Abstract
In this study, we sought to establish a novel method to prospectively and dynamically identify live human oligodendrocyte precursor cells (OPCs) and oligodendrocyte lineage cells from brain dissociates and pluripotent stem cell culture. We selected a highly conserved enhancer element of the Sox10 gene, known as MCS5, which directs reporter expression to oligodendrocyte lineage cells in mouse and zebrafish. We demonstrate that lentiviral Sox10-MCS5 induced expression of GFP at high levels in a subpopulation of human CD140a/PDGFαR-sorted OPCs as well as their immature oligodendrocyte progeny. Furthermore, we show that almost all Sox10-MCS5:GFPhigh cells expressed OPC antigen CD140a and human OPCs expressing SOX10, OLIG2, and PDGFRA mRNAs could be prospectively identified using GFP based fluorescence activated cells sorting alone. Additionally, we established a human induced pluripotent cell (iPSC) line transduced with the Sox10-MCS5:GFP reporter using a Rex-Neo cassette. Similar to human primary cells, GFP expression was restricted to embryoid bodies containing both oligodendrocyte progenitor and oligodendrocyte cells and co-localized with NG2 and O4-positive cells respectively. As such, we have established a novel reporter system that can track oligodendrocyte commitment in human cells, establishing a valuable tool to improve our understanding and efficiency of human oligodendrocyte derivation.
Keywords: SOX10, enhancer, pluripotent, lentivirus
INTRODUCTION
Stem cell therapies have long been proposed as a vector to treat demyelinating disease (Ben-Hur, 2011, Duncan, et al., 2011). Although the selection of optimal cell type for transplantation remains a largely unanswered question, of the currently assessed human phenotypes, only oligodendrocyte progenitor cells (OPCs) have shown the capacity to rescue an otherwise lethal myelin defect and generate large quantities of myelin in vivo (Windrem, et al., 2008). In contrast, human fetal or embryonic stem cell-derived neural stem cells have been largely incapable of oligodendrocyte differentiation (Chandran, et al., 2004, Joannides, et al., 2007). Recently, human neural precursors that have undergone extended periods of expansion in culture have been found to differentiate as oligodendrocytes and generate myelin in vivo (Uchida, et al., 2012).
The techniques necessary to drive oligodendrocyte progenitor fate from neural and pluripotent stem cells are currently inefficient, time consuming, and typically generate mixed cultures containing neuronal elements that are inappropriate for cell therapy in patients. The identification of immature stages of oligodendrocyte development remains technically challenging, especially in the human, and is currently reliant on the expression of cell surface antigens that necessitates end point rather than continuous study. As such, there is an important unmet need to identify oligodendrocyte lineage commitment in live cells and to be able to track oligodendrocyte fate in continuous culture (both dissociates of primary tissue and pluripotent cell cultures). The capability to routinely identify live OPCs would permit us to better understand the temporal regulation of cell fate in human oligodendrocyte development and allow optimization of approaches to induce OPC fate.
The existing surface antigen-based antibody strategies for isolation of human OPCs have significant limitations; 1) cell sorting techniques require a single cell suspension which is typically obtained following enzymatic dissociation. Proteolysis of the target antigen during dissociation can prevent or limit detection and requires optimization for each cellular source (Panchision, et al., 2007). 2) Surface antigen targets such as platelet-derived growth factor alpha receptor (PDGFαR), which is recognized by CD140a antibody (Sim, et al., 2011), can be internalized when cells are cultured in the presence of cognate growth factor ligand thus preventing detection or requiring that media conditions be altered prior to isolation. Finally, 3) age, region, and species-dependent differences in antibody specificity can lead to isolation of heterogeneous populations in human preparations. For example, while rat A2B5-sorted cells were essentially pure OPCs (Barres, et al., 1993), human brain A2B5-sorted cells isolated from 15 to 18 week gestation age fetal brain comprise only 10% OLIG2+ and generate <5% O4+ oligodendrocytes (Cui, et al., 2010). Furthermore, longitudinal studies of lineage commitment cannot be readily performed using antibody-based approaches. Here, we sought to establish a reporter system which tracks commitment to oligodendrocyte lineage as a function of extracellular perturbations such as growth factors or small molecules. In addition, we sought to develop a method that could identify and facilitate extraction of OPCs from mixed cultures while avoiding the problems associated with antibody-based techniques.
Several previous studies have used plasmid and adenoviral vectors to isolate neural stem and progenitor cells from human brain (Keyoung, et al., 2001, Roy, et al., 1999, Roy, et al., 2000, Wang, et al., 2010). Initially, these studies were limited by the low efficiency of plasmid transfection and resulted in poor cellular yields that precluded their routine use for identification of cell fate induction or subsequent study. Later studies, utilizing adenoviral infection to improve transgene delivery, were capable of isolating neural stem and progenitor cells, expressing either nestin, musashi-1, or SOX2, from fetal dissociates. However due to the transient nature of adenoviral gene delivery, these approaches were limited to identification and isolation of cell populations from fresh tissue dissociates. As such they were not appropriate for identification of cell fate induction from longer-term studies involving pluripotent stem cell culture.
In order to overcome the shortcomings faced by the above strategies, we generated a lentiviral-based GFP reporter utilizing a well characterized OPC-specific species-conserved enhancer element identified upstream of the Sox10 gene. Multiple species conserved (MCS) enhancer elements that regulate Sox10 expression have been identified in both zebrafish (Antonellis, et al., 2008) and mice (Kuspert, et al., 2011, Werner, et al., 2007). MCS5 enhancer activity was identified in cultured melanocytes and Schwann cells but not fibroblasts (Antonellis, et al., 2008). Unlike other Sox10-MCS enhancers in developing zebrafish, Sox10-MCS5 directed GFP expression was relatively restricted to dorsal root ganglia and myelinating glia of PNS and CNS. In transgenic mice, the Sox10-U2 enhancer, which encompasses MCS5, was the only enhancer element found to direct LacZ expression to oligodendrocyte lineage cells in the developing spinal cord (Werner, et al., 2007). Interestingly, Sox10-U2 activity was not found in early neuroepithelial cells expressing Sox10 at E12.5 but only in emigrating OPCs that had already left the ventricular zone (Kuspert, et al., 2011). Moreover, Sox10-MCS5/U2 activity appears to be restricted to OPCs as expression was down-regulated in NKX6.2-expressing mature oligodendrocytes in postnatal spinal cord.
As such, we selected the MCS5 enhancer, using it to generate a series of lentiviral reporter constructs to identify OPCs from mixed human cultures. We have characterized reporter activity in human fetal brain dissociates and cultures of induced pluripotent stem cells (iPSCs). GFP expression was restricted to human primary OPCs and immature oligodendrocytes and absent in astrocytes and neurons. Using multicolor FACS, more than 90% of GFP-defined positive cells were CD140a/PDGFαR positive OPCs. This enabled us to prospectively identify OPCs and could be used alone to enrich for OPC and oligodendrocytes cells by FACS. Furthermore, we generated and selected a human iPSCs line containing Sox10-MCS5:GFP that was used to follow OPC fate from pluripotent stem cells. Thus, the Sox10-MCS5:GFP reporter virus represents a valuable new tool to identify OPC fate and will permit the optimization of techniques for the generation of OPCs from pluripotent human stem cells.
MATERIALS AND METHODS
Lentiviral cloning and generation
The pXIG-Sox10-MCS5 plasmid contains Sox10 multiple species conserved enhancer element 5 (MCS5, mouse CH15:79029307-CH15:79028761) coupled with a c-fos basal promoter and eGFP (as described in Antonellis, et al., 2008). The Sox10 dual reporter lentivirus was generated by inserting the Sox10-MCS5:cfos:eGFP cassette from pXIG-Sox10-MCS5 into pTRIP-EF1α-mCherry lentivirus (gift of Abdel Benraiss, University of Rochester). The Sox10 cassette was PCR amplified and TA-cloned using modified primers containing 5′ SpeI restriction enzyme sites into pTOPO (Invitrogen) and sequence verified. The insert was then cloned into a unique SpeI site of pTRIP to generate a dual reporter lentivirus as shown in Fig. 1A. The single reporter lentivirus was generated by cloning Sox10-MCS5:cfos enhancer/promoter cassette into the aMHC-eGFP-Rex-Neo lentiviral reporter (AddGene, #21229) (Kita-Matsuo, et al., 2009). Briefly, the Sox10-MCS5:cfos cassette was removed using XhoI/AgeI digestion, and directionally cloned into aMHC-eGFP-Rex-Neo plasmid following digestion with XhoI/AgeI, thereby replacing the aMHC promoter. The single reporter, which lacks the Rex-Neo selection cassette, was generated by digesting this plasmid with KpnI and SpeI to remove Rex-Neo, then creating blunt ends using the Quick Blunting Kit (New England BioLabs), and blunt-end ligation (Fig. 1F). All three lentiviruses were generated using triple transfection of lentiviral backbone with helper plasmids pLP/VSVG (Invitrogen) and psPAX2 (AddGene, #12260) into 293T cells using calcium phosphate precipitation. Viral supernatants were collected in OPTI-MEM (Invitrogen) at 48 and 72 hours and yielded a titer of at least 106 GFP-transducing U/ml.
Figure 1. Sox10-MCS5 reporter GFP expression in human fetal cells.
Human fetal brain cells were infected with dual reporter virus containing both Sox10-MCS5:GFP and constitutive EF1α-mCherry reporters (A). CD140a-sorted OPCs were allowed to differentiate in serum-free media following infection (n=3, fetal brain preparations). At 3–4 days, CD140a+ cells expressing GFP were co-labeled with immature oligodendrocyte marker O4 (B) and oligodendrocyte transcription factor OLIG2 (C). All GFP+ cells expressed OLIG2 (arrow) but not all OLIG2+ cells co-expressed GFP (*). CD140a-depleted cells were similarly infected with dual reporter and fixed at the same time point. Although both infected GFAP+ astrocytes (D) and TuJ1/βIII-tubulin+ neurons (E) expressed mCherry under the constitutive EF1α promoter, Sox10 reporter GFP was either weakly expressed or not detected. To permit flow cytometry, a single reporter virus was also constructed that lacked constitutive mCherry expression (F). To confirm the specificity of GFP expression using this vector, CD140a+ OPCs were similarly infected and allowed to differentiate for 4 days in vitro (n=3, fetal preparations). G, cultures were visualized for native GFP fluorescence and immunostained with O4 (red) and NG2 (cyan), and counterstained with DAPI (blue). Brightly labeled GFP cells (GFPhigh) co-expressed immature oligodendrocyte marker O4 (#) and/or OPC marker NG2 (‡). In addition, several weakly positive GFP cells were observed that did not co-express either NG2 or O4 (arrow). Scale Bars: 50 μm.
Cell and tissue samples
Fetal brain samples (17–22 weeks gestational age) were obtained from patients who consented to tissue use under protocols approved by the State University of New York at Buffalo institutional review board. Cortical tissue, including ventricular and sub-ventricular zone, was collected, minced and dissociated using papain and DNase as previously described (Windrem, et al., 2002). Following dissociation, cells were suspended into DMEM/F12/N1 serum-free media (as detailed in Sim, et al., 2011), with 10ng/mL FGF2 (PeproTech, Rocky Hill, NJ).
Magnetic sorting for CD140a+ oligodendrocyte progenitor cells
Magnetic sorting of CD140a cells was performed, as described (Conway, et al., 2012). Briefly, cells were recovered and stained with CD140a PE-conjugated purified mouse IgG2a antibody (BD Pharmingen, San Diego, CA). Cells were washed and rat anti mouse IgG2a+b secondary antibody added according to manufacturer’s instructions (Miltenyi Biotech, Auburn, CA). Magnetic sorting was performed using LS column selection and a sample of positive cells collected for subsequent flow cytometry-based analysis of purity. For sorts with low purity, cells were passed over a second column to achieve a threshold of >80% CD140a+. CD140a cells were infected with lentivirus 24hr post seeding.
Immunocytochemistry
Live cultures were immunostained for oligodendrocyte marker O4, as described (Sim, et al., 2006). O4 supernatant (gift of Dr. James Goldman, Columbia University) was used at a dilution of 1:25, applied for 30 minutes at 37°C. Cultures were fixed in 4% paraformaldehyde and immunostained using primary antibodies against NG2 (1:250, clone 9.2.27, Millipore, Billerica, MA), OLIG2 (1:2000, Millipore), GFAP (1:400, clone G-A-5, Sigma-Aldrich, St. Louis, MO) and βIII-tubulin (clone TuJ1, 1:1000, Covance). Secondary antibodies Alexa-488, 594 and 647 conjugated goat-anti mouse IgM, rabbit and rat antibodies were used at a dilution of 1:500 (Invitrogen, La Jolla, CA). Cultures were visualized using an inverted epi-fluorescence microscope (Olympus IX51) and imaged using a cooled CCD camera (Hamamatsu).
Flow cytometry and cell sorting
Flow cytometry and fluorescence activated cell sorting (FACS) were performed using a BD FACSAria. Unsorted cells were infected with Sox10 reporter lentivirus 7–8 hours post seeding maintained in serum-free media containing PDGF-AA and FGF-2 (both 10ng/ml) for 3–4 days. 24hrs prior to flow cytometry, PDGF-AA was removed from media to permit CD140a antigen expression. Fetal brain dissociates were recovered using calcium-free media and stained with CD140a PE-conjugated antibody, O4 IgM hybridoma supernatant (gift of Dr. James Goldman, Columbia University), and goat anti-mouse IgM F(ab′) APC-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA). Matched fluorescence minus-one controls were used to set appropriate gates following forward and side scatter-based gating and doublet discrimination to exclude dead cells and doublets respectively.
For GFP cell sorting, 1 × 106 cells dissociated cells were infected with Sox10-MCS5:GFP single reporter lentivirus 3–6 hours post-seeding and maintained in serum-free media containing PDGF-AA and FGF-2 (both 10ng/ml). GFP-based FACS was performed 3–5 days later.
RNA isolation and quantitative RT-PCR
Immediately following FACS, RNA was extracted from sorted fractions using E.Z.N.A Total RNA Kit I (Omega Bio-Tek, Norcross, GA) according to manufacturer’s protocols and cDNA prepared using random hexamers (Superscript III kit; Invitrogen, Carlsbad, CA1). Human-specific primers for SYBR green-based PCR were designed using Primer Express (v1, Applied Biosystems, Foster City, CA) (Table 1). For SOX10 and OLIG2, pre-designed primers and Taqman® probes were ordered directly from Invitrogen and real-time PCR performed according to manufacturer’s instructions. Samples were run in duplicates for real-time PCR (MyiQ; Bio-Rad, Hercules, CA) and gene expression calculated by ΔΔCt analysis. Gene expression was normalized to the control gene GAPDH.
Table 1.
Primers detecting human OPC markers
| Primer | Sequence (5′ to 3′) |
|---|---|
| GAPDH | Fwd: GTGAAGGTCGGAGTCAACGG Rev: CCTGGAAGATGGTGATGGGA |
| PDGFRA | Fwd: CCTGGTGCTGTTGGTGATTG Rev: ATACCTCGGTTTCTGTTTCCAAAT |
| SOX10 | Invitrogen Assay - Hs00366918_m1 |
| OLIG2 | Invitrogen Assay - Hs00300164_s1 |
Human iPSC culture and OPC induction
Undifferentiated human iPSCs (iPS-DF19-9-7TA; WiCell) were cultured on Matrigel (BD) and fed daily with mTeSR1 (Stemcell Technologies). To generate stably integrated Sox10 reporter lines, recently passaged undifferentiated iPSCs were infected with Sox10-MCS5:GFP single reporter lentivirus in the presence of polybrene (4μg/ml) for 24 hours. Four days after virus infection, hiPSCs were treated with G418 (400μg/ml) for 36 hours, rinsed twice with DPBS to remove drugs, and cultured for three days in mTeSR1 with daily media change to permit recovery. Recovered cells were then treated with G418 a second time and allowed to recover as before until colonies reached a sufficient size and density for passage.
To generate embryoid bodies (EBs), colonies of undifferentiated Sox10-MCS5-eGFP-Rex-Neo iPSCs were grown to 80% confluence, treated with dispase (BD) for 7 minutes and gently scraped from the culture dish. Cell clusters were titrated to small aggregates and plated at an approximate density of 5000 cells/mm2 on ultra-low well cluster dishes (Corning) in Neurobasal/DMEM-F12 (“N2/B27”) media supplemented with ROCK inhibitor Y-27632 (10μM; Tocris). Following overnight recovery, EBs were differentiated to neural epithelia for 5 days (Stage 1) in N2/B27 media supplemented with dorsomorphin (2μM; Tocris) and SB431542 (10nM; Tocris) in suspension with daily media changes. On days 6–10, EBs were patterned to ventral progenitors (Stage 2) with exposure to retinoic acid (10μM; Sigma) and purmorphamine (1μM; Cayman Chemicals) in N2B27 basal media. Induction of pre-oligodendrocytes (Stage 3) was accomplished by incubating EBs in N2B27 media supplemented with bFGF (20ng/ml), retinoic acid (0.2μM), and purmorphamine (1μM) for 10 days. On day 20, EBs were collected and plated onto poly-L-ornithine (0.002%, vol/vol; Sigma) and fibronectin (100ug/ml; Invitrogen) coated 6 well dishes and exposed to N1B27 media supplemented with purmorphamine (1μM), PDGF-AA (10ng/ml), IGF-1 (10ng/ml), and NT-3 (10ng/ml) with daily media changes for an additional 15 days. Cells were observed on a daily basis during differentiation for eGFP expression using a Zeiss Axiovert 200 inverted fluorescence microscope. Cultures were selected for live and fixed immunocytochemistry at various stages of oligodendrocyte differentiation as described above.
RESULTS
Sox10-MCS5 enhancer directs GFP expression in human OPCs
A series of multiple species conserved (MCS) enhancers have been characterized that coordinate Sox10 gene expression in zebrafish and mouse CNS (Antonellis, et al., 2008). Of these, MCS5, also known as U2, was identified as an enhancer capable of driving expression in OPCs in the developing mouse spinal cord (Werner, et al., 2007). To test whether MCS5 enhancer activity was capable of identifying OPCs from human sources, we generated a lentiviral construct containing GFP driven by a c-fos minimal promoter coupled with the MCS5 enhancer (referred to as Sox10-MCS5:GFP). In addition, to determine infection efficiency, we included a second expression cassette containing a constitutive promoter EF1α driving expression of the mCherry protein (Fig. 1A).
To determine whether the Sox10-MCS5 enhancer could activate GFP expression in human OPCs, we isolated PDGFαR/CD140a+ cells from fetal human brain dissociates (Sim, et al., 2011). CD140a+ sorted OPCs cells were infected with dual reporter lentivirus construct 24 hours post-seeding. Following 3–4 days of differentiation in serum-free medium (SFM) lacking growth factors, a large fraction of cells expressed mCherry indicating successful viral transduction. Among lentiviral-infected mCherry-expressing cells, 51.0 ± 1.3% exhibited very high levels of GFP expression (n=3, fetal brain preparations, 19–22 wks gestational age). In 4 days, 20–30% of CD140a+ OPCs undergo oligodendrocyte differentiation to O4+ immature oligodendrocytes (Sim, et al., 2011). In matching conditions, we observed numerous O4+ immature oligodendrocytes all of which expressed both mCherry and GFP reporter genes (Fig. 1B) (n=3, fetal preparations). Importantly, all cells expressing high levels of GFP were also co-labeled with OLIG2 a marker of oligodendrocyte lineage cells (Fig. 1C). However the converse was not true and a significant subset of OLIG2+ cells were observed that did not drive high levels of Sox10 enhancer dependent GFP expression.
To better assess the specificity of GFP expression, we next examined GFP expression among CD140a depleted cells which largely comprise immature neurons and some astroglial cells. CD140 negative cells were plated into the same media conditions, infected and then immunostained for neuronal (Tuj1) and astrocytic (GFAP) fate (Fig. 1D–E, n=3 fetal preparations). We found that no Tuj1 or GFAP positive cell expressed high levels of GFP. We additionally stained these cultures for OLIG2 and found that all of bright GFP cells were OLIG2+ even among this OPC depleted population (data not shown). As a relatively large number of cells expressed relatively low levels of GFP, we hypothesized that while the c-fos minimal promoter may drive weak GFP expression in a non-specific manner GFP high expressing cells were restricted to oligodendrocyte lineage.
CD140a− and O4-defined human OPCs differentially drive Sox10-MCS5 enhancer activity
In order to quantitatively assess the phenotype of Sox10-MCS5:GFP expressing cells via flow cytometry, we generated a simplified lentiviral reporter construct that lacked constitutive mCherry expression (Fig. 1F). Similar to the dual reporter virus, in fixed cultures high levels of GFP expression was restricted to NG2 and O4-expressing cells (Fig. 1G), while weakly GFP+ cells did not co-localize with either NG2 or O4 (indicated with arrow) (n=3, fetal brain preparations). We next asked whether MCS5 enhancer activity was enriched within specific sub-populations of human oligodendrocytes and oligodendrocyte progenitors by flow cytometry.
Fetal brain dissociates were infected 7–8 hours post seeding. At 3–4 days, following expression of detectable GFP, cells were prepared for O4 and CD140a multicolor flow cytometry (Fig. 2A/B). We have previously shown that among these fractions both CD140a-defined populations are mitotic OPCs capable of oligodendrocyte differentiation in vitro (Conway, et al., 2012), while CD140a−O4+ cells are largely post-mitotic oligodendrocytes. Consistent with our results on fixed cells, we observed two populations of low and high GFP expressing cells. Given that low GFP expression was relatively non-selective in fixed cells, we hypothesized that weak GFP expression was due to basal activity from the c-fos minimal promoter. We constructed an alternative vector lacking the Sox10-MCS5 enhancer and infected fetal dissociates. The frequency of weakly GFP expressing, GFPlow, cells was similar between cells infected with c-fos basal promoter virus and Sox10-MCS5 virus (data not shown). In contrast, the incidence of GFPhigh cells was essentially abolished in c-fos only virus infected cells, 0.07 ± 0.01%, representing a reduction of >19 fold to matched Sox10 reporter-infected cultures (n=3, 17–18 week gestational age tissue samples). As such, we specifically gated highly expressing GFP+ cells, hereby referred to as GFPhigh. Strikingly, we observed that almost all GFPhigh defined cells were CD140a+ OPCs (90.3 ± 3.2%, mean ± SEM; n=3, fetal brain preparations). Indeed, as endogenous (Sim, et al., 2011) and media derived PDGF-AA can induce PDGFαR internalization (data not shown), the proportion of CD140a+ cells among GFPhigh may likely be an underestimation. In contrast, the proportion of O4+ oligodendrocytes among GFPhigh cells was much lower (6.1 ± 2.4%) but rather reflected the O4+ frequency among the entire CD140a+ population (5.4 ± 1.5%). Among all CD140a+ cells, only 35 ± 9% were GFPhigh cells (n=3, fetal brain preparations). The lower than expected yield was in part due to incomplete viral transduction at 1 MOI (approximately 80–90% cells are transduced with a similar constitutive EF1a-driven promoter). However, together with the dual reporter experiments described above, the low yield suggested that CD140a-defined cells were indeed heterogonous with respect to Sox10-MCS5 enhancer activity and that only a sub-population was capable of driving high GFP expression.
Figure 2. Fetal CD140a/O4-defined OPCs drive Sox10-MCS5 enhancer activity.

Unsorted human fetal brain dissociates, 19–22 weeks gestational age, were infected with single Sox10 reporter virus 7–8 hours following plating. Multicolor flow cytometry performed was 3–4 days post-infection with CD140a and O4 (n=3, fetal brain preparations). A–B, Representative dot plots and cell-type incidence (%) showing GFP reporter expression (x-axis) plotted against CD140a-PE (A) or O4-APC (B) fluorescence (y-axes) (19 week, 10,000 events shown). C, mean GFP fluorescence intensity among individual CD140a/O4 defined cell populations following normalization to CD140a−O4− cell intensity (n=3, mean ± SEM). * indicates significantly higher GFP expression among CD140a+O4+ defined OPCs (p<0.05, Tukey’s multiple comparison test p-value vs. double negative cells).
As such, we analyzed GFP intensity in individual cells and determined the relative level of GFP expression among O4-defined subpopulations of CD140a+ OPCs (CD140a+O4− and CD140a+O4+ cells) and in CD140a−O4+ oligodendrocytes (Fig. 2C). Both CD140a+ populations expressed GFP at more than 10 fold higher levels than double negative cells. However, CD140a+O4+ cells expressed approximately 2 fold more GFP on a per cell basis suggesting differential enhancer activity in O4+ subpopulation of OPCs. Interestingly, GFP expression was significantly lower in CD140a−O4+ oligodendrocytes compared to OPCs (repeated measures 1-way ANOVA, F(3,8)=5.077, Tukey’s multiple comparison test p<0.05, n=3 fetal preparations). Together, these data suggested that GFP expression alone may be sufficient to identify a subpopulation of OPCs present in fetal brain dissociates.
Sox10-MCS5 reporter may be utilized to prospectively isolate human OPCs
Having established that >90% of GFPhigh cells expressed CD140a antigen, we next asked whether GFP expression alone might facilitate isolation of OPCs from fetal human brain isolates. GFP-based FACS was performed 3–5 days following infection (Fig. 3A). As described above, we could distinguish three populations of cells; null, low and high GFP+ cells. We found that 46 ± 6% of total cells did not express GFP (null; n=9, fetal preparations). GFP-expressing cells were subdivided on the basis of fluorescence intensity into GFP ‘low’ (36% ± 4) and a smaller fraction of GFP ‘high’ cells (8.0 ± 1.7%) (Fig. 3B). To determine, the cellular identity of each population, we extracted RNA from 5,000–15,000 collected cells. Real time quantitative RT-PCR analysis revealed strong enrichment of SOX10, PDGFRA, and OLIG2 mRNAs specifically in GFPhigh cells (Fig. 3C). For example, we observed a 28 fold enrichment of SOX10 mRNA in GFPhigh cells versus GFPlow, and 44 fold vs. GFPnull cells (paired t-test, p<0.05). These results suggest that GFPhigh cells represent OPCs, corresponding with the subpopulation of CD140a+ cells described above, and indicate that the Sox10-MCS5:GFP reporter may be used to prospectively identify human OPCs from mixed fetal brain dissociates.
Figure 3. Sox10-MCS5 GFP expression prospectively identifies OPCs from human fetal dissociates.
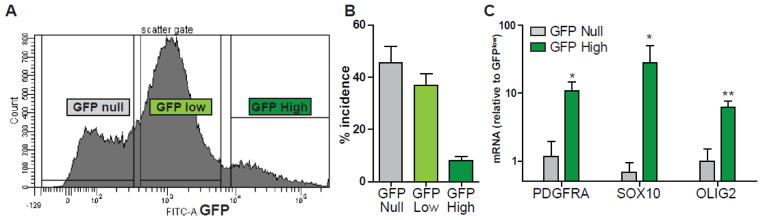
To determine whether the Sox10 reporter could be used to identify and isolate OPCs from mixed cultures, we infected dissociates of fetal human brain with Sox10 single reporter virus (n=9, fetal brain preparations; 18–22 gestational weeks). Following detectable GFP expression, at 3–5 days, FACS was performed to separate cells according to GFP expression (A) (21 week, 200,000 events shown). B, the incidence of GFPnull, GFPlow and GFPhigh defined populations was quantified (mean ± SEM, n=9). Immediately following sort, RNA was extracted from each fraction and OPC marker gene expression measured using real-time quantitative RT-PCR (C). Expression ratios were calculated following normalization to GAPDH expression and to the GFPlow population (ΔΔCt analysis, mean ± SEM, n = 4, fetal preparations). GFPhigh cells were found to express very high levels, >10 fold higher, of PDGFRA, SOX10 and OLIG2 mRNA relative to the other populations. * and ** indicate p<0.05 and p<0.01 (paired t-test GFPhigh vs. GFPnull, n = 4).
Sox10-MCS5 enhancer recognizes human iPSC-derived oligodendrocytes
We next asked whether the lentiviral Sox10-MCS5:GFP reporter could identify human OPCs and oligodendrocytes derived from human pluripotent cells. To this end, we modified the single Sox10-MCS5:GFP lentivirus to include a Rex promoter-driven neomycin resistance (Rex-Neo) cassette. The Rex-1 gene encodes a zinc finger transcription factor that is highly expressed by pluripotent stem cells and is rapidly downregulated upon differentiation (Thompson and Gudas 2002). As such, antibiotic selection based on Rex1 promoter allows the selection of only iPSCs successfully infected with the lentiviral reporter construct and not their differentiated progeny (Kita-Matsuo et al. 2009). While maintaining 19-7-7t iPSCs in the pluripotent state (see Methods), we infected cells with Sox10-MCS5:GFP/REX:NEO virus and selected a new cell line termed ‘Sox10eGFP’ using G418. We used a modified version of the protocols described by (Hu, et al., 2009, Jang, et al., 2011) to induce oligodendrocyte fate. Sox10eGFP embryoid bodies (EBs) were differentiated to neural epithelia in the simultaneous presence of SB431542 and dorsomorphin (DM), small molecule inhibitors of Activin/Nodal and BMP respectively. Cells were subsequently directed towards ventral spinal cord with retinoic acid (RA) and the sonic hedgehog (SHH) mimetic purmorphamine and induced to OPC fate with concurrent addition of bFGF. These progenitor cells were further maintained in the pro-oligodendrocyte lineage growth factors, PDGF-AA, NT-3 and IGF1. Immediately following induction to EBs, we observed weak GFP expression in numerous cells throughout stage I (data not shown). However, upon EB exposure to retinoic acid and purmorphamine in stage II, GFP expression was rapidly extinguished and did not reappear until days 8–10 of stage III (not shown). To determine the fate of GFP-expressing cells, we plated EBs onto poly-ornithine/laminin-substrate and allowed cells to migrate out for 3–4 weeks (Fig. 4A/B). Interestingly, not all EBs contained GFP+ cells (Fig. 4E) and, within individual GFP+ EBs, only a subpopulation of cells expressed high levels of GFP. Cultures were stained with NG2 and O4 to detect OPCs and immature oligodendrocytes, respectively. Interestingly, no NG2+ cells were found growing from EBs which lacked GFP-expression (Fig. 4F). In contrast, numerous NG2+GFP+ co-labeled cells were found migrating out from GFP+ EBs and displayed a characteristic bipolar morphology similar to primary OPCs (Fig. 4C/D and insert). A large number of NG2+ cells lacked GFPhigh expression suggesting that, as in primary cultures, Sox10-MCS5 reporter activity was restricted to a subpopulation of OPCs. We removed growth factors from parallel cultures to encourage oligodendrocyte differentiation. Importantly, we observed the production of several highly branched O4+ cells all of which expressed high levels of GFP (Fig. 4G–L). These results indicate that Sox10-MCS5 enhancer expression retains its fidelity in oligodendrocyte lineage cells and may also be used to recognize oligodendrocyte fate from induced pluripotent stem cells.
Figure 4. Sox10-MCS5 reporter identifies OPCs generated from human iPSCs.
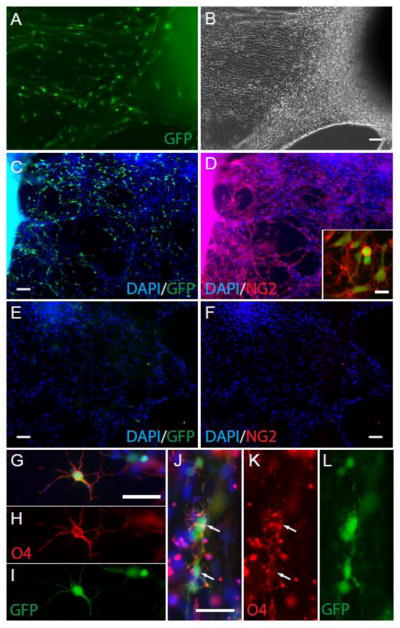
Sox10-MCS5 lentiviral transduced 19-7-7t iPSCs were induced to ventral spinal cord fate as embryoid bodies as described. EBs grown in OPC media were plated onto fibronectin on day 34, grown in PDGF, IGF and NT-3, and imaged at 60 days for GFP expression (A) and phase microscopy (B). Numerous bipolar GFP+ cells were observed migrating from individual EBs. Those EBs containing GFP+ cells were found to contain a high number of NG2-expressing cells (C–D). Almost all GFP+ cells co-expressed NG2 (D, inset). Of note, not all EBs contained GFP positive cells (E) and strikingly in those GFP− EBs we never observed NG2 or O4 staining (F). To encourage oligodendrocyte differentiation, we removed growth factors for 7 days from wells containing GFP+ cells. O4+ immature oligodendrocyte cells (red) were observed in the periphery of GFP+ EBs (G–L). O4+ cells exhibited multi-branched oligodendrocyte-type morphology and almost all co-expressed Sox10-MCS5 reporter GFP. Scale Bars: 100μm in (A–F), 20μm (D, insert), and 50μm in (G–L).
DISCUSSION
In this study, we describe a novel means to track and identify oligodendrocyte progenitor fate from mixed human cultures. In the selection of appropriate markers of oligodendrocyte progenitors, we considered OLIG1/2, NKX2-2, PDGFRA, CSPG4 (NG2) and SOX10. Although a recent study identified several enhancer elements located near OLIG1/2, these enhancers exhibited ectopic expression in irrelevant non-neural cell lines or were expressed in adult neurons in the cerebral cortex (Friedli, et al., 2010). Additionally, as OLIG2 is natively expressed by neural progenitor cells at early stages of development in cells capable of generating various neuronal and astrocytic fates as well as oligodendroglia fate, we did not consider OLIG2 further as a useful candidate for an OPC-specific reporter (Furusho, et al., 2006, Ligon, et al., 2006, Masahira, et al., 2006). Similar to OLIG2, NKX2-2 is expressed by early neuronal progenitors in the spinal cord and not restricted to oligodendrocyte lineage cells (Briscoe, et al., 1999). Also, the only described NKX2-2 enhancer has only been characterized in embryonic spinal cord and not in OPCs (Lei, et al., 2006). Lastly, promoter/enhancer elements active in OPCs for either PDGFRA or CSPG4 have not yet been described. In contrast, Sox10 is required for neural stem cell differentiation into oligodendrocytes and expression in the CNS is restricted to oligodendrocyte lineage cells (Stolt, et al., 2002). Relatively short (<1.5kb) size multiple species-conserved enhancer elements of Sox10 have been described which direct gene expression to OPCs and oligodendrocyte in both zebrafish and mice (Antonellis, et al., 2008, Werner, et al., 2007). Of these, we selected the Sox10-MCS5 enhancer as activity was relatively restricted to myelinating glia in the PNS and CNS.
We characterized the activity of the Sox10-MCS5 enhancer in human primary brain and induced pluripotent stem cell-derived culture. As enhancer activity alone is not sufficient to drive reporter expression without a promoter, we coupled Sox10-MCS5 with a c-fos basal promoter. Among the fetal brain dissociate, we found a relatively large number of cells expressed low levels of GFP following infection. However in addition to these weakly expressing cells, we observed a discrete population of highly GFP+ cells which we termed GFPhigh. We found that GFPhigh cells were OLIG2 expressing cells and co-localized with markers of OPCs and immature oligodendrocytes such as NG2 and O4. In unsorted dissociates, >90% of GFPhigh cells co-expressed CD140a/PDGFαR suggesting that almost all of the Sox10-MCS5 population comprise OPCs in 19–22 week gestational age brain. Furthermore, when GFPhigh cells were isolated via FACS we found that these cells were >10 fold enriched in transcription factors that are highly expressed by OPCs.
The relatively high number of weakly GFP-expressing cells suggested that the c-fos basal promoter retains residual transcriptional activity, which was confirmed when we used the c-fos promoter alone. Several improvements could be made to the current system. We expect that a reduction in non-specific activity of the Sox10 reporter can be achieved by utilizing an alternative basal promoter. Both β-globin and hsp68 promoters have been used in conjunction with cell-specific enhancers to isolate defined human cells from tissue dissociates (Roy et al. 2004; Roy et al. 2000; Wang et al. 2010). Although β-globin exhibits less endogenous activity than hsp68 in vivo (Timmer et al. 2001), the increased specificity is often at the expense of sensitivity due to lower fluorescent reporter expression. In addition to selection of an optimal basal promoter, increased reporter specificity in iPSC cultures could be obtained by inclusion of an mir-294 microRNA target site that effectively silences non-specific expression in pluripotent cells (Diekmann et al. 2012). However, our current data show that the existing construct is already sufficient to prospectively identify OPCs in mixed fetal brain and iPSC culture.
Using the dual reporter lentivirus, we found that around half of the infected CD140a+OLIG2+ cells expressed high levels of GFP. Likewise, less than half of CD140a+ OPCs were identified as GFPhigh by flow cytometry. Why was the apparent yield of the Sox10-MCS5 enhancer so low? Although the presence of c-fos driven GFPlow cells and incomplete viral transduction may contribute, it is likely that human CD140a+ OPCs are themselves heterogeneous with respect to enhancer activity. In the embryonic mouse spinal cord, Sox10-expressing cells resident in the VZ do not activate the MCS5/U2 enhancer (Kuspert, et al., 2011). However once Sox10-expressing OPCs emigrate from the VZ and into the spinal cord parenchyma, and until they differentiate to oligodendrocytes, they drive MCS5/U2 enhancer activity. CD140a+ cells are present in both VZ and parenchyma in the developmental period assessed in this study (Sim, et al., 2011). As such, fetal brain dissociates contain both VZ resident and parenchymal OPCs populations. Thus, not all CD140a+ cells are expected to activate MCS5/U2 and the frequency of Sox10-MCS5 driven GFPhigh cells among CD140a+ cells will likely vary based on the exact tissue composition of the received tissue sample. Human CD140a/PDGFαR+ cells represent a highly enriched population of myelinogenic progenitor cells. However, it is intriguing that Sox10-MCS5 GFPhigh cells represent a subfraction of CD140a+ cells that may exhibit a differential capacity for oligodendrocyte differentiation and myelination.
In mouse development, Sox10-MCS5/U2 enhancer activity is repressed in mature oligodendrocytes during mouse spinal cord development (Kuspert, et al., 2011). We determined the relative activity in CD140a and O4 double-sorted human oligodendroglial cells. Consistent with restricted OPC activity, GFP expression was very highly enriched among CD140a+ populations. The level of GFP fluorescence on a per-cell basis was greatest in CD140a+O4+ OPCs and significantly down-regulated in more mature CD140a−O4+ oligodendrocytes suggesting that the enhancer was less active in differentiated cells as observed in mouse. Yet, in fixed cultures of CD140a+ cells we observed GFPhigh expression among O4+ morphologically differentiated oligodendrocytes. Given the relatively long half-life of GFP protein, OPCs infected at day 0 would be expected to drive Sox10 enhancer prior to oligodendrocyte differentiation. As such, these cells would likely retain GFP expression for several days. The use of a destabilized GFP variant, such as eGFP-MODC (Li, et al., 1998), would permit us to better determine the point at which MCS5 enhancer activity is lost during human oligodendrocyte differentiation. In contrast, the cultures used for flow cytometry were maintained in PDGF/FGF containing media to prevent differentiation. Thus, although we have not directly assessed whether Sox10-MCS5 enhancer remains active in more mature stages of oligodendrocyte lineage development, the quantitative flow cytometry data and mouse transgenic data suggest that MCS5 activity would be lost in myelinating human oligodendrocytes. Together with Sox10-MCS7, whose expression is restricted to mature oligodendrocytes in zebrafish, a combination of lentiviral reporters could permit the development of lentiviral microarrays to monitor oligodendrocyte fate in real-time (Tian, et al., 2010).
Sox10-MCS5 driven GFPhigh expression and FACS was sufficient to prospectively identify and isolate human OPCs from fetal brain dissociates. GFPhigh cells were enriched for cells expressing OPC markers, PDGFRA, OLIG2, and SOX10, indicating that GFP-based identification alone was capable of identifying human OPCs. Previously, a promoter approach was taken to identify adult human OPCs using a large 5kb CNPase promoter (Roy, et al., 1999). However in that study, plasmid-based delivery to primary human cells greatly limited isolation efficiency. Thus, the Sox10 viral approach represents a novel way of identifying human OPCs from fetal human brain in addition to antigen-based approaches such as A2B5 (Windrem, et al., 2004) and PDGFRA/CD140a (Sim, et al., 2011). Antibody-based identification strategies remain the ideal approach for the isolation of OPCs from fetal brain as sorting can be performed on the day of dissociation and does not involve viral infection. However, CD140a-based identification of human OPCs from continuous culture is limited by PDGF-dependent receptor internalization. In comparison, Sox10-based isolation of human OPCs can be performed in complete media containing PDGF-AA and using standard dissociation protocols. Furthermore, as antibody approaches typically are typically end-point in nature and cannot readily be performed on the same cell population, the viral approach described herein will allow for the longitudinal study of OPC fate from primary human neural stem cells as well as from pluripotent cell derived populations.
To illustrate the capacity of the Sox10-MCS5 enhancer as a means to identify OPC cell fate, we generated an iPSC line transduced with Sox10-MCS5 reporter lentivirus and selected cells stably integrating the cassette using antibiotic resistance. This selection cassette has been used to generate lines from established embryonic stem cell lines (Kita-Matsuo, et al., 2009). The benefit of this approach is that a line containing the reporter can be generated in a short period of time (~2–3 weeks) using a simple antibiotic selection strategy that obviates the need for expensive and technically challenging approaches using homologous recombination or zinc-finger nucleases. Using Sox10eGFP iPSCs, we found that GFP expression was absent in the pluripotent state and transiently induced at low levels as cells were induced to neural lineages using the dual SMAD inhibition protocol. Upon induction to OPC fate, we observed high level of GFP expression in presumptive OPCs that were later confirmed using NG2 and O4 markers of oligodendrocyte lineage. Consistent with our observations in primary human cells, a subset of NG2+ cells activated the Sox10-MCS5:GFP reporter that likely correspond with Sox10+ VZ emigrants observed in developing mouse spinal cord (Kuspert, et al., 2011). We observed heterogeneous GFP expression between individual EBs. Heterogeneous neural differentiation from individual EBs has been associated with variable initial size (Bauwens, et al., 2008), and OPC derivation from human ES has been based on selection of aggregates based on their visual appearance (Nistor, et al., 2005). As such, the Sox10-MCS5 enhancer will permit high fidelity selection of OPC-induced EBs based on GFP expression. This proof-of-principle experiment indicates that the Sox10 reporter virus permits the dynamic study of OPC fate, and as such will allow us to study several important questions regarding human OPC specification. For example, at which time point following media-based induction is GFP first induced? At this stage, are GFPhigh cells committed to oligodendrocyte differentiation? Does the timing of development of iPSCs derived from patients with leukodystrophies or other myelin disorders differ from normal human iPSCs? In addition, Sox10 enhancer based tracking of OPC fate will also provide a valuable tool for screening of small molecules capable of inducing oligodendrocyte cell fate. We are actively using this new reagent to address these and related questions.
We show that Sox10-MCS5 activity was restricted to oligodendrocyte lineage cells in primary brain dissociate and CNS-induced cultures from pluripotent stem cells. However, it is important to note that this enhancer would likely be active in both human Schwann and melanocyte cell lineages (Antonellis et al. 2008). As such, in for example, CNS remyelination, the combination Sox10-MCS5:GFP with another CNS-specific antigen would be necessary to distinguish oligodendrocyte from Schwann cell fate. In summary, we describe the generation of three lentiviral reporters capable of identifying human OPCs. 1) A dual reporter that can reliably assess absolute incidence of OPCs by indicating infected cell with a constitutive EF1α-driven reporter. 2) A simple single reporter which permits direct FACs-based analysis and can be used to routinely identify OPC fate from mixed cultures, and 3) a Sox10-MCS5:GFP and Rex-Neo virus which permits the rapid establishment of reporter cell lines from pluripotent stem cells for the study of directed differentiation.
Highlights.
We generated lentiviral vectors to track oligodendrocyte progenitor fate.
Sox10-MCS5 enhancer activity was restricted to a subpopulation of human OPCs.
Lentiviral Sox10-MCS5:GFP may be used to identify and isolate live OPCs.
Induction of OPC fate in iPSCs may be followed in vitro using the Sox10 reporter.
Acknowledgments
This work was supported by the Empire State Stem Cell Fund through New York State Department of Health Contracts C026413 and C026428. Opinions expressed here are solely those of the author and do not necessarily reflect those of the Empire State Stem Cell Board, the New York State Department of Health, or the State of New York. Additional support was provided by the National Institute of Neurological Disease and Stroke (NS062972) to ASM. We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo. We thank Dr. Morrison of the Buffalo WomenServices clinic and Dr. Poulos of the Human Fetal Tissue Repository at the Albert Einstein College of Medicine for assistance with tissue acquisition. We thank Dr. Abdel Benraiss at the University of Rochester Medical Center for the gift of pTRIP lentiviral plasmid.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonellis A, Huynh JL, Lee-Lin SQ, Vinton RM, Renaud G, Loftus SK, Elliot G, Wolfsberg TG, Green ED, McCallion AS, Pavan WJ. Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 2008;4:e1000174. doi: 10.1371/journal.pgen.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 3.Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Hur T. Cell therapy for multiple sclerosis. Neurotherapeutics. 2011;8:625–642. doi: 10.1007/s13311-011-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 6.Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia. 2004;47:314–324. doi: 10.1002/glia.20011. [DOI] [PubMed] [Google Scholar]
- 7.Conway GD, O’Bara MA, Vedia BH, Pol SU, Sim FJ. Histone deacetylase activity is required for human oligodendrocyte progenitor differentiation. Glia. 2012;60:1944–1953. doi: 10.1002/glia.22410. [DOI] [PubMed] [Google Scholar]
- 8.Cui QL, Fragoso G, Miron VE, Darlington PJ, Mushynski WE, Antel J, Almazan G. Response of human oligodendrocyte progenitors to growth factors and axon signals. J Neuropathol Exp Neurol. 2010;69:930–944. doi: 10.1097/NEN.0b013e3181ef3be4. [DOI] [PubMed] [Google Scholar]
- 9.Duncan ID, Kondo Y, Zhang SC. The myelin mutants as models to study myelin repair in the leukodystrophies. Neurotherapeutics. 2011;8:607–624. doi: 10.1007/s13311-011-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedli M, Barde I, Arcangeli M, Verp S, Quazzola A, Zakany J, Lin-Marq N, Robyr D, Attanasio C, Spitz F, Duboule D, Trono D, Antonarakis SE. A systematic enhancer screen using lentivector transgenesis identifies conserved and non-conserved functional elements at the Olig1 and Olig2 locus. PLoS One. 2010;5:e15741. doi: 10.1371/journal.pone.0015741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusho M, Ono K, Takebayashi H, Masahira N, Kagawa T, Ikeda K, Ikenaka K. Involvement of the Olig2 transcription factor in cholinergic neuron development of the basal forebrain. Dev Biol. 2006;293:348–357. doi: 10.1016/j.ydbio.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang J, Kang HC, Kim HS, Kim JY, Huh YJ, Kim DS, Yoo JE, Lee JA, Lim B, Lee J, Yoon TM, Park IH, Hwang DY, Daley GQ, Kim DW. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann Neurol. 2011;70:402–409. doi: 10.1002/ana.22486. [DOI] [PubMed] [Google Scholar]
- 14.Joannides AJ, Webber DJ, Raineteau O, Kelly C, Irvine KA, Watts C, Rosser AE, Kemp PJ, Blakemore WF, Compston A, Caldwell MA, Allen ND, Chandran S. Environmental signals regulate lineage choice and temporal maturation of neural stem cells from human embryonic stem cells. Brain. 2007;130:1263–1275. doi: 10.1093/brain/awm070. [DOI] [PubMed] [Google Scholar]
- 15.Keyoung HM, Roy NS, Benraiss A, Louissaint A, Jr, Suzuki A, Hashimoto M, Rashbaum WK, Okano H, Goldman SA. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- 16.Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C, Talantova M, Bajpai R, Calzolari D, Terskikh A, McCulloch AD, Price JH, Conklin BR, Chen HS, Mercola M. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuspert M, Hammer A, Bosl MR, Wegner M. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 2011;39:1280–1293. doi: 10.1093/nar/gkq951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei Q, Jeong Y, Misra K, Li S, Zelman AK, Epstein DJ, Matise MP. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic Shh-Gli signaling in the neural tube. Dev Cell. 2006;11:325–337. doi: 10.1016/j.devcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 20.Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- 21.Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 23.Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- 24.Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 26.Sim F, Lang J, Waldau B, Roy N, Schwartz T, Pilcher W, Chandross K, Natesan S, Merrill J, Goldman S. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation (vol 59, pg 763, 2006) Annals of Neurology. 2006;59:990–990. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 27.Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature Biotechnology. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian J, Alimperti S, Lei P, Andreadis ST. Lentiviral microarrays for real-time monitoring of gene expression dynamics. Lab Chip. 2010;10:1967–1975. doi: 10.1039/c003153d. [DOI] [PubMed] [Google Scholar]
- 30.Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, Nguyen T, Cummings BJ, Anderson AJ, Tamaki SJ, Tsukamoto A, Weissman IL, Matsumoto SG, Sherman LS, Kroenke CD, Back SA. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Chandler-Militello D, Lu G, Roy NS, Zielke A, Auvergne R, Stanwood N, Nicolis SK, Sim FJ, Goldman SA. Prospective identification, isolation, and profiling of a telomerase expressing subpopulation of human neural stem cells, using sox2 enhancer-directed FACS. J Neurosci. 2010;30:14635. doi: 10.1523/JNEUROSCI.1729-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner T, Hammer A, Wahlbuhl M, Bosl MR, Wegner M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 2007;35:6526–6538. doi: 10.1093/nar/gkm727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, 2nd, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 34.Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, 2nd, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 35.Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]



