Synopsis
Regenerative medicine has entered a rapid phase of discovery, and much has been learned in recent years about the lung’s response to injury. In this review, we first summarize the cellular and molecular mechanisms that damage the alveolar-capillary barrier, producing ARDS. We then turn our attention to the latest understanding of endogenous repair processes, highlighting the diversity of lung epithelial progenitor cell populations and their regulation in health and disease. Finally, we review the past, present and future of exogenous cell-based therapies for ARDS.
Keywords: ARDS, lung progenitor cells, MSCs
ARDS: Disruption of the Alveolar-Capillary Barrier
Acute Respiratory Distress Syndrome (ARDS) develops when the normal capacity of the alveoli to remain dry and participate in gas exchange is overwhelmed by a cascade of insults to the delicate alveolar-capillary barrier resulting in airspace fluid accumulation. In health, pulmonary capillary endothelial cells form a relatively tight membrane resistant to the paracellular movement of proteinacious fluid and inflammatory cells. This barrier depends on adherens junctions held together by VE-cadherin, as shown by studies specifically targeting this molecule with a metalloprotease.1 Endothelial adherens junctions can be disrupted by TNF-α, VEGF and other cytokines from activated leukocytes,2 as well as thrombin, complement activation, and toll-like receptor 4 signaling.3 In addition, lung endothelial cells can be damaged or killed by bacterial products,4 activated platelets,5,6 and neutrophils.7
Increased permeability of lung endothelium is necessary but not sufficient for the development of pulmonary edema. Clearance of extravasated fluid from the interstitial space by lymphatics is normally rapid.8 Similar to the lung endothelium, alveolar epithelial cells are joined together by tight junctions, but this barrier can be disrupted by toxic mediators from activated neutrophils9 or macrophages,10 pathogens including influenza,11 and excessive mechanical stretch.12 In addition, the alveolar epithelium is normally capable of actively transporting fluid from the alveolar lumen to the interstitial space as a final defense against alveolar flooding. The rate of alveolar fluid clearance can be increased by mild insults13 but has been shown to be reduced by high tidal volume mechanical ventilation, inflammatory cytokines, and infection.14 Not surprisingly, pathologic15 and clinical studies16 of patients with ARDS have revealed evidence of combined endothelial and epithelial dysfunction, including impaired alveolar fluid clearance. Furthermore, damage to alveolar type II cells along with extravasated plasma proteins and cellular debris disrupts the normal secretion and function of pulmonary surfactant.17
Endogenous Cell-Based Pathways for Recovery
General mechanisms of recovery
Returning the alveolus to a functional state is the obvious imperative for survival and recovery from ARDS. Repair and/or replacement of most damaged alveoli must occur in patients given the relatively mild pulmonary physiologic abnormalities measured in long-term survivors.18 The processes by which this occur remain largely unknown, but some key insights have been generated over the last several decades. Broadly speaking, there must be resolution of edema fluid, removal of inflammatory cells and debris, and repair of the structural integrity and function of the alveolar epithelium and lung endothelium.
As recovery begins, aided by the resolution of the triggering event and prevention of further mechanical injury with lung-protective ventilation, there is a shift away from pro-inflammatory signaling. Interleukin-10 (IL-10), secreted by CD4 T cells, macrophages, and dendritic cells, is present even early during acute inflammation and acts primarily on macrophages to reduce pro-inflammatory mediator secretion and antigen presentation while enhancing scavenger function and production of other anti-inflammatory molecules, such as IL-1 receptor antagonist (IL-1ra).19 Thus, IL-10 is thought to be critical in balancing pathogen clearance and tissue homeostasis. Its importance in this regard is highlighted by the existence of pathogen mimics such as Epstein-Barr virus encoded BCRF1.19 A subset of CD4 T-cells termed regulatory T cells (Tregs), major secretors of IL-10 in a variety of disease states,20 are present in the airspaces of patients with ARDS, and are critical in the resolution of endotoxin-induced acute lung injury in mice, in part through increasing the anti-inflammatory molecule TGF-β.21
IL-10, IL-1ra, and TGF- β notwithstanding, it had generally been thought that resolution of inflammatory injury occurs primarily due to the passive decline of dozens of pro-inflammatory mediators. More recently, however, a number of investigators have defined a new paradigm of active resolution of inflammation. A complex class of highly potent fatty-acid derivatives including lipoxins, resolvins, protectins, and maresins are now known to be generated during resolution. These lipid mediators bind to specific immune and resident cell receptors with high affinity and inhibit granulocyte recruitment and tissue activation, induce phagocytosis of apoptotic cells and bacteria, and aid in clearance of mucosal leukocytes.22 Apoptotic neutrophils and other cells are removed mostly by macrophages in a phagocytic process termed efferocytosis.23 Interestingly, the act of ingesting apoptotic cells is itself a stimulus to further anti-inflammatory signaling, helping to propel a feed-forward process of resolution.24
Endogenous lung progenitors
As the airspaces begin to clear, the damaged alveolar epithelium must replace lost cells and reform tight junctions. How this occurs is an active and controversial area of investigation, but has particular relevance to understanding the potential for stem and progenitor cell therapies in ARDS. As we move from proximal to distal in the lung, settled fact yields progressively to confusion and uncertainty, and so what follows is merely the current state of the evidence (see Table 1).
Table 1.
Summary of lung progenitor studies
| Reported stem/progenitor* |
Injury model | Injured cells | Finding | Ref |
|---|---|---|---|---|
| type II AECs in rats | nitrogen dioxide | AECs | electron microscopy and autoradiography suggested that type II AECs self-renew and produce type I AECs | 36 |
| type II AECs | bleomycin; targeted diphtheria toxin | type I & II AECs; type II AECs | SPC lineage tracing showed replacement of both types I and II AECs | 35 |
| fraction of type II AECs | hyperoxia | type I AECs | replacement of both types I and II AECs via EGFR/KRAS signaling | 37 |
| BASC expressing Scgb1a1 and SPC | naphthalene; bleomycin | clara cells; AECs | these cells at the junction of bronchioles and alveoli self-renewed and had multipotent differentiation in culture | 28 |
| BASC | naphthalene; hyperoxia | clara cells; terminal bronchioles and type I AECs | Scgb1a1 lineage tracing showed that BASCs replace airway but not alveolar epithelium | 29 |
| BASC | bleomycin | AECs | Scgb1a1 lineage tracing showed that BASCs produce types I and II AECs |
30 31 |
| basal cells | naphthalene | Clara cells | KRT5 lineage tracing showed basal cells self-renew and make new clara and ciliated cells in the airways | 26 |
| human c-kit expressing cells | cryo-injury | All epithelial cell types | c-kit+ cells engrafted into mouse lung and produce airways, alveoli, and blood vessels | 34 |
| P63-expressing bronchiolar cells | influenza | All epithelial cell types | massive wave of Krt-5 expressing cells appeared to migrate from airways and form new alveoli | 38 |
all murine unless otherwise specified
Abbreviations: AECs, alveolar epithelial cells; BASC, bronchioalveolar stem cell; Scgb1a1, secretoglobin 1A1; SPC, surfactant protein c; KRT5, keratin 5; EGFR, epidermal growth factor receptor.
Based primarily on studies in mice, there is now general agreement that in the large airways basal cells self-renew and produce both ciliated and secretory cell types following epithelial damage incurred by various insults, including acid and naphthalene.25,26 In the smaller intralobar airways, Clara cells, secretory cells that express secretoglobin 1a1 (Scgb1a1, or CCSP) can self-renew and also produce ciliated cells.27 The subset of Scgb1a1+ cells at the bronchiolalveolar duct junction (BADJ) that also express surfactant protein C (SPC) has been termed bronchioalveolar stem cells (BASCs). In 2005, Kim et al.28 reported that BASCs proliferated in situ following injury with naphthalene (kills Clara cells) or bleomycin (kills alveolar epithelial cells, AECs) and showed multipotency in clonal assays in vitro. Subsequently Rawlins and colleagues performed lineage tracing of cells expressing Scgb1a1 and reported no contribution to the alveolar epithelium following hyperoxia (which damages terminal bronchioles and alveoli).29 Interestingly, following bleomycin injury, cells expressing Scgb1a1 due indeed produce alveolar epithelial cells as reported by multiple investigators.30,31 This demonstrates, perhaps not surprisingly, that the injury model itself is crucial in identifying which progenitor populations become active and how repair occurs.
To add to the complexity, there are at least two other cell types that can reportedly produce alveolar epithelial cells:
Integrin α6β4-expressing alveolar cells (not expressing other known epithelial markers) generate airway and alveolar epithelia in vitro32 and impressively produce alveolar-like structures abutting vascular elements in lung “organoids” when implanted into the kidney capsule of adult mice.33
Kajstura and colleagues34 reported that c-kit expressing cells derived from adult human lungs and injected into a 2 mm2 region of mouse lung destroyed by cryo-injury produced airways, alveoli, and blood vessels bearing human lineage tracer; these results await confirmation.
Several other reports add to the theme of progenitor response being dependent upon injury-type. Barkausksas et al.35 found with lineage tracing that SPC-expressing type II AECs self-renew and produce types I and II AECs slowly during adult life but rapidly following specific ablation of type II AECs with diphtheria toxin. That type II AECs could repopulate alveoli had been suspected since the 1970s36 but these results provided the best evidence to date. Similarly, Desai and collleagues37 recently reported that type II AECs repopulate alveoli slowly during healthy adulthood but rapidly after hyperoxia-mediated alveolar injury.
In contrast to these relatively mild, mostly alveolar-specific injuries, Kumar et al.38 reported that H1N1 influenza in mice induced massive areas of lung destruction followed by the appearance of p63+, keratin-5+ (Krt5) pods of cells that appeared to migrate from airways into injured lung parenchyma and potentially give rise to new alveoli, though the ultimate fate of these cells has not yet been determined convincingly by lineage tracing. This phenomenon had not been reported following the comparatively milder injury models in common use and demonstrates that lung progenitor populations may respond in a graded fashion to injury.
Coordination of endogenous progenitor responses
With such flexibility in the response of lung epithelial progenitors, two recent reports deserve special attention because they illustrate potentially important regulatory mechanisms. In 2011, Ding and colleagues39 performed pneumonectomies (PTX) on adult mice, and reproduced the finding that the intact lobes of the lung undergo rapid expansion with apparent formation of new alveoli.40 By flow analysis, proliferating epithelial cells 3 days post-PTX were similar phenotypically to BASCs. Interestingly, disrupting VEGF signaling only within pulmonary endothelium blocked the epithelial progenitor response. In a series of elegant experiments, the authors showed that VEGF signaling in lung endothelium triggers the production of matrix metalloproteinase 14, which in turn releases EGF-receptor ligands that drive epithelial progenitor proliferation and alveologenesis.
Lee et al.41 cocultured single BASCs with primary lung or liver endothelial cells and found that only lung endothelia supported BASC multilineage differentiation into airway and alveolar epithelial cells. Thrombospondin 1 (Tsp1), an inhibitor of angiogenesis expressed developmentally during alveolization,42 was found to be central to this supportive role as mice deficient in this molecule had impaired epithelialization of airways (following naphthalene) and of alveoli (following bleomycin). Remarkably, alveolar repair in Tsp1 knockout mice could be rescued by the conditioned media of primary lung endothelial cells. Taken together, these results suggest an important role of the lung vasculature in guiding the expansion and differentiation of epithelial progenitors, similar to what is thought to occur in lung development43 as well as in other adult tissues harboring multipotent progenitors, including the brain44 and bone marrow.45 Given the intricate structural and functional relationships between alveoli and lung capillaries required for effective gas exchange, this interaction during repair is not surprising. Clearly, further insights into how these vascular and epithelial processes are coordinated will be important in optimizing endogenous lung repair and in developing exogenous repair strategies.
Enhancement of epithelial and endothelial barrier function
Once reconstituted as a tight membrane, the alveolar epithelial barrier can resume effective active edema fluid transport and clearance as well as surfactant secretion. Although little is known about endogenous mechanisms controlling epithelial barrier tightening, several key signaling pathways are now known to regulate endothelial barrier function. Garcia and colleagues have discovered an important role for the sphingolipid sphingosine-1-phosphate (S1P) in rapidly enhancing lung endothelial barrier function by altering the cytoskeleton to increase cell overlap, and inducing adherens and tight junction assembly.46 S1P or its synthetic analogs have shown therapeutic efficacy in murine47 and canine48 models of endotoxin-induced acute lung injury, ischemia-reperfusion,49 radiation-induced lung injury,50 and influenza.51 Angiopoeitin-1 is produced by a variety of cells and acts on endothelial Tie2 receptors to promote barrier integrity.52 Adrenomedullin binds calcitonin receptor-like receptor on lung endothelial cells and promotes intercellular adherence.53 Administration of adrenomedullin improves endothelial barrier function in rodent models of ventilator-induced54 and endotoxin mediated lung injury.55 Finally, London and colleagues56 reported that Slit acts on lung endothelial Robo4 receptors to reduce vascular leakage in response to intratracheal endotoxin and H5N1 influenza, likely by promoting VE-cadherin expression.
Exogenous cell-based pathways for recovery
As many of the mechanisms of lung injury have been worked out over the last several decades, researchers have tested a variety of targeted pharmacologic interventions in patients with ARDS, including anti-oxidants, beta-agonists, surfactant, and IL-10.57 However, the results have been uniformly disappointing, probably in part because ARDS is heterogeneous and is characterized by multiple injurious cascades operating simultaneously. Mortality has declined as lung protective ventilation and fluid management strategies have been implemented,58 and additional clinical benefits from paralysis59 and prone positioning60 may improve outcomes further. Nevertheless, there remains a compelling need to develop therapies that directly target the complex pathophysiology of ARDS. Exogenous cell-based therapies may hold special promise in this regard, as recent research has shown they are capable of affecting multiple pathways of lung injury and repair.
Endothelial progenitor cells (EPCs)
Given the derangement of endothelial barrier function known to characterize ARDS, these cells have intuitive appeal as a potential therapy. EPCs were originally described in the late 1990s as circulating CD34+ cells that differentiated into endothelial cells in vitro and localized to sites of angiogenesis in adult animals.61 In 2005, Yamada et al.62 found that circulating EPCs were increased in patients with bacterial pneumonia, and that lower EPC counts were associated with persistent lung fibrosis following pneumonia resolution. Burnham and colleagues63 then isolated EPCs in patients with ARDS, finding that the number of EPC colonies predicted improved survival. In 2008, Lam et al.64 reported that administering autologous EPCs to rabbits 30 minutes following oleic acid injury improved endothelial barrier function and reduced lung edema, hemorrhage, and inflammation. Mao and colleagues65 treated rats with autologous EPCs or saline 30 minutes after intravenous endotoxin, finding that EPC-treated rats had improved survival, reduced lung edema, and increased IL-10. Interestingly, there was evidence of modest engraftment into the injured lung endothelium up to 14 days later. Such engraftment may be model-specific, however, as these cells do not appear to contribute to lung endothelial expansion after pneumonectomy.66 Autologous EPCs are now the subject of clinical trials in cirrhosis (NCT01333228), ischemic stroke (NCT01468064), and critical limb ischemia (NCT01595776). However, given that EPCs circulate at low levels, autologous transplantation is unlikely to be an option in the acute phase of ARDS, and the safety of allogeneic EPC transplantation remains unknown.
Mesenchymal stem/stromal cells (MSCs)
In contrast to EPCs, MSCs are relatively immunopriveleged and known to be well-tolerated after allogeneic transplantation.67 These cells were first described in the 1960s as plastic-adherent, spindlelike cells, that can be isolated from bone marrow, fat, umbilical cord blood, placenta, and connective tissues.68 Although defined in part by the capacity to differentiate into osteoblasts, chondroblasts, and adipocytes, the overwhelming balance of evidence is that they rarely integrate and survive long-term in adult tissues after allogeneic transplantation.69 They have been studied extensively in models of acute inflammation in many different organ systems, and have been found to have remarkable therapeutic effects across a range of murine models of acute lung injury, including bleomycin,70 intratracheal71–73 or intraperitoneal74 endotoxin, cecal ligation and puncture,75,76 pseudomonal abdominal sepsis,77 and E-coli pneumonia.78 Recently, human bone marrow derived MSCs were shown to reduce inflammation and improve alveolar fluid clearance in ex vivo human lungs injured with live E-coli.79 At least some of their therapeutic properties can be recapitulated by the microvesicles they actively secrete in culture.80–82
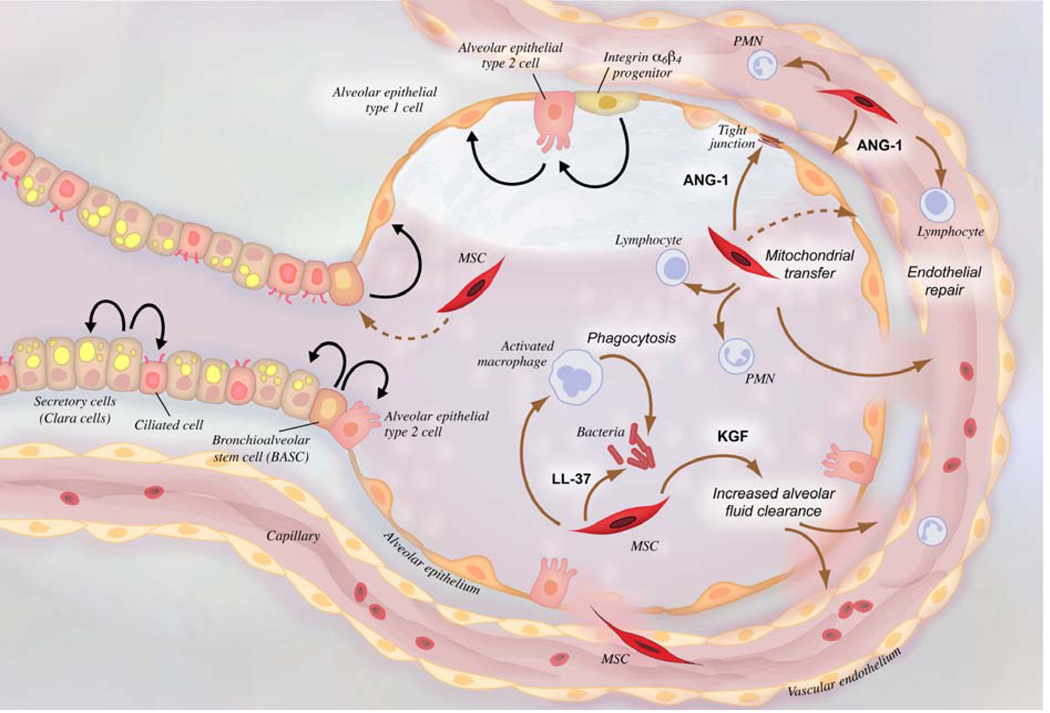
MSCs are thought to work by multiple mechanisms in these models (Fig. 2), including (a) reducing alveolar-capillary barrier permeability,72,75,76,83 in part by secretion of angiopoietin-1,84 (b) increasing alveolar fluid clearance, at least in part by secretion of keratinocyte growth factor,79,85 (c) shifting cytokines and resident macrophages from pro- to anti-inflammatory,86 (d) improving bacterial clearance by enhancing phagocytosis and secreting antibacterial peptides,77,78,83 and remarkably (e) transferring mitochondria to alveolar epithelial cells, rescuing ATP generation.73
Figure 2.
Schematic of an injured alveolus and adjacent alveolar duct. Potential mechanisms of MSC therapeutic effects in ARDS are shown with brown arrows. Black arrows depict lineage relationships during cell turnover. Abbreviations: MSC, mesenchymal stem cell; PMN, polymorphonuclear leukocyte; ANG-1, angiopoietin-1; LL-37, cathelicidin; KGF, keratinocyte growth factor.
Another intriguing possible mechanism has come to light recently. When postnatal rodents are exposed to high oxygen concentrations, they develop pulmonary hypertension due to a dramatic simplification of lung architecture, modeling human bronchopulmonary dysplasia (BPD). In 2009, MSCs were shown to largely normalize lung capillary and alveolar growth when given by airway or blood in mouse and rat BPD models, but without any evidence of significant engraftment.87,88 Interestingly, Tropea and colleagues30 reported in 2012 that MSCs increased BASCs in the BPD model by a paracrine mechanism. This work suggests that MSCs, like lung endothelium, may help orchestrate epithelial progenitor responses to injury. Indeed, in other experimental systems, MSCs have been shown to interact with endothelial cells to establish a hematopoietic microenvironment after heterotopic transplantation,89 to increase the proliferation and survival of hippocampal neural progenitors,90 and to increase c-kit+ cardiac stem cells in a porcine model of myocardial infarction.91
With this in mind, there is considerable optimism following the recently completed Korean phase 1 clinical trial of MSCs in neonates at high risk of BPD.92 In this dose-escalation study, 9 patients with mean gestational age of 25 weeks (mean birth weight 790 g) received either 1×107 or 2×107 allogeneic umbilical cord blood-derived MSCs/kg by airway at an average of 10 days after birth. There were no adverse events, and although the study was not designed to test efficacy, BPD severity appeared lower in treated patients than in a matched comparison group. A two year follow-up study of these patients is planned (NCT01632475).
Given the encouraging preclinical results from MSCs in rodent, sheep (Asmussen et al., in revision at Thorax) and ex vivo perfused human lung models of acute lung injury, an NHLBI-supported phase 1/2 (NCT01775774/NCT02097641) clinical trial of bone-marrow derived allogeneic MSCs in patients with moderate to severe ARDS is now underway. START (STem cells for ARDS Treatment) targets a total enrollment of 69 patients, 9 in phase 1, and 60 in phase 2. In phase 1, three cohorts of patients received 1, 5, or 10 × 106 cells/kg intravenously, and there were no significant adverse events at the highest dose. In phase 2, patients will be randomized 2:1 to receive 10 × 106 MSCs/kg or plasmalyte control. The primary endpoint of Phase 2 will be safety, but secondary endpoints will include the lung injury score, PaO2/FiO2, oxygenation index, SOFA score at day 3, ventilator-free days, 60 day mortality, plasma biomarkers of lung epithelial injury, endothelial injury, and inflammation, and protein in a mini-bronchoalveolar lavage at 48 hours.
Barriers to developing cell-based therapies in patients with ARDS
As with all trials in critical care, the complex nature of the patients and the importance of logistical speed pose formidable hurdles in the design and implementation of exogenous cell-based therapies for ARDS. Beyond these difficulties, additional challenges, many summarized in a recent report from an NIH-NHLBI workshop,93 include:
Ensuring consistent cell therapy product. This involves strict screening of donors and Good Manufacturing Practice for sterility, use of animal derived products, and passage number.
Quality control. Standard testing for cultured cell products includes screening for bloodborne pathogens, post-thaw cell viability, bacterial endotoxin, and cytogenetics.
Assessing potency. Ideally a rapid, simple, reliable assay that can be run at multiple sites and correlates well with observed therapeutic effects. This is a challenging but critical barrier to up-scaling any new promising cell-based therapy for widespread use (as for phase 3 trials and beyond); an effective potency assay is essential in fine-tuning donor selection, manufacturing processes, and final preparation of the product.
Developing pre-clinical (animal) data that adequately mirror the phase 1 safety studies required by the FDA. This might require a shift in mindset for basic science investigators, as it requires careful attention to logistics including cell storage, shipment, packaging, freezing and thawing procedures (including method of cryoprotection), dilution, washing, and method and speed of administration. In addition, supportive animal studies must follow Good Laboratory Practice (GLP) guidelines.
Determining the optimal dosage, route, and timing of cell delivery. Intratracheal administration bypasses the vasculature and theoretically offers more direct access to injured lung tissue,92 though may pose additional hazards to gas exchange in the acute and often dynamic respiratory failure that characterizes ARDS.
Careful consideration should be given to employing large animal models given that such studies may (a) provide important additional information on efficacy, (b) permit monitoring of salient physiological safety endpoints, especially during and immediately after cell administration.
Filing an Investigational New Drug application (IND). For this challenging process, it is helpful to elicit initial feedback from the FDA94 during the planning stages of the animal experiments, and to obtain institutional support for the writing and submission of this highly technical document.
Remaining questions and research priorities
Regenerative medicine has entered an era of intense discovery, as evidenced by exponential growth in clinical trials targeting a wide range of human diseases with cellular therapies; this promise holds true for those of us engaged in developing better therapies for ARDS. However, key questions remain, and many obstacles may yet prevent effective cell-based therapies from becoming a reality.
Understanding the endogenous response
The importance of the type of injury in determining the endogenous progenitor cell response (highlighted in detail above) cannot be overstated. Going forward, it will be increasingly important to utilize clinically relevant models of lung injury, including bacterial and viral pneumonia, which together account for the majority of cases of ARDS.95 These models are challenged by a tendency to be variable in the severity of lung injury, to cause severe systemic illness, and to produce a robust immune response with a complex cellular infiltrate, all of which complicate the kinds of lineage tracing studies that are now the accepted scientific standard.
Looking forward, we must advance the level of evidence of endogenous repair from a simple qualitative demonstration of expression of mature epithelial markers to a richer anatomical and temporal understanding of how newly formed epithelial-lined structures interface with lung capillaries and become (or fail to become) functional alveolar units. It may well be that simultaneous mechanisms are at work, including the diffuse growth of uninjured lung (as occurs following pneumonectomy39) and the more dramatic progenitor migratory events reported after influenza.38 Insights generated from this research have obvious relevance for ARDS but also hold promise for improving our understanding of endogenous repair processes in diseases as diverse as COPD and interstitial lung disease.
Safety of exogenous cell-based therapies
The perils of developing new classes of therapies for patients are well-known from gene therapy trials in the 1990s.96 Potential complications of allogeneic cell therapy include infections (due to contaminated product, immunomodulation, or even zoonoses, as prions can theoretically be carried by cell culture reagents), worsened inflammation from immune rejection of transplanted cells, and for intravenous administration, embolic load on the right ventricle (since most cells deposit at least temporarily in the lung97). A recent review98 reported that MSC therapy in children and adults with left heart failure, myocardial infarction, spinal cord injury, stroke, hematologic malignancies, and Crohn’s disease appears to be safe with only transient fever being occasionally noted. Given the concern for possible embolic insult to a pulmonary vasculature and right heart already stressed from the acute hypoxemia of ARDS, it is reassuring that a recent placebocontrolled RCT of MSCs in moderate to severe COPD99 showed no acute changes in hemodynamics or oxygenation, and no measurable difference in diffusing capacity, ambulatory oxygenation saturation, or echocardiographic estimate of right-sided pressures through two-years of follow-up. The recent phase 1 data from MSC administration by airway to neonates at risk for BPD92 and intravenously to patients with moderate to severe ARDS (NCT01775774) are similarly reassuring.
Neoplasia is another significant safety concern. In the MSC literature, there is some evidence that murine bone marrow-derived MSCs have genetic instability even at low passage number, with reports of tumor formation following intravenous administration in models of myocardial infarction and diabetic neuropathy.100 Fortunately, this appears to be unique to murine MSCs, as human MSCs cultured for prolonged periods do not appear to transform.101 A review of over 500 large animals treated with MSCs for therapy of myocardial infarction failed to reveal any evidence of malignancy out to 3 months.102 Finally, autopsy material from heavily immunosuppressed patients who had previously received allogeneic MSCs revealed minimal long-term engraftment and no evidence of ectopic tissue formation.103
“Off-target” effects
Given that ARDS is frequently associated with multiorgan failure, might exogenous cell therapies prove to have favorable effects outside the lung, for example in acute kidney injury104 or sepsis?105 Ideally, trial design for cell therapy in critical illness will incorporate clinical and biological endpoints that can help inform both efficacy and mechanism of action across related organ systems and scientific disciplines. Could cell therapies that effectively dampen the acute inflammatory response in ARDS unexpectedly impair the endogenous lung repair processes highlighted above? Or might they accelerate them? These and other questions should become more tractable in coming years as more data from clinical trials become available, and as the basic science research toolkit continues to expand.
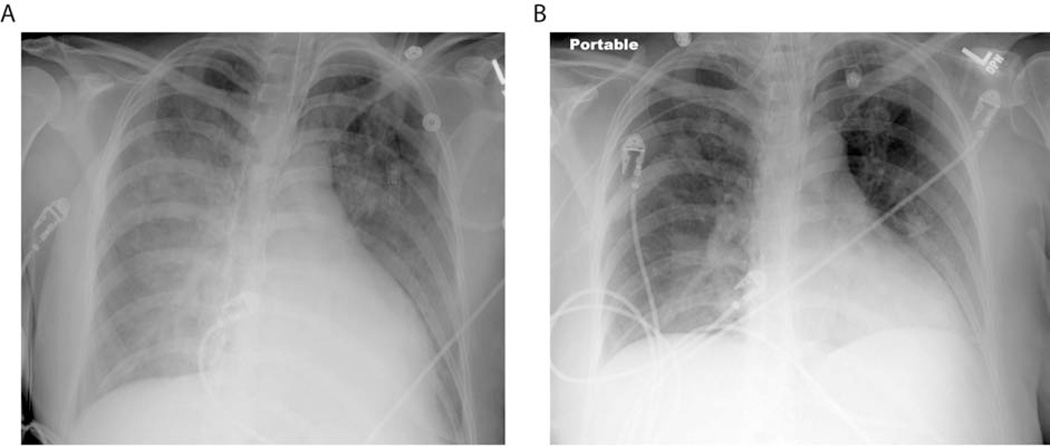
Figure 1.
Resolution of ARDS. Typical chest radiography findings in a patient with ARDS include patchy bilateral airspace opacities (A). During resolution, these changes improve significantly (B).
Table 2.
Challenges to the implementation of exogenous cell-based therapies for ARDS
| Potential barrier | Details |
|---|---|
| consistency of the cell product | cell handling, passage number, reagents (especially animal-derived) |
| quality control | screening for bloodborne pathogens, endotoxin limits, viability after thaw, cytogenetics |
| potency | assay should be simple, fast, reliable, and predictive of therapeutic effects in patients |
| IND-enabling animal data | GMP and GLP practices, standardized procedures for shipping, storing, freezing, thawing, diluting, washing, and administering; use of large animal models |
| best delivery route | airway or intravenous |
| IND submission | highly technical and labor intensive |
Abbreviations: IND, investigational new drug; GMP, good manufacturing practice; GLP, good laboratory practice.
Key Points.
ARDS occurs when protein-rich fluid accumulates in the airspaces due to a breakdown of the alveolar capillary barrier following endothelial and epithelial damage and dysfunction.
Endogenous lung progenitor populations are mobilized differentially in various animal models of lung injury.
Exogenous cell therapies for ARDS hold substantial promise for improving upon the endogenous response, and clinical trials are ongoing.
Acknowledgements
This work was supported by R37HL51856, R01HL51854, and F32HL117549. We thank Diana Lim for her excellent assistance preparing Fig. 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant financial disclosures.
References
- 1.Schulz B, Pruessmeyer J, Maretzky T, et al. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102(10):1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19(1):8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Müller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener-Kronish JP, Pittet J-F. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32(2):228–235. doi: 10.1055/s-0031-1275535. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JT, Gozal E, Roberts AM. Platelet-mediated vascular dysfunction during acute lung injury. Arch Physiol Biochem. 2012;118(2):72–82. doi: 10.3109/13813455.2012.665463. [DOI] [PubMed] [Google Scholar]
- 6.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheiermann C, Kunisaki Y, Jang J-E, Frenette PS. Neutrophil microdomains: linking heterocellular interactions with vascular injury. Curr Opin Hematol. 2010;17(1):25–30. doi: 10.1097/MOH.0b013e328333d2a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiener-Kronish JP, Broaddus VC, Albertine KH, Gropper MA, Matthay MA, Staub NC. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J Clin Invest. 1988;82(4):1422–1429. doi: 10.1172/JCI113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40(5):519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1191–L1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 11.Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 12.Davidovich N, DiPaolo BC, Lawrence GG, Chhour P, Yehya N, Margulies SS. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am J Respir Cell Mol Biol. 2013;49(1):156–164. doi: 10.1165/rcmb.2012-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garat C, Rezaiguia S, Meignan M, et al. Alveolar endotoxin increases alveolar liquid clearance in rats. J Appl Physiol Bethesda Md 1985. 1995;79(6):2021–2028. doi: 10.1152/jappl.1995.79.6.2021. [DOI] [PubMed] [Google Scholar]
- 14.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol. 2006;35(1):10–19. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 17.Zasadzinski JA, Stenger PC, Shieh I, Dhar P. Overcoming rapid inactivation of lung surfactant: analogies between competitive adsorption and colloid stability. Biochim Biophys Acta. 2010;1798(4):801–828. doi: 10.1016/j.bbamem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 19.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol. 2012;40(2):186–204. doi: 10.1177/0192623311430693. [DOI] [PubMed] [Google Scholar]
- 21.D’Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10):2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen WJ, Henson PM. Cellular regulation of the inflammatory response. Toxicol Pathol. 2012;40(2):166–173. doi: 10.1177/0192623311428477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wansleeben C, Barkauskas CE, Rock JR, Hogan BLM. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2(1):131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 26.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161(1):173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CFB, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tropea KA, Leder E, Aslam M, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107(4):1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman HA, Li X, Alexander JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121(7):2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anversa P, Kajstura J, Leri A, Loscalzo J. Tissue-specific adult stem cells in the human lung. Nat Med. 2011;17(9):1038–1039. doi: 10.1038/nm.2463. [DOI] [PubMed] [Google Scholar]
- 35.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- 37.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar PA, Hu Y, Yamamoto Y, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding B-S, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowan MJ, Crystal RG. Lung growth after unilateral pneumonectomy: quantitation of collagen synthesis and content. Am Rev Respir Dis. 1975;111(3):267–277. doi: 10.1164/arrd.1975.111.3.267. [DOI] [PubMed] [Google Scholar]
- 41.Lee J-H, Bhang DH, Beede A, et al. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell. 2014;156(3):440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sozo F, Hooper SB, Wallace MJ. Thrombospondin-1 expression and localization in the developing ovine lung. J Physiol. 2007;584(Pt 2):625–635. doi: 10.1113/jphysiol.2007.138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thébaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology. 2007;91(4):291–297. doi: 10.1159/000101344. [DOI] [PubMed] [Google Scholar]
- 44.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14(11):1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colmone A, Sipkins DA. Beyond angiogenesis: the role of endothelium in the bone marrow vascular niche. Transl Res J Lab Clin Med. 2008;151(1):1–9. doi: 10.1016/j.trsl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Abbasi T, Garcia JGN. Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handb Exp Pharmacol. 2013;(216):201–226. doi: 10.1007/978-3-7091-1511-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43(4):394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szczepaniak WS, Zhang Y, Hagerty S, et al. Sphingosine 1-phosphate rescues canine LPS-induced acute lung injury and alters systemic inflammatory cytokine production in vivo. Transl Res J Lab Clin Med. 2008;152(5):213–224. doi: 10.1016/j.trsl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okazaki M, Kreisel F, Richardson SB, et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2007;7(4):751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 50.Mathew B, Jacobson JR, Berdyshev E, et al. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25(10):3388–3400. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319(9):1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, et al. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol. 2006;126(3):305–316. doi: 10.1007/s00418-006-0174-5. [DOI] [PubMed] [Google Scholar]
- 54.Müller HC, Witzenrath M, Tschernig T, et al. Adrenomedullin attenuates ventilator-induced lung injury in mice. Thorax. 2010;65(12):1077–1084. doi: 10.1136/thx.2010.135996. [DOI] [PubMed] [Google Scholar]
- 55.Itoh T, Obata H, Murakami S, et al. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L446–L452. doi: 10.1152/ajplung.00412.2005. [DOI] [PubMed] [Google Scholar]
- 56.London NR, Zhu W, Bozza FA, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2(23):23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 60.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 61.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 62.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60(5):410–413. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172(7):854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 64.Lam C-F, Liu Y-C, Hsu J-K, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology. 2008;108(3):392–401. doi: 10.1097/ALN.0b013e318164ca64. [DOI] [PubMed] [Google Scholar]
- 65.Mao M, Wang S-N, Lv X-J, Wang Y, Xu J-C. Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010;34(2):196–204. doi: 10.1097/SHK.0b013e3181d49457. [DOI] [PubMed] [Google Scholar]
- 66.Voswinckel R, Ziegelhoeffer T, Heil M, et al. Circulating vascular progenitor cells do not contribute to compensatory lung growth. Circ Res. 2003;93(4):372–379. doi: 10.1161/01.RES.0000087643.60150.C2. [DOI] [PubMed] [Google Scholar]
- 67.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 68.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. [PubMed] [Google Scholar]
- 69.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14(9):2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol Baltim Md 1950. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 72.Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4(9):e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 75.Mei SHJ, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 76.Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med Berl Ger. 2010;88(1):5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krasnodembskaya A, Samarani G, Song Y, et al. Human Mesenchymal Stem Cells Reduce Mortality and Bacteremia in Gram Negative Sepsis in Mice in Part by Enhancing the Phagocytic Activity of Blood Monocytes. Am J Physiol Lung Cell Mol Physiol. 2012 doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y-G, Feng X-M, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Dayt Ohio. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol JASN. 2009;20(5):1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sdrimas K, Kourembanas S. MSC Microvesicles for the Treatment of Lung Disease: A New Paradigm for Cell-Free Therapy. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells Dayt Ohio. 2010;28(12):2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285(34):26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prockop DJ, Youn Oh J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol Ther J Am Soc Gene Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180(11):1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 90.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. J Pediatr. doi: 10.1016/j.jpeds.2013.12.011. (In press). [DOI] [PubMed] [Google Scholar]
- 93.Matthay MA, Anversa P, Bhattacharya J, et al. Cell therapy for lung diseases. Report from an NIH-NHLBI workshop, November 13–14, 2012. Am J Respir Crit Care Med. 2013;188(3):370–375. doi: 10.1164/rccm.201303-0522WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feigal EG, Tsokas K, Zhang J, Cromer MV, Whittlesey KJ, Werner MJ. Perspective: communications with the Food and Drug Administration on the development pathway for a cell-based therapy: why, what, when, and how? Stem Cells Transl Med. 2012;1(11):825–832. doi: 10.5966/sctm.2012-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matthay MA, Zemans RL. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu Rev Pathol. 2010 doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Couzin J, Kaiser J. Gene therapy. As Gelsinger case ends, gene therapy suffers another blow. Science. 2005;307(5712):1028. doi: 10.1126/science.307.5712.1028b. [DOI] [PubMed] [Google Scholar]
- 97.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lalu MM, McIntyre L, Pugliese C, et al. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PloS One. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeong J-O, Han JW, Kim J-M, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108(11):1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hatzistergos KE, Blum A, Ince T, Grichnik JM, Hare JM. What is the oncologic risk of stem cell treatment for heart disease? Circ Res. 2011;108(11):1300–1303. doi: 10.1161/CIRCRESAHA.111.246611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van der Spoel TIG, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of preclinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91(4):649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 103.Von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells Dayt Ohio. 2012;30(7):1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 104.Erpicum P, Detry O, Weekers L, et al. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014 doi: 10.1093/ndt/gft538. [DOI] [PubMed] [Google Scholar]
- 105.Kusadasi N, Groeneveld ABJ. A perspective on mesenchymal stromal cell transplantation in the treatment of sepsis. Shock. 2013;40(5):352–357. doi: 10.1097/SHK.0000000000000039. [DOI] [PubMed] [Google Scholar]