Abstract
Background
Instruments that are intended to measure change over time need to emphasize sensitivity to change as a central property. The aims of this report are to test whether the MOODS-SR, a measure of mood spectrum symptomatology, is sensitive to changes during acute and continuation treatment of depression and whether residual mood spectrum symptoms predict relapse in the subsequent 6 months.
Methods
The study sample includes 316 patients with nonpsychotic depression participating in the protocol ‘Depression: the search for treatment-relevant phenotypes’. Patients were initially randomized to selective serotonin reuptake inhibitors or interpersonal psychotherapy and then treated for 9 months using an algorithm-based protocol. Measures of mood symptomatology included the self-report version of the structured clinical interview for mood spectrum (MOODS-SR), the Quick Inventory for Depressive Symptomatology and the Hamilton Rating Scale for Depression.
Results
Repeated-measures ANOVA indicates that during the acute phase MOODS scores decrease significantly from baseline to weeks 6 and 12. This decrease was significantly different (p < 0.001) between those who remitted and those who did not remit on the depressive, the rhythmicity component and the total score. Nonrelapsing subjects had stable scores across the continuation phase, while among relapsing subjects, a significant increase was found in the depressive component (p < 0.001), the rhythmicity component (p = 0.024) and the total score (p < 0.001), at 2 months, followed by a decrease from 2 to 6 months. Scores on the depressive component at the entry into continuation predicted relapse in the subsequent 6 months.
Conclusions
Our findings suggest that the MOODS-SR is sensitive to change in depression status and may help the clinician to detect symptoms and signs not considered by established symptom severity scales.
Keywords: Mood, Sensitivity to change, Spectrum, Major depression, Predictive validity, Relapse, Remission, Residual symptoms, Subthreshold
Introduction
Although high levels of reliability are emphasized in the construction of many measures of psychopathology, instruments that are intended to measure change in clinical state need to emphasize sensitivity to change as a central and primary property [1].
The mood spectrum assessments [2, 3] are based on a dimensional approach to psychopathology that considers as clinically relevant not only threshold level manifestations of unipolar and bipolar mood psychopathology, but also atypical symptoms (i.e. symptoms not mentioned in the DSM-IV or ICD-10), behavioral traits and temperamental features that are associated with established diagnostic constructs. A novel aspect of the ‘spectrum model’ is that these symptoms and traits may occur in isolation, rather than as part of a temporally circumscribed clinical syndrome. Items are organized into three dimensions: a manic-hypomanic component and a depressive component, each exploring mood, energy and cognition, plus a component that explores disturbances in rhythmicity (i.e. changes in mood, energy and physical well-being according to the weather, the season, the phase of menstrual cycle etc.) and in vegetative functions, including sleep, appetite and sexual function.
Research has indicated that mood spectrum features are associated with considerable suffering and disability, even in the absence of threshold level psychiatric disorders. Cassano et al. [4] found that lifetime manic-hypo-manic spectrum symptoms were associated with increased depressive symptomatology, suicidal ideation and delusions in patients with unipolar depression with no history of manic or hypomanic episodes. Severity of lifetime mood spectrum psychopathology has also been associated with an increased likelihood of suicide attempts in patients with schizophrenia and mood disorders [5], with poorer quality of life in individuals with rheumatoid arthritis [6], with a higher likelihood of developing a depressive episode during interferon treatment in patients with chronic hepatitis [7] and with a history of self-induced vomiting and suicidality in patients with anorexia nervosa [8].
The mood spectrum assessment was initially developed as a structured interview that proved to have excellent test-retest reliability (intraclass correlations from 0.88 to 0.97) and discriminant validity in patients with mood disorders and normal controls [2]. Then, a self-report life-time version (MOODS-SR) was created that proved to be equivalent to the interview [3]. More recently, a last-month self-report version has been developed to be used in clinical trials and other longitudinal studies, including the study on which the present report is based. In this study patients with unipolar depression are treated for 9 months with selective serotonin reuptake inhibitors (SSRI) and/or interpersonal psychotherapy. The study consists of an acute phase lasting at least 12 weeks and a continuation phase lasting 6 months. The aims of this report are to test whether the MOODS-SR scores are sensitive to changes during acute and continuation treatment and whether residual mood spectrum symptoms at the entry into the continuation phase predict relapse in the subsequent 6 months. We hypothesized that last-month mood spectrum scores would decrease significantly during the acute phase, that the change in scores would be higher in patients who meet criteria for clinical remission compared to those who do not remit, and that subsequently scores would increase in those who relapse compared to those who do not relapse. Finally, we hypothesized that subjects endorsing a higher number of mood spectrum symptoms at remission would be at higher risk for relapse.
Methods
Study Design and Treatment Protocol
The study sample consists of patients with unipolar depression recruited between February 2002 and March 2007 at the outpatient clinics of the Departments of Psychiatry of the Universities of Pisa and Pittsburgh in the framework of the study ‘Depression: the search for treatment-relevant phenotypes’.
Inclusion criteria were age between 18 and 66 years, being able and willing to give informed consent, currently being in an episode of nonpsychotic major depression as defined by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID), and by a rating of ≥ 15 on the 17-item Hamilton Rating Scale for Depression (HRSD) and not currently receiving effective treatment. Females of childbearing potential had to practice an acceptable form of birth control. Subjects with suicidal ideation were eligible as long as outpatient treatment was deemed safe.
Exclusion criteria were a history of manic or hypomanic episodes, a history of schizophrenia or schizoaffective disorder, current primary diagnosis of eating disorders, drug and/or alcohol dependence or abuse, current psychosis, antisocial personality disorder, organic affective syndrome, kidney or liver disease, epilepsy, cardiovascular disease and any uncontrolled illness. Patients with a well-documented history of an inability to tolerate one of the study treatments or currently receiving treatment with an effective antidepressant were also excluded.
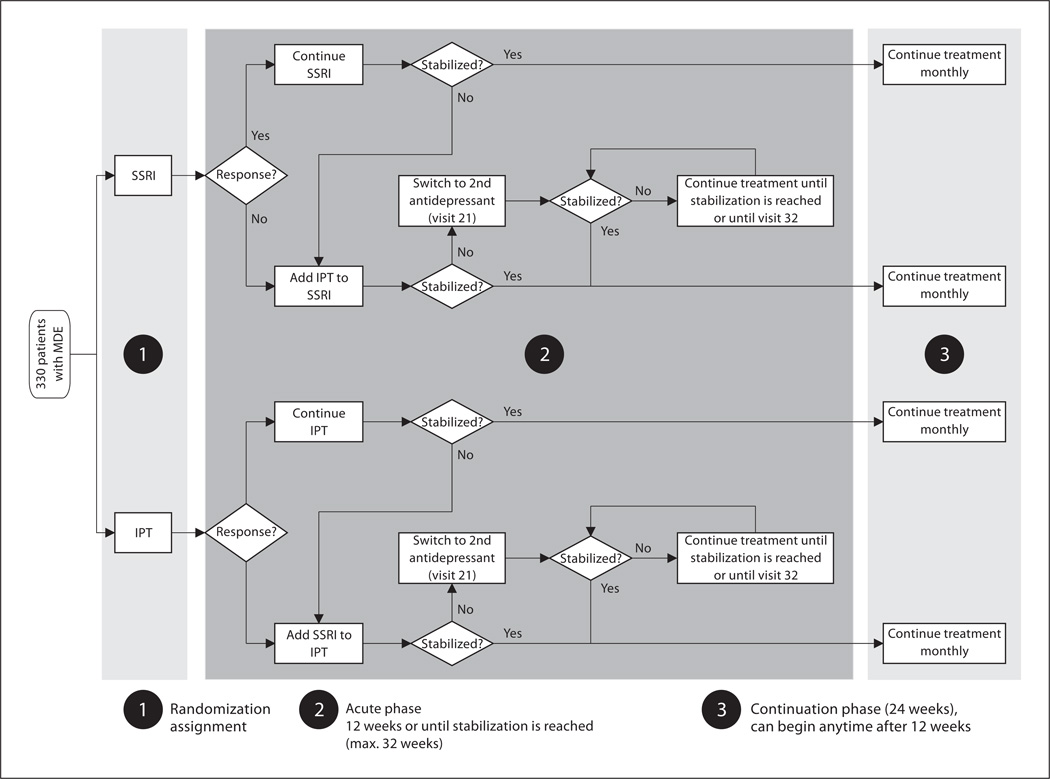
In the initial phase of treatment (fig. 1), the subjects were randomly assigned to pharmacotherapy (the SSRI, citalopram during the pilot phase or escitalopram during the full study phase) or psychotherapy (interpersonal psychotherapy) [9]. The acute treatment phase of the study involved 3 assessment and triage points, at weeks 6, 12 and 20. Those participants assigned to pharmacotherapy who did not evidence a response, defined as a 50% reduction of baseline score on the HRSD, first were increased from the initial daily dose of citalopram (20 mg) or escitalopram (10 mg) to a dose of 40 or 20 mg, respectively, at week 3. Subjects who did not evidence a response by week 6 were given psychotherapy in addition to pharmacotherapy, or beginning at week 12 if they were not taking 20 mg/day by week 6, as a result of initial improvement followed by worsening or difficulties with side effects.
Fig. 1.
Study design. MDE = Major depressive episode; IPT = interpersonal psychotherapy.
Those patients assigned to psychotherapy who did not evidence a response after 6 weeks of acute treatment (week 6), had pharmacotherapy added to their treatment. Patients who showed an initial response at week 6, but then worsened, had a second opportunity to receive pharmacotherapy augmentation at week 12. All patients still on monotherapy at week 12 who had not met the criteria for remission (average HRSD ≤ 7 over 3 weeks) had the other treatment (pharmacotherapy or psychotherapy) added to their treatment regimen.
Patients who had not remitted with combined psychotherapy and pharmacotherapy at week 20 continued interpersonal psychotherapy and switched from citalopram or escitalopram to a second antidepressant following the guidelines provided by the Texas Medication Algorithm Project [10].
Patients who relapsed were offered additional or alternative treatment and continued to be followed in the study through the end of 6 months of continuation treatment.
Assessments
At both sites, the diagnostic assessment was conducted using the SCID-I for DSM-IV diagnoses by clinicians trained and certified to the use of the interviews when high levels (>0.90) of inter-rater reliability of their diagnoses with the trainer were achieved. All interviewers had long-standing experience in the administration of standardized interviews. Other study assessments included established interview-based and self-report assessments of the severity of depressive symptoms, including the HRSD [11] and the Quick Inventory of Depressive Symptomatology (QIDS) [12] and the more recently developed and validated assessment of mood spectrum, the MOODS-SR. This questionnaire was derived from the corresponding structured interview [2] and is focused on the presence of manic and depressive symptoms, traits and lifestyles that may characterize the ‘temperamental’ affective dysregulation that makes up both fully syndromal and subthreshold mood disturbances. The latter include symptoms that are either isolated or clustered in time and temperamental traits that are present throughout an individual’s lifetime. The MOODS-SR consists of 161 items coded as present or absent for one or more periods of at least 3–5 days in the time frame investigated (the subject’s lifetime or the last month, according to the version of the instrument). The instrument can be downloaded from the web site www.spectrum-project.org.
In the present study we administered the lifetime version of the MOODS-SR at baseline and the last-month version at 7 time points: baseline, weeks 6, 12 and 20 of the acute treatment phase, entry into the continuation phase, and after 2 and 6 months of continuation treatment.
Demographic and Clinical Characteristics of Study Participants
Of the 344 subjects who entered the study, 316 filled out and returned the MOODS-SR questionnaire at baseline. Completers did not differ from noncompleters on age (t = 1.427, d.f. = 342, p = 0.155), gender (χ2 = 0.014, d.f. = 1, p = 0.906), educational level (t = 0.014, d.f. = 342, p = 0.989) or severity of depression at study entry (t = 0.412, d.f. = 342, p = 0.681) and therefore constitute an unbiased subsample of all study participants.
The characteristics of the final sample of 316 subjects who provided MOODS-SR data, broken down by site, are provided in table 1. Subjects were on average 39 years old, were more likely to be female, employed and had an educational level of 13 years.
Table 1.
Demographic and clinical characteristics of the study participants at baseline (n = 316)
| Pisa (n = 154) |
Pittsburgh (n = 162) |
Total (n = 316) |
|
|---|---|---|---|
| Age, years | 39.4 ± 12.0 | 39.1 ± 12.3 | 39.2 ± 12.1 |
| Female gender, % | 85.1 | 60.5 | 72.5 |
| Married, % | 45.4 | 35.8 | 40.5 |
| Education, years | 11.9 ± 3.6 | 15.5 ± 2.4 | 13.7 ± 3.5 |
| Employed, % | 65.0 | 66.0 | 65.5 |
| Median number of children | 1 | 2 | 1 |
| Mean age at onset, years | 31.0 ± 11.4 | 23.8 ± 12.4 | 27.2 ± 12.4 |
| Median number of depressive episodes | 2 | 3 | 2 |
| Median duration of illness, years | 3.5 | 11.0 | 8.0 |
| Lifetime MOODS-SR scores | |||
| Depressive component | 31.1 ± 11.7 | 37.3 ± 10.1 | 34.3 ± 11.4 |
| Manic component | 19.2 ± 9.6 | 20.2 ± 11.1 | 19.7 ± 10.4 |
| Rhythmicity | 13.3 ± 4.4 | 14.7 ± 4.5 | 14.1 ± 4.5 |
| Last-month MOODS-SR scores | |||
| Depressive component | 28.3 ± 12.5 | 28.7 ± 11.5 | 28.5 ± 12.0 |
| Manic component | 8.3 ± 6.3 | 7.0 ± 5.9 | 7.6 ± 6.1 |
| Rhythmicity | 10.3 ± 4.1 | 9.6 ± 4.2 | 10.0 ± 4.2 |
In patients who were randomized to SSRI or took the SSRI as adjunctive treatment, the mean dose of citalopram was 32.4 mg/day (SD 16.1, range 10–60) and that of escitalopram 16.8 mg/day (SD 5.3, range 5–40).
The remission rate at 12 weeks was 56.6%. Fifty-five patients were terminated or dropped out of the study during the acute phase and 36 during the continuation phase.
Data Analysis
The correlations between MOODS-SR scores and QIDS and HRSD scores were examined using Pearson’s correlation coefficient and Spearman’s correlation coefficient to take into account the departure from the gaussian distribution of the manic component. However, because the two types of coefficients were overlapping, we reported the former in the results. A repeated-measures analysis of variance was conducted in order to analyze differential changes in MOODS-SR scores in remitters at 12 weeks of treatment versus nonremitters during the acute phase of treatment. The assumption of sphericity underlying this analysis, i.e. that the variance of the differences between pairs of scores does not differ, was tested using Mauchly’s test statistics.
Changes during the continuation phase were also examined using the same analytical strategy. Logistic regression models were used to analyze the association of mood spectrum scores, HRSD score and QIDS score at entry into continuation with relapse. A forward stepwise procedure was used to enter the variables in the model. This approach was adopted to show whether the addition of subsequent variables to the model contributed to changing significantly the odds of relapse. All tests of significance were 2-tailed and the alpha level was set at 0.05. Effect sizes were computed for the mood spectrum scores, the QIDS and the HRSD. Sensitivity to change was assessed by computing the effect size for changes in scores from baseline, i.e. mean change (baseline – end-point) divided by the standard deviation of change, at 6 and 12 weeks and 9 months. SPSS, version 15.0, was used to perform the analyses.
Results
Completion Rates
Of the 316 patients entering the acute phase and providing baseline MOODS-SR data, at the time of analysis for this report data were available for 282 (89.2%) after 6 weeks of acute treatment, and 230 (72.7%) at 12 weeks of treatment. Of the 220 patients who had entered the continuation phase at the time of analysis, data were available for 196 after 2 months of continuation treatment and for 150 (68.2%) at study completion.
There were no statistically significant differences in standard demographic or clinical characteristics between the patients who did and did not complete the continuation phase. Mean scores on the MOODS-SR at the 6 study time points are provided in table 2. Because the assessment at 20 weeks was conducted only in the 33 patients for whom the acute phase was protracted because of non-response to combination treatment, data are not reported here.
Table 2.
Means ± SD of the MOODS-SR scores (last-month version) at 6 study time points
| Baseline (n = 316) |
Week 6 (n = 282) |
Week 12 (n = 230) |
Entry into continuation (n = 220) |
2 months (n = 196) |
6 months (n = 150) |
|
|---|---|---|---|---|---|---|
| Depressive component | 28.5 ± 12.0 | 18.5 ± 12.9 | 11.4 ± 11.4 | 9.6 ± 9.7 | 8.6 ± 9.8 | 8.7 ± 10.2 |
| Manic component | 7.6 ± 6.1 | 6.5 ± 6.8 | 5.3 ± 5.5 | 5.3 ± 5.8 | 4.3 ± 4.9 | 4.2 ± 4.7 |
| Rhythmicity | 9.5 ± 4.1 | 7.6 ± 4.3 | 5.8 ± 4.0 | 5.3 ± 3.9 | 4.8 ± 4.1 | 4.8 ± 3.9 |
| Total score | 45.6 ± 18.0 | 32.6 ± 18.5 | 22.5 ± 16.6 | 20.1 ± 15.1 | 17.8 ± 15.9 | 17.8 ± 15.6 |
| 17-Item HRSD | 18.8 ± 4.0 | 10.1 ± 5.9 | 6.9 ± 5.1 | 4.0 ± 2.1 | 4.9 ± 4.1 | 4.7 ± 4.2 |
Correlation between MOODS-SR, HRSD and QIDS Scores at Baseline
Pearson’s correlation coefficients at baseline between the MOODS-SR, last-month version scores and the HRSD and QIDS were positive and significant for the depressive component (HRSD: r = 0.51, p < 0.001; QIDS: r = 0.44, p < 0.001), the rhythmicity component (HRSD: r = 0.34, p < 0.001; QIDS: r = 0.31, p < 0.001), the total score (HRSD: r = 0.41, p < 0.001; QIDS: r = 0.38, p < 0.001), but not for the manic component (HRSD: r = −0.02, p = 0.741; QIDS: r = 0.06, p = 0.302). As expected, the correlations between these standard measures of depressive symptomatology were highest for the depressive component of MOODS-SR and moderate in absolute value, denoting a partial overlap in content of the instruments.
Remission Rates and Changes in Scores during the Acute Phase
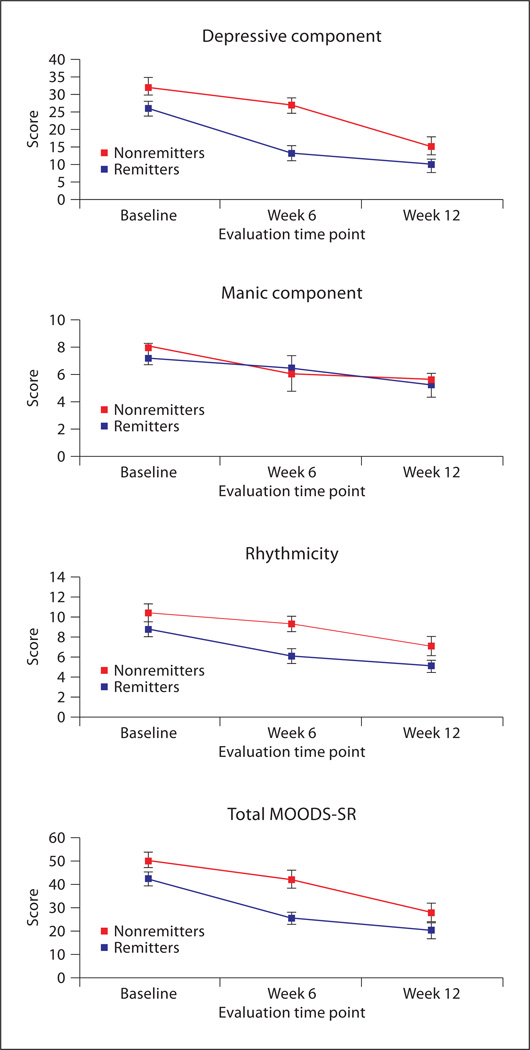
As noted above, in the present protocol remission was defined by an average HRSD score ≤ 7 over a period of 3 consecutive weeks. Remission by visit 12 was achieved by 179 subjects of the 316 subjects included in the present report (56.6%). The MOODS-SR scores during the acute phase in remitters and nonremitters are shown in figure 2. Repeated-measures ANOVA indicates that during the acute phase the depressive component, manic component, rhythmicity and total last-month MOODS scores all decrease significantly from baseline to week 6 and from week 6 to week 12 (main effect of time for the depressive component: F = 235.37, d.f. = 2, 446, p < 0.001; main effect of time for the manic component: F = 24.43, d.f. = 2, 446, p < 0.001; main effect of time for the rhythmicity component: F = 103.61, d.f. = 2,446, p < 0.001; main effect of time for the total score: F = 248.58, d.f. = 2, 446, p < 0.001). This decrease was significantly different between those who remitted and those who did not remit for the depressive component (interaction effect: F = 16.2, d.f. = 2, 446, p < 0.001), the rhythmicity component (interaction effect: F = 5.47, d.f. = 2, 446, p = 0.004), the total score (interaction effect: F = 10.98, d.f. = 2, 446, p < 0.001), but not for the manic component (interaction effect: F = 1.47, d.f. = 2, 446, p = 0.231; fig. 2).
Fig. 2.
Mean (and 95% confidence interval) of MOODS-SR scores in patients who remitted and patients who did not remit by 12 weeks of treatment.
Relapse Rates and Changes in Scores during the Continuation Phase
In the present study, relapse was declared when the subject presented with an HRSD score of ≥ 15 and was evaluated by an independent psychiatrist not otherwise involved in the study as being in an episode of major depression. Of the 220 subjects entering the continuation phase, 24 relapsed (10.9%) and 6 had not yet completed the continuation phase at the time of analysis for this report. At entry into the continuation phase, the subgroup of participants who subsequently relapsed differed significantly from those who did not on all but the manic component of the MOODS-SR [depressive component: 16.1 (SD = 11.4) vs. 8.7 (SD = 9.3), t = 3.568, d.f. = 218, p < 0.001; rhythmicity: 7.4 (SD = 3.7) vs. 5.0 (SD = 3.8), t = 2.928, d.f. = 218, p < 0.01; total mood score: 29.0 (SD = 16.1) vs. 18.9 (SD = 14.6), t = 3.157, d.f. = 218, p < 0.01]. Differences between participants who relapsed and those who did not relapse were not significant for the HRSD score at entry into continuation [4.3 (SD = 1.7) vs. 4.0 (SD = 2.1), t = 0.742, d.f. = 218, p = 0.459], but were significant for the QIDS score [5.0 (SD = 3.3) vs. 3.2 (SD = 2.3), t = 2.577, d.f. = 218, p = 0.016]. Using a logistic regression model with ‘relapse’ as the dependent variable and the mood spectrum scores and the QIDS score at entry into continuation treatment as the independent variables, only the depressive component of mood spectrum was retained in the model using a forward stepwise procedure. The odds ratio for the association of the depressive component with relapse was 1.066, with a 95% confidence interval of 1.026–1.107, p < 0.001, indicating that for each additional item endorsed, the likelihood of relapse increases by 6.6%. When we ran the logistic regression model using the HRSD in place of the QIDS, results were the same; however, this was expected since remission was defined by the HRSD.
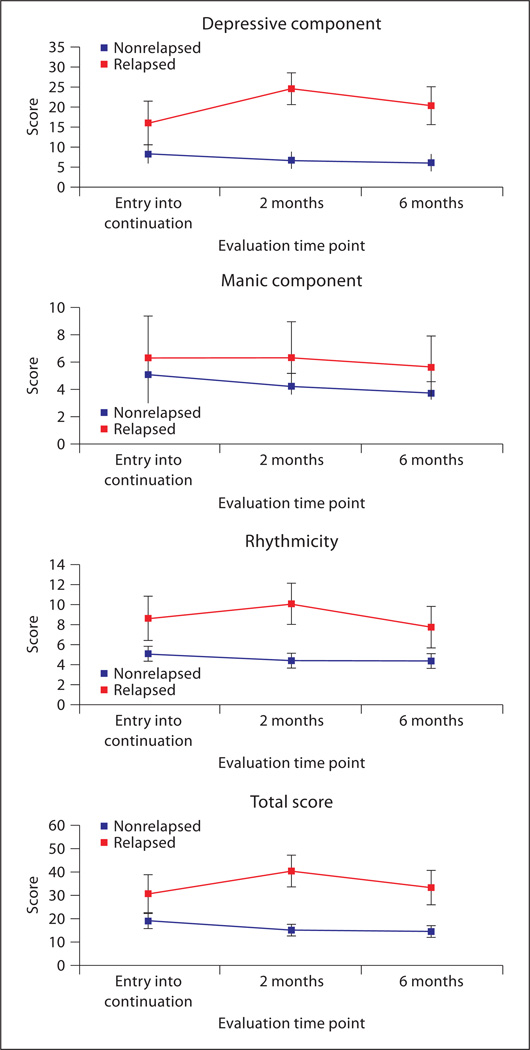
Nonrelapsing subjects had stable scores across the continuation phase, while among relapsing subjects we observed a significant increase in the depressive component (interaction effect: F = 10.81, d.f. = 2, 244, p < 0.001), the rhytmicity score (interaction effect: F = 3.81, d.f. = 2, 244, p = 0.024) and the total score (interaction effect: F = 9.94, d.f. = 2, 244, p < 0.001), at 2 months of continuation compared to entry into continuation, followed by a decrease from 2 to 6 months (fig. 3). The increase in scores at 2 months reflects the worsening of symptoms prior to relapse (relapses occurred at a median of 2.3 months after the start of the continuation phase) that was subsequently managed by adjusting treatment. In contrast, the manic component (interaction effect: F = 0.404, d.f. = 2, 244, p = 0.668) remained stable in this subgroup throughout the continuation phase. Using a receiver operating characteristic analysis, the optimal cutoff for predicting relapse using the depressive component of mood spectrum was 13 (sensitivity 62.5%, specificity 72.4%, positive predictive values 21.7%, area under the curve 0.689, 95% confidence interval 0.566–0.811).
Fig. 3.
Mean (and 95% confidence interval) of MOODS-SR scores in patients who relapsed and patients who did not relapse during the continuation phase.
Effect Size of the MOODS-SR, QIDS and HRSD Scores
Table 3 shows the effect size of the mood spectrum measures at 6 and 12 weeks and 9 months, expressed as change from baseline in standard deviation units. The depressive component had effect sizes, comparable to those of the total MOODS-SR score; the rhythmicity component achieved effect sizes of ≥ 0.5 or more at each of the 3 time points, while the manic component exceeded 0.5 only at 9 months. Compared to the QIDS, effect sizes for the depressive component and the total MOODS-SR scores were smaller at 6 weeks but larger at 12 weeks and 9 months. The HRSD had the highest effect sizes at each time point.
Table 3.
Effect size of the MOODS-SR scores, of the QIDS and of the 17-item HRSD at 6 and 12 weeks and 9 months
| Depressive component |
Manic component |
Rhythmicity | Total MOODS-SR score |
QIDS | 17-Item HRSD |
|
|---|---|---|---|---|---|---|
| Week 6 | 0.86 | 0.22 | 0.51 | 0.89 | 0.96 | 1.46 |
| Week 12 | 1.34 | 0.47 | 0.95 | 1.38 | 1.12 | 2.11 |
| 9 months | 1.56 | 0.69 | 1.08 | 1.54 | 1.45 | 2.89 |
Discussion
Researchers have recently emphasized ‘measurement-based care’ for depression [13] that requires the use of instruments to monitor patient progression, to adjust treatment and to make clinical decisions. Although the definition and the operational criteria for treatment course and outcome of major depressive disorders have been described in considerable detail [14–16], there is still a need for a valid, reliable and replicable way to assess the clinical course of patients with depression, during the different phases of treatment of the disorder [17–19]. Thus, the problem of a reliable definition of remission is still unresolved, firstly because remission – the primary goal of treatment – is still phenomenologically defined, in the absence of a biological marker [20]. The disparities in the definitions of basic concepts, such as response and remission, are reflected in the search for better instruments to identify those individuals who truly respond to treatment or remittance [13]. In our study, the MOODS-SR last-month version was administered together with standard outcome measures (HRSD and QIDS) during the acute and continuation treatment phases of a randomized clinical trial. We found that the instrument was able to capture changes in clinical status (in the acute phase) and to detect residual symptomatology among those patients who met HRSD criteria for remission (average HRSD ≤ 7 over 3 consecutive weeks). Patients in remission showed lower scores at weeks 6 and 12 not only on the MOODS-SR total score, but also on the depressive and rhythmicity components than patients who did not remit. Thus, the last-month MOODS-SR scores changed in the theoretically proposed direction following treatment. In terms of effect size, if one accepts an effect size of ≥ 0.5 as moderate and meaningful [21], those obtained for the depressive component, the rhythmicity component and the total MOODS-SR score meet this criterion at each of the time points considered.
Our finding suggests that the MOODS-SR is a valid method for the evaluation of change in depression status that may help the clinician to detect symptoms and signs not considered by classic symptom severity scales, such as the HRSD or the QIDS. These established scales for depression target primarily typical symptoms that are an essential part of the DSM criteria and do not consider as clinically relevant a large number of symptoms that often endure after the apparent resolution of a depressive episode. Our results suggest that a substantial number of patients, considered in stable remission according to the HRSD scores, report the presence of residual symptoms, not captured by the HRSD. Consistent with this observation, in our study we found that patients endorsed multiple MOODS-SR symptoms at entry into the continuation phase, such as being annoyed with everything, hypersensitivity to rejections, boredom, feeling like running away from current life and mood swings in relation to the weather, change of seasons and menstrual cycle.
Finally, in this study we found that patients who relapsed during the continuation phase endorsed on average 10 MOODS-SR items more than patients who did not relapse. Thus, higher MOODS-SR scores at remission were associated with a higher likelihood of relapsing in the subsequent 6 months of treatment. This result is consistent with a large number of previous studies in the last two decades. In fact, residual subthreshold depressive symptoms are a strong and reliable clinical marker of rapid and frequent relapse [22–24]. Such symptoms should be systematically assessed both because they are likely to affect the patients’ current well-being and predispose them to a new episode [25].
Our results should be interpreted taking into account the following limitations. First, while the MOODS-SR last-month version was included in the original protocol, testing the sensitivity to change of the instrument was not among the primary or secondary study aims; thus, the analyses presented here should be considered post hoc. Second, patients were initially randomized, but they were not blind to the type of treatment received (SSRI, interpersonal psychotherapy or combination of the two treatments) so one could argue that self-report measures, including the MOODS-SR, may be biased by the patients’ expectations of the treatment they received. Third, findings from this study may have limited generalizability because they are obtained from a sample constituted almost entirely of Caucasian patients, selected on the basis of a diagnosis of nonpsychotic, unipolar major depressive disorder. Fourth, in comparing the performance of the MOODS-SR with that of the QIDS, it should be noted that these self-report instruments have a different time frame (1 month vs. 1 week), that the MOODS-SR includes a larger number of items than the QIDS and that it has dichotomous (yes/no) rather than ordinal-level response options. While answering dichotomous items is easier than choosing 1 of 4 response options, the MOODS-SR is more time consuming, requiring on average 15 min to complete. Given these limitations, it is logical to ask whether the MOODS-SR should be administered in routine clinical settings and utilized as a standard outcome. On the other hand, the MOODS-SR carefully assesses all core criterion diagnostic depressive symptoms, and common important associated features, as well as a wide range of symptoms surrounding the core features of depression, in an easy-to-use format that is generally well accepted by patients. Findings from this study suggest that the mood spectrum assessment approach and, specifically, the depressive component of the MOODS-SR self-report instrument are able to offer clinicians a more clinically meaningful assessment of psychopathology than the established measures of depression severity, especially with respect to residual symptoms. Thus, the MOODS-SR may prove useful in many clinical and research contexts, including those requiring a more fine-grained measurement of outcome and monitoring of the course of illness. In the absence of a structured approach, the evaluation of subthreshold depressive features requires special clinical training and extensive clinical experience. With the carefully constructed, standardized assessment process provided by the MOODS-SR, the identification of these phenomena becomes a relatively easy process.
Future research should focus on the potential use of the depressive component of the mood spectrum as a stand-alone instrument in order to confirm its ability to predict relapse in both the short and longer term and on the development of an adaptive testing version of the full MOODS-SR that could significantly decrease the required administration time.
Acknowledgments
This work was supported by National Institute of Mental Health grants MH065376-01A1 (to E.F.) and MH030915-27 (to E.F.) and an investigator-initiated grant from Forest Research Institute, a Division of Forest Laboratories Inc. (to E.F. and G.B.C.) and Fondazione IDEA (to G.B.C.).
Dr. Miniati has received support from Lundbeck, Italia. Dr. Rucci has received support from Forest Research Institute and Fondazione IDEA. Dr. Cassano has served on the advisory boards of Eli Lilly, Lund-beck and Merck Sharp & Dohme, as a consultant for Pfizer Inc., Eli Lilly, Astra Zeneca, Lundbeck, Bristol Meyers Squibb and Janssen, and has received Investigator-initiated grants from Astra Zeneca, Merck Sharp & Dohme Italia, Organon Italia, Bayer, Pfizer Italia, Lundbeck Italia, Bristol Meyers Squibb, and Glaxo Smith-Kline. Dr. Kupfer has served on advisory boards of Eli Lilly & Company, Forest Pharmaceuticals Inc., Pfizer Inc. and Solvay/Wyeth Pharmaceuticals and also served as a consultant for Servier Amerique. Dr. Frank has served as a consultant to Pfizer, Eli Lilly and Novartis, as an advisory board member for Pfizer, Eli Lilly and Servier. She has received investigator-initiated research grant support from the National Institute of Mental Health, the Pittsburgh Foundation and Forest Research Institute, honoraria for teaching from Lundbeck and royalties from Guilford Press. Dr. Fagiolini has been in the speaker bureau and a consultant for Pfizer, Bristol Myers Squibb, Eli Lilly Italy, Novartis and Shire.
Footnotes
The data for this report were drawn from the pilot and full-study phases of a study entitled ‘Depression: the search for treatment-relevant phenotypes’. The investigators are: principal investigator: E. Frank (Pittsburgh); co-principal investigator: G.B. Cassano (Pisa); Pittsburgh co-investigators: B. Pollock, M.K. Shear, P. Pilkonis, A. Fagiolini, P. Rucci, H. Swartz, D. Kupfer, R. Bies; Pisa co-investigators: L. Dell’Osso, M. Mauri, S. Banti, A. Benvenuti, L. Maggi, M. Miniati, A. Papasogli, M. Saettoni.
Conflict of Interest
Dr. Oppo has no conflict of interest to declare.
References
- 1.Fava GA, Ruini C, Rafanelli C. Psychometric theory is an obstacle to the progress of clinical research. Psychother Psychosom. 2004;73:145–148. doi: 10.1159/000076451. [DOI] [PubMed] [Google Scholar]
- 2.Fagiolini A, Dell’Osso L, Pini S, Armani A, Endicott J, Maser JD, Rucci P, Grochocinski VJ, Bouanani S, Shear KM, Frank E, Cassano GB. Internal consistency and discriminant validity of a new instrument for assessing mood symptomatology: the structured clinical interview for mood spectrum (SCI-MOODS) Int J Methods Psychiatr Res. 1999;8:71–82. [Google Scholar]
- 3.Dell’Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, Shear MK, Grochocinski VJ, Maser JD, Endicott J, Cassano GB. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr Psychiatry. 2002;43:69–73. doi: 10.1053/comp.2002.29852. [DOI] [PubMed] [Google Scholar]
- 4.Cassano GB, Rucci P, Frank E, Fagiolini A, Dell’Osso L, Shear MK, Kupfer DJ. The mood spectrum in unipolar and bipolar disorder: arguments for a unitary approach. Am J Psychiatry. 2004;161:1264–1269. doi: 10.1176/appi.ajp.161.7.1264. [DOI] [PubMed] [Google Scholar]
- 5.Balestrieri M, Rucci P, Sbrana A, Ravani L, Benvenuti A, Gonnelli C, Dell’Osso L, Cassano GB. Lifetime rhythmicity and mania as correlates of suicidal ideation and attempts in mood disorders. Compr Psychiatry. 2006;47:334–341. doi: 10.1016/j.comppsych.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Piccinni A, Maser JD, Bazzichi L, Rucci P, Vivarelli L, Del Debbio A, Catena M, Bombardieri S, Dell’Osso L. Clinical significance of lifetime mood and panic-agoraphobic spectrum symptoms on quality of life of patients with rheumatoid arthritis. Compr Psychiatry. 2006;47:201–208. doi: 10.1016/j.comppsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Dell’Osso L, Pini S, Maggi L, Rucci P, Del Debbio A, Carlini M, Baldini A, Ferrari G, Manca E, Beverini E. Subthreshold mania as predictor of depression during interferon treatment in HCV+ patients without current or lifetime psychiatric disorders. J Psychosom Res. 2007;62:349–355. doi: 10.1016/j.jpsychores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Wildes JE, Gaskill JA, Ringham R, Marcus MD. Mood spectrum psychopathology in patients with anorexia nervosa. Compr Psychiatry. 2007;48:413–418. doi: 10.1016/j.comppsych.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Klerman GL, Weissmann MM. Interpersonal psychotherapy (IPT) and drugs in the treatment of depression. Pharmacopsychiatry. 1987;20:3–7. doi: 10.1055/s-2007-1017067. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi MH, Rush AJ, Crismon ML, Kashner TM, Toprac MG, Carmody TJ, Key T, Biggs MM, Shores-Wilson K, Witte B, Suppes T, Miller AL, Altshuler KZ, Shon SP. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry. 2004;61:669–680. doi: 10.1001/archpsyc.61.7.669. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 14.Frank E, Prien R, Jarrett RB, Keller MB, Kupfer DJ, Lavori P, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: response, remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 15.Prien RF, Carpenter LL, Kupfer DJ. The definition and operational criteria for treatment outcome of major depressive disorder: a review of the current research literature. Arch Gen Psychiatry. 1991;48:796–800. doi: 10.1001/archpsyc.1991.01810330020003. [DOI] [PubMed] [Google Scholar]
- 16.Rush AJ, Schatzberg AF, Gelenber AJ, Regier D, Kupfer DJ, Frank E, Sackeim H, Kraemer HC, Rosenbaum JF, Trivedi MH, Fava M, Michael M, Ray O, Ninan PT. Report by the ACNP Task Force on response and remission to major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 17.Blom MB, Jonker K, Dusseldorp E, Spinhoven P, Hoencamp E, Haffmans J, van Dyck R. Combination treatment for acute depression is superior only when psychotherapy is added to medication. Psychother Psychosom. 2007;76:289–297. doi: 10.1159/000104705. [DOI] [PubMed] [Google Scholar]
- 18.Maina G, Rosso G, Crespi C, Bogetto F. Combined Brief Dynamic Therapy and Pharmacotherapy in the Treatment of Major Depressive Disorder: a pilot study. Psychother Psychosom. 2007;76:298–305. doi: 10.1159/000104706. [DOI] [PubMed] [Google Scholar]
- 19.Bockting CL, Spinhoven P, Koeter MW, Ten Doesschate MC, Spijker J, Schene AH. Continuation and maintenance use of antidepressants in recurrent depression. Psychother Psychosom. 2008;77:17–26. doi: 10.1159/000110056. [DOI] [PubMed] [Google Scholar]
- 20.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289:3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences. ed 2. Hillsdale: Erlbaum Associates; 1988. [Google Scholar]
- 22.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 23.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual sub-threshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 24.Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Maser JD, Mueller T, Solomon DA, Keller MB. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry. 2000;157:1501–1504. doi: 10.1176/appi.ajp.157.9.1501. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80:135–144. doi: 10.1016/S0165-0327(03)00054-5. [DOI] [PubMed] [Google Scholar]