Abstract
Hemoglobin H-constant spring (Hb H-CS), the most common nondeletional alpha thalassemia in Asia is increasingly recognized in North America due to shifts in immigration patterns. In California, alpha (α)-thalassemia syndromes are the second most frequent finding among newborns screened for hemoglobinopathies with a two-fold increase compared to a decade earlier [1,2]. Though known to have a more severe anemia than Hb H disease, the other clinical findings of Hb H-CS are not well described. Moreover, beneficial therapies that have become available in the last decade are often not applied to their care. This analysis of 46 patients enrolled in the Thalassemia Clinical Research Network (TCRN) age 13+/− 10 years old, with Hb H-CS revealed moderate anemia (mean 8.7 ± 1.5 g/dl), regular transfusion therapy in 24% of patients, and splenomegaly or prior splenectomy in one-third of them. Serum transferin receptor (sTfr), was elevated; (44.4 ± 18 mcg/ml normal range 2.9–8.3 mcg/ml), reflecting ineffective erythropoiesis, which in turn leads to high iron absorption and increased ferritin levels in younger (median = 187 ng/ml) and older (median = 465 ng/ml) nontransfused patients. These findings along with moderate growth delay and low bone mass were more prevalent in Hb H-CS patients compared to deletional Hb H disease. Our results highlight the required monitoring of the extent of anemia, growth, splenomegaly, iron overload, gallstones, bone density and assessment of need for transfusions and specific treatments for disease complications.
The constant spring (CS) termination codon mutation (α142 STOP→Gln; TAA→CAA), is the most prevalent nondeletional α globin mutation in Southeast Asia (SEA) and southern China. DNA diagnosis of Hb H-CS, a combination of two cis a-gene deletions and one CS mutation is often required because the Constant Spring, a slow moving band produced in small quantities, can be missed by electrophoresis. It is inadvertently mistaken for the more common, three α-gene deletion—Hb H disease—typically a milder type of α thalassemia. In North America, clinical data on a thalassemia, in particular concerning Hb HCS, is lacking. Moreover, recent advances in technology for diagnosis and treatment of thalassemia-induced complications are therefore rarely considered for this patient population. We sought to characterize the clinical and hemato-logical findings in patients with Hb H-CS in North America, addressing findings that can impact on their clinical care. Genotyping of 836 thalassemia patients identified 106/836 (12.7%) with Hb H (three gene deletion) and 46/836 (5.5%) with a nondeletional mutation; 44 with Hb H-CS, and two (twin sibling) patients with Hb Dartmouth [3]. Among the patients with Hb H-CS, –SEA/αCSα was the most common genotype, detected in 86% of genotyped patients. (Table I). Mean Hb, available in a subset of patients, was lower in the nontransfused Hb H-CS patients; 8.7 g/dl ± 1.5, compared to patients with Hb H; 9.4 g/dl ± 0.8. Mean Hb in 2/5 patients with Hb H-CS who also carry an E beta globin mutation was lower 7.6 g/dl ± 0.9. All patients were prescribed folic acid supplementation (1 mg daily) and compliance was similar among Hb H and Hb H-CS individuals, at 80%.
TABLE I.
Patients Main Clinical and Hematological Characteristics
| Hb H-CS (N = 46) N (%) or mean (SD) | HbH (N = 106) N (%) or mean (SD) | P value | |
|---|---|---|---|
| Age (y), mean (SD) | 13 (10.6) | 9 (10.9) | 0.03 |
| Gender (male) | 24 (52%) | 52 (49%) | 0.72 |
| Adult (≥18 y) | 11 (24%) | 13 (12%) | 0.07 |
| Splenomegaly | 6 (13%) | 1 (1%) | 0.001 |
| Splenectomy | 6 (13%) | 2 (2%) | 0.01 |
| Regular transfusions (≥8/year) | 11 (24%) | 2 (2%) | <0.0001 |
| Ferritin (ng/ml) nontransfused | 375 (406) | 176 (304) | <0.0001 |
| Hb(g/dl) nontransfused | 8.7 (1.5) | 9.4 (0.8) | 0.09 |
| Alpha globin genotype | 37 (80%) | ||
| SEA deletion/CS | 32 | ||
| Filipino deletion/CS | 2 | ||
| 3.7 kb deletion/CS | 1 | ||
| SEA deletion/Hb darmouth | 2 | ||
| Beta gene phenotype | 35 (76%) | ||
| Heterozygous E mutation | 4 | ||
| Homozygous E mutation | 1 | ||
| No beta mutation | 30 | ||
| Pregnancy (females reproductive age) | 4/10 (40%) | 6/11 (55%) | |
| Cholecystectomy | 4 (9%) | – | |
| Known cholelithiasis | 4 (9%) | – | |
| Bacteremia/sepsis | 3 (6.5%) | 2 (1.9%) | 0.17 |
| Postsplenectomy thrombosis | 1 | – |
Splenomegaly or a prior splenectomy was common among the Hb H-CS patients, but rare in those with Hb H disease; 26% vs 3%, p = 0.0001. In the non transfused patients with Hb H-CS, splenectomy lead to a higher mean Hb level: 9.62 ± 2.44 g/dl vs. 8.40 ± 1.00 g/dl. Postsplenectomy portal vein thrombosis was reported in one subject with Hb H-CS. Cholelithiasis was common, detected in eight patients (18%), 4 symptomatic cases underwent cholecystectomy.
Patients with Hb H-CS had significantly higher levels of sTfr in comparison to the Hb H patient group; 44.4 ± 18 mcg/ml vs. 19.0 ± 9.6 mcg/ml (P < 0.0001, normal 2.9–8.3 mcg/ml). sTfr levels were lower in transfused Hb H-CS patients; 37.6 ± 33.7 mcg/ml.
Eleven of the 46 (24%) with Hb H-CS and 2 (1.8%) of the Hb H patients were placed on regular transfusions and chelation therapy. Mean age of initiation of transfusions was 3.5 ± 1.3 years (range 2–5 years). Among nontransfused patients, mean ferritin level was higher in Hb H-CS than in the Hb H (375.2 ± 406.1 ng/ml vs. 175.9 ± 304.2 ng/ml, P < 0.0001). Ferritin levels were higher in those 18 years or older (n = 9, mean age = 26.7 years) compared to the younger cohort (n = 22, mean age = 10.4 years): 490 ± 285 (median = 465 ng/ml, range:137–1153 ng/ml) vs. 328 ± 443 (median = 187 ng/ml, range: 37–1835 ng/ml). Among transfused Hb H-CS patients mean ferritin concentration was 2511 ± 2262 (median = 1833 ng/ml, range: 329–6852 ng/ml) and liver iron concentration obtained in five of them showed significantly elevated levels (27 ± 14 mg/gm dry wt). None of the patients were reported to have clinically evident iron-induced heart disease.
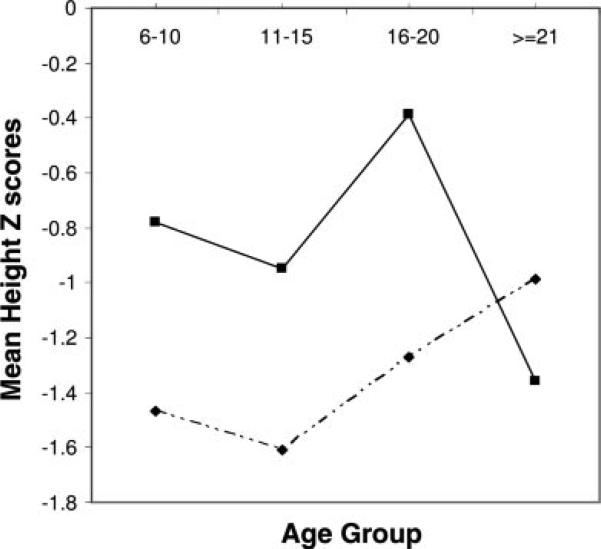
Growth delay was more apparent in the Hb H-CS patients (n = 19) in compared to 20 Hb H patients; Mean height Z score was −1.34 ± 0.98 for Hb H-CS vs. −0.82 ± 1.15 for Hb H (p = 0.16) (Fig. 1). Mean weight Z score was −1.15 ± 0.88 for Hb H-CS vs. −0.83 ± 1.61 for Hb H (p = 0.47). On average, patients with Hb H-CS had lower spine bone mineral density (BMD) Z-scores compared to those in Hb H patients: mean L1-L4 spine Z/T-score of −1.60 ± 0.86 vs. −0.93 ± 0.80 (p = 0.02). None of the Hb H-CS patients had evidence of growth hormone deficiency, diabetes mellitus, hypothyroidism or hypoparathyroidism. Four out of ten (40%) adult females had one or more successful pregnancies, most of whom required transfusion support during pregnancy.
Figure 1.
Height Z score grouped by age in Hb H and in Hb H-CS patients. Height Z-score (dashed line) was reduced (< −1 SD) in all Hb H-CS age groups while normal in Hb H patients <21 years old −(solid line). A discrepancy in height scores in subjects ≥21 years is noted, possibly reflecting different rates of improved growth velocity after reaching puberty.
The compound heterozygocity for a deletional mutation in combination with the CS mutation results in less α-globin mRNA production by the remaining functional α-gene in comparison to a three α-gene deletion. Therefore more α/β imbalance occurs and more Hb H (β4 tetramers) precipitates in the cell causing local oxidative damage, membrane dysfunction and shortened RBC survival [4]. In addition, direct membrane pathology is induced by αCS chains [5]. This results in increased ineffective erythropoiesis and hemolysis, causing a more severe phenotype than that of deletional Hb H as previously described [6–8] and as observed in this cohort of patients. Coinheritance of Hb E (also common in South-East Asia) with H-CS has resulted in a lower Hb; it was previously named AEBart's disease and noted to be more severe [9]. The finding of such coinheritance is useful in genetic counseling and prediction of clinical outcome.
Heterozygocity and H-CS Serum transferin receptor levels have not been assessed before in patients with Hb H-CS. High levels were reported in Hb H patients and in patients with β thalassemia intermedia [10–12]. However, the mean sTfr level in our cohort (44.4 ± 18.2 mcg/ml) was much higher than that reported in these studies; 5.6 ± 1.8 mcg/ml [10] and 25.5 ± 7 mcg/ ml [12], suggesting a higher extent of marrow activity and ineffective erythropoiesis. This concurs with a previous study demonstrating that despite maximal marrow erythropoiesis the compensation for the short RBC survival in Hb H-CS is inadequate [13]. In clinical practice precipitous drop in hemoglobin are frequently observed due to a hemolytic or aplastic crisis; this is frequently triggered by pyrexia or infection. It is likely that the marrow is unable to adequately respond to an abrupt fall in hemoglobin due to an already existing high rate of ineffective erythropoiesis. Episodes of precipitous drop in hemoglobin may influence the decision to initiate regular transfusion therapy and may be unnecessary in some cases. Eleven patients (24%) were started on regular transfusions, a higher rate than that of reports from Thailand and Hong Kong, where transfusion therapy was infrequent [4]. Hemoglobin concentration did not differ between younger and older patients (Table I) as also shown in a Mediterranean type of nondeletional Hb H disease in Sardinia [14]. Long-term studies are needed for evaluation f the change in hemoglobin over time and need for transfusion support during adolescence and in older patients. Several patients in this study have benefited from a splenectomy with increase in hemoglobin and reduced frequency of precipitous drop in hemoglobin. Post surgical portal vein thrombosis was seen in one patient and has been noted before [15], underscoring the higher risk for thrombotic events in some types of thalassemia after splenectomy.
Ferritin levels were moderately elevated in the nontransfused patients and a trend of increase with age was noted. High ferritin levels (841 ± 1,000 ng/ml) and a correlation with age in Hb H-CS has been reported before [6]. Increased iron absorption and subsequent increase liver iron has been described in 85% of patients with Hb H disease [16]. Despite high hepatic pancreatic and pituitary iron observed by MRI, cardiac hemosiderosis or overt endocrine dysfunction was uncommon [17]. A greater risk for iron-induced organ damage can be expected in Hb H-CS. Ferritin levels likely do not reflect the extent of organ hemosiderosis as shown before in nontransfused thalassemia patients (18). Monitoring of liver iron, cardiac and endocrine function might be indicated particularly as untrans-fused Hb H-CS patients reach adulthood. Iron chelation with deferiprone resulted in drop of ferritin levels in patients with Hb-H disease and improved liver iron concentration in 12 out of 16 [19], suggesting that oral chelation should be considered in this patient population as monitoring of iron overload is more often utilized.
Estimated mean spine BMD Z-scores of patients with Hb H-CS disease were worse than the measures in individuals with Hb H disease, at all ages [20]. However, there was no difference in the fracture rate, 2.5% and 2.3% in Hb H and in Hb H-CS, respectively [21]. Growth delay was reported in 13% of patients with Hb H and Hb H-CS in China [6]. We also found growth delay in our patients, but more so in the Hb H-CS subjects, mainly during adolescence (Fig. 1). Short stature was more frequent among the transfused patients (Table II). Hormonal deficiencies did not seem to play a role in the finding of growth delay in our study. Further longitudinal studies are needed to address growth velocity and its relation to anemia, ineffective erythropoiesis and changes of bone mass.
TABLE II.
Growth Parameters Among Transfused and Nontransfused Patients
| Height Z score Mean (SD) | Weight Z score Mean (SD) | |
|---|---|---|
| Hb H-CS (n = 19) | –1.34 (1.0) | –1.15 (0.9) |
| Hb H-CS transfused (n = 4) | –2.15 (0.95) | –1.88 (1.09) |
| Hb H-CS nontransfused (– = 15) | –1.13 (0.9) | –0.96 (0.74) |
| Hb H (n = 17) | –0.82 (1.1) | –0.83 (1.6) |
Transfused patients had lower Z scores suggesting a possible effect of the more severe iron overload noted in this subgroup, on growth and development. Alternatively, these patients may have had a worse clinical course that required imitation of regular transfusions.
In summary, this report highlights the specific complications of Hb H-CS in North American patients. We demonstrate a very high erythropoietic drive and marked ineffective erythropoiesis. Monitoring sTfr levels may prove to be useful in subjects with Hb H-CS and serve as a guide to initiate therapeutic intervention. Proper early diagnosis is needed, and Hb H-CS should be considered when presumed Hb H disease is more severe than expected. Regular monitoring of hemoglobin concentration and measurements of body iron loading can benefit patients, especially adolescents and adults. These parameters may help inform decisions for regular transfusion and chelation. Systematic assessments of bone mass and growth rate are also important in the routine clinical care for these patients.
Methods
Between 2001 and 2006 a total of 836 patients were enrolled and screened in the Thalassemia Clinical Registry Network (TCRN), a North American collaborative research group. The institutional review board of all participating institutions approved the protocol and written informed consent was obtained from all participants. Data on the patients with a DNA based diagnosis of Hb H-CS (or another structural mutation) was assessed and compared to that of patients with Hb H disease.
History of blood transfusions, splenectomy, average serum ferritin levels and liver iron concentration, use of chelation were recorded. Patients’ height was reviewed and evidence for growth delay documented. Growth delay was defined as height for age Z-score <−2.0 using the U.S. CDC growth charts, year 2000). Endocrinopathies; Diabetes, hypogonadal hypogonadism, growth hormone deficiency, hypothyrodism and hypoparathyrodism were assessed. BMD of the spine and whole body was measured by DXA (Hologic Delphi A) and Z-scores calculated. Information on occurrence of severe infections was collected. stFR concentrations were measured by an enzyme immunoassay (EIA; T-94, Ramco Laboratories, Houston, TX).
Descriptive statistics were reported as number and percent or mean and standard deviation (SD). Differences in categorical variables were tested by Chi-square or Fisher's exact test and differences in continuous variables were tested by Student's t-test. Ferritin level was not normally distributed and log-transformation was used. All analyzes were performed at the Data coordinating center (New England Research Institutes, Watertown, MA) with SAS statistical software (9.2, SAS Institute, Cary, NC). P-values less than 0.05 were considered statistically significant.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: U01-HL-65232; U01-HL-65233, U01-HL-65239, U01-HL-65244, U01-HL-65260, U01-HL-65238, M01-RR01271.
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Michlitsch J, Azimi M, Hoppe C, et al. Newborn screening for hemoglobinopathies in California. Pediatr Blood Cancer. 2009;52:486–490. doi: 10.1002/pbc.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorey F, Cunningham G, Vichinsky EP, et al. Universal newborn screening for Hb H disease in California. Genet Test. 2001;5:93–100. doi: 10.1089/109065701753145538. [DOI] [PubMed] [Google Scholar]
- 3.Mcbride KL, Snow K, Kubik KS, et al. Hb Dartmouth [alpha66(E15) Leu-->Pro (alpha2) (CTG-->CCG)]: a novel alpha2-globin gene mutation associated with severe neonatal anemia when inherited in trans with Southeast Asian alpha-thalassemia-1. Hemoglobin. 2001;25:375–382. doi: 10.1081/hem-100107874. [DOI] [PubMed] [Google Scholar]
- 4.Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: Not necessarily a benign disorder. Blood. 2003;101:791–800. doi: 10.1182/blood-2002-07-1975. [DOI] [PubMed] [Google Scholar]
- 5.Schrier SL, Bunyaratvej A, Khuhapinant A, et al. The unusual pathobiology of hemoglobin constant spring red blood cells. Blood. 1997;89:1762–1769. [PubMed] [Google Scholar]
- 6.Chen FE, Ooi C, Ha SY, Cheung BM, et al. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343:544–550. doi: 10.1056/NEJM200008243430804. [DOI] [PubMed] [Google Scholar]
- 7.Styles LA, Foote DH, Kleman KM, et al. Hemoglobin H-constant spring disease: An under recognized, severe from of alpha thalassemia. Int J Ped Hematol/Oncol. 1997;4:69–74. [Google Scholar]
- 8.Ne W, Harano K, Harano T, et al. Hb Constant Spring [alpha 142, Term-->Gln (TAA>CAA in alpha2)] in the alpha-thalassemia of anemic patients in Myanmar. Hemoglobin. 2008;32:454–461. doi: 10.1080/03630260802341588. [DOI] [PubMed] [Google Scholar]
- 9.Boonsa S, Sanchaisuriya K, Fucharoen G, et al. The diverse molecular basis and hematological features of Hb H and AEBart's diseases in Northeast Thailand. Acta Haematol. 2004;111:149–154. doi: 10.1159/000076523. [DOI] [PubMed] [Google Scholar]
- 10.Papassotiriou I, Traeger-Synodinos J, Kanavakis E, et al. Erythroid marrow activity and hemoglobin H levels in hemoglobin H disease. J Pediatr Hematol Oncol. 1998;20:539–544. [PubMed] [Google Scholar]
- 11.Amendola G, Danise P, Todisco N, et al. Lipid profile in beta-thalassemia intermedia patients: Correlation with erythroid bone marrow activity. Int J Lab Hematol. 2007;29:172–176. doi: 10.1111/j.1751-553X.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 12.Camaschella C, Gonella S, Calabrese R, et al. Serum erythropoietin and circulating transferrin receptor in thalassemia intermedia patients with heterogeneous genotypes. Haematologica. 1996;81:397–403. [PubMed] [Google Scholar]
- 13.Pootrakul P, Sirankapracha P, Hemsorach S, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia. Blood. 2000;96:2606–2612. [PubMed] [Google Scholar]
- 14.Origa R, Sollaino MC, Giagu N, et al. Clinical and molecular analysis of haemoglobin H disease in Sardinia: Haematological, obstetric and cardiac aspects in patients with different genotypes. Br J Haematol. 2007;136:326–332. doi: 10.1111/j.1365-2141.2006.06423.x. [DOI] [PubMed] [Google Scholar]
- 15.Tso SC, Chan TK, Todd D. Venous thrombosis in haemoglobin H disease after splenectomy. Aust N Z J Med. 1982;12:635–638. doi: 10.1111/j.1445-5994.1982.tb02655.x. [DOI] [PubMed] [Google Scholar]
- 16.Ooi GC, Chen FE, Chan KN, et al. Qualitative and quantitative magnetic resonance imaging in haemoglobin H disease: Screening for iron overload. Clin Radiol. 1999;54:98–102. doi: 10.1016/s0009-9260(99)91068-1. [DOI] [PubMed] [Google Scholar]
- 17.Au WY, Lam WW, Chu WW, et al. Organ-specific hemosiderosis and functional correlation in Chinese patients with thalassemia intermedia and hemoglobin H disease. Hematol Oncol. 2008;26(4):225–228. doi: 10.1002/hon.862. [DOI] [PubMed] [Google Scholar]
- 18.Pakbaz Z, Fischer R, Fung E, et al. Serum ferritin underestimates liver iron concentration in transfusion independent thalassemia patients as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer. 2007;49:329–332. doi: 10.1002/pbc.21275. [DOI] [PubMed] [Google Scholar]
- 19.Chan JC, Chim CS, Ooi CG, et al. Use of the oral chelator deferiprone in the treatment of iron overload in patients with Hb H disease. Br J Haematol. 2006;133:198–205. doi: 10.1111/j.1365-2141.2006.05984.x. [DOI] [PubMed] [Google Scholar]
- 20.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: A frequent and still unresolved problem. J Bone Miner Res. 2009;24:543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogiatzi MG, Macklin EA, Fung EB, et al. Prevalence of fractures among the Thalassemia syndromes in North America. Bone. 2006;38:571–575. doi: 10.1016/j.bone.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]