Abstract
Objective
To assess the time needed to achieve sustained, medication-free remission in a cohort of patients with juvenile dermatomyositis (DM) receiving a stepwise, aggressive treatment protocol.
Methods
Between 1994 and 2004, a cohort of 49 children with juvenile DM who were followed up at a single tertiary care children's hospital using disease activity measures according to a specific protocol received standardized therapy with steroids and methotrexate. If a patient's strength or muscle enzyme levels did not normalize with this initial therapy, additional medications were added in rapid succession to the treatment regimen. The primary outcome measure was time to complete remission. Additional outcome measures were onset of calcinosis, effect of treatment on height, and complications resulting from medications.
Results
Forty-nine patients were followed up for a mean ± SD of 48 ± 30 months. All but 1 patient received 2 or more medications simultaneously. Transient localized calcifications occurred in 4 patients (8%), and 2 additional patients (4%) had persistent calcinosis. Despite the aggressive therapy, complications associated with treatment were mild and were primarily attributable to steroids. No persistent effect on longitudinal growth was observed. A complete, medication-free remission was achieved in 28 patients; the median time to achievement of complete remission was 38 months (95% confidence interval 32–44 months). None of these patients experienced a disease flare that required resumption of medications during the subsequent period of observation (mean ± SD 36 ± 19.7 months).
Conclusion
Our findings suggest that aggressive treatment of juvenile DM aimed at achieving rapid, complete control of muscle weakness and inflammation improves outcomes and reduces disease-related complications. In more than one-half of the children whose disease was treated in this manner (28 of 49), a prolonged, medication-free remission was attained within a median of 38 months from the time of diagnosis.
Juvenile dermatomyositis (DM) is an idiopathic diffuse vasculopathy of the skin and muscles, characterized by proximal muscle weakness and typical rash. The inflammatory process primarily affects muscle and skin, but it can also involve numerous other organ systems, with significant mortality from cardiovascular, respiratory, and gastrointestinal sequelae of the disease. Prior to the introduction of steroids in the 1960s for treatment of the disease, one-third of patients with juvenile DM had permanent crippling morbidity and limitations, one-third died as a result of the disease, and only one-third of patients recovered with minimal or no sequelae (1).
Even with the introduction of steroids, ongoing disability and/or disease activity have persisted in a significant percentage of patients. Notable improvements in patient outcomes have been achieved with the additional treatment options of antiinflammatory medications such as methotrexate (MTX), cyclosporine, and intravenous immunoglobulin (IVIG) (2,3). In 2000, a report of a Canadian multicenter cohort study of juvenile DM patients (4) described long-term outcomes in children treated primarily with corticosteroids, with or without second-line agents. Overall, 63% of these patients received disease-modifying medications in addition to steroids; 37% demonstrated a monocyclic course, 11% demonstrated a polycyclic course, and 52% had chronic continuous disease activity. After a median followup of 7 years, 23% of patients had persistent weakness, and 35% continued to receive medications, 43% of whom continued regular treatment with systemic corticosteroids. Disruption of longitudinal growth was one of the significant long-term sequelae observed: almost one-third of patients were below their predicted height by >1 standard deviation (4).
In 2005, a report from a single Canadian center described the effectiveness of treating juvenile DM with MTX and aggressively tapered dosages of corticosteroids as compared with steroids alone (2). Both groups had similar improvements in strength and function, but the median duration of steroid therapy in patients receiving MTX was shorter (10 months versus 27 months). Though the steroid dosage could be tapered more rapidly in patients receiving MTX, prolonged, treatment-free remissions remained uncommon; children who received MTX and those who did not both had a 30% rate of disease flares by 40 months.
Despite advances in therapy, juvenile DM continues to be associated with considerable morbidity. In several reports, sizable percentages of patients are described as having persistently active disease and developing subcutaneous calcifications, as well as having significant growth retardation (4–8). In addition, juvenile DM patients with severe, prolonged disease and calcinosis appear to be at greater risk of developing lipodystrophy (9).
In 2002, we presented data regarding our institution's practice of stepwise, aggressive treatment aimed at achieving rapid, complete control of muscle inflammation in juvenile DM patients. We reported decreased long-term sequelae, including calcinosis, with the best outcomes being associated with a more rapid normalization of muscle inflammation (10).
In this report, we present the findings of our study of a cohort of patients followed up in our program for juvenile DM who were treated according to a prescribed prospective therapeutic algorithm. We sought to determine whether the rapid institution of steroid-sparing medications would improve outcomes and provide long-term disease control.
PATIENTS AND METHODS
Study population
Children with juvenile DM who were treated according to a prospective therapeutic regimen at Children's Hospital Boston between January 1994 and December 2004 were included in this analysis. Patients were diagnosed as having probable or definite juvenile DM based on the Bohan and Peter criteria for myositis (11), which include a typical skin rash and 2 or more of the following: symmetric proximal muscle weakness, increased serum muscle enzyme levels, electromyographic abnormalities, and muscle biopsy findings consistent with myositis. In most patients included in our cohort, the disease was also confirmed by magnetic resonance imaging (MRI). Patients were excluded if they had evidence of mixed connective tissue disease, scleroderma, systemic lupus erythematosus, or an overlap connective tissue disease syndrome; if their disease was managed at an outside institution; or if they exhibited no evidence of muscle weakness or inflammation (amyopathic DM).
The date of disease onset was defined as the earliest date that the patient's guardian reported symptoms consistent with juvenile DM. Medications, growth parameters, medication side effects, calcinosis, laboratory data, and Disease Activity Scores (DAS) for juvenile DM (12) were recorded from clinic and hospital records.
Criteria for assessment of disease activity
Strict criteria were used to define complete clinical response and clinical remission, based upon definitions of the International Myositis Assessment and Clinical Studies Group (13). Complete clinical response was defined as no evidence of active myositis for ≥6 months while receiving therapy. Clinical remission was defined as no evidence of active myositis for ≥6 months while not receiving any drug therapy (13).
Lack of evidence of myositis disease activity was defined as normal muscle strength, with normal muscle enzyme levels (including aldolase, alanine aminotransferase, aspartate aminotransferase, creatine kinase, lactate dehydrogenase, and von Willebrand factor antigen). In some cases, MRI of the upper thighs was obtained as well. A juvenile DM flare was defined as clinical or laboratory evidence of increasing muscle or skin disease requiring adjustment of medications.
Treatment protocol
Treatment was begun promptly upon diagnosis, according to a stepwise approach based on the extent of myositis at presentation. Patients with mild disease (isolated mild weakness that did not limit activities of daily living, and no involvement of the muscles of respiration or deglutition) received high-dose oral prednisone. Patients with moderate to severe disease (weakness that limited activities of daily living, and/or difficulties involving respiration or deglutition) were treated with 3 pulsed doses of intravenous methylprednisolone (MP) (30 mg/kg, with a maximum dose of 1 gm), followed by weekly pulse MP, MTX, and daily oral prednisone (2 mg/kg), until a complete clinical response was achieved. When muscle enzyme levels and strength normalized, medications were tapered, beginning with steroids (a mean of 4 months after the start of therapy). However, if a patient's strength or muscle enzyme levels did not normalize after 3 months of therapy, cyclosporine and IVIG were added sequentially. If needed, additional medications were added in rapid succession to further control inflammation. This study was approved by the Clinical Investigation Committee of Children's Hospital Boston.
Statistical analysis
Age and strength were compared using Student's t-test and expressed as the mean and SD. Medication data, months to remission, and diagnoses were compared using Wilcoxon's signed rank test and presented as the median and range. Categorical data and proportions were compared using Fisher's exact test. Kaplan-Meier survival analysis was applied to determine median time to outcome events of complete clinical response and clinical remission, with 95% confidence intervals (95% CIs) calculated using Greenwood's formula (14). Median time to complete clinical response and clinical remission was computed using the product-limit estimator for censored data. Repeated-measures analysis of variance using a mixed-model approach was utilized to assess differences in the age-adjusted height percentile between baseline and followup points in the time course through 48 months (15). Statistical analysis was performed with SPSS (version 16.0, 2007; SPSS, Chicago, IL). P values less than 0.05 were considered significant.
RESULTS
Demographics
Of 93 patients with juvenile DM followed up at our center from 1994 to 2004, 49 patients (23 boys, 26 girls) met the inclusion criteria for the study. Twenty-four patients were excluded because their initial disease management was outside of our institution, 10 were excluded because they moved out of state, and 10 were excluded because they had amyopathic DM.
The mean ± SD age at the time of diagnosis was 6.5 ± 3.1 years (range 1.6–13 years). Patients were followed up for a mean ± SD of 48 ± 30 months (range 9–151 months). The mean ± SD time from the onset of symptoms to initiation of treatment was 5.2 ± 11.7 months (range 1 week to 72 months) (Table 1).
Table 1.
Characteristics of the juvenile dermatomyositis (DM) patients at the time of presentation (n = 49)*
| Sex, no. (%) male | 23 (47) |
| Age at diagnosis, years | 6.5 ± 3.1 |
| Duration of symptoms, months | 5.2 ± 11.7 |
| Initial total DAS | 10.8 ± 3.7 |
| Muscle enzyme levels | |
| Aldolase, units/liter | 63 ± 221 (3–9.7) |
| Aspartate aminotransferase, units/liter | 293 ± 936 (2–40) |
| Alanine aminotransferase, units/liter | 128 ± 314 (3–30) |
| Creatine kinase, units/liter | 5,088 ± 21,185 (4–175) |
| Von Willebrand factor antigen, % | 211 ± 109 (50–160) |
| Lactate dehydrogenase, units/liter | 670 ± 979 (100–210) |
Except where indicated otherwise, values are the mean ± SD (normal range). DAS = Disease Activity Score.
Overall, all but 1 patient (98%) received 2 or more immunosuppressive medications concurrently. The median number of treatments patients received during their illness was 4, which included prednisone (98%), pulsed-dose steroids (84%), MTX (78%), hydroxychloroquine (45%), cyclosporine (27%), IVIG (20%), topical tacrolimus (20%), plasmapheresis (8%), and cyclophosphamide (4%).
Outcomes
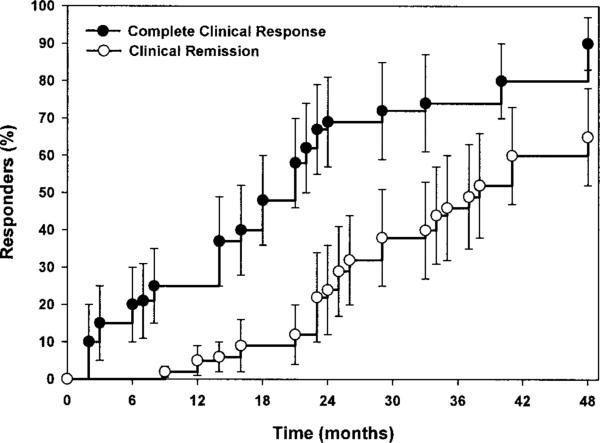
Response to treatment tended to be rapid, complete, and persistent. The mean ± SD initial DAS for juvenile DM was 10.8 ± 3.7, and the mean score subsequently improved to 3.7 and 1.9, at 12 and 24 months, respectively. Muscle enzyme levels, strength, and the presence of skin lesions normalized at a median of 14 months (range 1–67), 13 months (range 3–78), and 19 months (range 3–113), respectively. These data were skewed by the occurrence of disease flares in 5 patients. In the time period studied, complete clinical response was achieved in 37 of 49 patients within a median of 21 months (95% CI 18–24). Clinical remission (including a DAS of 0) was achieved in 28 of 49 patients within a median of 38 months (95% CI 32–44) (Figure 1). None of these patients experienced a disease flare that required resumption of medications during the subsequent period of observation (mean ± SD 36 ± 19.7 months).
Figure 1.
Time to complete clinical response and clinical remission in patients with juvenile dermatomyositis. Patients were considered to have had a complete clinical response once strength and muscle enzyme levels had normalized, with no evidence of active myositis for ≥6 months while receiving therapy. The median time to complete clinical response was 21 months (95% confidence interval [95% CI] 18–24 months), and the median time to complete remission was 38 months (95% CI 32–44 months). There was a lag between complete clinical response and clinical remission, since medications were tapered slowly, while normal strength and muscle enzyme levels were maintained. Values are the percentage of patients with clinical response or clinical remission at each time point; bars show the 95% CI.
Only 5 patients (10%) had disease flares/relapses after beginning therapy; clinical remission was achieved in 4 of these 5 patients by a median of 58 months (range 35–100). The time to complete remission in patients with a disease flare (n = 4) was significantly longer compared with that in the 24 patients in whom complete remission was achieved without a disease flare (Table 2). Clinical remission was not achieved in 1 patient (2%) with a chronic disease course. A total of 6 patients (12%) developed calcinosis, but this resolved in most. Only 2 patients (4%) had persistent calcinosis, which was superficial. There were no deaths during the period of study.
Table 2.
Characteristics of the patients in whom complete remission was achieved, with or without a disease flare (n = 28)
| Variable* | With flares (n = 4) | Without flares (n = 24) |
|---|---|---|
| Age, mean ± SD years | 5.5 ± 3.1 | 6.9 ± 3.7 |
| Sex, no. male/female | 1/3 | 12/12 |
| Concurrent medications, median (range) | 4 (3–5) | 3 (1–5) |
| Months to diagnosis, median (range) | 2(1–6) | 1 (0–72) |
| Calcinosis, no. (%) | 1 (25) | 4 (17) |
| Initial strength DAS, mean ± SD | 5.3 ± 3.8 | 4.6 ± 3.0 |
| Months to remission, median (range) | 58 (35–100)† | 26 (9–60) |
DAS = Disease Activity Score.
P < 0.01 versus patients without flares.
Medication side effects and growth
Despite the aggressive introduction of immunosuppressive medications into the patients’ treatment regimens, complications associated with treatment were mostly mild and self-limited. Moderate and severe side effects are listed in Table 3.
Table 3.
Moderate to severe side effects of aggressive treatment in the juvenile dermatomyositis patients (n = 49)*
| Abdominal distress/pain | 1 (2) |
| Acute psychosis | 1 (2) |
| Fractures† | 5 (10) |
| Hypertension | 3 (6) |
| Infections‡ | 2 (4) |
| Mood swings | 1 (2) |
Moderate to severe side effects were evaluated as grade 3 or higher according to the Common Toxicity Criteria of the National Cancer Institute (25). Values are the number (%) of patients.
One humeral, 1 radial, 2 tibial, and 1 vertebral compression fracture.
Rotavirus and diarrhea, requiring fluid resuscitation and admission to the intensive care unit, and axillary furuncle, requiring intravenous antibiotics.
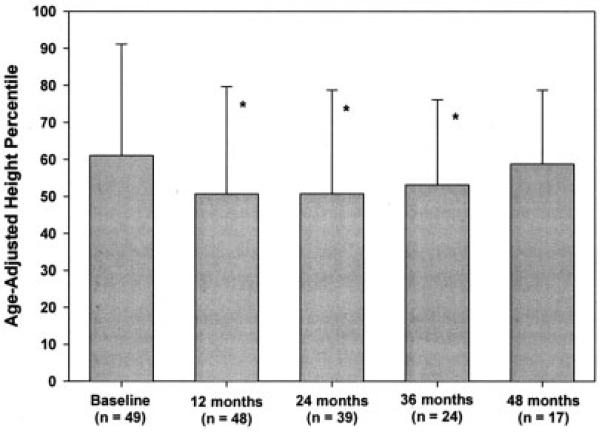
Growth assessment during the disease course revealed a decrease in height percentile during the first year of therapy, with subsequent recovery of height. The patients’ overall mean height never dropped below the 25th percentile, and the mean height had returned to baseline values by 48 months after the start of therapy (Figure 2).
Figure 2.
Height of the patients with juvenile dermatomyositis. Growth deceleration was demonstrated after the initiation of treatment, but height percentiles returned to pre-disease levels over time (mean of 4 years after diagnosis). Values are the mean and SD. * = P < 0.05 versus pre-disease level (baseline).
DISCUSSION
In this single-center cohort study, we demonstrated that aggressive treatment of juvenile DM may improve the rates of complete disease remission, limit disease flares, and reduce disease- and therapy-related complications as compared with historical controls. To our knowledge, this is the first study of a pediatric rheumatologic disease to demonstrate a high percentage of children with no evidence of disease years after discontinuing all medications. In addition, we found no significant long-term sequelae associated with treatment. Side effects were largely attributable to steroids and were generally mild, self-limited, and reversible. Furthermore, only 6 patients developed calcinosis, which persisted in only 2 (4%).
For the majority of rheumatologic diseases, the goal is disease control, and cures are thought to be largely unattainable. This is true for a variety of reasons, including the fact that the fundamental aberration causing most chronic inflammatory conditions is unknown. As a result, rheumatologists have tended to accept as inevitable disease chronicity in a sizable proportion of children. The occasional cases that seem to enter a prolonged, medication-free remission are termed “monocyclic,” in contrast to the majority of cases that are incurable and labeled “polycyclic” or “chronic” (1,4). The implication is that the difference is inherent and uncontrollable.
In fact, until recently, pediatric malignancies were regarded in a similar manner. Prior to 1960, essentially no children with acute lymphocytic leukemia survived past 5 years, and treatment efforts aimed at curing this condition were thought to be futile (16,17). With the availability of more potent multidrug chemotherapeutic regimens, effectiveness of therapy came to be viewed as the major determinant of prognosis in pediatric oncology, with characteristics of the tumor affecting the details of the regimen but generally not the goals (18). Success is now measured in cures, and 5-year survival rates in patients with juvenile acute lymphocytic leukemia have improved to >85% (19,20).
The results of the current study suggest that just such a change in paradigm should be the goal in rheumatologic diseases, and in fact, may now be applied to juvenile DM. Only with aggressive, complete control of all vestiges of active myositis and dermatitis can a sustained, drug-free remission be achieved in a majority of children. This is not just a desirable goal, but is apparently an urgent necessity, since the ability of medications to affect the aberrant autoimmunity that leads to disease may wane over time.
Even though autoimmune diseases may be initiated by loss of tolerance to a single antigen (21), results of animal studies and limited data in humans demonstrate that with chronicity, the immune response often broadens to include other epitopes of the same molecule and even other molecules (so-called “epitope spreading”) (22). While this process likely enhances the efficiency of protective immune responses in clearing infections, in autoimmune diseases, this process may contribute to the decreased responsiveness to treatment over time (23). As with malignancies, there may be a window of opportunity for curing juvenile DM during the early phases of the illness, when the disease burden is relatively limited. Consistent with this is our finding that patients with disease flares (4 of 49 patients) had significantly longer disease durations. Clinical remission was ultimately achieved in the majority of these patients with resumption of aggressive therapy, suggesting that we might have missed low-grade, subclinical disease activity, which was then controlled with additional treatments.
Disease flares occurred less frequently in our patient population as compared with that in a recent Canadian study in which MTX and steroids were used as initial therapy (10% [versus 30% at 40-month followup]) (2). This disparity is likely due in part to differences in treatment and tapering protocols. The Canadian protocol mandated the tapering of medications after 6 weeks of therapy if there were improvements in muscle enzyme levels and strength, with a predetermined schedule for tapering. In contrast, the protocol at our institution involved very gradual tapering that proceeded only when all markers of disease activity, including muscle enzyme levels and strength, were normal. Our reliance on intravenous MP could also have contributed to our improved outcomes, in light of recent pharmacokinetic findings in juvenile DM patients that suggested improved bio-availability of parenteral steroids compared with oral therapy (24).
The goal of therapy at our institution is to completely control all detectable evidence of disease activity or muscle inflammation in all patients. Thus, the deliberately slow tapering of medication is dictated by the requirement that all ongoing disease activity be eradicated. At the time of presentation of any patient with juvenile DM, it is not possible to predict whether the disease will be more or less aggressive or more or less responsive to treatment. Therefore, we varied the approach between patients based on the clinical severity of their disease at presentation, which is, at best, an inexact predictor of prognosis and of response to particular therapies.
Our results are limited by the relatively small number of patients in this study and by the restricted long-term followup (mean 36 months). Controls are historical, so differences between centers or secular trends could account for the improved outcomes in our patients. This seems unlikely to completely explain our findings, however, since outcomes of previous studies have been fairly consistent in all centers (1,2,4,8). Finally, neither the investigators nor the patients were blinded with regard to the treatments. However, outcomes included objective measures (muscle enzyme levels) as well as subjective measures (DAS for juvenile DM), making observer bias or a placebo effect an unlikely explanation for our findings.
In summary, using an aggressive, multidrug treatment regimen, clinical remission was achieved in a majority of children with juvenile DM within a median of 38 months. Furthermore, even patients who did not exhibit complete disease control demonstrated fewer complications and sequelae compared with patients described in previous reports. Complete clinical remission was maintained in our patients for a mean of 3 years of followup after medication discontinuation, demonstrating for the first time apparent disease resolution in a majority of patients with a pediatric autoimmune condition. With the expanding number of alternatives for controlling inflammatory myopathies, including ongoing studies of targeted anti–B cell therapy, and with the discovery of specific markers of disease activity and therapeutic responsiveness, further optimization of treatment and improvement in outcomes seems attainable.
Acknowledgments
Dr. Kim's work was supported by NIH grants T32-AI-007512 and KL2-RR-025757-01.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Kim, El-Hallak, Dedeoglu, Zurakowski, Fuhlbrigge, Sundel.
Acquisition of data. Kim, El-Hallak, Dedeoglu, Sundel.
Analysis and interpretation of data. Kim, El-Hallak, Zurakowski, Fuhlbrigge, Sundel.
REFERENCES
- 1.Bitnum S, Daeschner CW, Jr, Travis LB, Dodge WF, Hopps HC. Dermatomyositis. J Pediatr. 1964;64:101–31. doi: 10.1016/s0022-3476(64)80325-5. [DOI] [PubMed] [Google Scholar]
- 2.Ramanan AV, Campbell-Webster N, Ota S, Parker S, Tran D, Tyrrell PN, et al. The effectiveness of treating juvenile dermatomyositis with methotrexate and aggressively tapered corticosteroids. Arthritis Rheum. 2005;52:3570–8. doi: 10.1002/art.21378. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mayouf SM, Laxer RM, Schneider R, Silverman ED, Feldman BM. Intravenous immunoglobulin therapy for juvenile dermatomyositis: efficacy and safety. J Rheumatol. 2000;27:2498–503. [PubMed] [Google Scholar]
- 4.Huber AM, Lang B, LeBlanc CM, Birdi N, Bolaria RK, Malleson P, et al. Medium- and long-term functional outcomes in a multi-center cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–9. doi: 10.1002/1529-0131(200003)43:3<541::AID-ANR9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Bowyer SL, Blane CE, Sullivan DB, Cassidy JT. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882–8. doi: 10.1016/s0022-3476(83)80706-9. [DOI] [PubMed] [Google Scholar]
- 6.Cimaz R. Osteoporosis in childhood rheumatic diseases: prevention and therapy. Best Pract Res Clin Rheumatol. 2002;16:397–409. [PubMed] [Google Scholar]
- 7.Pachman LM, Maryjowski MC. Juvenile dermatomyositis and polymyositis. Clin Rheum Dis. 1984;10:95–115. [PubMed] [Google Scholar]
- 8.Sallum AM, Kiss MH, Sachetti S, Resende MB, Moutinho KC, Carvalho Mde S, et al. Juvenile dermatomyositis: clinical, laboratorial, histological, therapeutical and evolutive parameters of 35 patients. Arq Neuropsiquiatr. 2002;60:889–99. doi: 10.1590/s0004-282x2002000600001. [DOI] [PubMed] [Google Scholar]
- 9.Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A, et al. Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 2008;87:70–86. doi: 10.1097/MD.0b013e31816bc604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47:505–11. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]
- 11.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 12.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 13.Oddis CV, Rider LG, Reed AM, Ruperto N, Brunner HI, Koneru B, et al. for the International Myositis Assessment and Clinical Studies Group. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum. 2005;52:2607–15. doi: 10.1002/art.21291. [DOI] [PubMed] [Google Scholar]
- 14.Machin D, Cheung YB, Parmar M. Survival analysis: a practical approach. 2nd ed. Wiley; New York: 2006. [Google Scholar]
- 15.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 16.Holland JF. Hopes for tomorrow versus realities of today: therapy and prognosis in acute lymphocytic leukemia of childhood. Pediatrics. 1970;45:191–3. [PubMed] [Google Scholar]
- 17.Baum E, Sather H, Nachman J, Seinfeld J, Krivit W, Leikin S, et al. Relapse rates following cessation of chemotherapy during complete remission of acute lymphocytic leukemia. Med Pediatr Oncol. 1979;7:25–34. doi: 10.1002/mpo.2950070106. [DOI] [PubMed] [Google Scholar]
- 18.Donadieu J, Auclerc MF, Baruchel A, Leblanc T, Landman-Parker J, Perel Y, et al. Critical study of prognostic factors in childhood acute lymphoblastic leukaemia: differences in outcome are poorly explained by the most significant prognostic variables. Fralle group. French Acute Lymphoblastic Leukaemia study group. Br J Haematol. 1998;102:729–39. doi: 10.1046/j.1365-2141.1998.00818.x. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Progress against childhood cancer: the Pediatric Oncology Group experience. Pediatrics. 1992;89(4 Pt 1):597–600. [PubMed] [Google Scholar]
- 21.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 22.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 23.Mottonen T, Hannonen P, Korpela M, Nissila M, Kautiainen H, Ilonen J, et al. Delay to institution of therapy and induction of remission using single-drug or combination–disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46:894–8. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 24.Rouster-Stevens KA, Gursahaney A, Ngai KL, Daru JA, Pachman LM. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;59:222–6. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute Common toxicity criteria. URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.