Abstract
Antibody-mediated immunotherapy has gained significant momentum since the first FDA-approved monoclonal antibody (mAb) in 1997, namely, Rituximab (chimeric anti-CD20 mAb) for the treatment of B-NHL cells. Subsequently, over 20 approved mAbs have been in use clinically for the treatment of various cancers and several non-cancer related diseases. Further, the combination treatment of mAbs with chemotherapy, immunotherapy, proteaosome inhibitors and other inhibitors have resulted in synergistic anti-tumor activity with significant objective clinical responses. Despite their successful clinical use, the underlying mechanisms of rituximab in vivo activities remain elusive. Further, it is not clear why a subset of patients is initially unresponsive and many responding patients become refractory and resistant to further treatments; hence, the underlying mechanisms of resistance are not known, Attempts have been made to develop model systems to investigate resistance to mAb therapy with the hope to apply the findings in both the generation of new therapeutics as well as their use as new prognostic biomarkers. This review focuses on the development of resistance to Rituximab treatments and discusses possible underlying mechanisms of action, postulated mechanisms of resistance in model systems and suggested means to overcome resistance. Several prior reviews on the subject of Rituximab resistance have been published and the present review both complements as well as adds new topics of relevance.
I. Introduction
During the last decade, we have witnessed the emergence of anti-cancer targeted therapies, namely, of the use of monoclonal antibodies (mAbs) directed against surface tumor associated antigens. A major limitation of both conventional and targeted therapies is that a subset of patients does not initially respond to such therapies and another responding subset develops resistance to further treatments. Hence, many malignant cancers exhibit both intrinsic and acquired resistance.1 Nevertheless, the introduction of antibody-mediated therapy has resulted in significant clinical objective responses and, in many cases, responses in cancers that did not respond to conventional chemotherapies.
Historically, several decades ago, antibody-mediated therapy originated by the use of polyclonal antibodies derived from mice, rabbits or rats. Treatment of cancer patients with such foreign antibodies (antigenic) resulted in the development of a humoral antibody response against these foreign antibodies. Hence, the therapeutic antibodies were blocked and cleared and, therefore, limited their ability to be effective against the cancer. Immunotherapy by antibodies became practical following the milestone discovery of the generation of antigen-specific mAbs by Kohler and Milstein in 1975.2
In order to overcome the obstacle of the host response to the administered xenogenic antibodies, engineering of chimeric humanized and privatized antibodies were developed by linking mouse or primate antibody recognition regions with human back bone fragments.3,4 For example, humanized antibody is a human antibody consisting of the complementarity-determining regions (CDR) of non-human origin and human constant regions. The earliest clinically approved mAb was in Europe 1994 and consisted of Edrecolomab (Panorex®) for the treatment of patients with clororectal cancer. Subsequently, the first mAb approved in the USA for cancer therapy was in 1997 by the chimeric anti-CD20 mAb, Rituximab, Rituxan® for the treatment of low grade and follicular NHL.5,6 Subsequently, over 20 mAbs have been approved for the treatment of various cancers and non-cancer diseases.7
Rituximab is a chimeric anti-CD20 mAb. It is directed against cell surface membrane receptors, CD20, expressed on mature B cells but not on pre-B cells or plasma cells. The receptor CD20 is a tetramembrane spanning molecule of molecular weight 33–37 kDa and the gene is located on chromosome 11q12-q13.1. CD20 is resident in lipid raft domains of the plasma membrane.8 In this review, I’ll briefly summarize the findings reported on Rituximab treatment regimens both in vivo and in vitro, with emphasis on postulated mechanisms of actions, postulated mechanisms of resistance and suggested means to overcome resistance. Rituximab has been chosen as a putative prototype for other anti-cancer mAbs.
II. Rituximab
Rituximab-containing regimens have emerged as current therapeutics for NHLs and other lymphomas. Rituximab is routinely incorporated into the conventional treatment of follicular NHL, namely, in first line therapy, maintenance and salvage therapy.9–11 The treatment of patients with FL and diffuse large B-cell lymphoma (DLBCL), alone or in combination with chemotherapy, resulted in significant clinical responses and prolongation of survival.7 Rituximab off label uses in other malignant and non-malignant diseases were reported.12
Postulated mechanisms of action mediated by rituximab
Several mechanisms of action have been reported including ADCC, CDC, and apoptotic activities as well as its cell signaling-mediated effects that are responsible, in part, for its chemo and immuno-sensitizing activities. The combination of Rituximab and chemo-immuno-therapeutic drugs resulted in reversal of resistance and synergy. Below, a brief description of the various mechanisms of action by rituximab
A. Antibody-dependent cellular cytotoxicity (ADCC)
ADCC has been attributed a significant role on the in vivo mechanism of action of rituximab. ADCC consists of the ligation of the human Fc portion of rituximab in antibody-coated tumor cells to the Fc receptors expressed on the surface of NK cells, macrophages and neutrophils and resulting in triggering the cytotoxic cells for killing of the bound target cells. For instance, the reported depletion of B-CLL in patients-derived PBMCs (which contain circulating effector cells) was significantly augmented following treatment with rituximab (even more by rituximab combination with GMCSF.13 The treatment of patients with rituximab and low dose IL-2 resulted in clinical responses of 55% in patients with a relapsed and refractory FL.14
B. Complement-dependent cellular cytotoxicity (CDC)
It has been reported that rituximab-coated tumor cells bind C1q and activate the complement cascade for cytotoxicity.3 Sensitivity to CDC is dependent on the origin of lymphoma cells. Rituximab induces significant CDC killing of FL cells whereas it has only moderate cytotoxicity in MCL, DLBCL, and small lymphocytic leukemia (SLC) cells.15 Various agents have been shown to induce CDC activity in vitro. For example, dexamethasone enhances rituximab-mediated CDC but it has no effect on ADCC.16
C. Apoptosis
In certain NHL cell lines, rituximab has been reported to exert moderate apoptosis in vitro.17 However, cross linking of rituximab with a secondary anti-human IgG results in significant induction of apoptosis in resistant cells. Cross-linking is accompanied by the activation of tyrosine-kinases, Plc-2 phosphorylation, calcium influx, and caspase 3 activation. These various manifestations were inhibited by PP2, a selective inhibitor of the Src family kinases.18 Freshly isolated B-CLL coated with rituximab and cross-linked with anti-human IgG (Fab’2) resulted in a concentration and time-dependent apoptosis, independent of ADCC and CDC.19 The mechanisms of cross-linking induced apoptosis and were reported by Pedersen et al.19 and Mathias et al..20 In addition, the role of activation of the apoptotic mitochondrial pathway by cross linking was reported.21 These findings show the ability of rituximab to kill cells directly if cross-linking takes place.
D. CD20 redistribution to lipid rafts
Lipid rafts are heterogeneous lipid microdomains enriched in sphingomyelin, glycosphingo lipids and cholesterol. Lipid rafts serve as a platform for cell signaling. The binding of anti-CD20 antibody to B cells results in the rapid redistribution of CD20 molecules (up to 98%) to low density detergent insoluble lipid rafts.22 It appears that the redistribution of CD20 into membrane lipid rafts regulates the efficacy of anti-CD20 antibodies to induce CDC in lymphoma cells.23 The co-existence of CD20 and Src family kinases in the lipid rafts following rituximab treatment suggests a role of CD20-mediated cell signaling.
E. Cell signaling mediated by Rituximab
The findings that Rituximab inhibits cell proliferation and induces apoptosis suggested that it may trigger signal transduction. This was supported by the findings that anti-CD20 antibodies redistribute CD20 into the lipid drafts, a cell signaling site.24 The cytotplasmic domain of CD20 is not involved in transmembrane signaling and, thus, CD20 may be interacting with other molecules associated with signaling. Indeed, Rituximab treatment inhibits a Src kinase present in lipid drafts, namely, Lyn, and decreases both phospho-Lyn and phospho-Cbp/PAG.24 Bezombes et al.26 reported that treatment with Rituximab resulted in rapid, although transient, increase in acid sphingomyelinase activity with concomitant accumulation of cellular ceramide in the raft microdomains. Further, Dean et al.27 reported that inhibition of cell growth by Rituximab is mediated through a ceramide-triggered pathway in a MAPK-dependent mechanism. These findings were corroborated by us with the demonstration that treatment of B-NHL cells with Rituximab inhibited several intracellular/anti-apoptotic signaling pathways.28
We have initially reported that treatment of AIDS-related lymphoma (ARC) cell lines with Rituximab resulted in inhibition of the JAK/STAT pathway and inhibition of the targeted gene product IL10 which was, in part, responsible for resistance.29 Vega et al.30 and subsequently Jazirehi et al.31 reported that treatment of B-NHL cells with Rituximab inhibited the Raf/MEK/ERK pathway and downstream downregulated AP-1-dependent gene target transcripts. In addition, Rituximab treatment inhibited the NF-κB pathway.28 The PI3K/AKT pathway was also inhibited by Rituximab in B-NHL cells.32
The above findings demonstrated the Rituximab can signal the cells via the CD20 receptor. However, the role of the Fc fragment in participating in cell signaling via interaction with FcRs on the target membrane was not examined. To address this issue, Rituximab (Fab’2) was generated and examined for cell signaling in comparison to wild type Rituximab. The findings revealed that treatment with (Fab’2) Rituximab resulted in inhibiting cell signaling pathways similar to Rituximab, both qualitatively and quantitatively.33
III. Rituximab-mediated chemo-immuno-sensitization of resistant B-NHL cells to apoptosis by chemo-immuno-therapeutic drugs
The above findings on cell signaling inhibition of intracellular survival/anti-apoptotic pathways by Rituximab treatment suggested that the treated cells threshold of resistance must have been significantly compromised and, thus, may be more sensitive to cytotoxic stimuli. This hypothesis was tested by delineating for each of the inhibited pathways by Rituximab of its involvement in the regulation of resistance as well as underlying molecular mechanisms involved. Rituximab-mediated chemo-sensitization and immuno-sensitization are briefly summarized below.
A. Rituximab-mediated chemosensitization
1. Role of p38 MAPK
The role of phospho-STAT3 inhibition by Rituximab in sensitization to drugs was examined.29 Inhibition of p-STAT3 resulted in downstream inhibition of the IL-10 transcription factor SP-1 and inhibition of the transcription of its targeted gene product Bcl-2. The combination of treatment of Rituximab and CDDP resulted in the reversal of drug resistance of B-NHL cells and synergy in apoptosis was achieved. The synergistic activity was the result of activation of the type II mitochondrial apoptotic pathway. The direct role of Rituximab-mediated inhibition of p38 MAPK activity in sensitization was corroborated by the use of specific chemical inhibitors.30,34
2. Role of the Raf/MEK/ERK pathway
The inhibition of the Raf/MEK/ERK pathway in B-NHL cells by Rituximab was paralleled by inhibition downstream of the transcription factor AP-1 and AP-1 transcription gene products including the anti-apoptotic gene product Bcl-xl.35 The direct role each of the Raf/MEK/ERK pathway factors and Bcl-xl in chemosensitization was corroborated by the use of specific chemical inhibitors.
3. Role of the NF-κB pathway
The inhibition by Rituximab of the anti-apoptotic gene product Bcl-xl expression and the finding that the inhibition of Bcl-xl sensitizes the drug resistance B-NHL cells to apoptosis by various chemotherapeutic drugs28 suggested that the NF-κB pathway, which also regulates Bcl-xl, might have been involved.36,37 In fact, the NF-κB pathway was inhibited by Rituximab and its direct role in chemo-sensitization was corroborated by the use of various inhibitors of the pathway, which mimicked the Rituximab-mediated chemosensitization.28
4. Role of the PI3K/AKT pathway
In addition to the roles of the JAK/STAT3, Raf/MEK/ERK and NF-κB pathways, all of which regulate Bcl-xl, and all are inhibited by Rituximab, these findings suggested that, in addition, the PI3K/AKT pathway that also regulates Bcl-xl may be inhibited by Rituximab.38 Rituximab treatment mediated inhibition of the PI3K/AKT pathway and resulted in the inhibition of p-PI3K, p-PDK1 and p-AKT with no inhibition of the unphosphorylated proteins. In addition, downstream of the PI3K pathway, Rituximab inhibited phospho-Bad leading to augmentation of the association of Bad with Bcl-xl and, thus, resulting in the inhibition of Bcl-xl activity on the mitochondria. The direct role of PI3K inhibition by Rituximab in chemosensitization was corroborated by the use of specific chemical inhibitors and by the use of small interference RNA (siRNA) AKT.32
B. Rituximab-mediated immunosensitization
Immune cells such as CTL and NK mediate their cytotoxic activity by both necrotic and apoptotic mechanisms.39–41 Since rituximab treatment alone was shown to regulate the apoptotic pathways and leading to chemosensitization, we hypothesized that rituximab may also sensitize resistant B-NHL cells to immune-mediated apoptosis, namely, by the death ligands Fas-L and TRAIL.
1. Rituximab-mediated sensitization to Fas-L-mediated apoptosis
We have reported that treatment of B-NHL with rituximab sensitized the cells to recombinant Fas-L-induced apoptosis.42 The mechanism underlying sensitization was examined. Previous findings demonstrated that the transcription factor, Yin Yang 1 (YY1), negatively regulates Fas transcription and expression and inhibition of YY1 resulted in the upregulation of Fas expression and sensitization to Fas-L apoptosis.43 Since YY1 is a target gene product of NF-κB and we have shown that NF-κB is inhibited by rituximab, we expected that inhibition of NF-κB by rituximab will be accompanied by inhibition of YY1 and, therefore, sensitization of B-NHL to Fas-L apoptosis. Indeed, the findings corroborated this hypothesis. The rituximab-mediated sensitization to Fas-L apoptosis was the result of the activation of type II mitochondrial apoptotic pathway.42
2. Rituximab-mediated sensitization to
TRAIL apoptosis Like Fas-L above, we have found that treatment of TRAIL-resistant B-NHL cells with rituximab sensitized the cells to recombinant TRAIL-induced apoptosis. Based on previous findings demonstrating that YY1 negatively regulates the TRAIL receptor DR5 and regulates resistance to TRAIL, its inhibition sensitized the cells to TRAIL apoptosis along with upregulation of DR5.44 We have also found that rituximab sensitization to TRAIL apoptosis was accompanied by upregulation of DR5 along with inhibition of YY1. In addition, treatment of cells with YY1 siRNA sensitized the cells to TRAIL apoptosis and, thus, mimicking rituximab.45
IV. Resistance to Rituximab
Currently, the combination of Rituximab and chemotherapy (CHOP) is the approved protocol for the treatment of B-NHL. While the use of Rituximab for treatment has been successful, however, a subset of patients has an innate resistance. In follicular lymphoma only about 15% of patients respond to the initial treatment with Rituximab monotherapy.46 Furthermore, the majority of responders becomes refractory to Rituximab.47 The five year overall survival (OS) for patients with low grade follicular lymphoma who fail to respond to or develop resistance to Rituximab or Rituximab containing treatment regiments is 58%48, which is markedly a decrease compared to survival of all lymphoma patients.49
The mechanisms of resistance in vivo are not clear. Several mechanisms have been reported including inhibition of ADCC by deposition of C3 activating fragments50, polymorphism of the FcγRIIIa on cytotoxic cells,51,52 inhibition of CDC,53 loss of CD20 expression on the surface of subclones,47,54 overexpression of anti-apoptotic gene products (eg Bcl2)55, CD20 mutations,56 shedding of CD20 Rituximab complexes,57 the tumor micro-environment,58 distribution of Rituximab in vivo and its pharmacokinetics and failure to respond to Rituximab-mediated cell signaling. Briefly (below each) the postulated mechanisms are presented.
A. Poor ADCC
ADCC neutralizes the Fc fragment of bound Rituximab to interact with the FcR on cytotoxic cells (e.g. NK, macrophages, neutrophils) to initiate the cytotoxic process. Some patients showed expression of a variant FcγRIIIa and receptor expressing 158 V and 158 F cell types. The homozygosity of the FcγRIIIaA-158 V allotype was the single parameter associated with the clinical response of Rituximab at 12 months post treatment.51,59 Rituximab-resistant clones were also shown to be resistant to ADCC.55 These findings established the importance of ADCC in the clinical response to Rituximab.
B. Inhibition of CDC
Rituximab is capable of binding to C1q3 and thus activating the complement cascade. The major contributors to CDC resistance consists of CD46 (MCP), CD55 (DAF), and CD59. The levels of CD20 and complement inhibitors were determinants in the clinical response of isolated BCLL, prophylactic leukemia (PLC) and MCL. Blocking of the inhibitors enhanced CDC.60 However, a controversy exists on the relevance of CDC and inhibitors in response to Rituximab.52 Rituximab resistance clones were also resistant to CDC.55
C. Resistance to apoptosis
Rituximab treatment alone poorly displays apoptosis on B-NHL cells. However, cross-linking with secondary anti-Ig triggers a significant apoptotic response. Treatment of Rituximab-resistant clones, however, with Rituximab and secondary anti-Ig did not trigger apoptosis.55,61
D. CD20 modulation
The role of CD20 expression levels and response to Rituximab is not clear in vivo. Terner, et al.62 reported deletion at the C terminal region of the CD20 gene in a subset of tumor samples from patients with NHL. However, it is not clear what was the clinical response in those patients. Also, Pederson, et al.63 described a process “shaving” whereby complexes of CD20 and Rituximab are shed off from the cells by monocytes and, hence, the cells become unresponsive to Rituximab. We have also reported that the in vitro generation of Rituximab-resistant clones showed a reduction of cell membrane CD20 expression compared to wild type.51,61 Treatment of patients (relapsed and refractory) with a combination of Rituximab and Bortezomib resulted in ORRs of 49% and 43% respectively, for two different regimens.64 The loss of CD20 expression was observed following Rituximab treatment in a subset of patients. There were cases of CD20 deficient lymphoma relapses identified following treatment with Rituximab containing regimens in DLBCL.54 In addition, Hiraga et al.65 reported that 30% of patients with B-cell lymphoma and treated with Rituximab and chemotherapy, their tumor CD20 expression was lost. DNA demethylating agents restored CD20 expression suggesting an epigenetic mechanism responsible for loss of CD20.
E. Generation of Rituximab-resistant clones
A survey66 of 92 immortalized normal lymphoblastoid cell lines and separated for sensitive and resistant lines. The findings were as follows: (1) The level of CD20 protein and surface expression was decreased in the resistant lines. (2) The susceptibility was not correlated with mRNA that is post-transcriptional. They also have selected resistant cell lines that were cultured with Rituximab in various LCL and lymphoma cell lines. They found that levels of CD20 expression were reduced in all resistant cell lines. Also, they found CD20 mRNA spliced variants associated with resistance. In addition, ofabumumab was more active as compared to Rituximab in vitro.
In an effort to recapitulate what might be responsible, in part, for resistance to Rituximab in patients, we have generated Rituximab-resistant clones (RR) from several B-NHL cell lines. These were developed by exposing wild type cells to increasing concentrations of Rituximab in culture and followed by multiple rounds of limiting dilution assays. The resulting RR clones were grown and analyzed for their properties compared to wild type cells. Several key findings were obtained. In comparison with the wild type cells, the RR clones expressed lower surface CD20 expression, resistance to both ADCC and CDC, and did not respond to Rituximab-mediated inhibition of cell proliferation, apoptosis by cross-linking and were not chemo- or immuno-sensitized to drug apoptosis. The RR clones were not triggered by Rituximab to inhibit intracellular survival/anti-apoptotic pathways and, in contrast, the RR clones exhibited hyper activated survival pathways, such as the NF-κB and ERK1/2 pathways and overexpression of anti-apoptotic gene products, targets of above pathways such as Bcl2, Bclxl, and Mcl-1.55,61 Czucman et al.67 developed RR cell lines. They have found that the resistant cell lines have had changes in CD20 expression, decreased calcium mobilization and redistribution of CD20 in lipid grafts. There was a partial reversion of resistance by proteasome inhibitors (CCR 2008; 14156). In a subsequent study, Brem et al.68 reported that treatment with the BH3 mimetic Obatoclax induced cell death in both Rituximab sensitive and Rituximab resistant cell lines and also in primary tumor cells. Synergy was achieved by a combination of Obatoclax and chemotherapeutic drugs. Using the more selective proteasome inhibitor Carfilzomib, in comparison with the predecessor Bortezomib, showed significant augmentation of cytotoxicity in the resistant lines and reversed resistance to chemotherapy. There was upregulation of the apoptotic gene product Bak.69 Further, studies with the reversible proteasome inhibitor MLN2238 showed that it induces caspase independent cell death of the resistant cell lines and potentiated the cytotoxic activity of various chemotherapeutic drugs.70
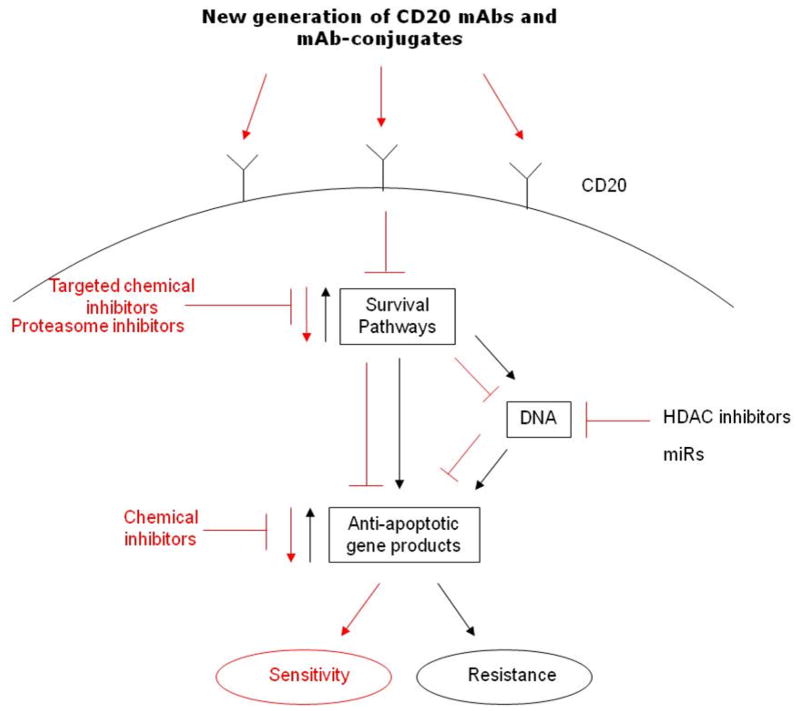
V. Overcoming Rituximab resistance (Schematic diagram-Figure 1)
Figure 1.
Schematic diagram of the molecular mechanisms that regulate resistance to rituximab and approaches to overcome resistance. Briefly, the unresponsive B-NHL cells exhibit hyperactivated survival/anti-apoptotic pathways which regulate downstream anti-apoptotic gene products that result in the development of resistance to both rituximab, chemotherapy and combination. The intervention to inhibit the survival pathways may be achieved by targeted chemical inhibitors, proteasome inhibitors, selective chemical inhibitors for the anti-apoptotic gene products, as well as HDAC inhibitors and microRNAs. In addition, the potential application for the development of a new generation of CD20 mABs, alone or conjugated with various agents to enhance their activities.
The development of RR clones and their unresponsiveness to Rituximab alone or in combination with drugs allowed the exploration of mechanisms underlying resistance and to examine means to overcome resistance. We have reported that the RR clones exhibited hyper-activated survival/anti-apoptotic pathways and we also reported that interference with the activity of such pathways in the Rituximab sensitive lines by various chemical inhibitors resulted in chemo-immuno-sensitization, mimicking Rituximab in combination with cytotoxic agents in wild type rituximab-sensitive B-NHL cells.55,61 Therefore, we examined if inhibition of intracellular pathways can sensitize RR clones to cytotoxic agents.
The inhibition of NF-κB and downstream Bclxl expressed by Rituximab treatment in sensitive lines was corroborated by the use of inhibitors such as the inhibitors of NF-κB (DHMEQ and Bay-7805) and impairment of the function of Bclxl (by 2MAM-A3). Treatment with these inhibitors sensitized the B-NHL cells to various chemo therapeutic drugs. In the RR clones, treatment with the proteasome inhibitor Bortezomib in combination with chemotherapeutic drugs sensitized the RR cells to apoptosis. Further, inhibition of NF-κB by DHMEQ also sensitized the RR cells to apoptosis by various drugs.33 Maiso, et al.71 have reported that HDAC inhibitors reversed drug resistance in MM. Valproic acid and romidepsin were used on B-cell lines and resulted in augmentation of CD20 expression, enhancing CDC activity, and in vivo in mice bearing tumor xenografts resulted in response to combination of Rituximab and HDAC inhibitors.
The enhancement of CD20 expression in cases where resistance to Rituximab was observed, due in part, to downregulation of CD20 expression may be attempted to reverse resistance. Shimizu, et al..72 reported that treatment of cells with HDAC inhibitors, valproic acid and romidepsin increased the expression of CD20 and enhanced Rituximab-mediated CDC cytotoxicity. This finding suggested that the epigenetic regulation of CD20 expression and clinical application of HDAC inhibitors in combination with drugs or Rituximab may be effective.
Resistance to Rituximab appears to be due, in part, as a result of the overexpression of anti-apoptotic gene products and as a consequence overactivation of survival pathways that regulate these gene products as has been reported in RR clones.55, 61 Bortezomib50 and Temsirolimus73 treatment of lymphoma cells sensitized the cells to Rituximab. Clinical trials have been run for the combination of Rituximab and Bortezomib for salvaged treatment for B-NHL.64
The Bcl2 pan inhibitor, Oblimersem, was clinically tested in a phase I and demonstrated inhibition of Bcl2 levels and an objective clinical response in heavily treated patients.24 In a phase II combination of Rituximab and Oblimersem, there was a modest activity in patients with residual disease and in patients with DLBCL. A higher RR resistance was eliminated in Fl. The combination of Rituximab and Oblimersem was effective in patients refractory to Rituximab.75 ADCC has been shown to play an important role in vivo in Rituximab. Thus, augmenting ADCC may improve Rituximab effects and may override resistance in a subset of resistant patients. In clinical trials, G-CSF and Rituximab produced a responsive rate of 42% in patients with indolent NHL,76 with a possible longer duration of response compared to Rituximab alone. Combination of IL2 and Rituximab in Rituximab-resistant indolent NHL did not produce a clinical response.77 Lenalidomide, an immuno-modulating drug, enhances ADCC and reverses immune suppression. Single agents have therapeutic activity in relapsed/refractory B-cell lymphoma. In a phase 2 trial, in patients resistant to rituximab, relapsed or refractory indolent B or mantle cell lymphoma. Lenalidomide in low dose with dexamethasone and rituximab were used. The combination achieved a high response rate with durable response. The over response rate increased from 29% after 2 cycles of lenalidomide and dexamethasone to 58% before the addition of rituximab.78
Combination of Rituximab and anti-CD22 mAb, epratuzumab
The fusion protein anti-CD20-hIFN-α, consists of anti-CD20 and hIFN-α, was engineered and shown to have a more potent activity and apoptosis on B-NHL cell lines in vitro and anti-tumor response in vivo.79 Preliminary findings indicated that treatment of RR clones with anti-CD20-hIFNα, but not with Rituximab-hFNα. or combination, resulted in inhibition of cell recovery, induction of apoptosis, and sensitization to chemotherapeutic drugs.80
Novel anti-CD20 mAbs
1. Ofatumumab: patients with rituximab resistant follicular
NHL and treated with ofatumumab resulted in response rate of 20–22% and patients resistant to single agent rituximab with response rate of 9% and patient treated with combination treatment with rituximab and chemotherapy.81 Ofatumumab is a fully humanized anti-CD20 mAb approved by the FDA.82, 83 Ofatumumab was found to induce signaling for CDC, more potent than rituximab. It shows activity in rituximab-refractory lymphoma.7
2. GA-101 (Type 2) with Fd engineered for higher affinity for FcR.83 There was no indication about the response in patients with rituximab resistance
The development of novel scaffolds of much smaller sequences and higher stability.85–87 The size of the scaffolds (12–15 kDa) is an order of magnitude smaller than the size of IgG (160 kDa). These scaffolds leads to good penetration, they are more stable in the circulation and could be taken orally. Also, they can penetrate the blood brain barrier.
IX. Conclusions
This mini-review described briefly the current status of rituximab and suggested mechanisms of innate and developed resistance in patients to both rituximab monotherapy and rituximab-containing chemotherapy regimens. There are also suggestions of various means to reverse resistance to rituximab treatment based on analysis of rituximab-resistant clones investigated in vitro. Clearly, some of the mechanisms of resistance of the rituximab-resistant clones may or may not be applicable to the mechanisms in vivo; however, they will need to be validated. Future directions require the development of various clinical trials to address some of the postulated mechanisms and their validation. In addition, further studies from patient-derived tumor tissues from unresponsive patients may be useful to examine gene products that regulate resistance and validate their prognostic significance as novel biomarkers.
Acknowledgments
The work reviewed in this chapter derived primarily from published work from my laboratory at UCLA and is in the result of scholarly publications by post doctoral fellows, doctoral students and collaborators during the last several years. I am indebted for their efforts and for their significant contributions. The followings have co-authored publications on Rituximab-mediated effects:-Doctors Alas, S.; Baritaki, S.; Demidem, A.; Hannah, N.; Hariharan K.; Huerta-Yepez, S.; Jazirehi, A.; Martinez-Panigua, M.; and Vega, M.I.
The studies reported in this review were supported in part by the Jonsson Comprehensive Cancer Center at UCLA, the Fundamental and Clinical Immunology Training Grant AI07126-23, UCLA AIDS Institute, the Fogarty International Center Fellowship (D43 TW00013), UC MEXUS-CONACYT. The author acknowledges the assistance of Melissa Cao and Anna Shvartsur in the preparation of the manuscript.
Footnotes
No financial disclosures or conflict of interest in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lippert TH, Ruoff HJ, Volm M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung. 2008;58:261–4. doi: 10.1055/s-0031-1296504. [DOI] [PubMed] [Google Scholar]
- 2.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibodu of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 3.Reff ME, Carner K, Chambers KS, Chinn PC, Leonar JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 4.Newman R, Alberts J, Anderson D, Carner K, Heard C, Norton F, et al. “Primatization” of recombinant antibodies for immunotherapy of human diseases: a macaque/human chimeric antibody against human CD4. Biotechnology. 1992;10:1455–60. doi: 10.1038/nbt1192-1455. [DOI] [PubMed] [Google Scholar]
- 5.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 6.Davis TA, White CA, Grillo-Lopez AJ, Velasquez WS, Link B, Maloney DG, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: results of a phase II trial of rituximab. J Clin Oncol. 1999;17:1851–7. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 7.Adler MJ, Dimitrov DS. Therapeutic antibodies against cancer. Hematol Oncol Clin North Am. 2012;26:447–81. doi: 10.1016/j.hoc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–74. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 9.Khouri IF, Saliba RM, Hosing C, Okoroji GJ, Acholonu S, Anderlini P, et al. Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2005;23:2240–7. doi: 10.1200/JCO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Neumann F, Harmsen S, Martin S, Kronenwett R, Kondakci M, Aivado M, et al. Rituximab long-term maintenance therapy after autologous stem cell transplantation in patients with B-cell non-Hodgkin’s lymphoma. Ann Hematol. 2006;85:530–4. doi: 10.1007/s00277-006-0113-5. [DOI] [PubMed] [Google Scholar]
- 11.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–6. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robak T. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 13.Vose JM. Antibody-targeted therapy for low-grade lymphoma. Semin Hematol. 1999;36:15–20. [PubMed] [Google Scholar]
- 14.Friedberg JW, Neuberg D, Gribben JG, Fisher DC, Canning C, Koval M, et al. Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin’s lymphoma. Br J Haematol. 2002;117:828–34. doi: 10.1046/j.1365-2141.2002.03535.x. [DOI] [PubMed] [Google Scholar]
- 15.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–54. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy AD, Solga MD, Shcuman TA, Chi AW, Lindorfer MA, Sutherland WM, et al. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101:1071–9. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 17.Demidem A, Hanna N, Hariharan H, Bonavida B. Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. FASEB J. 1995;9:A206. doi: 10.1089/cbr.1997.12.177. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–43. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood. 2002;99:1314–9. doi: 10.1182/blood.v99.4.1314. [DOI] [PubMed] [Google Scholar]
- 20.Mathas S, Rickers A, Bommert K, Dorken B, Mapara MY. Anti-CD20- and B-cell receptor-mediated apoptosis: evidence for shared intracellular signaling pathways. Cancer Res. 2000;60:7170–6. [PubMed] [Google Scholar]
- 21.van der Kolk LE, de Haas M, Grillo-López AJ, Baars JW, van Oers MH. Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils. Leukemia. 2002;16:693–9. doi: 10.1038/sj.leu.2402424. [DOI] [PubMed] [Google Scholar]
- 22.Polyak MJ, Tailor SH, Deans JP. Identification of a cytoplasmic region of CD20 required for its redistribution to a detergent-insoluble membrane compartment. J Immunol. 1998;161:3242–8. [PubMed] [Google Scholar]
- 23.Cragg MS, Morgan SM, Chan HT, Morgan BP, Filatov AV, Johnson PW, French R, Glennie MJ. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–52. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- 24.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signaling through lipid rafts. Immunology. 2002;107:176–82. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semac I, Palomba C, Kulangara K, Klages N, van Echten-Deckert G, Borisch B. Anti-CD20 therapeutic antibody rituximab modifies the functional organization of rafts/microdomains of B lymphoma cells. Cancer Res. 2003;63:534–40. [PubMed] [Google Scholar]
- 26.Bezombes C, Grazide S, Garret C, Fabre C, Quillet-Mary A, Muller S, et al. Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood. 2004;104:1166–73. doi: 10.1182/blood-2004-01-0277. [DOI] [PubMed] [Google Scholar]
- 27.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signaling through lipid rafts. Immunology. 2002;107:176–82. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-{kappa}B signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–76. [PubMed] [Google Scholar]
- 29.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 2001;61:5137–44. [PubMed] [Google Scholar]
- 30.Vega MI, Huerta-Yepaz S, Garban H, Jazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23:3530–40. doi: 10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- 31.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by rituximab. Cancer Res. 2004;64:117–26. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki E, Bonavida B. Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26:6184–93. doi: 10.1038/sj.onc.1210448. [DOI] [PubMed] [Google Scholar]
- 33.Vega MI, Huerta-Yepez S, Martinez-Paniagua M, Martinez-Miguel B, Hernandez-Pando R, González-Bonilla CR, et al. Rituximab-Mediated Cell Signaling and Chemo/Immuno-sensitization of Drug-Resistant B-NHL Is Independent of Its Fc Functions. Clin Cancer Res. 2009;15:6582–94. doi: 10.1158/1078-0432.CCR-09-1234. [DOI] [PubMed] [Google Scholar]
- 34.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non- Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–26. [PubMed] [Google Scholar]
- 35.Jazirehi AR, Gan XH, De Vos S, Emmanouilides C, Bonavida B. Rituximab (anti-CD20) selectively modifies Bcl-xL and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin's lymphoma B cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2003;2:1183–93. [PubMed] [Google Scholar]
- 36.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 37.Dixit V, Mak TW. NF-κB signaling. Many roads lead to Madrid. Cell. 2002;111:615–9. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 38.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer [review] Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 39.Arancia G, Malorni W, Donelli G. Cellular mechanisms of lymphocyte-mediated lysis of tumor cells. Ann Ist Super Sanita. 1990;26:369–84. [PubMed] [Google Scholar]
- 40.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–7. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 41.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–55. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 42.Vega MI, Jazirehi AR, Huerta-Yepez S, Bonavida B. Rituximab-induced inhibition of YY1 and Bcl-xL expression in Ramos non-Hodgkin's lymphoma cell line via inhibition of NF-kappa B activity: role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectively. J Immunol. 2005;175:2174–83. doi: 10.4049/jimmunol.175.4.2174. [DOI] [PubMed] [Google Scholar]
- 43.Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor Yin-Yang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J Immunol. 2001;167:75–81. doi: 10.4049/jimmunol.167.1.75. [DOI] [PubMed] [Google Scholar]
- 44.Huerta S, Baay-Guzman G, Gonzalez-Bonilla CR, Livingston EH, Huerta-Yepez S, Bonavida B. In vitro and in vivo sensitization of SW620 metastatic colon cancer cells to CDDP-induced apoptosis by the nitric oxide donor DETANONOate: Involvement of AIF. Nitric Oxide. 2009;20:182–94. doi: 10.1016/j.niox.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, et al. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood. 2007;110:4037–46. doi: 10.1182/blood-2007-02-076075. [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 47.Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–43. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 48.Abdollahi S, Chong EA, Olin RL, et al. The impact of rituximab resistance on overall survival rate in low-grade follicular lymphoma [abstract] Blood (ASH Annual Meeting Abstracts) 2008;112:3783. [Google Scholar]
- 49.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2009 (vintage 2009 populations) National Cancer Institute; Bethesda, MD: National Cancer Institute; 2012. [Accessed June 6, 2012.]. (based on the November 2011 SEER data submission, posted to the SEER web site April 2012). Available at: http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 50.Wang M, Han XH, Zhang L, Yang J, Qian JF, Shi YK, et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22:179–85. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]
- 51.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 52.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, et al. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007;67:10556–63. doi: 10.1158/0008-5472.CAN-07-1811. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy GA, Tey SK, Cobcroft R, Marlton P, Cull G, Grimmett K, et al. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br J Haematol. 2002;119:412–6. doi: 10.1046/j.1365-2141.2002.03843.x. [DOI] [PubMed] [Google Scholar]
- 55.Jazirehi AR, Vega MI, Bonavida B. Development of Rituximab-Resistant Lymphoma Clones with Altered Cell Signaling and Cross-Resistance to Chemotherapy. Cancer Res. 2007;67:1270–81. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- 56.Terui Y, Mishima Y, Sugimura N, Kojima K, Sakurai T, Mishima Y, et al. Identification of CD20 C-terminal deletion mutations associated with loss of CD20 expression in non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15:2523–30. doi: 10.1158/1078-0432.CCR-08-1403. [DOI] [PubMed] [Google Scholar]
- 57.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176:2600–9. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 58.Burger JA, Gandhi V. The lymphatic tissue microenvironments in chronic lymphocytic leukemia: in vitro models and the significance of CD40-CD154 interactions. Blood. 2009;114:2560–1. doi: 10.1182/blood-2009-06-228981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 60.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–8. [PubMed] [Google Scholar]
- 61.Jazirehi AR, Bonavida B. In: Cancer Cell Culture: Methods and Protocols. 2. Cree IA, editor. New York: Springer; 2011. pp. 407–19. [Google Scholar]
- 62.Ternant D, Henin E, Cartron G, Tod M, Paintaud G, Girard P. Development of a drug-disease simulation model for rituximab in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol. 2009;68:561–73. doi: 10.1111/j.1365-2125.2009.03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pederson AE, ungersen MB, Pedersen CD. Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology. 2011;133(2):239–45. doi: 10.1111/j.1365-2567.2011.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Vos S, Goy A, Dakhil SR, Saleh MN, McLaughlin P, Belt R, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27:5023–30. doi: 10.1200/JCO.2008.17.7980. [DOI] [PubMed] [Google Scholar]
- 65.Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood. 2009;14:113, 4885–93. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- 66.Small GW, McLeod HL, Richards KL. Analysis of innate and acquired resistance to anti-CD20 antibodies in malignant and nonmalignant B cells. PeerJ. 2013;1:e31. doi: 10.7717/peerj.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, Knight J, Starostik P, Deans J, Hernandez-Ilizaliturri FJ. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clincal Cancer Research. 2008;14 (5):1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 68.Bram EA, Thudium K, Khubchandani S, Tsai PC, Olejniczak SH, Bhat S, Riaz W, Gu J, Iqbal A, Campagna R, Knight J, Mavis C, Hoskin P, Deeb G, Gibbs JF, Fetterly G, Czuczman MS, Hernandez-Ilizaliturri FJ. Distinct cellular and therapeutic effects of obatoclax in rituximab-sensitive and – resistant lymphomas. British Journal of Hematology. 2011;153 (5):599–611. doi: 10.1111/j.1365-2141.2011.08669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu JJ, Hernandez-Illizaliturri FJ, Kaufman GP, Czuczman NM, Mavis C, Skitzki JJ, et al. The novel proteasome inhibitor carfilzomib induces cell cycle arrest, apoptosis and potentiates the anti-tumor activity of chemotherapy in rituximab-resistant lymphoma. Brit J Haematol. 2013;162(5 ):657–69. doi: 10.1111/bjh.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu JJ, Hernandez-Illizaliturri FJ, Mavis C, Czuczman NM, Deeb G, Gibbs J, Skitzki JJ, Patil R, Czuczman MS. MLN2238, a proteasome inhibitor, induces caspase-dependent cell death, cell cycle arrest, and potentiates the cytotoxic activity of chemotherapy agents in rituximab-chemotherapy-sensitive or rituximab-chemotherapy-resistant B-cell lymphoma preclinical models. Anticancer Drugs. 2013;24 (10):1030–1038. doi: 10.1097/CAD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maiso P, Carvajal-Vergara X, Ocio EM, López-Pérez R, Mateo G, Gutiérrez N, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66:5781–9. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 72.Shimizu R, Kikuchi J, Wada T, Ozawa K, Kano Y, Furukawa Y. HDAC inhibitors augment cytotoxic activity of rituximab by upregulating CD20 expression on lymphoma cells. Leukemia. 2010;24:1760–8. doi: 10.1038/leu.2010.157. [DOI] [PubMed] [Google Scholar]
- 73.Wanner K, Hipp S, Oelsner M, Ringshausen I, Bogner C, Peschel C, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitizes DLBCL cells to rituximab. Br J Haematol. 2006;134:475–84. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 74.Waters JS, Webb A, Cunningham D, Clarke PA, Raynaud F, di Stefano F, et al. Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2000;18:1812–23. doi: 10.1200/JCO.2000.18.9.1812. [DOI] [PubMed] [Google Scholar]
- 75.Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F, et al. Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. Br J Haematol. 2008;143:355–60. doi: 10.1111/j.1365-2141.2008.07353.x. [DOI] [PubMed] [Google Scholar]
- 76.van der Kolk LE, Grillo-Lopez AJ, Baars JW, van Oers MH. Treatment of relapsed B-cell non-Hodgkin’s lymphoma with a combination of chimeric anti-CD20 monoclonal antibodies (rituximab) and G-CSF: final report on safety and efficacy. Leukemia. 2003;17:1658–64. doi: 10.1038/sj.leu.2402995. [DOI] [PubMed] [Google Scholar]
- 77.Khan KD, Emmanouilides C, Benson DM, Jr, Hurst D, Garcia P, Michelson G, et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:7046–53. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- 78.Ahmadi T, Chong EA, Gordon A, Aqui NA, Nasta SD, Svoboda J, et al. Combined Lenalidomide, Low-Dose Dexamethasone, and Rituximab Achieves Durable Responses in Rituximab-Resistant Indolent and Mantle Cell Lymphomas. Cancer. 2014;120:222–8. doi: 10.1002/cncr.28405. [DOI] [PubMed] [Google Scholar]
- 79.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010 Apr 8;115(14):2864–71. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vega GG, Vega MI, Huerta-Yepez S, Jazirehi A, Mayani H, Martinez-Maza O, Morrison S, Bonavida B. Cytotoxic Activity of Anti-CD20-hIFN-α on Rituximab-Resistant B-NHL Clones and Synergy with Chemotherapy. Blood. 2011;118:3499. [Google Scholar]
- 81.Hagenbeek A, Fayad L, Delwail V, et al. Evaluation of ofatumumab, a novel human CD20 monoclonal antibody, as single agent therapy in rituximab-refractory follicular lymphoma. Blood. 2010;114:385, 6, 935. [Google Scholar]
- 82.Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, et al. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010;16:4331–8. doi: 10.1158/1078-0432.CCR-10-0570. [DOI] [PubMed] [Google Scholar]
- 83.Czuczman MS, Fayad L, Delwail W, Cartron G, Jacobsen E, Kuliczkowski K, et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698–704. doi: 10.1182/blood-2011-09-378323. [DOI] [PubMed] [Google Scholar]
- 84.Salles GA, Morschhauser F, Cartron G, et al. A phase I/II study of RO5072759 (GA101) in patients with relapsed/refractory CD20þ malignant disease. Blood. 2008;112:93, 234. [Google Scholar]
- 85.Dimitrov DS, Marks JD. Therapeutic antibodies: Current state and future trends -is a paradigm change coming soon? Meth Mol Biol. 2009;525:1–27. doi: 10.1007/978-1-59745-554-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skerra A. Alternative non-antibody scaffolds for molecular recognition. Curr Opin Biotechnol. 2007;18:295–304. doi: 10.1016/j.copbio.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Chen W, Dimitrov DS. Human monoclonal antibodies and engineered antibody domains as HIV-1 entry inhibitors. Curr Opin in HIV and AIDS. 2009;4:112–7. doi: 10.1097/COH.0b013e328322f95e. [DOI] [PMC free article] [PubMed] [Google Scholar]