Abstract
Background
The progressive nature of heart failure (HF) coupled with high mortality and poor quality of life mandates greater attention to palliative care as a routine component of advanced HF management. Limited evidence exists from randomized, controlled trials supporting the use of interdisciplinary palliative care in HF.
Methods
The Palliative Care in Heart Failure trial (PAL-HF) is a prospective, controlled, unblinded, single-center study of an interdisciplinary palliative care intervention in 200 patients with advanced HF estimated to have a high likelihood of mortality or re-hospitalization in the ensuing 6 months. The 6-month PAL-HF intervention focuses on physical and psychosocial symptom relief, attention to spiritual concerns and advanced care planning. The primary endpoint is health-related quality of life measured by the Kansas City Cardiomyopathy Questionnaire and the Functional Assessment of Chronic Illness Therapy with Palliative Care Subscale score at 6 months. Secondary endpoints include changes in anxiety/depression, spiritual well-being, caregiver satisfaction, cost and resource utilization, and a composite of death, HF hospitalization and quality of life.
Conclusions
PAL-HF is a randomized, controlled clinical trial that will help evaluate the efficacy and cost-effectiveness of palliative care in advanced HF using a patient-centered outcome as well as clinical and economic endpoints.
Keywords: palliative care, heart failure, end of life
Heart failure (HF) currently affects over 5 million Americans1. Despite recent therapeutic advances, patients with advanced disease suffer not only from physical effects, but also from psychosocial and spiritual distress2, 3. Selected patients are candidates for aggressive treatments such as cardiac transplantation or mechanical circulatory support4, but the application of these therapies to the broader HF population is limited by constrained resources and their untested efficacy in frail and elderly patients with significant co-morbidities. The progressive nature of HF coupled with high mortality rates and poor quality of life mandates greater attention to palliative care as a routine component of HF management5.
Palliative care is a multidisciplinary approach that focuses on providing patients with relief from the symptoms, pain, and stress of living with a serious illness, at any stage of that illness6. The goal is to improve quality of life for both the patient and family. Despite consensus-panel and guideline recommendations to combine palliative care with evidence-based HF therapies in the later stages of the disease7, 8, the integration of palliative care into the HF treatment paradigm has been limited by several challenges.
First, an unpredictable disease trajectory makes prognostication difficult. Despite validated multivariable models to predict survival in HF9, 10, physicians are frequently unsure whether they are caring for a patient near or far from the end of life. Patients have even a harder time and are typically overly optimistic about their survival11. Prognostic uncertainty poses a challenge as to when palliative care interventions should be implemented, particularly for physicians who equate palliative care with end of life care. Clinicians may experience turmoil from competing perspectives related to this prognostic uncertainty, current recommendations to institute palliative care earlier during the disease course and the fear of being viewed as “giving up” on life-prolonging care. Second, cardiologists typically lack formalized training in the principles of palliative care. Nurses are more likely to have formal training in palliative care and there has been a recent call to increase the involvement of nurse practitioners (NPs) in the palliative care team12, 13. Third, the design of care delivery in many health systems does not accommodate palliative care interventions that cross boundaries from acute settings to home 14. Finally, until recently, limited evidence was available regarding the benefit of palliative care interventions in HF15 and most studies focused on resuscitation preferences16-18. It was less clear which HF patients benefit from palliative interventions and which interventions improve quality of life and achieve outcomes desired by patients and family members7. However, multiple recent pilot studies in HF populations have suggested that palliative care may reduce symptom burden and improve quality of life19-21. These studies have highlighted the importance of symptoms such as anxiety and depression in HF patients in addition to the commonly recognized symptoms of fatigue, dyspnea and nausea. In addition, the publication of a randomized trial of palliative care in Stage 4 lung cancer that showed improved patient outcomes and reduced costs22 has led to increased interest of whether similar results can be found in other common diseases such as HF. These studies provide the foundation and rationale for a large-scale randomized HF trial sufficiently powered to assess the effect of palliative care on clinical outcomes.
To help create this body of evidence, the National Institute of Nursing Research (NINR) has funded the Palliative Care in Heart Failure trial (PAL-HF) to evaluate whether a multidimensional palliative care intervention improves health-related outcomes relative to usual care alone in advanced HF patients with a high expected short-term mortality (ClinicalTrials.gov Identifier: NCT01589601). This article describes the design and rationale of the PAL-HF trial (Figure). The authors are solely responsible for the design and conduct of this study, the drafting and editing of this paper and its final contents. Of note, the NINR is currently funding multiple clinical trials and research projects related to palliative care (see http://projectreporter.nih.gov).
Figure.
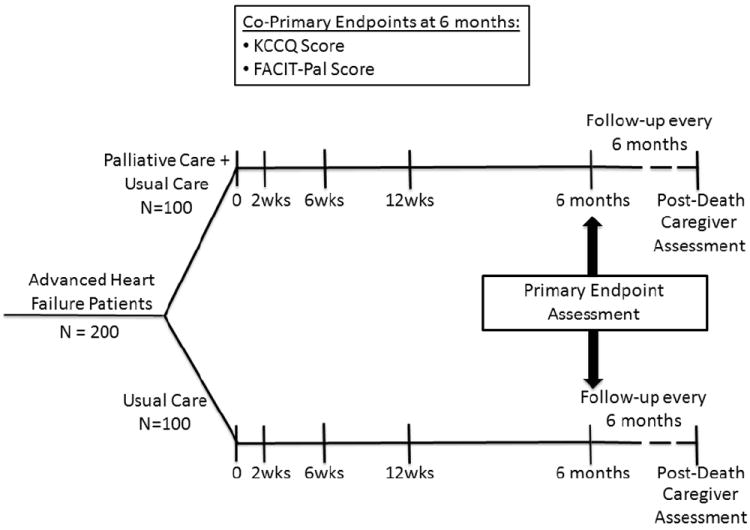
Trial design.
METHODS
Overall Design
PAL-HF is a prospective, controlled, two-arm, single-center clinical trial of 200 advanced HF patients with >50% predicted 6-month mortality randomized in a 1:1 ratio to usual contemporary HF care or usual care combined with the PAL-HF intervention. Subjects are assigned treatment using a complete randomization scheme23. The palliative care intervention focuses on symptom relief, attention to spiritual concerns, and advanced care planning. The trial is unblinded, since it is not possible to execute a double-blinded trial of the PAL-HF intervention given that the intervention is a multidisciplinary team. The duration of the intervention is 6 months, but patients in both groups are followed until death or the end of the study (~4 years). The primary endpoint is health-related quality of life.
Study population
The study will enroll patients currently hospitalized for acute HF (either systolic HF or HF with preserved ejection fraction) or within 2 weeks of discharge from hospitalization for acute HF. Inclusion and exclusion criteria are presented in Table 1. Entry criteria include previous HF hospitalization in the past year and an ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk score ≥4 indicating >50% predicted 6-month mortality (Supplemental Table)9. Since the risk for morbidity and mortality in advanced HF patients may be high in patients that do not meet these criteria, we also included patients in three high risk groups: chronic inotropes, ≥3 HF hospitalizations in the past 12 months or an ESCAPE score >4 if the patient has had no prior hospitalization for HF. Exclusion criteria include anticipated heart transplant or ventricular assist device within 6 months, and non-cardiac terminal illness.
Table 1.
Trial Entry Criteria.
| Inclusion Criteria |
|
| OR |
|
|
|
| Exclusion Criteria |
|
Usual Care
Patients are managed by a cardiologist-directed team with HF expertise. Until discharge, inpatient care is focused on symptom relief and use of evidence-based therapies24. Additional goals of care include treatment of co-morbidities and patient education designed to assist with self-management techniques. For instance, we routinely target sleep disordered breathing and mood disorders, and also encourage exercise training in our HF patients as recommended by guidelines8. For ethical reasons, inpatient palliative care consultation, at the discretion of the attending cardiologist, is not denied to Usual Care patients. After discharge, Usual Care patients generally receive outpatient follow-up with a HF cardiologist or NP focused on guideline-based medication titration, assessment of adherence to medical and dietary regimens, and serial monitoring of end-organ function. Duke’s outpatient palliative care practice has historically been based in the Duke Cancer Institute and was not readily available to Usual Care patients. As the value of outpatient palliative care in the HF population is undefined, we believe it is ethically acceptable for outpatients in this arm to receive only state-of-the-art usual cardiovascular care.
Palliative Care Intervention
An interdisciplinary, guideline-driven, multi-component palliative care intervention has been designed that is administered with contemporary HF management. The goal is a structured and reproducible approach to assessing and managing the multiple domains of quality of life for patients with advanced HF including physical symptoms, psychosocial concerns, spiritual concerns, and advanced care planning. At the core of the PAL-HF intervention team is a palliative care trained NP who coordinates this aspect of the patient’s care, a hospice and palliative care board-certified physician, and a trained counselor. Since the same cardiology team cares for patients in both the intervention and control groups, the palliative care intervention is performed in collaboration with the cardiology team, but does not involve specific cardiology-based palliative interventions. This approach was designed in an attempt to minimize the extension of palliative care-specific interventions into the usual care study group to maintain integrity of the study design. Notably, as palliative care is increasingly incorporated into routine practice, it will be necessary to have even greater multidisciplinary collaboration than afforded in a clinical trial.
Physical symptoms
At study enrollment and at pre-specified time-points (see below), the NP performs standardized assessments to determine the presence of commonly experienced symptoms, including dyspnea, fatigue, pain, anorexia, constipation, and insomnia. Symptoms are managed by the PAL-HF team using treatment algorithms to ensure standardization. In addition to periodic assessment and protocolized management of symptoms, patients in the intervention arm are provided with a PAL-HF Heart Failure Relief Medication handout that provides instruction on timely self-management of symptoms. The handout includes information on medications typically used for HF on an “as needed” basis by subjects for symptom relief at home, such as loop diuretics, sublingual nitroglycerin and low dose morphine elixir 6, 25. The handout also includes information on other medications that may be prescribed for managing anxiety, nausea, vomiting and constipation. The medications themselves are prescribed at the discretion of the PAL-HF clinical provider.
Psychosocial symptoms
Patients are screened for symptoms of anxiety and depression using the Hospital Anxiety and Depression Scale (HADS)26. The HADS has been widely used in clinical trials as a psychological screening test for the states of anxiety and depression27. Patients who screen positive for either anxiety or depression receive a more thorough assessment and management as specified by a treatment algorithm used to determine the need for a mental health referral and the possible use of antidepressants, anxiolytics, stress management techniques, and psychotherapy.
Spiritual concerns
The PAL-HF NP completes a spiritual assessment at the time of patient enrollment or at the first outpatient visit after hospitalization using the FICA Spiritual History Tool28. FICA is an acronym which serves as a guide and includes: F-Faith and Beliefs; I-Importance in life and to health; C-Community-Religious and Spiritual Community; A-How the patient would like spirituality addressed in medical care. The NP shares information gathered in the spiritual assessment with the PAL-HF team and addresses specific spiritual concerns as appropriate. If needed, the NP arranges for subsequent visits to address concerns raised during the initial assessment.
End-of-life preparation
To address end-of-life preparation, we use the Outlook Intervention29, which includes three 1-hour sessions, spaced one week apart. Session 1 focuses on life review, accomplishments, proudest moments, and cherished times. Session 2 focuses on issues of forgiveness, things the patient would have done differently, and things left unsaid or undone. Session 3 focuses on lessons learned, heritage, and legacy. To ensure replicability, the intervention follows questions outlined in the Outlook Intervention training manual. The Outlook Intervention is administered by the trained counselor.
Goals of care
The intervention includes communication designed to elicit patients’ goals of care; that is, determine what is most important to individual patients and use those goals to frame discussions of prognosis and the use of life-prolonging therapies. These conversations are facilitated by the PAL-HF NP and revisited periodically to document changes in preferences. To ensure standardization, the PAL-HF NP was trained in a 2-day intensive workshop based on the OncoTalk curriculum30, 31, originally designed to provide oncologists with communication skills to facilitate discussions with seriously ill patients. To assess this aspect of the palliative care intervention, we document code status, completion of advance directives, and preferences for life-prolonging therapies.
After the 6 month intervention period is completed, the PAL-HF NP continues to contact the patients in the intervention arm every 3 months to provide ongoing support and clinical care. In addition, the PAL-HF team is available for phone consultation to patients randomized to the intervention arm throughout the duration of the trial.
Measurements
The primary endpoint is health-related quality of life as measured by the KCCQ and the FACIT-Pal score at 6 months (Table 2). The KCCQ is a 23-item, disease-specific questionnaire scored from 0-100 with high scores representing better health status32. A 5-point change in the KCCQ overall summary score has been demonstrated to be the minimally detectable clinical difference33. The FACIT-Pal is a 46-item measure of self-reported quality of life that assesses several domains including physical well-being, social/family well-being, emotional well-being, and functional well-being34. The palliative care subscale also examines issues in advanced illness including symptoms, relationships with family and friends, life closure, and decision-making and communication abilities.
Table 2.
Trial Endpoints.
| Primary Endpoint: |
| Health-related quality of life – Measured by the Kansas City Cardiomyopathy Questionnaire and the Functional Assessment of Chronic Illness Therapy with Palliative Care Subscale score at 6 months |
|
|
| Secondary Endpoints: |
|
Secondary endpoints are changes in anxiety/depression (HADS), spiritual well-being (FACIT-Sp), caregiver satisfaction, cost and resource utilization, and a composite of death, HF hospitalization and quality of life.
The FACIT-Sp (Functional Assessment of Chronic Illness Therapy Spiritual Well-Being Scale) is a 12 item scale which assesses the role of faith in illness and meaning, peace, and purpose in life35, 36.
A structured interview with a pre-identified caregiver will be conducted following a study subject’s death using the After-Death Bereaved Family Member Interview–Hospice Version37. Six weeks following the patient’s death, the caregiver will be contacted by telephone. If the interviewee agrees to participate, they will provide verbal consent over the phone. Inclusion in the study does not specifically require a caregiver. The interview provides an assessment of patient-focused, family-centered care and assesses overall quality of care received in 7 domains36.
We will use administrative data from Duke University Health System to estimate costs and resource utilization. Based on previous work38, we plan to use the amount paid for care (e.g., by Medicare or private insurance) as the primary estimate of cost. We will conduct sensitivity analyses using the Duke Health System-estimated cost of providing care as the measure of resource-utilization. Any differences in resource utilization across the study arms will be investigated to determine not only the magnitude of cost differences, but the sources that produce the difference. At all follow-up points, patients will be asked if they received care outside of the Duke Health system. If so, they will be asked to estimate the number of visits and/or days in the hospital. The cost of such care will be estimated using the Medical Expenditure Panel Survey, and included in the aggregate cost of care from randomization until study completion. The primary resource allocation measure will be the median cost of care received by patients in each arm from initiation until the end of follow-up.
The composite endpoint of death, HF hospitalization and quality of life was the primary endpoint of the African-American Heart Failure Trial (AHeFT)39, which provides relative weighting of key clinical events: death = -3, first HF admission = -1, and changes in quality of life = -2 to +2. The potential scores range from -6 (an individual who has worsening quality of life, a hospitalization and subsequent death) to +2 (an individual whose quality of life improves and does not die or require hospitalization). This endpoint has been used in other contemporary clinical trials to capture the overall disease burden40. We wanted to capture a more traditional HF survival endpoint, but acknowledge that it is appropriately of secondary importance. The findings of a previous palliative care study in lung cancer patients22, which demonstrated improved survival in the palliative care arm, demonstrates the importance of including this endpoint. AHeFT used the Minnesota Living with Heart Failure (MLHF) Score as the quality of life measure. The PAL-HF study will examine this as a secondary endpoint using the KCCQ rather than the MLHF. This composite endpoint places greater weight on the quality of life component than on hospitalization or death, but also ensures that a palliative care intervention does not hasten death.
A complete schedule of assessments is given in the Appendix. Follow-up evaluations are expected to occur at the first post discharge HF clinic visit (within two weeks of discharge), at 6 weeks, 3 months, 6 months and every 6 months thereafter until the end of the study or until death. As necessary, we will mail questionnaires to subjects and/or complete questionnaires over the telephone to help ensure the collection of primary endpoint data.
Statistical Analysis
We chose the co-primary endpoints of KCCQ and FACIT-Pal to assess quality of life by multiple validated metrics and determined the sample size calculations based on previous work33. Assuming a standard deviation of 12 points for the KCCQ instrument, a sample size of 200 subjects (100/group) will provide 80% power to detect a difference of 4.8 points. For the FACIT-Pal co-primary endpoint, the sample size of 200 subjects will provide 90% power to detect a difference of 0.36 points assuming a standard deviation of 0.76. These calculations are based on a 2-sample t-test with a type I error rate of 0.05. The primary analysis of the longitudinal data from the KCCQ will be based on a linear mixed model with an indicator variable for the treatment group. The treatment effect will be summarized using the estimated treatment difference and the 95% confidence interval. In general, for continuous outcomes measured over time, a mixed model repeated measures analysis will be used to describe patterns of response for the treatment groups.
We are anticipating a mortality rate of approximately 50% by 6 months. To account for the anticipated high-rates of mortality, we plan to conduct a secondary analysis based on the approach of Hayden et al41. With this approach, we assume that death will be informative but not directly influenced by treatment assignment. In particular, we will base our estimates for the Survival Average Causal Effects in KCCQ and FACIT-Pal on the cohort of individuals who would have survival to 6 months, for example, had they been assigned to either treatment group. Both co-primary endpoints will be tested at the two-sided 0.05 level because the KCCQ and FACIT-Pal are measuring two different dimensions that are both important to patients. The primary analyses will be based on the intention-to-treat principle.
Trial Organization
A Data and Safety Monitoring Board (DSMB) has been appointed to review the conduct and results of this trial at 2 enrollment landmarks (after 33% and 66% of the patients have enrolled). The DSMB is charged with reviewing safety composite data in both arms of the trial. The DSMB will be empowered to stop the study for evidence of harm, but not for evidence of lack of efficacy. The DSMB is also asked to offer perspective on any therapeutic or diagnostic testing advances that may occur during the course of the trial that may influence the outcome. If protocol modifications are warranted, close consultation among the DSMB, the NINR staff, and the study leadership will be required.
A Clinical Events Committee (CEC) composed of HF physicians based at the DCRI Coordinating Center will review blinded data on hospital admissions at regular intervals throughout the study to determine their relationship to the diagnosis of HF.
Summary
Advanced HF creates significant physical, psychosocial and spiritual burdens for patients and their families. Palliative care may represent an important component of the holistic management of patients with advanced HF. Further evidence from randomized trials of a palliative care intervention in HF is required to identify which patients may benefit from specific interventions. PAL-HF aims to provide empiric data to support the efficacy and cost-effectiveness of palliative care to improve the health-related quality of life in end-stage HF patients. Such evidence could provide impetus to overcome current challenges related to limited access to, and adoption of, palliative care services for HF patients.
Acknowledgments
Funded by the National Institute of Nursing Research: R01NR013428. R.J.M. was supported by T32GM086330 from the NIGMS.
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Selman L, Beynon T, Higginson IJ, Harding R. Psychological, social and spiritual distress at the end of life in heart failure patients. Curr Opin Support Palliat Care. 2007;1(4):260–6. doi: 10.1097/SPC.0b013e3282f283a3. [DOI] [PubMed] [Google Scholar]
- 3.Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643–8. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol. 2011;26(3):232–6. doi: 10.1097/HCO.0b013e328345aff4. [DOI] [PubMed] [Google Scholar]
- 5.Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA. 2004;291(20):2476–82. doi: 10.1001/jama.291.20.2476. [DOI] [PubMed] [Google Scholar]
- 6.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54(5):386–96. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 7.Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–9. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872–8. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156(4):662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299(21):2533–42. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips JL, Piza M, Ingham J. Continuing professional development programmes for rural nurses involved in palliative care delivery: an integrative review. Nurse Educat Today. 2012;32(4):385–92. doi: 10.1016/j.nedt.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Heinle R, McNulty J, Hebert RS. Nurse Practitioners and the Growth of Palliative Medicine. Am J Hosp Palliat Care. 2014;31(3):287–291. doi: 10.1177/1049909113489163. [DOI] [PubMed] [Google Scholar]
- 14.Lynch S. Hospice and palliative care access issues in rural areas. Am J Hosp Palliat Care. 2013;30(2):172–7. doi: 10.1177/1049909112444592. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz ER, Baraghoush A, Morrissey RP, Shah AB, Shinde AM, Phan A, et al. Pilot study of palliative care consultation in patients with advanced heart failure referred for cardiac transplantation. J Palliat Med. 2012;15(1):12–5. doi: 10.1089/jpm.2011.0256. [DOI] [PubMed] [Google Scholar]
- 16.Levenson JW, McCarthy EP, Lynn J, Davis RB, Phillips RS. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000;48(5 Suppl):S101–9. doi: 10.1111/j.1532-5415.2000.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, et al. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation. 1998;98(7):648–55. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- 18.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20(9):1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista LS, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Liao S. Examining the Effects of an Outpatient Palliative Care Consultation on Symptom Burden, Depression, and Quality of Life in Patients With Symptomatic Heart Failure. J Card Fail. 2012;18(12):894–899. doi: 10.1016/j.cardfail.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelista LS, Liao S, Motie M, De Michelis N, Lombardo D. On-going palliative care enhances perceived control and patient activation and reduces symptom distress in patients with symptomatic heart failure: A pilot study. Eur J Cardiovasc Nurs. 2014;13(2):116–123. doi: 10.1177/1474515114520766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison R, Penrod JD, Cassel J, et al. Cost savings associated with us hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 22.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 23.Vickers AJ. How to randomize. J SocIntegr Oncol. 2006;4(4):194–8. doi: 10.2310/7200.2006.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure--a pilot study. Eur J Heart Fail. 2002;4(6):753–6. doi: 10.1016/s1388-9842(02)00158-7. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu DS, Thompson DR, Yu CM, Oldridge NB. Assessing HRQL among Chinese patients with coronary heart disease: angina, myocardial infarction and heart failure. Int J Cardiol. 2009;131(3):384–94. doi: 10.1016/j.ijcard.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 28.Borneman T, Ferrell B, Puchalski CM. Evaluation of the FICA Tool for Spiritual Assessment. J Pain Symptom Manage. 2010;40(2):163–73. doi: 10.1016/j.jpainsymman.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Steinhauser KE, Alexander SC, Byock IR, George LK, Olsen MK, Tulsky JA. Do preparation and life completion discussions improve functioning and quality of life in seriously ill patients? Pilot randomized control trial. J Palliat Med. 2008;11(9):1234–40. doi: 10.1089/jpm.2008.0078. [DOI] [PubMed] [Google Scholar]
- 30.Fryer-Edwards K, Arnold RM, Baile W, Tulsky JA, Petracca F, Back A. Reflective teaching practices: an approach to teaching communication skills in a small-group setting. Acad Med. 2006;81(7):638–44. doi: 10.1097/01.ACM.0000232414.43142.45. [DOI] [PubMed] [Google Scholar]
- 31.Back AL, Arnold RM, Baile WF, Fryer-Edwards KA, Alexander SC, Barley GE, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167(5):453–60. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 32.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 33.Flynn KE, Lin L, Ellis SJ, Russell SD, Spertus JA, Whellan DJ, et al. Outcomes, health policy, and managed care: relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J. 2009;158(4 Suppl):S64–71. doi: 10.1016/j.ahj.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative care (FACIT-Pal) scale. J Pain Symptom Manage. 2009;37(1):23–32. doi: 10.1016/j.jpainsymman.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bekelman DB, Parry C, Curlin FA, Yamashita TE, Fairclough DL, Wamboldt FS. A comparison of two spirituality instruments and their relationship with depression and quality of life in chronic heart failure. J Pain Symptom Manage. 2010;39(3):515–26. doi: 10.1016/j.jpainsymman.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 37.Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of Toolkit After-Death Bereaved Family Member Interview. J Pain Symptom Manage. 2001;22(3):752–8. doi: 10.1016/s0885-3924(01)00331-1. [DOI] [PubMed] [Google Scholar]
- 38.Abernethy AP, Kassner CT, Whitten E, Bull J, Taylor DH., Jr Death service ratio: a measure of hospice utilization and cost impact. J Pain Symptom Manage. 2011;41(6):e5–6. doi: 10.1016/j.jpainsymman.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Jr, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 40.Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N Engl J Med. 2013;369(15):1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 41.Hayden D, Pauler DK, Schoenfeld D. An estimator for treatment comparisons among survivors in randomized trials. Biometrics. 2005;61(1):305–10. doi: 10.1111/j.0006-341X.2005.030227.x. [DOI] [PubMed] [Google Scholar]


