Abstract
Despite the increasing use of stem cells for regenerative-based cardiac therapy, the optimal stem cell population(s) remains in a cloud of uncertainty. In the past decade, the field has witnessed a surge of researchers discovering stem cell populations reported to directly and/or indirectly contribute to cardiac regeneration through processes of cardiomyogenic commitment and/or release of cardioprotective paracrine factors. This review centers upon defining basic biological characteristics of stem cells used for sustaining cardiac integrity during disease and maintenance of communication between the cardiac environment and stem cells. Given the limited successes achieved so far in regenerative therapy, the future requires development of unprecedented concepts involving combinatorial approaches to create and deliver the optimal stem cell(s) that will enhance myocardial healing.
Keywords: adult stem cell, aging, cardiochimera, cardiocluster, commitment, communication, diversity, microenvironment, proliferation, survival
Myocardial regeneration: what exactly is the problem?
Cardiomyocyte turnover in the mammalian heart is limited and has prompted extensive research in the field of cardiac regeneration. DNA synthesis in cardiomyocytes is highest during the embryonic stage, which drops dramatically in adulthood. The potential for cardiomyocyte division decreases by 20-times in ventricular cardiomyocytes 1 day post-birth and 4000-times in adult rodent models (0.0005% of total cardiomyocytes) measured by 3H-thymidine incorporation [1-3]. Interestingly, complete heart regeneration occurs after apical resection of the left ventricle or after myocardial infarction (MI) injury if the damage occurs before 7 days post-birth [4,5]. Cardiomyocyte cell cycle withdrawal prevents essential regeneration of the heart after damage, but this discovery has supplied researchers an invaluable tool to investigate novel signaling pathways and miRNAs that regulate cardiomyocyte turnover during this regenerative window [5,6]. Additionally, researchers have shown that final cardiomyocyte numbers are constrained to preadolescence regulated in part by a temporal surge of metabolic hormones [7]. However, MI increases cardiomyocyte cell cycle entry observed in 0.0055–0.0083% of total cardiomyocytes bordering the infarct zone or 11- to 16-fold relative to basal proliferative indices [1-3]. In the adult heart, mature cardiomyocytes primarily respond to damage by undergoing hypertrophy, remodeling of sarcomeres, compromised hemodynamics and cardiac function as early as a few days after MI [8]. Systemic disease and aging affects the myocardial environment promoting fibrosis, increases in inflammatory cells, leading to cellular senescence and telomere attrition of cardiomyocytes and non-myocyte populations. Competition with inflammation and fibroblast proliferation contributes to decreased stem cell numbers and function by encouraging cell proliferation over commitment and diminution of stem cell niches. The intrinsic battle to support cardiac regeneration through stem cell-dependent means falls critically short of the needed levels for restoration of function as ongoing controversy to the contribution of endogenous c-kit+ cardiac progenitor cells (CPCs) to new cardiomyocyte formation is still under investigation [9-11]. Collectively, the field continues to struggle with uncertainties regarding the potency of cardiac-derived progenitor cells to mediate meaningful cardiac repair during development or after myocardial damage.
Cellular therapeutic approaches: the past, present & where we need to go for the future
Not surprisingly given the controversies and inefficiencies of endogenous repair, researchers and clinicians have turned their attention toward the isolation and expansion of primary stem cell populations from both embryonic and adult tissue to promote regeneration after adoptive cell transplantation. Embryonic stem cells (ESCs) derived from the inner cell mass are attractive due to their pluripotent nature and ability to differentiate into cells belonging to the cardiovascular system [12]. The next-generation counterpart of ‘induced pluripotent stem cells’ (iPSCs) do not cause adverse immune responses due to the extraction of somatic cells from same patient and an acquired tolerance after transplantation and differentiation in vivo [13,14]. Cell culture protocols involving addition of growth factors (BMP4 and activin A), DNA modifiers such as 5-azacytidine and trichostatin A and thyroid hormones help enrich for ES/iPSC-derived cardiomyocytes (70–90%), with accumulation of differentiated cell properties reminiscent of young cardiomyocytes including mono-nucleation with the potential to divide [15-19]. Cardiomyocyte purity and efficiency of ESC differentiation is of critical importance as undifferentiated ESCs may form teratomas and prevent ESC-cardiomyocyte maturation in vivo [17,20]. Unfortunately, reports as to the safety and efficacy of purified ESC-derived myocyte delivery show a reduced capacity to electrically couple with the existing myocardium in both pig and non-human primates despite suppression of arrhythmias in small animal models [17,21]. These studies indicate that protocols for the derivation of ESC-cardiomyocytes need to be improved before proceeding to human clinical trials [22]. Somatic cell transdifferentiation also known as directed reprogramming has been reported with mouse and human fibroblasts into cardiomyocytes like cells in vitro and in vivo [23-25]. Cardiomyocyte-directed reprogramming bypasses a pluripotent stem cell state by transfection with cardiomyogenic-specific transcription factors that can be delivered after myocardial injury to support the conversion of fibrotic scar into nascent cardiomyocytes [23]. The transdifferentiation efficiency of fibroblasts is reported to be low, confirming the difficulty in changing the epigenetic landscape of adult somatic cells for iPSC development and lineage-specific cell formation required for tissue regenerative practices [25-27].
Despite the ongoing conundrum of which stem cells to use, the ubiquitous approach of expanding desired cells ex vivo to deliver cells above the limited quantity found presently in the endogenous cardiac niche has been widely adopted. To effectively design cellular therapy for cardiac regeneration, commonly considered issues remain: the cell population to be used, the number of cells to be delivered, timing of delivery after damage and the route of cell delivery into the myocardium. Placing the stem cell into the clinical setting often moves quickly without a fundamental understanding of the biology of the stem cells, confirmation of the optimal stem cell characteristics or whether a particular stem cell population is even suitable for patients of varying ages and genetic backgrounds. Individualized cellular therapy, although inherently difficult to validate, will eventually become recognized as essential for determining which patients are likely to benefit from intervention and which cell types should be used on a case-by-case basis to cure heart failure. Unfortunately, consideration of the optimal stem cell population has been overlooked in favor of what cell type is the easiest to isolate such as in the case of bone marrow-derived stem cells as these cells have been historically the most widely used for clinical therapy [12]. Popularity of bone marrow-derived therapy arises from the relative ease and efficiency of isolation of whole bone marrow, straightforward enrichment of mononuclear bone marrow cells and mesenchymal stem cells (MSCs) from the patient, as well as delivery by both autologous and allogeneic means [28-30]. However, there are inconsistencies in clinical trial design, randomization and statistics to support the efficacy of these cells for use in the USA despite common clinical practice across Europe. The results from these trials cast doubts over the value of bone marrow-derived cells, which admittedly provide for modest myocardial recovery even under the best of circumstances [31]. Future research of stem cell biology will need to build on a foundation of critical knowledge of potency status, properties of self-renewal, survival and the mechanisms that dictate obligatory asymmetric division to repopulate the damaged myocardium and outweigh scar formation. These traits will need to be considered in combination with ease of merging into clinical practices where cell numbers, timing and delivery approaches will be considered once the validity of the stem cell population is established.
Several researchers have established their standing as leaders in the field and are certainly interested in preventing and treating heart disease. However, the complex long-term logistics to improve cellular therapy are often overlooked by ‘flash in the pan’ experiments performed in small animal models. The idea that a single cell population is carefully attuned to accept the challenge of complete heart regeneration although attractive from a simplicity standpoint is idealistic. Despite established isolation techniques, there appears to be a lack of understanding of the native stem cell biology in culture. The remainder of this review highlights the modern use of stem cells being advanced. In addition, presentation of adult stem cells will be described in relation to the optimal properties that encompass a composite stem cell in the context of cell autonomous traits such as survival and proliferation, commitment and ability to communicate with the endogenous cardiac environment (Figure 1, bottom). Conversely, genetics and inevitable aging of the patient population will place a large burden on the heart environment and diminish efficient communication while also driving variability in the characteristics of stem cells that are isolated from the donor (Figure 1, top). Ultimately, a picture of the ideal stem cell population emerges from our assessment and overview, but predictably the collective characteristics needed indicate that such a population might not exist under natural conditions to mediate cardiac repair. Therefore, we posit that a combination of stem cells or a single stem population created by ex vivo engineering will be required to advance the next generation of cell-based therapy. We will also explore the possibility to imitate the natural environment of stem cells through creation of an artificial niche in vitro that possesses the heterogeneity inherently important for cardiac regeneration in vivo. Our goal is to point toward new avenues and novel transformative therapeutic angles that embrace the ideal properties of adult stem cells that are presented in this review.
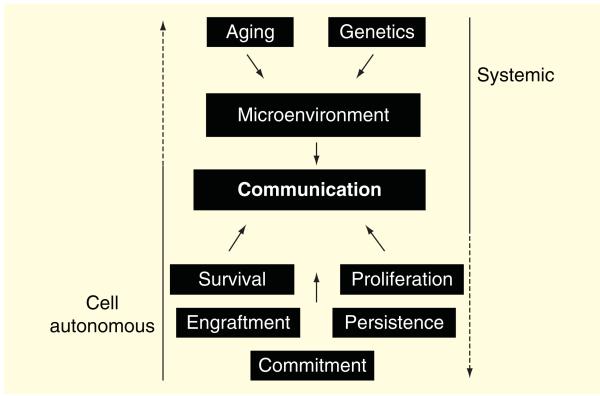
Figure 1. Enhancing stem cell therapy using pluripotent and multipotent stem cells requires the understanding of cell autonomous traits such as survival, proliferation, traits that will both impact on persistence and engraftment after delivery in the myocardium and allow for the final stage of cellular commitment in vivo.
These traits in stem cells will facilitate in creating a dialogue with the cardiac environment supplied by the donor and recipient of cells after damage. The systemic effects of aging, genetics and disease state of the patient should also be considered as they can negatively affect the microenvironment and stem cell effectiveness post delivery. Conversely, stem cell traits can contribute to systemic aging and diminished cell to system communication if characteristics of cells are less than optimal.
Aging & genetics: tyranny of the cardiac environment
Systemic aging is an unavoidable process linked to genetics and diversity, environmental stress and lifestyle habits such as smoking, alcohol consumption and obesity [32]. Genetics increases susceptibility to ischemic diseases, arrhythmias and heart failure due to mutations in sarcomere and contractile genes that may or may not be familial linked [33-35]. Aging is associated with impairment of cellular function as aging accelerates a decline in stem cell function. Senescent myoblasts from aged mice transplanted into young recipients fail to restore the endogenous stem cell niche or support nascent fiber formation implicating a cell autonomous property and irreversible senescence of aged stem cells from skeletal muscle [36]. In a top down rationale, the effects of systemic aging and more specifically cardiac aging are characterized by loss of cardiac function, increases in fibrosis and inflammation and impaired systemic circulation [37,38]. Cumulative effects of aging are detrimental to cardiac niches, prematurely pulling stem cells from supportive hypoxic environments resulting in fewer stem cell numbers, reduced self-renewal, survival and commitment potential [38]. However, natural exhaustion of endogenous stem cells aside from systemic aging or genetics also occurs through the ongoing sensitivity of the cardiac cells to their environment and recruitment as house-keeping cells for tissue regeneration. Cardiogenic adult stem cells are responsible for maintenance of homeostatic repair in the metabolically taxing myocardial environment that can result in exhaustion, which coincides with symptoms of overall cardiac senescence [39]. Synergy between physiological, biological and molecular signaling appears to exert bidirectional effects between the organism and the single cell level. Presumably cardiac aging differentially affects cardiomyocytes or stem cells, in fact due to vastly distinct properties of cell proliferation as well as exposure to the cardiac microenvironment and systemic factors. Indeed, phenotypic characteristics of aging such as impairment in tissue formation and vasculogenesis can be partially overcome by chronic administration of systemic factors from a young mouse forcibly merged with an aged recipient in the experimental surgical model of parabiosis [40]. The ‘youthful’ benefits from systemic mixing and circulation of beneficial factors may only be transient and essentially little is known if these factors target mature and transit amplifying cells homogenously. Furthermore, such parabiosis findings also suggest that factors in aged mice negatively affects younger mice, with the existence of senescence-associated factors chronically expressed with advanced age that impact upon organismal biology [40]. Stem cells of the heart are subjected to adverse stimuli of early apoptosis and necrosis after myocardial damage just as their neighboring cardiomyocytes, smooth muscle cells, fibroblasts and supporting cells of the myocardium. The adult mammalian heart, although not purely a post-mitotic organ, has not evolved to cope with insults that occur during aging or acute injury pathogenesis to efficiently regenerate and repair. At least half of the CPCs in the heart are less than competent to show myogenic potential, supporting the need to characterize stem cell populations that can combat senescence and inflammatory associated secretomes [41].
Transcriptional analysis of adult stem cells to customize cellular therapy
Genetics and severity of disease can outweigh the benefits of autologous clinical cell therapy, as decreases in blood circulation, diabetes and chronic heart failure impair adoptive stem cell regenerative potential [42]. Recently, micro-array platforms identified global transcriptional differences between cardiac derived c-kit+ and stem cell antigen-1 (sca-1+) cells relative to bone marrow-derived cells, highlighting the need to focus on the true ability of stem cells [43]. Cardiogenic cells have enriched expression for cardiac-specific developmental markers, whereas bone marrow cells have increased expression of genes for neutrophil maintenance, cell cycle progression and extracellular matrix (ECM) secretion [43]. Utilization of sequencing tools and bioinformatics to support specific mechanisms of adult stem-based therapy derived from human patients can be used to improve regeneration across all patients by recognizing variability in background or age. In the current age of RNA-sequencing, transcriptional variation of iPSCs created from human fibroblasts correlate with the background of donors (age, disease, personal habits), although the overall phenotypic traits of human iPSCs are similar ex vivo [44]. In the future, autologous stem cells should be analyzed for optimal phenotypic and traits related to extensive mRNA analysis to optimize clinical therapy in humans. After delivery of stem cells into the heart, there is going to be a significant uphill battle with intrinsic cellular limitations coincident with trying to understand the cardiac environment. In order to combat systemic issues, approaches to define markers and signaling networks could help select cells primed for proliferation and survival potential [45]. Therefore, proliferative and survival abilities of adult stem cells will be examined in the next section with an eye toward how these traits will help improve cardiac parameters after damage or disease.
Identifying stem cells for optimal proliferation & survival
Optimal cell proliferation and enhanced survival are indispensable traits in an adult stem cell population tailored for cellular therapy. Ischemic events and cardiac disease result in cardiomyocyte death, inflammation, impaired proteolysis and diminished clearance creating a challenging environment for transplanted stem cell proliferation and survival [46,47]. Stem cell populations that are efficient in DNA replication but sensitive to low levels of oxidative stress and inflammatory insults could be augmented by increasing the number of delivered cells to balance cell death during acute damage. However, limited persistence of stem cells 24 h after intramyocardial injection reduces the initial population to less than 10% of cells as quantified by sensitive PCR methods [48,49]. Increasing the number of cells to a higher threshold will only create higher rates of competition between injected stem cells and the microenvironment depleting stem cell resourcefulness. Future approaches will need to consider the ability of cells to thrive in the damaged myocardium by empowering proliferation and survival characteristics observed ex vivo and prior to delivery.
The coveted cardiac-derived stem cell has been traced as early as the developmental stage that defines the mesoderm. Analysis of the early embryo reveals distinct first and second heart fields, which provide a sufficient regulatory niches for multipotent and early adult CPCs (Mef2c+ and Nkx2.5+) [50]. In the past decade, researchers have come to recognize differential CPCs due to the heterogenic nature of the heart, such as LIM homeodomain transcription factor Islet1 cells (Isl1) found primarily in the embryonic atria, sca-1+ cells, side population cells and non-adherent cardiospheres [51]. The receptor tyrosine kinase, Kit is highly expressed in the bone marrow, a marker of hematopoietic stem and progenitor cells and was subsequently used to discover stem and progenitor cell populations in the heart. Cardiac-derived stem cells were identified using a transgenic mouse model to express the green fluorescent protein under regulatory control of the c-kit promoter to track progeny through development and trace lineages consistent with myocardial and vascular lineages [10,11,52,53]. Use of c-kit cells in the clinical trial SCIPIO supported increases in ejection fraction by 12% and an average decrease in fibrotic scar by 30% based on a 1-year follow-up in patients [8]. Additionally, quality of life improved in patients receiving CSC treatments based on New York Heart Association (NYHA) functional parameters and the Minnesota Living with Heart Failure (MLHF) Questionnaire score, which was downgraded in most patients to NYHA Class I and MLHF score between 20 and 30 1 year following treatment [8]. C-kit binding with stem cell factor results in downstream activation of PI3K family known to regulate processes such as cell proliferation, survival signaling, migration, secretion and differentiation [54]. The myocardial progenitor cell milieu is diverse as evidenced by discovery of adult stem cells from cardiogenic origin based on extracellular expression of sca-1 and platelet-derived growth factor receptor α, both markers correlated with enhanced commitment into vascular smooth muscle and endothelium [53,55,56]. Furthermore, the anatomic location of stem cells can influence proliferative status of cardiac-derived MSCs displaying high notch activity distinctly found in the epicardial space prior to injury [57,58]. Similarly, adult bone marrow cells and cardiogenic stem cells remain mostly quiescent until activated by injury or aging, increasing the presence of heterogenic stem cells in the cardiac environment [43].
Defining youthful adult stem cells based on biomarkers
Cardiogenic or bone marrow-derived adult stem cells regardless of the marker composition can be transiently activated from a quiescence state to perform functions such as paracrine secretion and differentiation if solicited properly by appropriate triggering of receptor and downstream signaling cascades. Stem cell expression of the receptor tyrosine kinases such as c-kit and insulin growth factor receptor as well as IL-6 family of receptors are known to influence downstream signaling related to survival and proliferation in stem cells [59-61]. Isolation of insulin growth factor receptor+ cells co-labeled with c-kit from aged human heart have increased regenerative potential and are a superior choice for mediation of cardiac repair relative to c-kit+ cells alone [59]. Growth properties of adult stem cells ex vivo are an important metric to predict cardiac repair as studies that correlate an increase in wall thickness after infarction also had the most robust properties in stem cell proliferation [62]. Optimal proliferation and survival traits for cellular therapy can be assessed through biomarkers such as receptor expression, internal signaling and most notably telomere length and telomerase activity. Although, hypoxia is often associated with ischemia, endogenous CPCs preferably inhabit hypoxic niches and display longer telomeres due to selective activation and contribution to myocyte renewal [39]. Isolation of the primitive stem cell that is quiescent during most of the organism lifespan characterized by longer telomeres and high telomerase activity are critical for optimal cardiac regeneration, as critically short telomeres lead to decline in stem cell function and accelerate systemic aging by decreasing homeostatic tissue repair [38,39,62,63]. Future isolation protocols can consider selecting stem cells closer to a youthful state so as to not deplete proliferative and survival status, exacerbate telomere shortening and promote desensitization of protective molecular signaling associated with persistent ligand-receptor binding.
Viral technology to improve stem cell characteristics
Exogenous expansion of adult stem cells is necessary to deliver the adequate number of cells to support regeneration after damage, especially in view of the inevitable losses of cells observed following adoptive transfer. Methodologies to invigorate stem cells by improving proliferation and survival capabilities for transplantation studies have employed genetic engineering with viral vectors (adeno-, retro- and lentiviruses). Boosting stem cell vigor has been accomplished with the serine threonine kinase Akt and downstream activator, Pim-1 a constitutively active protein implicated in cardioprotection after pathological stimulus and promotion of endogenous CPC cycling and cardiomyogenic differentiation from both mouse and human [64-67]. Bone marrow cells (BMCs) migrate to the heart after myocardial damage, yet do very little to maintain cardiac integrity with limited evidence of transdifferentiation [68]. Pim-1 overexpressing BMCs enhanced structural integrity of the heart after damage compared with non-genetically modified stem cells possibly through increased persistence and secretion of paracrine factors from BMCs well into the third month after delivery [69]. Similarly, mouse and human CPCs support cardiac regeneration directly up to 8 months after delivery through means of engraftment and differentiation into mature cardiogenic cells [65,69]. Pim-1 overexpression increases growth kinetics and provides for transient lengthening of telomeres in mouse CPCs [70]. Routine protocols in our laboratory has allowed for isolation of c-kit+ CPCs from patients exhibiting symptoms of severe heart failure before left ventricular assisted device implantation [60]. Isolated human CPCs with slow proliferative indices correlate with shorter telomeres and increased susceptibility to oxidative stress [60,71]. Genetic enhancement of human CPCs with Pim-1 increases telomerase activity, telomere lengths and prevents premature cell cycle arrest in culture [60]. Senescent properties of CPCs can be reversed using Pim-1 and further broaden the use of CPCs from patients regardless of suboptimal stem cell traits. Additionally, our group has identified nucleostemin (NS), a downstream target of Pim-1 [72]. NS is known to play a role in regulation of ribosomal biogenesis, proliferation and growth in proliferative stem cells making NS an ideal biomarker for identifying youthful CPCs derived from the heart [71,73]. These studies suggest that rejuvenation of adult stem cells can be conferred by genetic modification of cells enhancing classic signaling pathways, protection from cellular death, increasing the proliferative potential and telomere length to defer senescence and promote a youthful phenotype without having to tediously isolate pristine stem cells from the heart [60]. Stem cell therapy would benefit by isolation of young, proliferative and survival competent cells, but also cells that are receptive to molecular strategies to supplement inherent limitations in the reparative potential of the human heart.
Myocardial mechanical stress to impact stem cell engraftment & commitment
Issues with stem cell engraftment and commitment have been met with multiple novel approaches to improve the transplantation of cardiogenic adult stem cells. Since the discovery of in vitro cell culture in 1912, the methods for maintenance of growth and differentiation have not changed substantively for bulk expansion of cells. Heterogeneity of stem cell populations after isolation observed in vivo is believed to occur concurrently with differential chromosome segregation during mitotic division [74]. Therefore, single cell isolation and analysis is not necessarily the key to understanding distinct stem cells but appreciation for the population as a whole, which reflects the existence of multiple cells within tissues. Advancement of cell culture approaches has led to studying the effects of mechanical stress, changes in substrate geometries and microfluidics to predict stem cell orientation and engraftment of delivered cells [75-77]. Stretch-activated channels on the cell surface allow for influx of calcium known to regulate stem cell behavior as seen with cyclic stretch of human CPCs leading to a decrease in proliferation and increase in growth and expression of cardiomyogenic markers [76,78]. Biomechanosensing of CPCs can be activated in a microenvironment during stress such as MI, further facilitated by an upregulation of signaling pathways Wnt/β-catenin and Hippo pathway factors YAP and TAZ to support communication between the environment and delivered cells [79,80]. Mechanotransduction through stretch-activated channels on stem cells can better translate cardiac environmental stiffness, tensile or shear and properly tune temporal and spatial differentiation patterns of both human CPCs and ESCs ex vivo and in vivo [79,81]. Furthermore, electric stimulation such as mono- and biphasic electrical pulses mediate human CPC expression of the calcium channel Cav1.3 [82]. Cell culture strategies to support synergism between biochemical and mechanical perturbations will help foster stem cells to an environment closer to the native myocardium, facilitate engraftment and encourage cardiogenic commitment.
Re-creation of the myocardial environment: communication is key
The 3D spontaneous aggregation of heterogeneous stem cells into structures termed ‘cardiospheres’ has been promulgated as an approach to enhance connections between cardiogenic stem cells and the cardiac environment after adoptive transfer [83,84]. Unfortunately, clinical application of cardiospheres as seen in the CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction trial requires dissociation that destroys the 3D architecture and communication of a cardiosphere prior to delivery of the resulting single cell suspension termed ‘cardiosphere-derived cells’ (CDCs) [85]. Human CDCs reportedly conferred improvements in myocardial structure, but left ventricular volumes and ejection fraction were unchanged relative to standard of care in patients who did not receive CDCs [85,86]. Although the NYHA classification was unchanged in patients treated with CDCs, the MLHF score was decreased correlating with improved physical activity, increased walking distance during 6-min walk test and peak oxygen consumption relative to control treated patients [85]. The regenerative potential of stem cells is often evaluated in a co-culture with cardiomyocytes, wherein CDCs increase neonatal cardiomyocyte proliferation dependent on integrin binding and paracrine secretion reminiscent of an MSC-like phenotype [86]. Co-culture with cardiomyocytes is a valid approach to determine efficacy of stem cells prior to adoptive transfer to the heart. However, synergistic interactions between various stem cells, mature fibroblasts and endothelial cells should be considered to enhance stem cell communication and improve the integrity of transplanted cells [80,87-89].
Fibroblasts are the most abundant cell type within the heart, and provide for integral structural and paracrine signaling toward myocytes, vasculature and stem cells within the heart [80,87-89]. Fibroblasts are implicated in wound healing, direct ECM production and growth factor secretion to attract proinflammatory cells [87]. Delivery of CPCs and bone marrow-derived cells into border zone areas surrounding the infarct (a critical area for scar formation and proliferation of fibroblasts) blunts scar formation, degrades ECM and prevents fibroblast to myofibroblast conversion [89]. A critical role for cardiac fibroblasts in myocardial formation is confirmed by developmental studies that show genetic deletion of cardiac fibroblasts resulting in developmental and morphological defects of the four cardiac chambers [88]. Although true ‘cardiac-specific’ fibroblasts markers have not been validated, fibroblasts express high levels of vimentin and cell surface receptor discoidin domain receptor 2 to regulate fibroblast function in vivo [87]. Cardiac fibroblasts share similar markers and transcriptional profiles to stromal cells such as MSCs and co-exist in several tissue types [88]. Collectively, these observations support the premise that fibroblasts are a complex multifaceted contributory cell in myocardial homeostasis and deserve consideration when developing therapies to support myocardial regenerative medicine. Extracellular matrices supplied by the microenvironment and stromal cells (MSCs and fibroblasts) of the heart are of crucial importance for improving cell delivery. ECM proteins such as fibronectin and laminin provide for signaling that enhances stem cell recruitment and persistence in the wake of MI injury [90]. Biomaterials created in vitro are commonly used to understand stem cell properties such as cellular fate prior to injection [91]. Therefore, co-injection of engineered ECM-based scaffolds with small molecules and adult stem cells has been developed to improve efficacy of delivery [92]. Biomaterials can not only mediate adult stem cell delivery in the damaged heart, but also provide protective signaling to the endogenous environment. These studies indicate that the complex interplay between multiple cell types and the natural ECM in the heart holds virtually limitless possibilities for cell-to-cell communication after ischemic injury. Myocytes release cytokines and factors that support epicardial cell recruitment and fibroblast proliferation, endothelial secretion of angiogenic factors, bone marrow-mediated inflammation and much more collectively converge upon delivered adult stem cells. In turn, delivered stem cells must learn to adapt and contribute to productive facilitation of all these processes [80]. Understanding the cardiac environment and translating the language of repair communicated by heterogeneous stem cells will potentiate the ability to improve regenerative-based therapy. Therefore, novel and creative approaches to engineer microenvironments outside the body are needed to more faithfully recapitulate mechanical and biochemical stressors of the heart.
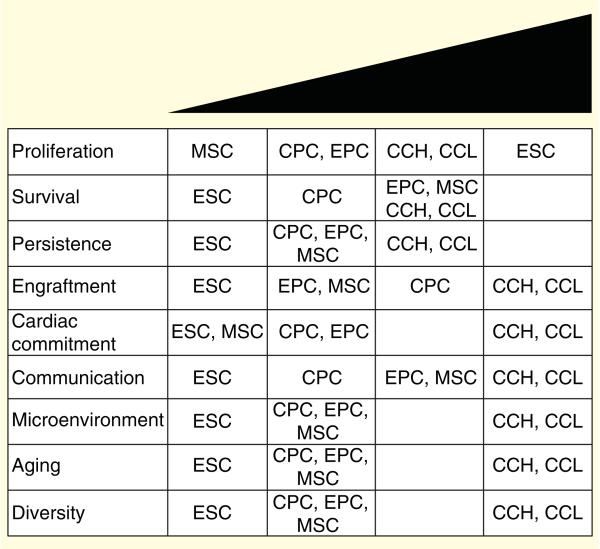
The strengths and weaknesses of ESCs, CPCs, endothelial progenitor cells (EPCs) and MSCs are highlighted relative to optimal traits required for efficient cellular therapy based on both in vitro and in vivo assessments (Figure 2). In summary, ESCs show extreme proliferative properties, however, ESC transplantation requires chronic administration of immunosuppressive agents that compromise cell survival, persistence, engraftment and communication in vivo [93,94]. In order to prevent teratoma formation, ESCs must undergo extensive pre-differentiation protocols but there are still significant issues with ESC-cardiomyocyte coupling and production of arrhythmias in vivo [17,93]. CPCs show an intermediate proliferative phenotype, a moderate resistance to cell death, but an increased ability to undergo cardiac-specific commitment [95]. EPCs support cardiac repair by differentiation into vascular endothelium [96] and can be similarly subjected to phenotypic and functional assessments in vitro like CPCs. CPCs and EPCs benefit as a cell population that can be carefully selected with biomarkers to counteract adverse traits supplied by the microenvironment, aging and/or diversity of the patient [59,62,97]. MSCs undergo premature replicative senescence, but like EPCs secretion of autocrine, paracrine and immunomodulatory factors enhance cell survival, persistence and communication [28]. Furthermore, MSCs can be supplied to the heart by allogeneic transplantation, supporting MSCs applicability to a diverse patient population [30]. In vitro evaluation of proliferation and cell death can be determined by measuring cell doubling time and cell death after treatment with oxidative stress agents. Persistence and engraftment will be highly dependent on the number of cells that survive after injection into the acutely damaged heart. Cardiac commitment would be evaluated with in vitro assays such as after co-culture experiments with cardiomyocytes. More convincingly, cells should be assessed for cardiomyogenic commitment in vivo. Communication would be measured by the efficient cross-talk between stem cells through paracrine secretion, supplying a microenvironment with suppressed inflammation, reduced scar formation, increased recruitment of endogenous stem cells and the presence of healthy cardiomyocytes. The enhanced paracrine milieu provided by delivered stem cells would modulate the aging or senescent environment by stabilizing and preventing telomere attrition. Additionally, the optimal stem cell population would be amenable to patient variability by exhibiting immune tolerance when delivered to a broad patient population. The remainder of this review describes advantages of a 3D stem cell cluster composed of CPCs, MSCs and EPCs termed as CardioCluster as well as unique stem cell hybrids created after cell fusion of distinct stem cells called CardioChimeras. As seen in Figure 2, CardioClusters and CardioChimeras represent two future strategies to enhance individual stem cell properties and mechanisms to support regeneration of the heart.
Figure 2. Characterization of cardiac progenitor cells, mesenchymal stem cells, endothelial progenitor cells and embryonic stem cells related to optimal traits reported in the literature.
CardioClusters and CardioChimeras are included to highlight the gains in traits with novel combinatorial cell delivery approaches.
CCH: CardioChimeras; CCL: CardioClusters; CPC: Cardiac progenitor cells; EPC: Endothelial progenitor cell; ESC: Embryonic stem cell; MSC: Mesenchymal stem cells.
CardioClusters: next-generation approach to deliver multiple adult stem cells into the heart
Current methodological challenges in studying and manipulating stem cells have spurred intense development of microengineering of stem cells in an ex vivo environment to support communication between cell types [91,98]. Study of endogenous cardiac niches may help dissect the complex molecular interplay of stem cells that exists in vivo helping researchers better predict cell behavior once introduced into the cardiac environment. Ex vivo cardiac microenvironments such as cardiospheres allow for enhanced cellular communication and physical proximity [84], but direct cell-to-cell communication possibly enhanced in the 3D cardiosphere is destroyed to allow for injection of CDCs. Consequently, CDCs derived from cardiospheres exhibit negligible direct contribution to regeneration and are rapidly cleared from the myocardium, with the purported mechanism of action relegated to activation of endogenous reparative processes in the myocardium arising from short-lived paracrine activity [85]. To overcome these apparent limitations of cardiosphere technology, our group has pioneered the rational design of a structure dubbed a ‘CardioCluster’. CardioClusters are a 3D microenvironment created using defined cell populations from the human heart: CPCs, MSCs, EPCs and fibroblasts. CardioCluster cellular composition is distinctly distinguished from cardiospheres that are spontaneously composed of a cardiac-derived heterogeneous population expressing CD150 and CD90, mostly MSC-like, with minimal contribution from c-kit cells [84]. Additionally, the ability of CDCs to directly contribute to vascularization depends upon a comparatively low fractional percentage of CD31 or CD133 EPCs [83,99]. CardioClusters are layered to promote efficient myocardial repair with planned participation of fibroblasts. CardioClusters represent a combinatorial application of stem cells that have distinct roles to enhance endogenous mechanisms as well as promotion of delivered stem cell survival and differentiation potential.
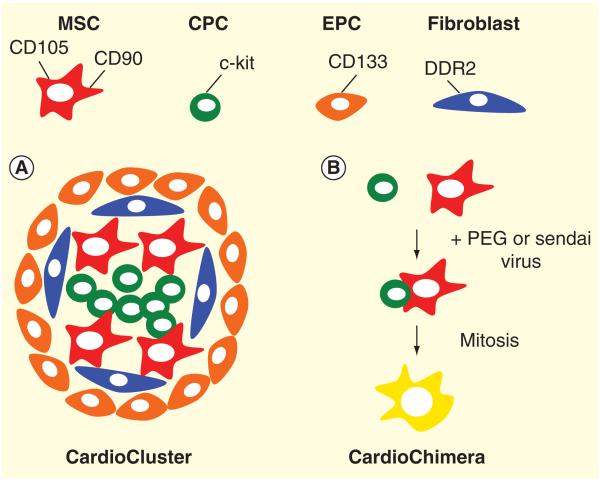
Mitigation of acute cell death and replacement of scar tissue in the wake of infarction injury requires involvement of multiple myocardial and stem cell types. MSCs from the bone marrow are of interest because of their ability to secrete an assortment of paracrine factors, although they lack marked ability to transdifferentiate into highly differentiated functional cardiac muscle [100-102]. Additionally, MSCs may also provide for immunomodulatory effects in vivo, reducing inflammatory responses and stimulating ECM turnover [89]. EPCs transplanted in vivo create microvessels, followed by regression in the absence of stromal cells to support vessel maturity [100]. Each of these cell types alone has beneficial properties to augment regeneration, yet achieving long-lasting myocardial benefits likely requires interaction with additional cell types rather than any single cellular ‘agent of change’. Specifically, added value of concurrent adoptive transfer of two cell types such as CPCs and MSCs together in a pig model of injury significantly enhances reparative efficacy [100], but to date the injection of multiple stem cells explanted solely from the human heart has not been reported. In consideration of additional cell types that could be employed in a combinatorial fashion, resident CPCs have distinct advantages over other cell types for cardiac cell therapy as they are pre-committed to the cardiovascular fate and produce new cardiogenic cells without inducing arrhythmias [103]. CPCs would be ideal for the core of a CardioCluster as CPCs are acclimated to hypoxic conditions in the heart [18,104]. Strategically, CPCs in the CardioCluster core will be mixed with MSCs and fibroblasts that will secrete cell adhesion molecules such as integrins and cadherins to help maintain the CardioCluster structure [105]. EPCs forming the outer ‘shell’ layer of the CardioCluster provide for endothelial-specific differentiation and production of tubular networks [100,106]. The size of the CardioCluster is compact enough to be delivered safely (<50 μM) without the necessity of dissociation from the 3D architecture (Abstract #22209 ‘Enhanced Myocardial Repair with CardioClusters’ Circulation Research 2013). Use of ECM or scaffolds will be considered to secure uniformity of the structure size and prevent breakdown of the cluster after injection (Figure 2 & Figure 3A). Delivery of CardioClusters is an ideal approach to replace the diminished cardiac niche seen in disease models and cardiac aging. In our opinion, CardioClusters will allow for distinct regenerative cells to thrive, wherein structure can enhance the function of multiple stem cells in a single entity.
Figure 3. Cardiac-derived MSCs (CD105+ and CD90+), CPC (c-kit+), EPC (CD133+) and fibroblasts (DDR2+) can be used for novel niche formations and dual stem cell fusion for the creation of A. CardioClusters and B. CardioChimeras respectively. A. CardioClusters are represented with four cardiac cell types presented in this review to have optimal traits required for improved communication, work synergistically and promote efficient cardiac regeneration.
B. CardioChimeras represent the blending of two distinct cell types to create hybrid cells that exhibit dual stem cell traits in a single cell population. CardioChimeras will ensure that the properties of survival, proliferation, paracrine secretion abilities and commitment are constrained to a single and unique cell type without the involvement of combinatorial cell delivery.
CardioChimeras: from wildly imaginative to cardiac relevance
Cell fusion for reprogramming of somatic cells has undergone a form of renaissance in the regenerative medicine era, sparking new interest in defining the optimal genetic factors to activate dormant cell types [107-109]. Fusion of slow growing MSCs with an immortal cell line produces hybrids that escape replicative senescence and increase MSC-specific secretion [108,109]. Hematopoietic stem cells (HSCs)-hepatocyte hybrids observed in vivo results in silencing of transcription factors associated with HSC maintenance, acquisition of hepatocyte chromatin regulators and overexpression of growth factor genes in the bone marrow nuclei overall leading to liver-specific commitment in combination with enhanced bone marrow-derived features [110]. In the cardiac context, proliferative stem cells fused with cardiomyocytes result in hybrids with constitutive expression of DNA synthesis markers and maintenance of well-defined sarcomeres [107]. These studies validate fusion between a proliferative parent cell and a slow growing, committed or senescent cell population to create hybrids with more youthful phenotypes, pre-committed cells with an ability to proliferate and optimal properties to overcome an aging cardiac environment. Fortunately, cell fusion is being discussed at a critical time for improving cellular-based approaches, as stem cell transplantation to support cardiomyocyte replacement occurs through a process of uncontrolled fusion of stem cells with the myocardial milieu in order to salvage damaged tissue and improve cardiac function [11,111,112]. However, there has been a dearth of studies to delineate the most favorable stem cell types to be used in a cell fusion-based protocol ex vivo to support repair of the myocardium. Therefore, our group has derived a strategy to create unique fused cell types among stem cell populations such as between CPCs, MSCs or EPCs to produce a novel and improved cell type for myocardial repair known as a CardioChimera (Figure 3B). Utilization of MSCs for fusion is of critical importance as bone marrow cells have desirable traits such as mobility and paracrine secretion to improve cell-to-cell communication [51]. On the other hand, CPCs retain an undeniable ability to undergo differentiation into cardiomyocytes, smooth muscle cells, vascular endothelium and fibroblasts [113]. CardioChimeras, relative to combinatorial cell delivery, secure equal spatial and temporal distribution of stem cell-specific mechanisms regardless of distinct migratory, proliferative, survival and cellular fate choices by the stem cell in the myocardium (Figure 2). With these examples, fusion is a validated technique to overcome undesirable characteristics such as slow growth rate or susceptibility to oxidative stress of stem cells. In comparison, combinatorial cell delivery is a limited approach to support cellular communication because of marked differences in proliferation rates and survival after injection into the damaged heart. Furthermore, combinatorial cell delivery of CPCs and MSCs would have limited interactions up to the peak of inflammation and clearance (3–4 days after MI). After fusion of MSCs with CPCs, the chimera could persist and productively contribute to endogenous and exogenous repair in a single cell. In large animal models and humans, allogeneic bone marrow MSC transplantation strategies have allowed for successful cellular engraftment across major histocompatibility complex barriers, allowing for long-term multilineage hematopoietic-derived chimerism [114-116]. Hybrids arising from MSC fusion provide for positive immunomodulatory effects and support allogeneic transplantation of CardioChimeras. However, derivation and characterization of chimeras from two distinct stem cell populations from the heart and investigation of their potential regenerative, paracrine and immunomodulatory capabilities has not been previously explored.
Currently, our group is performing fusion with bone marrow-derived MSCs, which have limited potential for cell proliferation and undergo irreversible cell cycle arrest six passages outside the mouse bone marrow. In order to maintain positive attributes of MSCs such as paracrine secretion, without constraints of proliferative senescence, CPC and MSC fusion was performed to produce hybrids of dual phenotypic and functional properties provided by both parent cells (Figure 3B). The CPC-MSC specific CardioChimeras show similar or increased cellular proliferation relative to parent CPCs and do not exhibit adverse susceptibility to oxidative stress. Furthermore, CardioChimeras show enhanced communication with cardiomyocytes in co-culture, decreasing cellular death of myocytes during acute stress. Conversely, cardiomyocytes support indirect and direct regenerative potential of CardioChimeras, as evidenced by increases in secreted anti-inflammatory factors and cardiomyogenic commitment. CardioChimeras are reported to have increased cellular size, consistent with an MSC-like property, which will improve the retention of cells delivered into the border zone area of the myocardium. Preliminary data suggest that the CardioChimera has increased positive properties relative to individual stem cell delivery by increasing proliferative traits, cardiac-specific differentiation, growth factor release to enhance persistence and engraftment in the heart (Abstract #21998 ‘Enhanced Myocardial Repair with CardioChimeras’ Circulation Research 2013). Additionally, CardioChimeras represent a single population that does not test the limits of cellular size as CardioChimeras display comparable cell size as MSCs (Figure 2). Application of CardioChimeras, a fused combinatorial delivery approach, provides for unique cell variability that is not unlike the inherent heterogeneity observed in tissue [43,74]. In the bone marrow niche, hematopoietic stem cells undergo cellular fusion creating genetic variation without compromising mitotic ability or clonogenicity of stem cells [117]. Mosaic aneuploidy, or abnormal chromosome number, is observed in the inner cell mass and neural progenitor cells, a well-known characteristic of stem cells that leads to somatic variation in several tissue types [118,119]. Overall, these reports support the existence of chromosomal heterogeneity in stem cell populations, yet the contribution of hybrids as a cell therapeutic geared toward cardiac tissue regeneration has yet to be unraveled. Future studies will test the regenerative capacity of CardioChimeras by injection of cells into a mouse model of myocardial injury. Cardiac functional recovery in addition to CardioChimera cell engraftment, persistence and differentiation will be compared relative to single and combined parent stem cell populations. Collectively, CardioChimeras represent a future technique to combine any two stem cell populations. In this case, our ideal therapy would be to combine the beneficial properties of CPCs to undergo cardiac-specific commitment as well as MSCs that foster an improved microenvironment with protective paracrine secretion.
Expert commentary
Cellular therapy has relied heavily upon the existence of techniques that allow for ease in isolation but not necessarily efficacy after transplantation. Researchers involved in these studies have been pleased to support that clinical application of adult stem cells is feasible, but real-world outcomes have proven to be disappointing relative to the increases in cardiac structural remodeling and function observed in animal models. Evaluation of biomarkers such as cell surface receptors, telomere length, proliferation and survival are valid attributes that can be used to gauge regenerative potential in adult stem cells. However, it is becoming increasingly apparent that no single cell population is truly exceptional in promoting the best and most efficient cardiac repair, and that combinatorial approach increases the ability of the two cells together inferring that there are inherent synergistic mechanisms between injected cell types to support engraftment and cardiac repair. However, most understanding of adult stem cell therapy has to be gained through analysis of endogenous mechanisms that are greatly linked to the diversity and age of the organism. Microenvironments supply the cues to establishing optimal survival and proliferation of cells, which will lead to better persistence and engraftment, and cardiogenic commitment and promote cardiac regeneration.
The field of adult stem cell therapy is filled with a broad range of stem cells from both the heart and the bone marrow. This review highlights the limiting mechanisms to support adult stem cell fate or paracrine secretion in vivo, as each adult stem cell population, although effective, has narrow functions that may or may not be dependent on cell fate or commitment. Biochemical properties and markers such as cell surface receptors lead to identification of c-kit+ CPCs, cells that show enhanced proliferative patterns ex vivo and cardiomyogenic commitment after transplantation into the damaged heart [62]. Bone marrow-derived populations are considered to be the most popular cell type used for various cardiac injury and disease states, however, the presumption is that they are inadequate in persistence, although transcriptional profiles have indicated a large role in DNA replication [29,43]. In turn, this allows us to question bone marrow-derived cells survival tactics once in the damaged or diseased heart. Non-cellular therapy for severe cardiac dysfunction has been approached with growth factor stimulation such as with IGF, HGF, VEGF and stromal-derived growth factor delivered systemically to promote endogenous stem cell recruitment and aid in cardiac repair [120,121]. Bone marrow cells serve as a vehicle for a protective paracrine milieu, but with a short existence of cells early after injection, do these cells actually do more harm than good? In the clinic, if patients were given the choice to infuse growth factors or undergo transplantation with cells that disappear in a matter of days, more commonly patients would choose the less invasive therapy.
Increasing communication between stem cells and the cardiac environment has been suggested with CDCs and combinatorial cell delivery of c-kit+ CPCs and MSCs [85,122]. However, the term heterogeneous is not pre-meditated for CDC delivery, as these cells have MSC-like properties and not surprisingly disappear shortly after delivery, yet there is advancement into clinical trials [85]. Cardiac lineage commitment of c-kit+ cells is enhanced by creation of a structure called a ‘CardioStem sphere’ prior to differentiation protocols supporting the formation of artificial niches to enhance individual stem cell traits [113]. Based on observations of the endogenous cardiac stem cell niches, our group has created a CardioCluster, an idyllic microenvironment to boost individual stem cell phenotypes and genetic traits outside the body prior to repopulation of the damaged heart. In the CardioCluster, supporting cell types such as fibroblasts and MSCs create natural matrices and provide for secretory mechanisms initiating cross-talk between cardiomyogenic CPCs and EPCs. On the other hand, the creation of a CardioChimera was introduced as unique cell that has merged properties of distinct stem cell types. Clonally derived CardioChimeras result in a cell population that would never exist in nature, an exceptional biological anomaly that can be carefully selected to support regeneration of the heart despite genetic diversity and aging. Stories of mythological chimeras as composites of ideal phenotypic features to generate a more robust animal or human are wildly imaginative. Despite implausibility, studies of mythological creatures exemplify the true strengths and weaknesses of animal and human traits that can apply to the way we study adult stem cells geared for cellular therapy. The formation of novel structures and combinations can be performed in an optimal fashion if we truly understand adult stem cell function. Next-generation cell models that place the most favorable stem cell traits right and center to improve regeneration of the heart from all angles is needed in the field.
Five-year view
Five years from now, we should have better understanding of the biology of adult stem cells beyond focusing on presumed actions of adult stem cells to mediate cardiac repair. Stepping back from the mounting field of markers has led to an egotistical grab and push of favorite stem cells into clinical trials. Rather, the future should concentrate on the transcriptional variation of adult stem cells for autologous cell therapy, and determine which populations are optimal for diverse and aged patients. This will require transcriptional profiling of adult stem cells using techniques like RNA-sequencing to effectively move us beyond characterizing phenotypic traits of cells. An era comprising bioinformatics and computational analyses of cells will create easier ways for patients to be diagnosed and treated with the right cell population, combination or formation of cells that is not based on ease but necessity. This review presents a perspective on the adaptation of new approaches to advance the bioengineering aspect of adult stem cell cultures. Instead of pre-treating cells with viruses, we can subject cells to mechanical stretching, microfluidics or co-injection with ECM that help maintain native proliferative and survival potentials outside the body as well as drive cardiac commitment after transplantation. Herein, two novel approaches are described that may improve the use of existing single cell or combinatorial delivery approaches through the use of CardioClusters, using desirable cell types that are pre-defined and known to interact and understand the language of the heart, as well as CardioChimeras to force communication between two chosen stem cell types resulting in creation of hybrids with blended phenotypic traits and genetic material derived from the parental cell lines prior to fusion. The field of cellular therapy will benefit from more ‘out of the box’ thinking and approaches to more effectively take advantage of the unique traits of adult stem cells for the advancement of cardiac regeneration.
Key issues.
Cardiomyocyte number is defined shortly after birth, which is modestly upregulated after injury and disease and cannot contribute to sufficient cardiac replacement.
Cardiac progenitor cells minimally contribute to the formation of new cardiomyocytes and promotion of endogenous regeneration.
Cardiac therapy using embryonic stem cells, induced pluripotent stem cells and adult stem cells from both the heart and the bone marrow has arisen as a response to combat limitations in endogenous regeneration.
Aging and diversity impairs the cardiac environment to be receptive to cellular therapy and in turn negatively impacts the regenerative capacity of stem cells.
Selection of stem cells for optimal proliferation and survival can supply against the negative effects of aging and diversity in individuals allowing for increased persistence and engraftment of cells.
Cardiac stem cell commitment is influenced by the microenvironment, structure and communication among heterogeneous cell populations of the heart.
Creation of optimal cardiac niches called CardioClusters ex vivo can be used to repopulate depleted stem cell sources and cover optimal stem cell traits for more efficient cardiac regeneration.
CardioChimeras represent a mononucleated hybrid cell resulting from the fusion of distinct stem cells to provide for increases in cardiac commitment and paracrine secretion mechanisms after injury.
Acknowledgements
The authors would like to thank members of the Sussman Laboratory for the constructive comments.
Financial & competing interests disclosure
P Quijada is supported by NIH grant F31HL117623 and Rees Stealy Research Foundation and Achievement Rewards for College Scientists (ARCS). MA Sussman is supported by NIH grants R01HL067245, R37HL091102, R01HL105759, R01HL113656, R01HL113647, R01HL122525 as well as an award from the Fondation Leducq Transatlantic Network and is a founder and co-owner of CardioCreate Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–6. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 3.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porrello ER, Mahmoud AI, Simpson E, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA. 2013;110:187–92. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoud AI, Kocabas F, Muralidhar SA, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–53. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naqvi N, Li M, Calvert JW, et al. A Proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Nadal-Ginard B, Ellison GM, Torella D. The absence of evidence is not evidence of absence: the pitfalls of cre knock-ins in the C-kit locus. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.304676. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10*.Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42. doi: 10.1016/j.cell.2013.07.039. Transient increases in c-kit+ CPCs in the mouse heart are essential in mediating global cardiac damage induced by isoproteronol. Ablation of c-kit+ cells after damage suggest that cells are necessary to maintain cardiac function after injury.
- 11*.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit cells minimally contribute cardiomyocytes to the heart. Nature. 2014 doi: 10.1038/nature13309. Epub ahead of print. Recent study using genetic lineage tracing of c-kit labeled cardiac progenitor cells (CPC) during development and after myocardial infarction. The study shows that c-kit cells are capable of forming cardiomyocytes and a variety of undefined interstitial cells in the murine heart.
- 12.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isobe KI, Cheng Z, Nishio N, et al. iPSCs, aging and age-related diseases. N Biotechnol. 2014 doi: 10.1016/j.nbt.2014.04.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.de Almeida PE, Meyer EH, Kooreman NG, et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat Commun. 2014;5:3903. doi: 10.1038/ncomms4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 17**.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014 doi: 10.1038/nature13233. Epub ahead of print. Clinical advancement of embryonic stem cells differentiated into cardiomyocytes (ES-CMs) prior to delivery into the damaged hearts of Rhesus Maques. ES-CMs developed maturity in vivo but produced significant arrhythmogenic events in the heart.
- 18.Yang X, Rodriguez M, Pabon L, et al. Triiodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–5. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundy SD, Gantz JA, Pagan CM, et al. Pluripotent stem cell derived cardiomyocytes for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:319. doi: 10.1007/s11936-014-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiba Y, Filice D, Fernandes S, et al. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J Cardiovasc Pharmacol Ther. 2014;19:368–81. doi: 10.1177/1074248413520344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson ME, Goldhaber J, Houser SR, et al. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ Res. 2014;115:335–8. doi: 10.1161/CIRCRESAHA.114.304616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu JD, Stone NR, Liu L, et al. Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State. Stem Cell Reports. 2013;1:235–47. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Chen JX, Krane M, Deutsch MA, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–5. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher SA, Brunskill SJ, Doree C, et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2014;4:CD007888. doi: 10.1002/14651858.CD007888.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–38. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 30.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowbar AN, Mielewczik M, Karavassilis M, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumanasekera WK, Tran DM, Sumanasekera TU, et al. Cigarette smoke adversely affects functions and cell membrane integrity in c-kit+ cardiac stem cells. Cell Biol Toxicol. 2014;30:113–25. doi: 10.1007/s10565-014-9273-6. [DOI] [PubMed] [Google Scholar]
- 33.Dichgans M, Malik R, Konig IR, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke. 2013;45:24–36. doi: 10.1161/STROKEAHA.113.002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duygu B, Poels EM, da Costa Martins PA. Genetics and epigenetics of arrhythmia and heart failure. Front Genet. 2013;4:219. doi: 10.3389/fgene.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill TJ, Ashrafian H, Watkins H. Genetic cardiomyopathies causing heart failure. Circ Res. 2013;113:660–75. doi: 10.1161/CIRCRESAHA.113.300282. [DOI] [PubMed] [Google Scholar]
- 36.Cosgrove BD, Gilbert PM, Porpiglia E, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond-Barbosa D. Stem cells, their niches and the systemic environment: an aging network. Genetics. 2008;180:1787–97. doi: 10.1534/genetics.108.098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumpel S, Rudolph KL. The role of telomere shortening in somatic stem cells and tissue aging: lessons from telomerase model systems. Ann N Y Acad Sci. 2012;1266:28–39. doi: 10.1111/j.1749-6632.2012.06547.x. [DOI] [PubMed] [Google Scholar]
- 39.Sanada F, Kim J, Czarna A, et al. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ Res. 2013;114:41–55. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–4. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torella D, Ellison GM, Mendez-Ferrer S, et al. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 42.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–30. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Dey D, Han L, Bauer M, et al. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–62. doi: 10.1161/CIRCRESAHA.112.300779. Transcriptional profiling of stem and progentor cells isolated from cardiac and bone marrow origin.
- 44.Rouhani F, Kumasaka N, de Brito MC, et al. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10:e1004432. doi: 10.1371/journal.pgen.1004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cesselli D, Beltrami AP, D’Aurizio F, et al. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–66. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2013;63:185–95. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Hong KU, Li QH, Guo Y, et al. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. Study that quantifies the number of surviving cells early after transplantation of c-kit+ cells using a sensitive PCR method.
- 49.Hong KU, Guo Y, Li QH, et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–77. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 52.Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–13. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fransioli J, Bailey B, Gude NA, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–24. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo J, Jie W, Shen Z, et al. Li T, Zheng S. SCF increases cardiac stem cell migration through PI3K/AKT and MMP2/9 signaling. Int J Mol Med. 2014;34:112–18. doi: 10.3892/ijmm.2014.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chong JJ, Reinecke H, Iwata M, et al. Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem Cells Dev. 2013;22:1932–43. doi: 10.1089/scd.2012.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bailey B, Fransioli J, Gude NA, et al. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res. 2012;111:750–60. doi: 10.1161/CIRCRESAHA.112.274662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–40. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell JL, Goetsch SC, Gaiano NR, et al. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res. 2011;108:51–9. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Amario D, Cabral-Da-Silva MC, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–81. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60*.Mohsin S, Khan M, Nguyen J, et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–79. doi: 10.1161/CIRCRESAHA.113.302302. Pim-1 overexpression in aged adult CPC decreases phenotypic and biochemical senescence by increasing survival after oxidative stress, preventing replicative senesnce and preserving telomere lengths.
- 61.Brady JJ, Li M, Suthram S, et al. Early role for IL-6 signalling during generation of induced pluripotent stem cells revealed by heterokaryon RNA-Seq. Nat Cell Biol. 2013;15:1244–52. doi: 10.1038/ncb2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.D’Amario D, Leone AM, Iaconelli A, et al. Growth properties of cardiac stem cells are a novel biomarker of patients’ outcome after coronary bypass surgery. Circulation. 2014;129:157–72. doi: 10.1161/CIRCULATIONAHA.113.006591. Study that correlates positive properties of cardiac stem cells isolated from patients with increased recovery of patients suffereing from specific cardiac injury and surgery.
- 63.Kajstura J, Bai Y, Cappetta D, et al. Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium. Circ Res. 2012;111:894–906. doi: 10.1161/CIRCRESAHA.112.273649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Fischer KM, Cottage CT, Wu W, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–87. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Mohsin S, Khan M, Toko H, et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–87. doi: 10.1016/j.jacc.2012.04.047. Application of human CPC that are genetically modified to have increases in proliferation and survival prior to adoptive transfer. The study showed that human CPCs have increases in persistence and support cardiomyogenic commitment.
- 66.Cottage CT, Bailey B, Fischer KM, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–75. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 68.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 69.Quijada P, Toko H, Fischer KM, et al. Preservation of myocardial structure is enhanced by pim-1 engineering of bone marrow cells. Circ Res. 2012;111:77–86. doi: 10.1161/CIRCRESAHA.112.265207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cottage CT, Neidig L, Sundararaman B, et al. Increased mitotic rate coincident with transient telomere lengthening resulting from pim-1 overexpression in cardiac progenitor cells. Stem Cells. 2012;30:2512–22. doi: 10.1002/stem.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siddiqi S, Sussman MA. Cell and gene therapy for severe heart failure patients: the time and place for Pim-1 kinase. Expert Rev Cardiovasc Ther. 2013;11:949–57. doi: 10.1586/14779072.2013.814830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siddiqi S, Gude N, Hosoda T, et al. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hariharan N, Sussman MA. Stressing on the nucleolus in cardiovascular disease. Biochim Biophys Acta. 2013;1842:798–801. doi: 10.1016/j.bbadis.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Altschuler SJ, Wu LF. Cellular heterogeneity: do differences make a difference? Cell. 2010;141:559–63. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang D, Jin Z, Shi J, et al. The anistropy of field effect mobility of CVD graphene grown on copper foil. Small. 2014;10:1761–4. doi: 10.1002/smll.201303195. [DOI] [PubMed] [Google Scholar]
- 76.Kurazumi H, Kubo M, Ohshima M, et al. The effects of mechanical stress on the growth, differentiation, and paracrine factor production of cardiac stem cells. PLoS ONE. 2011;6:e28890. doi: 10.1371/journal.pone.0028890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehling M, Tay S. Microfluidic cell culture. Curr Opin Biotechnol. 2014;25:95–102. doi: 10.1016/j.copbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Shah N, Morsi Y, Manasseh R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem Funct. 2014;32:309–25. doi: 10.1002/cbf.3027. [DOI] [PubMed] [Google Scholar]
- 79.Mosqueira D, Pagliari S, Uto K, et al. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS Nano. 2014;8:2033–47. doi: 10.1021/nn4058984. [DOI] [PubMed] [Google Scholar]
- 80.Deb A. Cell-cell interaction in the heart via Wnt/beta-catenin pathway after cardiac injury. Cardiovasc Res. 2014;102:214–23. doi: 10.1093/cvr/cvu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang F, Wasserman SM, Torres-Vazquez J, et al. The role of Hath6, a newly identified shear-stress-responsive transcription factor, in endothelial cell differentiation and function. J Cell Sci. 2014;127:1428–40. doi: 10.1242/jcs.136358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pietronave S, Zamperone A, Oltolina F, et al. Monophasic and biphasic electrical stimulation induces a precardiac differentiation in progenitor cells isolated from human heart. Stem Cells Dev. 2013;23:888–98. doi: 10.1089/scd.2013.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 84.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 85.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie Y, Ibrahim A, Cheng K, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014 doi: 10.1002/stem.1736. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70C:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furtado MB, Costa MW, Pranoto EA, et al. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res. 2014;114:1422–34. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramkisoensing AA, de Vries AA, Atsma DE, et al. Interaction between myofibroblasts and stem cells in the fibrotic heart: balancing between deterioration and regeneration. Cardiovasc Res. 2014;102:224–31. doi: 10.1093/cvr/cvu047. [DOI] [PubMed] [Google Scholar]
- 90.Konstandin MH, Toko H, Gastelum GM, et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013;113:115–25. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc. 2013;88:884–98. doi: 10.1016/j.mayocp.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menasche P. Embryonic stem cells for severe heart failure: why and how? J Cardiovasc Transl Res. 2012;5:555–65. doi: 10.1007/s12265-012-9356-9. [DOI] [PubMed] [Google Scholar]
- 94.Foldes G, Mioulane M, Kodagoda T, et al. Immunosuppressive agents modulate function, growth, and survival of cardiomyocytes and endothelial cells derived from human embryonic stem cells. Stem Cells Dev. 2013;23:467–76. doi: 10.1089/scd.2013.0229. [DOI] [PubMed] [Google Scholar]
- 95.Ellison GM, Galuppo V, Vicinanza C, et al. Cardiac stem and progenitor cell identification: different markers for the same cell? Front Biosci (Schol Ed) 2009;2:641–52. doi: 10.2741/s91. [DOI] [PubMed] [Google Scholar]
- 96.Palii CG, Vulesevic B, Fraineau S, et al. Enhances Vascular Repair by Injected Human Endothelial Progenitors through Increasing the Expression of TAL1-Dependent Genes. Cell Stem Cell. 2014;14:644–57. doi: 10.1016/j.stem.2014.03.003. et al. [DOI] [PubMed] [Google Scholar]
- 97.D’Amario D, Fiorini C, Campbell PM, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–61. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobel S, Lutolf M. High-throughput methods to define complex stem cell niches. Biotechniques. 2010;48:ix–xxii. doi: 10.2144/000113401. [DOI] [PubMed] [Google Scholar]
- 99.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–65. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 100.Lee WY, Wei HJ, Wang JJ, et al. Vascularization and restoration of heart function in rat myocardial infarction using transplantation of human cbMSC/HUVEC core-shell bodies. Biomaterials. 2012;33:2127–36. doi: 10.1016/j.biomaterials.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 101.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 102.Wang W, Itaka K, Ohba S, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–15. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 103.Hwang HJ, Chang W, Song BW, et al. Antiarrhythmic potential of mesenchymal stem cell is modulated by hypoxic environment. J Am Coll Cardiol. 2012;60:1698–706. doi: 10.1016/j.jacc.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 104.Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638–47. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 106.Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–57. doi: 10.22203/ecm.v022a19. discussion 257. [DOI] [PubMed] [Google Scholar]
- 107.Acquistapace A, Bru T, Lesault PF, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–24. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Islam MQ, Panduri V, Islam K. Generation of somatic cell hybrids for the production of biologically active factors that stimulate proliferation of other cells. Cell Prolif. 2007;40:91–105. doi: 10.1111/j.1365-2184.2007.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Islam MQ, Ringe J, Reichmann E, et al. Functional characterization of cell hybrids generated by induced fusion of primary porcine mesenchymal stem cells with an immortal murine cell line. Cell Tissue Res. 2006;326:123–37. doi: 10.1007/s00441-006-0224-2. [DOI] [PubMed] [Google Scholar]
- 110.Quintana-Bustamante O, Grueso E, Garcia-Escudero R, et al. Cell Fusion Reprogramming Leads to a Specific Hepatic Expression Pattern during Mouse Bone Marrow Derived Hepatocyte Formation In Vivo. PLoS ONE. 2012;7:e33945. doi: 10.1371/journal.pone.0033945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 112.Takei S, Yamamoto M, Cui L, et al. Phenotype-specific cells with proliferative potential are produced by polyethylene glycol-induced fusion of mouse embryonic stem cells with fetal cardiomyocytes. Cell Transplant. 2005;14:701–8. doi: 10.3727/000000005783982693. [DOI] [PubMed] [Google Scholar]
- 113.Smith AJ, Lewis FC, Aquila I, et al. Isolation and characterization of resident endogenous c-Kit(+) cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9:1662–81. doi: 10.1038/nprot.2014.113. [DOI] [PubMed] [Google Scholar]
- 114.Gleit ZL, Fuchimoto Y, Yamada K, et al. Variable relationship between chimerism and tolerance after hematopoietic cell transplantation without myelosuppressive conditioning. Transplantation. 2002;74:1535–44. doi: 10.1097/00007890-200212150-00010. [DOI] [PubMed] [Google Scholar]
- 115.Mapara MY, Pelot M, Zhao G, et al. Induction of stable long-term mixed hematopoietic chimerism following nonmyeloablative conditioning with T cell-depleting antibodies, cyclophosphamide, and thymic irradiation leads to donor-specific in vitro and in vivo tolerance. Biol Blood Marrow Transplant. 2001;7:646–55. doi: 10.1053/bbmt.2001.v7.pm11787527. [DOI] [PubMed] [Google Scholar]
- 116.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–8. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 117.Skinner AM, Grompe M, Kurre P. Intra-hematopoietic cell fusion as a source of somatic variation in the hematopoietic system. J Cell Sci. 2012;125:2837–43. doi: 10.1242/jcs.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peterson SE, Westra JW, Paczkowski CM, Chun J. Chromosomal mosaicism in neural stem cells. Methods Mol Biol. 2012;438:197–204. doi: 10.1007/978-1-59745-133-8_16. [DOI] [PubMed] [Google Scholar]
- 119.Peterson SE, Westra JW, Rehen SK, et al. Normal human pluripotent stem cell lines exhibit pervasive mosaic aneuploidy. PLoS One. 2011;6:e23018. doi: 10.1371/journal.pone.0023018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacArthur JW, Jr, Purcell BP, Shudo Y, et al. Sustained release of engineered stromal cell-derived factor 1-alpha from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation. 2013;128:S79–86. doi: 10.1161/CIRCULATIONAHA.112.000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Konoplyannikov M, Haider KH, Lai VK, et al. Activation of diverse signaling pathways by ex-vivo delivery of multiple cytokines for myocardial repair. Stem Cells Dev. 2013;22:204–15. doi: 10.1089/scd.2011.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122**.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. First study to use a combinatorial cell delivery approach of human-derived CPC and bone marrow-derived mesenchymal stem cells. Combinatorial cell delivery increased engraftment, ejection fraction and reduced the formation of scar compared with single cell delivery alone in a pig model of ischemia.