarettes, I am thinking about the next time I will use cigarettes, I want to buy cigarettes, I have the urge or desire to use cigarettes, and If I was offered a cigarette I could resist. These items have been used in previous studies to measure craving for alcohol and drugs of abuse with appropriate adjectival modification (Johnson, Chen, Schmitz, Bordnick, & Shafer, 1998; Preston & Jasinski, 1991).
To simulate a typical clinic setting, no extraordinary measures (other than study compensation) were used to motivate participants to remain in the study.
Results
Treatment Enrollment and Attrition
NRTO
Of the 42 eligible participants in the NRTO, Group 7 (17%) dropped out before their first treatment session. Of the 35 participants who started treatment in the NRTO group, 25 (71%) completed the 10-week treatment.
VRST
Of the 44 eligible participants in the VRST, Group 8 (18%) dropped out before their first treatment session. Of the 36 participants who started treatment in the VRST group, 21 (58%) completed the 10-week treatment. Using a χ2 analysis, there was no significant difference in the percentage of dropouts between groups.
Analysis of participants who dropped out at both stages of the study (during treatment or at follow-ups) showed no significant differences between groups on demographic (e.g., age) or smoking-related (e.g., cigarettes smoked per day) variables.
Table 1 summarizes the demographic and smoking characteristics of the study participants who completed 10 weeks of treatment. There were no significant differences between treatment groups at baseline on demographic or smoking-related variables.
Table 1.
Baseline Demographic and Smoking Characteristics by Treatment Group
| Variable | NRTO N = 25 |
VRST N = 21 |
|---|---|---|
| Age | ||
| M | 46.2 | 47.9 |
| SD | 8.4 | 10.4 |
| Gender (n, %) | ||
| Male | 15, 60 | 9, 43 |
| Female | 10, 40 | 12, 57 |
| Ethnicity (n, %) | ||
| Caucasian | 4, 16 | 1, 5 |
| African American | 20, 80 | 19, 90 |
| Hispanic | 1, 4 | 1, 5 |
| FTND score | ||
| M | 5.9 | 6.6 |
| SD | 2.6 | 1.9 |
| QSU score | ||
| M | 4.5 | 4.2 |
| SD | 1.5 | 2.0 |
| CCVAS | ||
| M | 52.1 | 50.8 |
| SD | 18.4 | 23.8 |
| SCQ | ||
| M | 39.8 | 37.5 |
| SD | 19.5 | 16.9 |
| SASE | ||
| M | 4.0 | 3.8 |
| SD | 0.6 | 0.8 |
| Number of years smoking | ||
| M | 14.8 | 19.1 |
| SD | 10.8 | 9.1 |
| Cigarettes smoked/day | ||
| M | 26.4 | 24.5 |
| SD | 10.0 | 6.3 |
Note. CCVAS = Cigarette Craving Visual Analogue scale; FTND = Fagerstrom test for nicotine dependence; M= mean; NRTO = nicotine replacement therapy only; QSU = questionnaire of smoking urges; SASE = smoking abstinence self-efficacy; SCQ = Smoking Confidence Questionnaire; SD= standard deviation; VRST = virtual reality skills training.
Analysis of Preliminary Treatment Outcome
The following results are those from participants who completed 10 weeks of treatment (n = 46). A mixed analysis of variance was used for all analyses with group assignment (VRST treatment, NRTO control) as between-subjects variables, while time points (week of treatment) were included as within-subjects variables. Post quit data were analyzed in the same manner, however, analyses at each follow-up were conducted on available data. Because of the preliminary nature of this study, no intent-to-treat or survival analyses were conducted.
End of Treatment
Cigarettes smoked
At the end of the 10-week treatment program, participants were asked about their smoking using a time line follow back procedure for the previous 7 days. The VRST treatment group reported smoking significantly fewer cigarettes in the previous 7 days compared to the NRTO control group, F(1, 44) = 4.4, p < .05, which was confirmed with expired CO levels taken at the same time point, NRTO (M = 2.4, SD = .3.2) and VRST (M = .65, SD = 1.2), F(1, 44) = 4.24, p < .05, ηp2 = .14. See Table 2.
Table 2.
Cigarettes Smoked by Treatment Group
| Variable | NRTO | VRST |
|---|---|---|
| Intake | ||
| M | 21.8 | 23.2 |
| SD | 6.2 | 7.9 |
| N | 25 | 21 |
| End of treatment | ||
| M | 2.4 | 0.65 |
| SD | 3.2 | 1.2 |
| N | 25 | 21 |
| 1 month | ||
| M | 3.3 | 0.63 |
| SD | 4.0 | 1.2 |
| N | 25 | 16 |
| 2 months | ||
| M | 3.0 | 0.74 |
| SD | 3.6 | 1.4 |
| N | 21 | 15 |
| 3 months | ||
| M | 3.3 | 1.0 |
| SD | 3.9 | 2.5 |
| N | 18 | 13 |
| 6 months | ||
| M | 7.4 | 0.41 |
| SD | 7.3 | 0.86 |
| N | 11 | 11 |
Note. M =mean; NRTO =nicotine replacement therapy only; SD =standard deviation; VRST =virtual reality skills training.
Craving
At end of treatment, participants in the VRST treatment group (M = 1.3, SD = 0.5), compared to the NRTO group (M = 2.1, SD = 1.1), had significantly lower craving, F(1, 44) = 6.9, p = .012, ηp2 = .37, as measured by the QSU-Brief taken presession.
Measures of self-efficacy
At the end of treatment, both groups were assessed for self-efficacy using the SASE. The NRTO group had significantly less self-efficacy (M = 2.5, SD = 1.2) than the VRST group (M = 1.7, SD = .72), F(1, 44) = 6.3, p < .02, ηp2 = .125.
Long-Term Follow-Up
Confidence to resist smoking
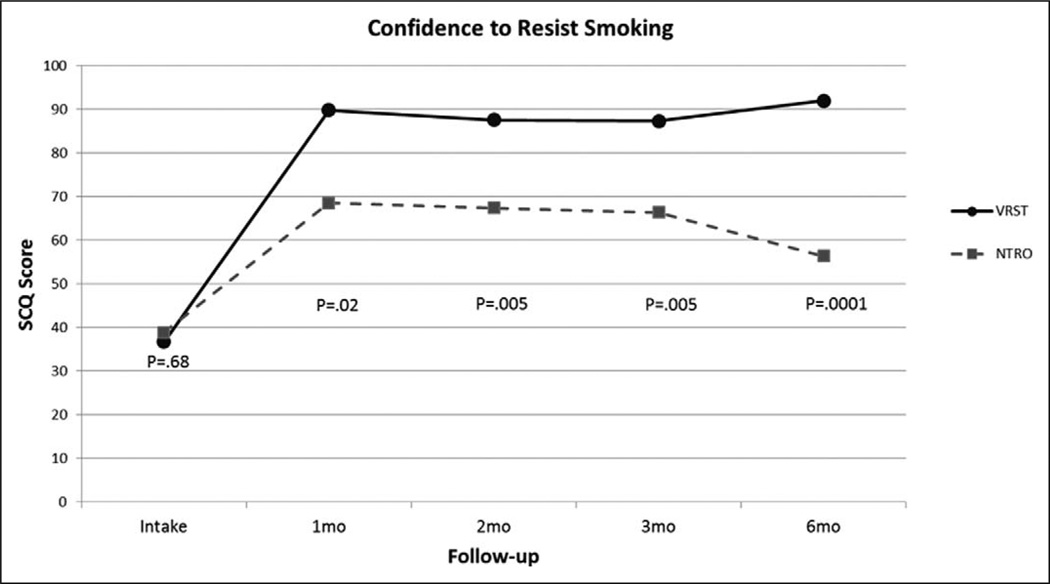
Participants were assessed for confidence to avoid smoking (SCQ) at intake, 1, 2, 3, and 6 months posttreatment. Treatment and control groups did not significantly differ in these measures at intake; however, the VR treatment group had significantly greater confidence to avoid smoking at 1-month posttreatment, F(1, 41) = 5.9, p < .05, 2 months posttreatment, F(1, 35) = 8.9, p < .01, 3 months posttreatment, F(1, 31) = 9.4, p < .01, and 6 months posttreatment, F(1, 23) = 17.8, p < .001, see Figure 3. This analysis includes all participants who were available to provide data at each time point.
Figure 3.
Smoking Confidence Questionnaire (SCQ) scores between groups at long-term follow-up.
Cigarettes smoked
Participants were assessed at each follow-up point using time line follow back for the number of cigarettes smoked in the preceding 7 days, which was converted into mean number of cigarettes smoked per day. The VR treatment group had smoked significantly fewer cigarettes at 1-month posttreatment, F(1,39) = 6.6, p < .05, 2 months posttreatment, F(1, 34) = 5.2, p < .05, and 6 months posttreatment, F(1, 20) = 9.9, p < .01 (see Table 2). Note that there was a trend toward significantly fewer cigarettes smoked at 3 months posttreatment, F(1, 29) = 3.5, p = .07. This analysis includes all smokers who were available to provide data at each time point.
Conclusions
The focus of this study was to examine the feasibility of a novel VR skills training intervention for smoking cessation treatment. The treatment attrition data showed a modest drop-out rate for both the NRTO and VRST groups, with the VRST group having slightly more dropouts than the NRTO group. This difference may be due to the variable time demands on the participants. The NRTO group had only a brief counseling session at each treatment point, while the VRST group was required to complete a full training session. Overall, these data suggest the VR training sessions were well tolerated and did not pose an undue burden on the participants.
The preliminary results demonstrate that smokers who received VRST had significantly lower craving and self-reported smoking compared to those who received NRTO when assessed at the end of treatment and at 6 months follow-up. Moreover, self-efficacy was significantly higher in the VRST group compared to the NRTO group at posttreatment and at 1, 2, 3, and 6 months follow-up.
Differences in self-reported smoking rate, as verified by CO level, craving level, self-efficacy, and coping skills (SCQ) in the VRST participants may be due to the ability to practice active coping skills and relapse strategies during treatment sessions in realistic high-risk situations. The interactive VR environments were developed to provide exposure to realistic smoking social interactions and contexts that parallel situations smokers encounter in their daily lives outside the clinic while smoking, thus allowing clinicians to provide exposure and coping skills training across a variety of scenarios. Follow-up data provide initial support that skills acquired in VRST generalize to the real world. Assessment of self-efficacy suggested that participants in the VRST group had increased confidence in the use of coping skills in the real world which was evident at 6 months posttreatment.
These findings should be tempered with the following limitations. At present, it cannot be determined whether the VR component of the additional training provided to the VRST group was the primary factor in their success. That is, the same effects might have been achieved through traditional coping skills counseling. Additional studies are needed to replicate these findings and compare VRST to traditional coping skills training to explore whether any additional benefit exists for using VR-based coping skills training.
In conclusion, the use of VRST appears to be a viable method to reduce smoking in nicotine-dependent adults. The data presented provide initial evidence of the feasibility of VRST-based treatment, and may offer advancements in treatment, by improving exposure to realistic relapse scenarios in the clinical setting above what current counseling approaches offer. Going beyond the current feasibility study, VRST can offer social work, psychiatry, psychology, nursing, and counseling an innovative tool to enhance current CBT-based interventions in substance abuse treatment and research. Future research focusing on the unique components of VRST beyond traditional counseling, combined with long-term follow-up assessments, is warranted.
Acknowledgments
The authors are thankful to Mirtha Ferrer, James Carroll, and Emlyn Murphy for VR development and programming of the scenarios.
Funding
The authors disclosed receipt of the following financial support for the research and/, and/or publication of this article: This work was supported by the National Institutes of Health (STTR Grant R42DA016085-03).
Footnotes
Dr. Bordnick, Dr. Traylor, and Dr. Carter do not have conflicts of interest or financial interests in Virtually Better, Inc. Mr. Graap is employed by CNS Response and he states no conflict of interest in this research.
Declaration of interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: Author; 2000. [Google Scholar]
- Araujo RB, Olivera MS, Pedroso RS, Castro MG. Coping strategies for craving management in nicotine dependent patients. Revista Brasileira de Psiquiatria. 2009;31:89–94. doi: 10.1590/s1516-44462009000200002. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Copp HL, Brooks J, Ferrer M. VR cue reactivity assessment in cigarette smokers. CyberPsychology & Behavior. 2005;8:487–492. doi: 10.1089/cpb.2005.8.487. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Copp HL, Brooks JS, Ferrer M, Logue B. Utilizing virtual reality to standardize nicotine craving research: A pilot study. Addictive Behaviors. 2004;29:1889–1894. doi: 10.1016/j.addbeh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses-United States. Morbidity & Mortality Weekly Report (MMWR) 2008;57:1226–1228. [PubMed] [Google Scholar]
- Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. Journal of Consulting Clinical Psychology. 1981;49:648–658. doi: 10.1037//0022-006x.49.5.648. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Curry S, Marlatt GA. Unaided quitters’ strategies for coping with temptations to smoke. In: Shiffman S, Wills TA, editors. Coping and substance use. New York, NY: Academic Press; 1985. pp. 243–265. [Google Scholar]
- Diclemente CC, Prochaska JO. Processes and stages of self-change: Coping and competence in smoking behavior change. In: Shiffman S, Wills TA, editors. Coping and substance abuse. New York, NY: Academic Press; 1985. pp. 319–344. [Google Scholar]
- Ferguson SG, Shiffman S. The revlvance and treaement of cue-induced cravings in tobacco depdence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders—Patient edition (SCID-I/P, version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Garcia-Vera MP. Clnical utility of the cobination of cogntive-behavioral techniques with nicotine patches as a smoking-cessation treatment: Five year results of the ‘‘Ex-Moker’’ program. Journal of Substance Abuse Treatment. 2004;27:325–333. doi: 10.1016/j.jsat.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YR, Schmitz J, Bordnick P, Shafer A. Cue reactivity in cocaine-dependent subjects: Effects of cue type and cue modality. Addictive Behaviors. 1998;23:7–15. doi: 10.1016/s0306-4603(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Kanfer FH, Phillips JS. Behavior therapy: A panacea for all ills or a passing fancy? Archives of General Psychiatry. 1966;15:114–128. [Google Scholar]
- Kanfer FH, Schefft BK. Guiding the process of therapeutic change. Champaign, IL: Research Press; 1988. [Google Scholar]
- NIAAA. Cognitive-behavioral coping skills therapy manual. Vol. 3. Rockville, MD: Department of Health and Human Services; 1995. [Google Scholar]
- Preston KL, Jasinski DR. Abuse liability studies of opioid agonist-antagonists in humans. Drug and Alcohol Dependence. 1991;28:49–82. doi: 10.1016/0376-8716(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Spiga R, Rhoades HM, Fuentes F, Grabowski J. Smoking cessation in women with cardiac risk: A comparative study of two theoretically based therapies. Nicotine & Tobacco Research. 1999;1:87–94. doi: 10.1080/14622299050011191. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Coping with tempations to smoke. Journal of Clinical and Consulting Psychology. 1984;52:261–267. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol timeline follow-back users manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Stevens VJ, Hollis JF. Preventing smoking relapse, using an individually tailored skills-training technique. Journal of Consulting & Clinical Psychology. 1989;57:420–424. doi: 10.1037//0022-006x.57.3.420. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire of smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Traylor AC, Bordnick PS, Carter BL. Assessing craving in young adult smokers using virtual reality. American Journal on Addictions. 2008;17:436–440. doi: 10.1080/10550490802268876. [DOI] [PubMed] [Google Scholar]