Abstract
Elucidating the gene regulatory networks that control kidney development can provide information about the origins of renal birth defects and kidney disease, as well as insights relevant to the design of clinical interventions for these conditions. The kidney is composed of functional units termed nephrons. Renal malfunction often arises from damage to cells known as podocytes, which are highly specialized epithelial cells that comprise the blood filter, or glomerulus, located on each nephron. Podocytes interact with the vasculature to create an elaborate sieve that collects circulatory fluid, and this filtrate enters the nephron where it is modified to produce urine and balance water homeostasis. Podocytes are an essential cellular component of the glomerular filtration barrier, helping to protect nephrons from the entry of large proteins and circulatory cells. Podocyte loss has catastrophic consequences for renal function and overall health, as podocyte destruction leads to nephron damage and pathological conditions like chronic kidney disease. Despite their importance, there is still a rather limited understanding about the molecular pathways that control podocyte formation. In recent years, however, studies of podocyte development using the zebrafish embryonic kidney, or pronephros, have been an expanding area of nephrology research. Zebrafish form an anatomically simple pronephros comprised of two nephrons that share a single blood filter, and podocyte progenitors can be easily visualized throughout the process of glomerular development. The zebrafish is an especially useful system for studying the mechanisms that are essential for formation of nephron cell types like podocytes due to the high genetic conservation between vertebrate species, including humans. In this review, we discuss how research using the zebrafish has provided new insights into the molecular regulation of the podocyte lineage during kidney ontogeny, complementing contemporary research in other animal models.
Keywords: Kidney, Podocyte, Glomerulus, Nephron, Pronephros, Zebrafish, Retinoic acid, Wt1a, foxc1a, rbpj, Wt1, FoxC2, Notch
Introduction
Kidney structure and function
The kidneys are important organs with a set of physiological roles that are essential for life [1]. Kidneys are made up of functional units called nephrons that are epithelial tubes responsible for producing urine and balancing water and salt levels [1]. One central task of the renal system is to interact with the vascular system to collect plasma from the bloodstream in order to excrete metabolic waste from the body [1]. To accomplish this task, nephrons are comprised of unique working parts located along their length. Typically, nephrons have a blood filter on one end, a duct at the opposite end that drains the urine, and an intervening segmented tubule in which domains of different cell types progressively modify the filtrate and thereby regulate solute reabsorption and secretion [1].
During development, vertebrates form up to three kidney structures of increasing complexity: the pronephros, the mesonephros, and metanephros [2]. In higher vertebrate species like mammals, the pronephros is a vestigial structure that exists transiently in the developing embryo, while the subsequent kidney forms become functional during juvenile and/or adult stages [2,3]. Other vertebrates like fish and amphibian species form only two kidney structures, and the pronephros is functional until a mesonephros is formed in juvenile stages [2,3]. Common among all of these vertebrate kidney iterations is a structural composition based on nephrons [2,3]. In addition, each subsequent renal structure that forms within any given species is more complex due to the increasing number of nephrons they contain, as well as the overall anatomical organization of nephrons. For example, early kidney structures are often comprised of parallel rows of nephrons that drain into a common duct, while later organ variations typically have branched or otherwise arborized arrays of nephrons [3].
Progress in understanding renal cell type development can be used to gain valuable medical insights for the nephrology field. Applications of such knowledge include the design of novel therapeutic approaches for kidney birth defects, as well as new interventions to treat or prevent acute and chronic kidney diseases. Defects that arise in the blood-filtering unit of the nephron, known as the glomerulus, are a prevalent initiating event common to many renal conditions [4–6]. Proper glomerulus development and maintenance of glomerular structural-functional integrity are crucial for nephron health and overall organ fitness. The glomerulus is the site where fluid is collected from the circulation using an intricate molecular sieve [7]. If this sieve is compromised, large proteins/protein complexes and circulating cells leak into the nephron tubule, a condition termed proteinuria. Proteinuria can cause damage that culminates in attrition of the entire nephron, and can initiate a vicious chronic cycle of inflammation and renal fibrosis that spreads within the kidney [4–6]. Thus, as the matrix of components at the glomerulus selectively gathers blood plasma, it serves a vital barrier function to protect the downstream components of the nephron and stave off chronic injury.
The glomerular blood filter apparatus and roles of podocyte cells
The glomerulus is made up of unique cellular and extracellular matrix materials [7]. The renal epithelial cells located at this apparatus are called podocytes. Podocytes intimately surround the capillary tuft, forming a cellular fence that restricts fluid egress from the circulation into the nephron. Podocytes extend foot processes from their basal surface that interdigitate with the foot processes of adjacent podocytes, forming unique cell-to-cell junctions called the slit diaphragm. The interlocking network of podocytes, with these slit diaphragm junctions, are an important physical restriction on the entry of large macromolecules and hematopoietic cells into the nephron. Between the epithelial podocytes and the fenestrated endothelial cells that comprise the capillary loops is a thick Glomerular Basement Membrane (GBM) that is assembled from secreted protein contributions of each flanking cell type [8]. Together, these sandwiched layers of cells and the intervening basement membrane form an elaborate selective barrier to the entering filtrate. Genetic defects that lead to structural disruptions in any of these components can alter the integrity of the entire structure, causing proteinuria that leads to kidney disease [4–6]. There has been tremendous progress in identifying how defects in particular proteins disrupt the structure of the blood filter (both in extracellular (GBM) and cellular compartments) and trigger pathological conditions [4–6]. Nevertheless, many aspects of glomerular cell biology remain poorly understood [7], such as the identity of the genes that specify the podocyte lineage during nephrogenesis.
Animal models of podocyte development
To date, insights into the genetic pathways that regulate podocyte development have been made through research using kidney structures in various vertebrate animal models, such as the mouse [9], due to the broad conservation of genes that exists between these species and humans. The zebrafish, Danio rerio, is a small, tropical freshwater fish that has been widely used in research to study aquatic pollutants [10] and has come to the forefront of current biomedical research [11,12] due to their substantial conservation with humans and other vertebrate species [13]. Kidney research using the zebrafish has steadily expanded in recent years due to the features of this model that enable complementary research pursuits to work in other vertebrate model systems [14–17]. The zebrafish embryo utilizes a functional pronephros, which is an anatomically simple structure comprised of two nephrons that share a common glomerulus [14–16]. The nephrons develop quickly during embryogenesis: they form and begin blood filtration during the first two days of life, thus enabling rapid interrogation of gene function tests by loss and gain of function approaches [14–16]. Further, zebrafish nephrons have a segmental organization and contain numerous cell types that are quite similar to mammals, including podocytes as well as proximal and distal tubule segment epithelial cells [17,18].
Due to these fundamental parallels, the zebrafish is a useful model organism to delineate the regulatory networks that are responsible for directing formation of several different nephron cell types [14–22], perhaps first and foremost being podocytes. Zebrafish podocytes exhibit morphological and gene expression characteristics that are very similar with mammalian podocytes [17–21]. The structural simplicity of the zebrafish pronephros provides the benefit of being able to label and visually track podocytes during development (Figure 1) [16]. Furthermore, integrity of the glomerulus can be assessed in the zebrafish embryo, which has facilitated the systematic assessment of factors that are essential to form or maintain the filtration barrier through the utilization of gene knockdown tools (like morpholinos) and gain of function studies (such as cDNA over-expression) [16]. Thus, the continued study of podocyte development using zebrafish has immense potential.
Figure 1.
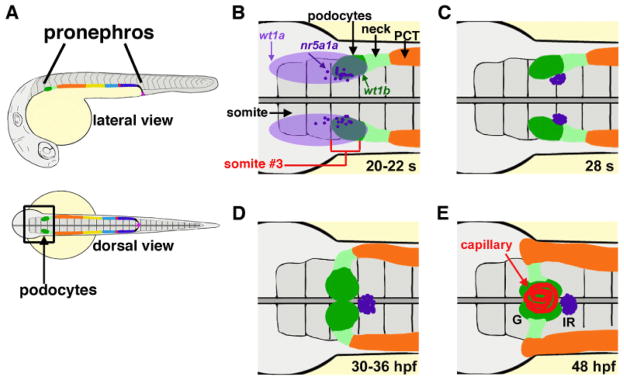
Glomerular development in the zebrafish. (A) The zebrafish pronephros is comprised of a pair of segmented nephrons, and podocytes (dark green) occupy the rostral-most position. (B–E) Anatomy of the cell types that develop in proximity to podocytes over development: (B) 20–22 somite stage; (C) 28 somite stage; (D) 30–36 hours post fertilization; (E) 48 hours post fertilization. Gene expression of wt1a (light purple) is broad, while wt1b transcripts (dark green) are restricted next to somite (s) three, and interrenal precursors marked by nr5a1a transcripts are interspersed in this region. The neck (light green) is located caudal to the podocytes, followed by the proximal convoluted tubule (PCT, orange). Morphogenesis of these populations is progressive from the 20 s stage through to 48 hours post fertilization (hpf), when the podocytes have migrated to the midline and recruited capillaries to form a single glomerulus (G). The interrenal gland (IR) is situated just caudal to the glomerulus. (Images adapted from Ref [16] with author rights).
To date, a number of studies have provided insights on the requirements for normal podocyte and/or glomerular development in zebrafish [18–31]. Here, we discuss in depth several contemporary research studies that have used the zebrafish pronephros to elucidate new information about the molecular regulation of podocyte formation during nephrogenesis, with the central focus on a recent study that analyzed the genetic interactions and biochemical activities of the Wilms’ tumor suppressor-1 (Wt1) gene during podocyte differentiation.
Podocyte conservation among vertebrates and the regulation of wt1a/Wt1 homologs by RA signaling
Glomerular podocytes in zebrafish have numerous similarities to mammals, including their ultrastructure, gene expression, and function [17–31]. For example, transmission electron microscopy of zebrafish podocytes has demonstrated that they extend elaborate foot processes and interact with a trilaminar glomerular basement membrane, similar to their mammalian counterparts [17,29]. Further, the gene expression profile of zebrafish podocytes has been shown to mirror that of mammalian podocytes [18,21,23–31].
Among this gene list is Wt1, which encodes a mammalian zinc-finger transcription factor and RNA binding protein that is essential for normal renal development and one of the earliest markers of podocytes in vertebrates [7]. Mature and developing podocytes in zebrafish express the Wt1 paralogs wt1a and wt1b [18,21,30,31]. In zebrafish, wt1a expression is detected first in a broad domain [17,18,26,30], and then transcripts encoding wt1b are expressed in a subset of cells within the wt1a domain. The dual wt1a/wt1b-expressing cells are the podocyte progenitors (Figure 1) [26,30]. Intermingled in the wt1a-expressing field are cells that form the interrenal gland, which produces steroid hormones like its mammalian counterpart, the adrenal gland (Figure 1) [28]. Loss of function studies have demonstrated that knockdown of wt1a disrupts formation of the glomerulus by leading to a reduction in the number of podocytes that develop [28,31]. The role of wt1b has not been fully characterized, and conflicting loss of function studies have been reported to date. Knockdown of wt1b has been associated with high incidence of edema (>70%), a phenotype that can indicate renal failure; other researchers have reported that wt1b may be dispensable for podocyte development due to redundant roles with wt1a, based on knockdown studies in which dual loss of wt1a/1b was not more severe than wt1a knockdown alone [21,31].
During zebrafish pronephros formation, the expression of both wt1a and wt1b in renal progenitors is contingent on the presence of Retinoic Acid (RA) signaling [18,19]. RA is a well-established morphogen that elicits dose-dependent effects on target tissues, and RA gradients are essential in many developing tissues [32]. RA leads to changes in gene transcription through binding to heterodimeric complexes of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) [32]. Normal renal progenitor development in the zebrafish pronephros requires RA [18,19]. RA is secreted by paraxial mesodermal cells located adjacent to the intermediate mesodermal field of renal progenitors [18,19]. This source of RA is necessary and sufficient for the patterning of proximal cell fates when the renal progenitors develop, which include both podocytes and the proximal tubule segments [18,19]. Embryos that are deficient in expression of the RA-biosynthesis enzyme aldehyde dehydrogenase 1a2 (aldh1a2), or have been treated with chemical inhibitors to block RA production or downstream signaling, fail to form podocytes or proximal segments [18,19]. For example, side-by-side examination of wt1b expression in aldh1a2 mutants compared to their wild-type siblings shows that the mutants fail to express wt1b in the developing glomerulus (Figure 2). Further, both podocyte and proximal tubule development in aldh1a2 mutant embryos could be rescued by exogenous treatment with all-trans RA [19]. Interestingly, RA treatment was associated with elevated levels of wt1a transcripts in the intermediate mesoderm based on whole mount in situ hybridization analysis [18]. However, these studies did not determine whether RA acts directly or indirectly to influence renal progenitors.
Figure 2.
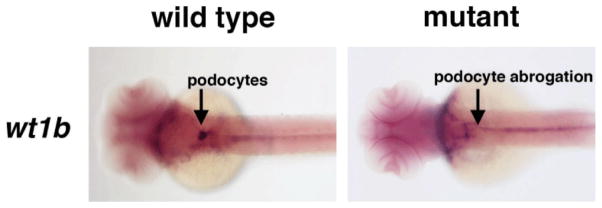
RA deficiency leads to absent or reduced numbers of podocytes in the zebrafish pronephros. Whole mount in situ hybridization was performed with a riboprobe to wt1b (purple) at the 48 hpf stage of development, when the podocytes have migrated to the midline to form a single glomerulus. Wild type embryos display strong expression in two fused, oval clusters of podocytes at the midline, while aldh1a2 mutant embryos lack podocytes at this stage.
A direct link was subsequently established between RA and the promoter of wt1a/Wt1 [20]. Analysis of the wt1a promoter region in zebrafish revealed an upstream enhancer that is highly conserved with the genomic region upstream of Wt1 in human, mouse, and Xenopus [20]. Through biochemical studies, this wt1a/Wt1 enhancer sequence was shown to be bound by complexes of the RARβ/RXRα, but not by either protein alone [20]. These experiments provide compelling proof of principle evidence that the RAR/RXR proteins can regulate wt1a/Wt1, thus suggesting that direct interactions occur between RA and the podocyte gene wt1a/Wt1.
The essential interactions between Wt1, FoxC1/2 transcription factors and Notch signaling components during podocyte development
Recently, insights into the formation of the podocyte lineage, and how wt1a/Wt1 cooperates with other essential podocyte factors have been elucidated through combinatorial loss of function experiments in zebrafish embryos along with biochemical assays of various zebrafish proteins and their mammalian counterparts [21]. In the subsequent sections, we discuss these studies.
Spatiotemporal resolution of podocyte precursors within the renal progenitor field
To characterize podocyte development in the zebrafish pronephros, researchers analyzed gene expression from the podocyte progenitor stage until differentiation into a mature podocyte [21]. Whole mount in situ hybridization was used to localize the mRNA transcripts of transcription factors characteristic of both early and late stages of podocyte development. The expression patterns of early podocyte markers wt1a, wt1b, mafba, hey1, lhx1a, pax2a were determined from 15 somites to 48 hours post fertilization (hpf). Markers that are expressed in mature podocytes, such as nephrin, podocalyxin, podocin, and integrinα3 were also localized at later time points in development (24 hpf, 36 hpf, and 48 hpf). In sum, expression patterns of these markers suggested that podocyte progenitors appear around the 15 somite stage marked specifically by wt1b transcripts, and that terminal differentiation proceeds at 24 hpf due to the appearance of transcripts encoding slit diaphragm components like nephrin and podocalyxin. Additionally, the maturation of podocytes coincides with an upregulation of wt1a and a downregulation of lhx1a, hey1, and pax2a transcripts.
To gain further insight into the podocyte development process, researchers investigated podocyte formation alongside the neighboring neck, proximal tubule, and interrenal gland tissues [21]. The expression domains of markers characteristic of the three different cell types were analyzed at 24 hpf and 36 hpf. Detection of wt1b transcripts was used to label and visualize the podocytes, pax2a for the neck region, cdh17 for the non-podocyte renal epithelia, slc20a1a for the proximal convoluted tubule, and nr5a1a used to label the interrenal gland cells. The results showed non-overlapping cellular domains, thus establishing the respective spatial domains of each cell group at the rostral end of the pronephros (Figure 1).
Since podocyte progenitors initially express pax2a, the origin of podocyte progenitors was determined by a comparison of the expression patterns of pax2a and the podocyte marker wt1a in a very early stage of development [21]. In the 8 somite stage embryo, the cells expressing both of these markers are the putative podocyte progenitors. Whole mount in situ hybridization results showed the most co-expression in the cells of the intermediate mesoderm adjacent to somite 3. By the 15 somite stage, these cells produce a population of podocyte and neck progenitors. In support of these results, laser ablation of one side of the intermediate mesoderm adjacent to somite 3 resulted in reduced pax2a expression and close to a complete absence of wt1b-expressing podocytes on the ablated side of the embryo at 36 hpf, but normal expression of nephrin on the unablated side. This suggests that most of the podocytes originate from the intermediate mesoderm adjacent to somite 3 during zebrafish development (Figure 1).
To further analyze the candidate factors responsible for podocyte specification, expression of several members of the Notch signaling pathway and other transcription factors were analyzed along with podocyte markers [21]. Whole mount in situ hybridization of 8 somite stage embryos revealed that the Notch ligands jag1b and jag2a are expressed in the intermediate mesoderm along with the transcription factor foxc1a. The expression domains of these factors overlap with the podocyte markers wt1a and wt1b in the region of intermediate mesoderm adjacent to somite 3, suggesting that these factors likely have a contribution to podocyte formation.
Genetic analysis of podocyte patterning
Morpholino knockdowns of wt1a, foxc1a, and the Notch mediator rbpj were used to delineate their roles in podocyte specification [21]. Under single knockdown conditions it was found that morphant embryos deficient in expression of any one of these three transcripts exhibited a reduced number of wt1b-expressing podocyte progenitors at the 15 somite stage and also later at 24 hpf. Although there were wt1b-expressing podocytes in the wt1a, rbpj, and foxc1a morphants at 24 hpf, they were only abrogated by 36 hpf in the wt1a morphants, and did not express normal levels of other markers characteristic of mature podocytes, such as nephrin and podocalyxin. These results indicate that wt1a plays roles in podocyte maturation and survival. In contrast, rbpj and foxc1a knockdowns displayed reductions in nephrin or podocalyxin expression in podocytes at 36 hpf, suggesting that deficiency of these factors alone does not significantly disturb podocyte differentiation. These findings are consistent with amphibian development studies in which the single elimination of wt1 or foxc2 was not sufficient to abrogate podocyte development in the pronephros [33]. Interestingly though, dual knockdowns of wt1 and foxc2 resulted in the loss of podocytes in amphibians [33].
In keeping with the notion that combinations of these factors may be essential for podocyte specification and/or differentiation, dual knockdowns of wt1a/rbpj, foxc1a/wt1a, and foxc1a/rbpj in zebrafish embryos all displayed an absence of early and late podocyte marker expression, suggesting that podocyte specification was abrogated [21]. The lack of podocytes was confirmed to be a result of the morpholino knockdown, and not simply due to a loss of the podocyte progenitor cell population of the intermediate mesoderm, because staining for pax2a and myod1 did not show a truncated intermediate mesoderm, and there were no elevated levels of apoptosis in this region.
Further, the effect of various single and double knockdowns on neck and interrenal gland cell fate in the zebrafish pronephros was also evaluated [21]. rbpj/foxc1a deficient embryos developed a fairly normal pax2a-expressing neck segment, but the wt1a/rbpj and wt1a/foxc1a morphants exhibited a noticeably reduced pax2a expression in the neck segment at 48 hpf. No change in proximal tubule development was observed in the double knockdown embryos. Interestingly, singly deficient wt1a and rbpj morphants showed an increase in nr5a1a-expressing interrenal progenitor cells. In the double wt1a/rbpj knockdown, the increase in cell number was even more apparent, suggesting that wt1a and rbpj may each play a role in the suppression of interrenal gland formation. foxc1a morphants did not show a similar enlargement of the interrenal progenitor field, but foxc1a/rbpj double deficient embryos exhibited an interrenal gland of modestly increased size. The foxc1a/wt1a double knockdowns had an interesting phenotype, in which there was an absence of nr5a1a-expressing interrenal gland cells. This suggests that foxc1a and wt1a may act redundantly to modulate interrenal cell development. The results of the combinatorial knockdowns of wt1a, rbpj, and foxc1a indicate that the interplay between these three factors governs the development of podocytes, the neck segment, and the interrenal gland.
The expression of a downstream target of the Notch pathway was analyzed under single and combinatorial knock down conditions of the three factors of interest [21]. All double knockdown morphants had no hey1 expression, and single knockdowns had significantly reduced hey1 expression. This finding supports a model whereby wt1a, foxc1a, and rbpj regulate common targets during podocyte development.
Elucidation of physical interactions between wt1a/Wt1, foxc1a/FoxC2, and Notch pathway components in zebrafish and mammals
In order to gain insight into the protein-protein interactions between wt1a, foxc1a, and Notch signaling factors, Glutathione S-transferase (GST) tagged in vitro pull-down assays were also completed [21]. GST-rbpj was able to bind NICD3, the intracellular domain of Notch 3, as well as wt1a, and foxc1a, which was unexpected. Interestingly, protein-protein interactions between GST-foxc1a and NICD3 could not be detected. In addition, GST-wt1a did not directly interact with NICD3. Despite the lack of interaction with NICD3, both these GST tagged proteins could bind the reciprocal protein partner. These results suggest rbpj connects NICD3 with wt1a and foxc1a using protein-protein interactions during podocyte development, however the data cannot rule out several distinct protein complexes instead of one large multimeric complex. These results were confirmed by co-immunoprecipitation in HEK293T cells, due to the lack of viable zebrafish antibodies. By overexpression of the murine homologs of these proteins, followed by co-immunoprecipitation studies, the results from the pull-down assay were confirmed, suggesting these protein-protein interactions are conserved in mammals.
To investigate the effects of these complexes on promoter activation with the zebrafish genes and their respective mammalian counterparts, cells with the Hey1 promoter luciferase reporter were co-transfected with NICD1, foxc1a, FoxC2, or Wt1 [21]. Each of these independent experiments showed a 4-fold, or less, induction of luciferase compared to control. Excess fox1a, Fox2C, or Wt1 combined with NICD1 increased the luciferase activation to between 7 and 11 fold above controls. Similar results were achieved when fox1a or Fox2C were combined with Wt1, without exogenous NICD1. The most synergistic result occurred with triple transfections of NICD1, fox1a/Fox2C, and Wt1, which increased activity of the Hey1 promoter 13–15 fold. To test this synergy, a synthetic Notch reporter line with several Rbpj binding sites upstream of a minimal promoter was used. NICD1 activated this promoter, while co-transfection of NICD1 and foxc1a or Wt1 inhibited transactivation, thus suggesting these factors antagonize Notch signaling under these conditions. Furthermore, the promoter of a more mature gene expressed in podocytes, Podocalyxin, was tested. NICD1 had very little effect, suggesting the role of Notch occurs early in podocyte development. Conversely, Wt1 and Foxc1a induced Podocalyxin promoter activity over controls. Furthermore, the transfection of these factors seems to have dose dependent effects as a 2:1 ratio of foxc1a over Wt1 shows additive effects, however a 5-fold excess of Foxc1 suppresses activation of the promoter. These results suggest the ratio of these factors is important in the regulation of podocytes during development.
Taken together, these biochemical studies reveal previously unknown physical interactions between wt1a/Wt1 with foxc1a/FoxC2, Rbpj, and NICD. Further, these studies suggest that different physical interactions of these proteins are capable of binding genomic targets, and that switches in the complex components over time may orchestrate transcriptional alterations that proceed during podocyte differentiation. These data provide new molecular insights into podocyte development and emphasize the necessity of further biochemical analyses to reveal how transcriptional factors control renal progenitor lineage choices and cell type maturation.
Conclusion
Progress understanding podocyte development is highly significant to the biomedical community because the loss of podocytes in humans leads to kidney disease. Podocyte structure and molecular composition is conserved between zebrafish and higher vertebrates, such as mice and humans. Therefore, identification of the genetic pathways that are required for podocyte formation in the zebrafish are likely to be broadly applicable to understanding development of this cell type across numerous species. As presented in this commentary, recent cross-species research has provided evidence that RA regulates wt1a/Wt1 expression, and that wt1a/Wt1 can interact physically with different co-factors to modulate the transcriptional activity of essential podocyte genes like podocalyxin. Parallels between various genetic and biochemical attributes of Wt homologs across zebrafish, amphibian, and mouse indicates that research with these systems can provide insights into conserved as well as species-specific renal programs of development. Thus, additional podocyte research with the zebrafish model is poised to make useful contributions to this area of nephrology in the years ahead.
Continued work to identify Wt1 targets [34] and to ascertain the full transcriptional profile of podocytes [35,36], is necessary to solve the remaining enigmas of Wt1 function in podocyte ontogeny and identify players in podocyte gene regulatory networks, respectively. Further, ongoing identification of disease-associated loci through genome wide association studies can triage potentially causative genetic components in kidney disease [37]. The zebrafish pronephros model provides a valuable system in which the functional role of such genes can be rapidly assessed with loss and gain of function experiments, as discussed herein. For example, morpholino studies in zebrafish have been implemented to assign critical roles for a number of genes associated with podocyte differentiation or physiological maintenance [38–47]. Insights from such avenues of research can be applied to better understand how podocytes might be generated in vitro or in vivo for the treatment of chronic kidney disease. The ability to coax human induced pluripotent cells to the podocyte lineage has been reported [48], and further progress on this and similar research endeavors may lead to innovative regenerative therapies for patients with renal conditions.
Acknowledgments
This work was supported in whole or part by funding to the Wingert lab from the following sources: National Institutes of Health grants K01DK083512, DP2OD008470, and R01DK100237; March of Dimes Basil O’Connor Starter Scholar grant award #5-FY12-75; startup funds from the University of Notre Dame College of Science and Department of Biological Sciences; and a generous gift to the University of Notre Dame from Elizabeth and Michael Gallagher on behalf of the Gallagher Family to foster stem cell research. The funders had no role in manuscript design, preparation or decision to publish. We thank the staffs of the Department of Biological Sciences for their support, and the Center for Zebrafish Research at Notre Dame for their outstanding care of our zebrafish colony. Finally, we express our gratitude to the members of our lab for support and discussions of the ideas presented in this review.
References
- 1.Reilly RF, Bulger RE, Kriz W. Diseases of the Kidney and Urinary Tract, Chapter 1: Structural-functional relationships in the kidney. 8. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 2.Saxen L. Organogenesis of the kidney. Cambridge University Press; Cambridge, UK: 1987. [Google Scholar]
- 3.McCampbell KK1, Wingert RA. Renal stem cells: fact or science fiction? Biochem J. 2012;444:153–168. doi: 10.1042/BJ20120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CK, Inagi R. Glomerular diseases: genetic causes and future therapeutics. Nat Rev Nephrol. 2010;6:539–554. doi: 10.1038/nrneph.2010.103. [DOI] [PubMed] [Google Scholar]
- 5.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62:1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Chen YM, Miner JH. Glomerular basement membrane and related glomerular disease. Transl Res. 2012;160:291–297. doi: 10.1016/j.trsl.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 8.Miner JH. Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis. 2011;7:75–82. doi: 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laale HW. The biology and use of zebrafish, Brachydanio rerio in fisheries research. A literature review. J Fish Biol. 1977;10:121–173. [Google Scholar]
- 11.Lieschke GJ1, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 12.Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. 2012;122:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 15.Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens. 2011;20:416–424. doi: 10.1097/MNH.0b013e3283477797. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2:559–585. doi: 10.1002/wdev.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 18.Wingert RA, Selleck R, Yu J, Song HD, Chen Z, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 2011;240:2011–2027. doi: 10.1002/dvdy.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollig F, Perner B, Besenbeck B, Köthe S, Ebert C, et al. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development. 2009;136:2883–2892. doi: 10.1242/dev.031773. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien LL, Grimaldi M, Kostun Z, Wingert RA, Selleck R, et al. Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev Biol. 2011;358:318–330. doi: 10.1016/j.ydbio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Cheng CN, Verdun VA, Wingert RA. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev Biol. 2014;386:111–122. doi: 10.1016/j.ydbio.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–229. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Majumdar A, Drummond IA. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol. 2000;222:147–157. doi: 10.1006/dbio.2000.9642. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- 26.Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233–2241. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- 27.Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492–497. doi: 10.1016/s0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- 28.Hsu HJ, Lin G, Chung BC. Parallel early development of zebrafish interrenal glands and pronephros: differential control by wt1 and ff1b. Development. 2003;130:2107–2116. doi: 10.1242/dev.00427. [DOI] [PubMed] [Google Scholar]
- 29.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bollig F, Mehringer R, Perner B, Hartung C, Schäfer M, et al. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn. 2006;235:554–561. doi: 10.1002/dvdy.20645. [DOI] [PubMed] [Google Scholar]
- 31.Perner B, Englert C, Bollig F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol. 2007;309:87–96. doi: 10.1016/j.ydbio.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White JT, Zhang B, Cerqueira DM, Tran U, Wessely O. Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development. 2010;137:1863–1873. doi: 10.1242/dev.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartwig S, Ho J, Pandey P, MacIsaac K, Taglienti M, et al. Genomic characterization of Wilms’ tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development. 2010;137:1189–1203. doi: 10.1242/dev.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He L, Sun Y, Patrakka J, Mostad P, Norlin J, et al. Glomerulus-specific mRNA transcripts and proteins identified through kidney expressed sequence tag database analysis. Kidney Int. 2007;71:889–900. doi: 10.1038/sj.ki.5002158. [DOI] [PubMed] [Google Scholar]
- 36.Lindenmeyer MT, Eichinger F, Sen K, Anders HJ, Edenhofer I, et al. Systematic analysis of a novel human renal glomerulus-enriched gene expression dataset. PLoS One. 2010;5:e11545. doi: 10.1371/journal.pone.0011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, et al. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 2012;8:e1002584. doi: 10.1371/journal.pgen.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, et al. Amino acid transporter LAT3 is required for podocyte development and function. J Am Soc Nephrol. 2009;20:1586–1596. doi: 10.1681/ASN.2008070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, et al. Cofilin-1 inactivation leads to proteinuria--studies in zebrafish, mice and humans. PLoS One. 2010;5:e12626. doi: 10.1371/journal.pone.0012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, et al. Mutations in the gene that encodes F-actin binding protein anillin cause FSGS. J Am Soc Nephrol. 2014;25 doi: 10.1681/ASN.2013090976. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller T, Rumpel E, Hradetzky S, Bollig F, Wegner H, et al. Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney Int. 2011;80:1055–1063. doi: 10.1038/ki.2011.256. [DOI] [PubMed] [Google Scholar]
- 42.Arif E, Kumari B, Wagner MC, Zhou W, Holzman LB, et al. Myo1c is an unconventional myosin required for zebrafish glomerular development. Kidney Int. 2013;84:1154–1165. doi: 10.1038/ki.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao J, Wang D, Mataleena P, He B, Niu D, et al. Myo1e impairment results in actin reorganization, podocyte dysfunction, and proteinuria in zebrafish and cultured podocytes. PLoS One. 2013;8:e72750. doi: 10.1371/journal.pone.0072750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishibori Y, Katayama K, Parikka M, Oddsson A, Nukui M, et al. Glcci1 deficiency leads to proteinuria. J Am Soc Nephrol. 2011;22:2037–2046. doi: 10.1681/ASN.2010111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, et al. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol. 2009;334:1–9. doi: 10.1016/j.ydbio.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Raschperger E, Neve EP, Wernerson A, Hultenby K, Pettersson RF, et al. The coxsackie and adenovirus receptor (CAR) is required for renal epithelial differentiation within the zebrafish pronephros. Dev Biol. 2008;313:455–464. doi: 10.1016/j.ydbio.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 47.Hyvärinen J, Parikka M, Sormunen R, Rämet M, Tryggvason K, et al. Deficiency of a transmembrane prolyl 4-hydroxylase in the zebrafish leads to basement membrane defects and compromised kidney function. J Biol Chem. 2010;285:42023–42032. doi: 10.1074/jbc.M110.145904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]


