Abstract
Light has profoundly influenced the evolution of life on earth. As widely appreciated, light allows us to generate images of our environment. However, light, through the atypical intrinsically photosensitive retinal ganglion cells (ipRGCs; Box 1), also influences behaviors that are essential for our health and quality of life, yet are independent of image formation. These include the synchronization of the circadian clock to the solar day, tracking of seasonal changes, and regulation of sleep. Irregular light environments lead to problems in circadian rhythms and sleep, which eventually cause mood and learning deficits. Recently, it was found that irregular light can also directly impact mood and learning without producing major disruptions in circadian rhythms and sleep. Here, we will discuss the indirect and direct influence of light on mood and learning and provide a model for how light, the circadian clock, and sleep interact to influence mood and cognitive functions.
Introduction
The retina, located in the back of the eye, detects light for the formation of images and for object tracking1. However, our eyes are also essential for light detection for the regulation of several behavioral and physiological functions that are independent of image formation, collectively termed non-image forming (NIF, see glossary) visual functions. These NIF functions include the adjustment of the internal circadian clock to light (circadian photoentrainment, see glossary) and alterations in sleep and alertness. Therefore, light is essential for both image and non-image forming functions.
The rotation of the earth about its axis results in periodic changes in the light-dark environment. This predictable change in the light environment allows organisms to confine their activity-rest rhythms and physiology to specific times of the day-night cycle. To anticipate changes in the light-dark environment, organisms have evolved an internal biological clock that runs with a period close to 24-hours in the absence of environmental influences such as light2. The circadian clock drives many outputs, which include the sleep/wake and metabolic cycles as well as hormonal changes. Proper alignment between light, the circadian clock, and output behaviors produces a temporal order in organisms that is essential for survival3.
The circadian clock partitions sleep to occur at a particular time of the day-night cycle, whereas a homeostatic mechanism tracks sleep need. This homeostatic drive accumulates during periods of wakefulness and diminishes with sleep. The combination of circadian mechanism and homeostatic sleep drive determines the length of sleep4. It was assumed for many years that light influences sleep only secondarily through changes in circadian photoentrainment. However, several studies have now demonstrated that light directly affects both sleep onset and homeostatic sleep drive5–9. In this way, light, the circadian clock and sleep may closely interact to allow organisms to adapt to their environments.
This interaction can be used to explain why changes in the light environment, such as those associated with shift-work, shortened day lengths in winter, and transmeridian travel are associated with general changes in health including mental health issues such as seasonal affective disorder, depression, and cognitive dysfunction10. The effects of light on the circadian system have been thoroughly studied, with a focus on how changes in the light environment lead to changes in circadian rhythms that, in turn, influence sleep and contribute to alterations in mood and cognitive function. We will refer to this as the indirect pathway by which changes in the light environment lead to mood and cognitive alterations.
In addition to the indirect pathway, several new findings show that light can also have direct effects on mood and cognition. Furthermore, aberrant light stimulation can lead to mood and cognitive deficits independent of circadian arrhythmicity or sleep deprivation. We will refer to this as the direct pathway by which light leads to changes in mood and cognition. Therefore, in addition to the interdependence between light, circadian rhythms, and sleep, affect now emerges as a new function that is influenced by these three factors (Figure 1).
Figure 1. Model of the direct and indirect influences light on mood and cognition.
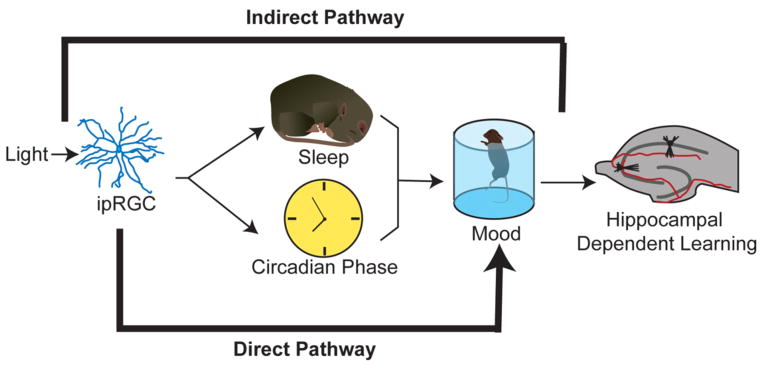
Light can regulate mood and learning secondarily by first modulating sleep and circadian rhythms (indirect pathway), or light can directly affect mood without disrupting sleep or causing circadian arrhythmicity (direct pathway). The effects of light on circadian rhythms, sleep and mood are mediated by ipRGCs.
In this Review, we discuss recent work that has investigated the influence of light on mood regulation through these two pathways. We highlight studies in humans and rodent models that have led to advancements in our understanding of the retinal and brain circuits involved in these pathways. Finally, we discuss the implications of these findings and consider how they can be applied in future investigations of the mechanisms underlying mood disorders and in the design of improved treatments for these disorders.
Mammalian light detection machinery
The solar day synchronizes circadian rhythms and sleep/wake cycles in animals and limits their activity to the correct temporal niche. Under normal conditions, organisms experience a 24-hour pattern of light/dark, and the circadian system of most animals uses the day to night transitions to align to environmental time. In non-mammalian vertebrates, in addition to the eyes, extraocular photoreceptors also detect light for circadian photoentrainment as well as other NIF functions11. In mammals, however, the eye is the only organ capable of detecting light for NIF functions12 indicating that the extraocular photoreception that is observed in non-mammalian vertebrates, is centralized to the eyes in mammals.
At the back of the eye, the retina contains the classical photoreceptors rods and cones, which transform photon energy into an electrical signal1. This information is then conveyed to the brain through retinal ganglion cells (RGCs), the output neurons of the retina (Figure 2a)13. A mere decade or so ago, rods and cones were considered the only photoreceptive cells in the mammalian retina. However, early work characterizing the spectral sensitivity of the circadian system provided the initial evidence of an additional photoreceptive system in the retina14. This was then supported by the intriguing discovery of a group of blind humans who were unable to form images but were nonetheless capable of detecting light for regulation of melatonin secretion15. The presence of an additional photoreceptive system was confirmed when genetically modified mice lacking rods and cones, were likewise able to detect light for circadian photoentrainment as well as other NIF functions16. Together, these studies suggested that a non-rod/non-cone photoreceptor in the mammalian eye is important for mediating non-image responses to light. The discovery that the photopigment melanopsin is present in only a small minority of RGCs in rodents (initially thought to amount to 1–2% of total RGCs) suggested that this subset of RGCs might be the elusive third class of photoreceptor17. These melanopsin-expressing RGCs were shown to respond to light intrinsically in the absence of rod/cone signaling and so they were called intrinsically photosensitive (ip)RGCs. However, like all ganglion cells, ipRGCs also receive light information secondarily from rods and cones18–21. Therefore, ipRGCs can detect light on their own through the melanopsin protein and can also mediate rod/cone input (Box 1).
Figure 2. Retinal and brain circuits underlying the effects of light on non-image forming visual functions.
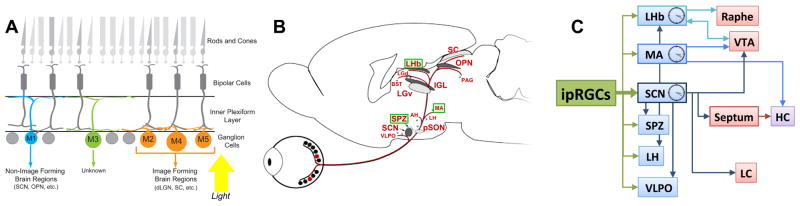
A. Schematic view of the retina showing the organization of different neuronal populations and their synaptic connections. Rods and cones are confined to the photoreceptor layer. Light detected by rods and cones is processed and signaled to retinal ganglion cells (RGCs) through horizontal, amacrine and bipolar cells. RGCs are the only output neurons from the retina to the brain. A subset of RGCs (2–5% of the total number of RCGs) are intrinisically photosensitive RGCs (ipRGCs). There are at least 5 subtypes of ipRGCs (M1–M5) with different morphological and electrophysiological properties, which show widespread projection patterns throughout the brain.
B. ipRGCs project to numerous brain regions, including many that have a role in driving light-mediated behaviors, including circadian photoentrainment and sleep. In addition, ipRGCs also innervate nuclei involved in depression and/or anxiety, such as the MA, LHb and SPZ (highlighted in green) indicating a possible direct role of light on mood.
C. Several of the ipRGCs targets (blue), including the SPZ, VLPO, LH and LHb also receive innervation from the SCN, raising the possibility that in addition to its pacemaker function, the SCN can also act as a conduit for light information. Interestingly, the MA and the LHb are also brain peripheral clocks that receive direct retinal innervation. Areas involved in mood regulation (VTA and raphe) and cognition (LH) can be influenced by light either via the SCN or in parallel via the MA and LHb.
LHb: lateral habenula; MA: medial amygdala; SCN: suprachiasmatic nucleus; SPZ: subparaventricular zone; VLPO: ventrolateral preoptic area; VTA: ventral tegmental area; HC: hippocampus; LH: lateral hypothalamus; LC: locus coeruleus.
Box 1. Intrinsically photosensitive retinal ganglion cells (ipRGCs).
Retinal photoreceptors transduce light energy into electrical signals that initiate vision. The classical photoreceptors, rods and cones, possess modified cilia that consist of stacks of membranes in which photopigments (rhodopsin and cone opsins) are concentrated. Rods are exquisitely sensitive and are able to detect even a few photons. Rods are therefore used for night vision. Cones are less sensitive than rods and are used for day and color vision. Color vision is mediated by cone photoreceptors that express cone-opsins with sensitivity peaks at different wavelengths (colors) of light. Humans have three cone types: short, mid and long wavelength sensitive cones (for simplicity, we will refer to these as blue, green and red cones, respectively). Rods and cones relay photic information through multisynaptic pathways to retinal ganglion cells (RGCs), which innervate different areas in the brain for complex visual processing13.
A surprising discovery showed that a subpopulation of RGCs is intrinsically photosensitive and express the photopigment melanopsin. These cells were thus termed ipRGCs17–19. The melanopsin gene (Opn4) was originally cloned from Xenopus laevis dermal melanophores, and was shown to have orthologs in many mammalian species, including humans141. Sequence analysis shows that melanopsin shares more homology with invertebrate opsins than with vertebrate opsins, suggesting that melanopsin may use a different mechanism for light signaling than that used by the photopigments present in the rods and cones of vertebrates142. ipRGCs do not have modified membranes in which the photopigment can be concentrated: thus, melanopsin protein is expressed uniformly throughout the soma, dendrites, and the initial segment of the axon143. The lack of membrane specialization makes ipRGCs less sensitive to light than rods and cones. However, ipRGCs are able to incorporate light signals over extended period of time, resulting in an increase in their sensitivity during prolonged light stimulation. ipRGCs are most sensitive to wavelengths of light that are in the blue region of the light spectrum144, 145. As ganglion cells, ipRGCs also convey light information from rods and cones in addition to their intrinsic melanopsin-dependent pathway and can control a variety of light-mediated behaviors30.
Originally, ipRGCs were thought to comprise a uniform population, however, recent discoveries revealed that ipRGCs are highly diverse, comprising at least five distinct subtypes (M1-M5) in rodents based on morphological and electrophysiological analyses22–29. The originally identified population is now known as M1 ipRGCs and project predominantly to brain regions involved in non-image forming visual functions, whereas the non-M1 ipRGCs show widespread projections to areas in the brain important for image formation. ipRGC subtypes express varying levels of the melanopsin protein and have different patterns of dendrite stratification in the inner plexiform layer (IPL)27, 28, 146, 147, indicating that each subtype could play a particular role in detecting light intrinsically and in signaling rod and cone information to the brain.
Initially, ipRGCs were thought to constitute a uniform population whose predominant role is to influence circadian rhythms, as they project to the suprachiasmatic nucleus (SCN), the central circadian pacemaker. However, in depth investigations have revealed the existence of at least 5 subtypes of ipRGCs with different morphological and electrophysiological properties in rodents22–29. Together, these constitute 4–5% of the total number of RGCs (Figure 2a)30. In addition to their projections to the SCN, ipRGCs show widespread projection patterns throughout the rodent brain, targeting regions such as the subparaventricular zone (SPZ) and intergeniculate leaflet (IGL), important for regulation of circadian rhythms, the ventrolateral preoptic area (VLPO) and lateral hypothalamus (LH), important for the regulation of sleep, and the medial amygdala and lateral habenula, implicated in mood regulation (Figure 2b)30. Therefore, ipRGCs emerge as leading candidates for mediating the effects of light on several behaviors such as circadian rhythms, sleep, alertness and mood.
The SCN, as a circadian pacemaker, drives rhythms in several downstream targets, which include brain regions implicated in sleep regulation such as the VLPO and the LH31–38, and mood and motivational states such as the locus coeruleus, amygdala, lateral habenula and ventral tegmental area (VTA)39–41 (Figure 2). It is of interest that some brain targets receive input from both ipRGCs and the SCN (Figure 2c)42–47. This means that light can influence these areas through direct projections from ipRGCs, but also that the SCN, in addition to its pacemaker function, can possibly act as a conduit for light input to these areas (Figure 2). Therefore, the convergence of environmental light information directly from ipRGCs and indirectly through the SCN at specific times of the circadian cycle may influence the physiological functions of these brain regions such as inducing sleep or influencing mood.
The indirect pathway
The involvement of sleep and circadian rhythms in the etiology of mood disorders is supported by well-documented alterations in sleep in patients with psychological alterations. Most patients with depression note poor quality of sleep, which begins to appear weeks before the recurrence of depression symptoms48. This is generally characterized by a disruption in sleep continuity.
Sleep can be divided into stages: REM (rapid eye movement; also known as paradoxical sleep) and non-REM sleep. Non-REM sleep is further divided into several stages (termed N1–N3) of which N3 is the stage during which slow wave or deep sleep occurs49. As described above, sleep timing, depth, and duration are regulated by homeostatic and circadian factors4. Proper alignment between the circadian and homeostatic mechanisms improves the quality of sleep. Sleep recordings in patients with depression show a marked decrease in slow wave sleep, decreased latency to REM sleep, and an increase in the density of REM bouts50. In bipolar disorder, in which patients fluctuate between mania and depression, a striking reduction in the duration of sleep precedes the recurrence of a manic episode, and a primary characteristic of the manic state is a decrease in sleep need51. In addition to sleep disturbances, disruption to other circadian rhythms, such as alterations in daily rhythmic fluctuations of hormones, (including melatonin and cortisol) is also evident in mood disorders52–54.
Since light has profound effects on circadian rhythms and sleep, work in the field has focused on the changes in circadian rhythms and sleep that occur as a result of altered light environments and their involvement in the induction of mood disorders. This has led to a model in which mood and cognitive disorders associated with changes in light exposure are caused by disruptions to circadian rhythms and sleep, essentially suggesting that the effects of light on mood and cognitive functions are secondary. In line with this hypothesis, several mood-related changes occur under different light environments in humans.
Seasonal changes in day length
Seasonal affective disorder (SAD) is a form of depression in which the onset of symptoms is coincident with decreasing day length during fall and winter months55. The prevalence of SAD is greater at higher latitudes where the seasonal changes in day length are more extreme55. Two major hypotheses have been put forth to explain this seasonal form of depression: altered melatonin daily rhythms and circadian phase shift. Melatonin is a hormone that is released by the pineal gland. In humans, this release increases at night coincident with the onset of sleep and is thought to be an important factor in circadian and sleep regulation56. The presentation of light at night, which increases alertness, suppresses melatonin release in humans57–60. Studies have found altered melatonin rhythms in patients with SAD. However, there is also evidence that melatonin varies seasonally in healthy individuals, suggesting that further investigation of the possible role of melatonin in SAD is required54, 61, 62. Furthermore, the fact that melatonin release is also directly affected by light and that retinal sensitivity changes in SAD63 suggest that it will be important to re-evaluate whether the depression occurs secondarily to changes in sleep or is a direct manifestation of reduced total light levels due to the shorter day length.
According to the circadian phase shift hypothesis, sunrise occurring later during the winter months causes a delay in circadian rhythmicity64, 65. This is thought to lead to a dissociation between sleep-wake cycles and other peripheral circadian rhythms that are more tightly coupled to the central circadian oscillator. Strong support for this delayed phase shift hypothesis lies in the antidepressant effects of morning exposure to bright light, which causes an advance in the phase of the clock64–67. Although phase delays have been observed in most SAD patients, a minority of these patients show phase advances, indicating that they should respond better to evening light treatment. Indeed, some studies have shown that evening light treatment is as effective as an antidepressant as morning treatment68–70. Thus, there are perhaps two groups of SAD patients – phase advanced and phase delayed – that would benefit from light treatment at different times of the day71, 72.
Seasonal mood changes are not restricted to SAD. In fact, seasonal fluctuations in mood have been observed in many bipolar patients. A profound switch in mood between periods of mania and depression characterizes bipolar disorder73. These mood swings can happen very quickly, and the triggers for these changes are largely unknown. However, shifts to the depressive phase have been observed to begin in autumn as day length decreases and often persist throughout the winter74, 75. By March, when day length begins to increase, manic episodes become more prevalent, a phenomenon nicknamed “March madness”74, 76, 77. The explanation for these seasonal changes is similar to those suggested for SAD. Strong support for this lies in bright light treatments, which have therapeutic effects in bipolar patients with seasonal fluctuations78. Additionally, bright light treatment can have a manic inducing effect, providing further support for the influence of light on mood79, 80.
Transmeridan travel
Many individuals have experienced the general malaise, including poor mood and cognitive impairments, that is associated with transmeridian travel. The ability to travel rapidly across time zones has the unfortunate consequence of desynchronizing the circadian system81. The involvement of glucocorticoids in resetting the phase of the circadian clock has highlighted their possible contribution to the mechanisms underlying the effects of jet-lag82. Indeed, altered cortisol rhythms have been observed subsequent to transmeridian travel83. Additionally, it was found that chronic transmeridian travel occurring at least once per week for one to four years resulted in cognitive deficits, possibly due to chronic circadian disruption and/or chronic elevated cortisol levels84. A recent study showing that fluoxetine (Prozac) modulates levels of corticosterone to alleviate depression in mice maintained under irregular light schedules further suggests that this mechanism could underlie the depression associated with jet-lag85.
Shift work
The advent of artificial lighting has allowed us to utilize more hours of the day and create disruptive schedules such as shift work. Under these conditions, individuals are exposed to excessive nighttime light and alter their sleep-wake schedule, which causes asynchrony between the circadian and sleep systems. The attempt to remain awake at night and sleep during the day can lead to sleep deprivation, as daytime sleep tends to be more fragmented86. The risks associated with these disruptions include safety hazards and extensive health problems ranging from mood disorders to cancer87. Particular focus has been given to understanding the role of sleep deprivation in the genesis of these health problems. Sleep deprivation has been shown to result in cognitive function deficits including negative effects on learning and memory, alertness, and concentration87, 88. The sleep disruptions that precipitate an episode of depression have been well characterized48, 50. Interestingly, acute sleep deprivation has a robust antidepressant effect; however, the chronic sleep deprivation associated with shift work leads to mood disturbances89–91.
Together, evidence from these three situations in which the light environment is altered provide evidence that light could play an essential role in modulating mood, probably through changes in the circadian and sleep systems. However, it is possible that light could modulate each function independently, and hence, that irregular light patterns could influence mood directly. Indeed, irregular light schedules that do not cause circadian arrhythmicity and sleep deprivation were recently shown to induce mood changes85.
Evidence from rodent research in mood disorders
Many labs have used rodents to study the mechanisms underlying the ability of light to influence mood through the circadian and sleep systems. Using rodents allows researchers to model mood disorders and examine the underlying physiological changes (Box 2). In these studies the light environment has been altered and the effects on mood-related behaviors and cognitive functions assessed (Table 1).
Box 2. Validity of animal models of depression.
Human depression is a complex disorder with a heterogeneous collection of symptoms that vary from patient to patient. This has made it difficult to identify the underlying mechanisms responsible for depression, a necessary step for the development of more effective treatments. Currently, it is not possible to completely model human depression in animals, and in particular it is difficult to model symptoms such as suicidality, excessive guilt, and sadness148. However, there are animal models of some aspects of depression. Clearly it is important for these animal models to reflect the human condition in order to be valid. Indicators of such validity include similar symptom profiles (face validity), improvement in response to drugs that are effective in the human disorder (predicative validity), and involvement of similar neurological processes (construct validity)149. For instance, exposure to chronic stress can induce depression in humans and depression-related behaviors in animal models. Chronic unpredictable stress, chronic social defeat stress, and learned helplessness are some the most widely used paradigms to induce depression-related behaviors in animals. These paradigms lead to changes in the animals that are reminiscent of human depression such as the inability to derive pleasure (termed anhedonia), which is considered one of two cardinal symptoms obligatory for the diagnosis of depression in humans. Anhedonia can be assessed by measuring sucrose preference when animals are given a choice between a bottle containing plain water and another containing water and sucrose. Animals normally prefer the sucrose-containing water; however, animals showing increased depression-related behavior exhibit a reduction in this preference task. Furthermore, these changes can be rescued with administration of antidepressants.
For decades, animal models have been vital in furthering our understanding of the mechanisms underlying antidepressant efficacy and identifying potentially new, more effective antidepressants. A prime example has been in mechanistic understanding of a rapidly acting antidepressant, ketamine. Although ketamine has the potential for abuse in humans due to its psychomimetic side effects and hence cannot be prescribed, ketamine use in animal models has furthered our understanding of the molecular mechanisms that could underlie its rapid antidepressant effects150, 151. This could lead to the production of new antidepressants that benefit from the mechanistic insights obtained from the ketamine studies.
The ability to model disorders such as depression in rodents allows the use of genetic, tracing and molecular tools that are widely available. These models and the available tools are essential to delineate and refine the underlying mechanisms that cause the physiological changes that influence mood regulation. In fact, in combination with optogenetics, we are now able to dissect the neural circuits involved in mood regulation. For example, optogenetic manipulation revealed the importance of the firing pattern of VTA dopaminergic neurons in the regulation of depression-related behaviors152, 153.
Table 1.
Rodent research in mood disorders using different light schedules.
| Model | Animals used | Diurnal or Nocturnal | Circadian Rhythms, locomotor activity and/or sleep architecture | Behavioral responses observed | CORT levels | References |
|---|---|---|---|---|---|---|
| Constant light (LL) | Mice | Nocturnal |
|
|
↓ | 159–161 |
| Wistar rats | Nocturnal |
|
|
NT | 162 | |
| Constant dark (DD) | S-D rats | Nocturnal |
|
|
NT | 163 |
| Dim Night-time Light exposition | Nile grass rats | Diurnal |
|
|
↑ | 89 |
| Siberian hamsters | Nocturnal |
|
|
↓ | 91, 164–166 | |
| Mice | Nocturnal |
|
|
= | 159 | |
| Short daylight | Sand rats | Diurnal |
|
|
NT | 61,62 |
| Nile grass rats | Diurnal |
|
|
NT | 167 | |
| “Jet lag” | Golden hamsters | Nocturnal |
|
|
↑ | 71 |
| phase shift protocol | Siberian hamsters | Nocturnal |
|
|
NT | 106 |
| Long Evans rats / Albino rats | Nocturnal |
|
|
NT | 70,72 | |
| T7 cycle | Mice | Nocturnal |
|
|
↑ | 52 |
SA: sucrose anhedonia; FST: forced swim test; OF: open field test; MWM: Morris water maze; NOR: nobel object; EPM: elevated plus-maze.
For cortiol levels, ↑ indicates increased levels, ↓ indicates decreased levels or blunted effect, = indicates that no differeces were found, and NT indicates that, at least to our knowledge, it has not been tested.
A short or long photoperiod can replicate seasonal light conditions and can be used to assess the behavioral effects of these seasonal light changes. Researchers using a variety of diurnal and nocturnal rodent species have found that short photoperiods, like those experienced during the winter months, induce increased depression-related behavior and hippocampal learning deficits92–96. Corresponding alterations in hippocampal cell structure and long-term potentiation indicate functional deficits in this region in response to the shortened day length96.
To model jet-lag, researchers expose mice to shifts in the light/dark cycle that are similar to those that occur when flying across time zones97 (Supplementary Figure 1). Shifting the light/dark cycle results in the re-synchronization of the circadian system to the new time. This temporarily disrupts the rhythmic expression of circadian related genes within the SCN98 and decouples the SCN from peripheral oscillators99, 100. As in human studies, rodents show learning deficits in response to these light/dark cycle shifts. Studies have found that repeated shifts of the light/dark cycle result in impaired learning and memory in a series of behavioral tasks101–104. Furthermore, a recent study showed that one shift of the light/dark cycle was sufficient to attenuate recall in a contextual fear-conditioning task105. Together, these studies suggest that synchronization of the circadian system is important for cognitive function.
Some light paradigms are capable of disrupting the circadian system to the point of causing circadian arrhythmicity (Table 1). For example, a light paradigm consisting of light pulses and phase delays was used to cause circadian arrhythmicity in hamsters. These hamsters showed hippocampal learning deficits, providing further evidence that the circadian system is required for normal learning and memory106. Similar results were observed in mice housed in conditions that simulated a 20-hour day consisting of a repeating 10 hours of light followed by 10 hours of dark. Mice are unable to synchronize their rhythms to this light/dark cycle and instead show disrupted circadian rhythms107. This resulted in metabolic changes, and although these mice were able to learn a spatial memory task, they lacked flexibility when required to re-learn within the same task. These mice also showed decreased dendritic branching in the prefrontal cortex and mood-related behaviors associated with lesions to this region. To model the prolonged light exposure experienced as a result of artificial lighting, researchers have examined the effects of housing mice in constant light. This environment, depending upon the light intensity, can cause circadian arrhythmicity, disrupt sleep/wake cycles, and lower activity. Mice housed in constant light showed increased depression-related behavior and deficits in spatial learning and memory108. Interestingly, providing mice with an opaque tube in which to escape from constant light exposure rescued the increase in depression-related behavior.
It is important to note that circadian arrhythmicity induced by surgical or genetic techniques causes antidepressant or manic effects109, 110 that are opposite to the effects of light-induced circadian arrhythmicity (Table 1). This suggests that the effects of constant light on mood are not solely an outcome of circadian rhythm disruption. Rather, it is possible that light has a direct, clock independent, role in influencing these functions. The effects of light may still require an SCN-dependent mechanism through which light information is either conveyed by or alters coupling of the SCN to downstream brain regions (Figure 2). Alternatively, there may be a role for additional brain regions that are directly innervated by ipRGCs. Regardless of the brain circuit involved, light would disrupt normal regulation of brain regions important for mood, either by altering SCN firing patterns or providing additional cues that would potentially disrupt normal activity.
These studies show that light-induced circadian rhythm disruption has a profound effect on multiple aspects of learning as well as mood-related behaviors in animal models. Additional work into the circadian, sleep, and activity changes that occur under these environments will help to delineate the roles of each of these factors and produce a comprehensive model of how they interact together to influence affect (Figure 1).
The direct pathway
Although the role of light, sleep, and circadian rhythms in mood regulation and cognitive function are seemingly interdependent, recent evidence for direct light regulation of physiology may provide additional cellular mechanisms by which irregular light cycles lead to mood disorders.
Evidence for a direct pathway in humans
Initial studies on the effects of bright light on behavior in humans found enhanced alertness and vigilance performance in response to light111–113. Functional imaging studies showed a correlation between increased alertness in response to light exposure and activation of the corresponding cortical and thalamic regions114–116. These neural responses were short-lived, declining minutes after light offset implying a direct influence of light on these regions. Using different light spectra, studies have found that regions of the brain involved in attention, alertness, and emotional processes respond preferentially to 480nm, blue light, the wavelength that maximally activates melanopsin (Box 1)117, 118. Furthermore, this light treatment also modulates cognitive function and emotional responses117, 118. Evidence showing that the same effects of light are detected in blind subjects provided additional evidence for a role for ipRGCs in cognitive function119 and are in line with other studies suggesting a role of ipRGCs in signaling light for non image forming functions in humans120, 121. In SAD patients, blue light enhanced responses to emotional stimuli in the posterior hypothalamus122. These patients also showed greater activation of regions of the brain involved in depression and reward such as the locus coeruleus and dorsal raphe nucleus122. This suggests that light, in particular blue shifted light, is capable of modulating mood and cognitive functions, and that patients with mood disorders may have alterations in their responsiveness to this light information.
New discoveries in rodent models
Rodent models have also been used to understand the direct role of light on cognitive function and mood-related behaviors, although it should be noted that rodents cannot engage in human-like higher cognitive processes. A series of papers showed that exposure to dim light throughout the night resulted in increased depression-related behavior123–125. However, the effects of dim light on hippocampal structure and function as well as the underlying mechanism differed depending upon the rodent model used. The diurnal species, Nile grass rats, showed impaired spatial learning and reduced dendritic length in the dentate gyrus and CA1 regions of the hippocampus124. In hamsters, dim light at night reduced spine density in the CA1 region of the hippocampus126. Hamsters also showed a decrease in locomotor activity in response to dim light at night. Therefore, it is possible that this lowered activity could contribute to the observed effects.
Although light influences mood, the circadian clock can also gate its effects, rendering light input beneficial or disruptive only at specific times of the daily activity. Indeed, this has been shown in a study in which light exposure during the late portion of the night influenced despair related behavior in the forced swim test127. However, future work is needed to understand this apparent influence of the time of day on mood related behaviors and the possible role of the circadian system.
A recent study examined the effects of a light paradigm similar to shift work on mood related behaviors and learning. Mice housed under an aberrant light paradigm were exposed to light during their active and inactive (sleep) phases but maintained intact circadian rhythms and normal sleep in terms of both amount and distribution of sleep stages (REM and non-REM sleep)85. These mice showed increased depression-related behaviors and a corresponding increase in baseline serum corticosterone. However, they showed no change in anxiety-related behaviors. Mice housed in this aberrant light cycle also showed hippocampal learning defects and a decreased ability to elicit long-term potentiation85. This study presents a new model that can be used to better understand the more direct role of light on mood and learning without disruption to the circadian system or sleep (Figure 1).
Mechanisms underlying the direct effect of light
Although there is strong evidence implicating direct light input in the modulation of mood and cognitive functions, little is known about the retinal circuits responsible for signaling this light information to the brain. Studies in humans suggested that melanopsin may have a role in this process because it is preferentially activated by blue shifted light118, but there was no direct evidence to support the role of melanopsin or ipRGCs in these functions. Additional studies found polymorphisms in the melanopsin gene and decreased retinal sensitivity in the winter months in SAD patients128. In rodents, light has been suggested to enhance the responses to subsequent stimulations in a cued fear conditioning protocol. This response was present if light was only administered at recall (that is, after learning) and was dependent on the presence of rods and cones129. Since rods and cones can signal light information through both ipRGCs and conventional RGCs, whether ipRGCs or conventional RGCs are responsible for conveying this information to the brain remained unknown.
To study the function of ipRGCs both as photoreceptors and as RGCs, ipRGCs were genetically ablated in mice through the expression of diphtheria toxin (aDTA) from the melanopsin locus (Opn4aDTA/aDTA mice)130. In the Opn4aDTA/aDTA mice, conventional RGCs were not affected, and animals were able to form images similar to wild type controls. However, although Opn4aDTA/aDTA mice maintained intact circadian rhythms, they were completely incapable of circadian photoentrainment130. The intact rhythms, which have a period of slightly less than 24 hours, caused Opn4aDTA/aDTA mice to have regular bouts of activity that were not aligned to the light dark environment. Thus, this study identified ipRGCs as the cells that are capable of signaling light information for circadian photoentrainment, independent of vision and provided a great opportunity to study how irregular light schedules may affect mood and leads to sleep disturbances. Remarkably, mice lacking ipRGCs remained unaffected by an aberrant light cycle (an ultradian cycle consisting of 3.5-h light and 3.5-h dark; Supplementary Figure 1). By contrast, wildtype mice showed increased depression-related behaviors and learning deficits85. This demonstrated a new role for the ipRGCs in conveying light information to the brain to mediate mood regulation and cognitive function. Importantly, it also showed that vision and conscious perception of light in the environment may have little role in mood-related behaviors associated with irregular light environments. Therefore, light could act as a central modulator of circadian rhythms, sleep and mood-related functions for enhanced physiological outcome.
Perspectives
Circuits involved in aberrant light induced-depression
Remarkable advances in gene targeting methods and the discovery of sensitive reporter systems have set the stage for dissecting complex neuronal circuits. Several mice that express different reporters from the endogenous melanopsin locus (such as the tauLacZ reporter or Cre recombinase) have revealed diverse projection patterns from ipRGCs to several brain targets, that are involved in depression and/or anxiety19, 27, 131, 132. At present, five subtypes of ipRGC have been identified based on their morphology and electrophysiological properties30. The principal target of M1 ipRGCs is the circadian pacemaker located in the SCN. However, ipRGCs (M1 and all non-M1 cells) also project to preoptic and lateral hypothalamic areas such as the VLPO and the ventral subparaventricular zone, which control sleep induction and general activity levels, respectively. In addition, ipRGCs innervate some limbic regions such as the lateral habenula and the medial amygdala, highlighting the possible direct role of light in the regulation of mood and cognitive functions (Figure 2).
How could one uncover the brain region(s) that underlie the direct effects of light on mood? A recent discovery characterized that ipRGCs can be molecularly differentiated based on the expression of the Brn3b transcription factor133. Brn3b is expressed in all non-M1 cells and in the majority of M1 cells. In animals in which all Brn3b-positive ipRGCs (all non-M1 and a subpopulation of M1 cells) were ablated, it was shown that the remaining M1 Brn3b-negative cells, which represent ~10% of total ipRGCs, provide the major input to the SCN133, with Brn3b-positive ipRGCs providing only a minor input. In addition, the Brn3b-negative ipRGCs do not project to areas outside the SCN that regulate mood. Therefore, these animals, if placed under aberrant light conditions, could be used to determine the role of the SCN, versus other brain targets that receive ipRGCs input, in mood regulation (Figure 2). A complementary animal model in which only the SCN projecting ipRGCs are ablated will be essential for understanding the roles of other brain regions in mood regulation.
Defining a single population of retinal neurons that if activated at the wrong time of the day causes depression-like symptoms and learning deficits affords us the possibility of identifying new areas important for these functions. An approach that might be taken to achieve this goal would involve the expression of synaptically-tagged channel rhodopsins in the ipRGC population and stimulation of ipRGC terminal fields in the brain at different times of the day and night to examine whether depression symptoms and learning deficits will be observed.
Genetic mouse models provide evidence whether a specific brain is required for light modulation of mood-related behaviors. Research using imaging technology in humans will confirm whether similar brain regions are activated in response to light. Recent observations show dynamic changes in activity in regions involved in mood and cognitive functions in response to light in humans114–118, 134. The role of melanopsin in mediating these light-dependent changes is supported by imaging studies performed in blind subjects and by using wavelength of light that maximally activates this photopigment116–119. Research in this area is also beginning to provide new insight into the changes in light-dependent neural responses in mood disorders like SAD122. The findings that light-dependent modulation of brain activity can depend on the circadian system and sleep135, 136 makes it key to dissect the influence of light on these brain regions in conditions where the circadian clock and sleep are perturbed such as in persons with shift work schedules or people experiencing jet-lag.
Future investigations should be directed toward understanding the cellular and molecular mechanisms through which aberrant light causes these symptoms. Does aberrant light change the expression of genes, synaptic connectivity or neurotransmitter composition in specific brain regions? Does it affect neurogensis in the brain? How do these changes lead to depression symptoms and learning deficits? If combined, these studies will expand the understanding of how light modulates mood and learning for improved physiology.
Light effects on central and peripheral clocks
Different environmental stimuli may play a role in inducing depression-like behaviors. Light (particularly artificial illumination at night) constitutes one of the strongest of these stressors (Box 3). The circadian timing system utilizes light to adjust the SCN and synchronize it with the external environment. The integration of external signaling with the autonomous clock properties of the central pacemaker results in the transmission of timing information to peripheral clocks and brain areas involved in complex behaviors. Could irregular light schedules lead to asynchrony between the phases of brain and peripheral oscillators that then lead to depression? For such a dissociation to occur, brain regions that influence affect, have an endogenous clock, are controlled by the central pacemaker and receive light input directly from ipRGCs would have to exist. Two such areas satisfy these requirements: the amygdala and the lateral habenula. The amygdala projects to the ventral tegmental area and the hippocampus; two brain regions known to have a role in depression. The lateral habenula also projects to the ventral tegmental area and the raphe nucleus, and forms a node of connection between limbic nuclei, hypothalamic brain regions, and brainstem monoamine neurons137. A comprehensive evaluation of the phase relationship between the peripheral clocks that influence mood and the central pacemaker should be undertaken to understand the mechanisms by which irregular light causes depression.
Box 3. Irregular light as a risk factor for disease.
Light has a profound impact on physiology. It exerts a potent influence on the circadian system, which coordinates and appropriately times physiological functions including hormone secretion, metabolism, and sleep3. Light has also been found to directly influence sleep, alertness, and cognitive function5, 6, 115, 117. Given the strong effects light can have on physiology and behavior, regular light exposure is important for the proper maintenance of physiological processes.
The advent of artificial lighting has allowed us to utilize more hours of the day. This has initiated the restructuring of the workday and shift away from activity in alignment with the solar day. Although this has been thought to increase productivity, it comes at a significant cost, which is irregular, nighttime light exposure. The impact that light has on physiological functions makes irregular light exposure potentially detrimental to health and wellness. Indeed, irregular light environments can be implicated in the manifestation of health problems observed, for example, in shiftwork and jet lag. These range from cardiovascular disease to mood disorders. Irregular light exposure, thus, becomes an important risk factor for developing health problems.
This risk has the potential to be exacerbated in the presence of additional factors, such as chronic stress or genetic susceptibility factors for mood-related disorders. Several genetic factors are implicated to underlie psychiatric diseases and chronic stress is known to influence circadian rhythms and sleep and has deleterious health effects154–158. Compounded with the presence of irregular light exposure, these factors could interact and lead to higher incidence of depression and other neurological disorders that are prevalent in society.
Despite the importance of light, it is surprising how little is understood regarding the mechanisms through which light affects physiological functions. A better understanding of the underlying mechanisms of how light affects mood-related behaviors will have major impact on further understanding of mood and cognitive deficits and it would provide impetus to change the way we expose ourselves to light during the day/night cycles for the enhancement of health.
Environmental lighting conditions
Extreme lighting conditions or schedules can affect mood and behavior. Common examples of this are shift work schedules or transmeridian travel, as described above. A fundamental question is how ipRGCs signal light information to regulate mood and cognitive functions. The use of genetically modified mouse lines will allow us the possibility to study the relative contributions of classical photoreceptors, rods and cones, versus the intrinsic melanopsin-related response of ipRGCs, under aberrant light conditions.
Understanding the changes in light exposure, cell circuits, and pathways that lead to mood and cognitive changes will be helpful to create new strategies for treating mood disorders. Work in this area has so far sought to use behavioral interventions to alter the circadian system and restore normal mood. Perhaps the most well-known of these treatments is bright light therapy. The use of light treatment, particularly for SAD, has been shown to be effective in ameliorating depression symptoms. In fact, blue light therapy has been found to be particularly effective in the treatment of SAD138, 139. Bright light therapy has also been found to be useful for non-seasonal depression and bipolar disorder. These disorders are widely treated with medication; however, a significant lag time (usually ~2 weeks) exists between the start of antidepressant treatment and response to treatment. Bright light therapy was found to decrease this latency when combined with antidepressant treatment140. It is thought that the morning is the most effective time for bright light treatment. However, this is not true in all cases, and the best approach is individualized to take into account a particular patient’s circadian rhythms. Determining the pathways by which ipRGCs affect mood would shed light on how better lighting conditions (wavelength, intensity, and exposure) can create an effective treatment. Understanding the role of melanopsin-based phototransduction in light induced-depression will be important in order to design lighting conditions enriched in red wavelength at night that still allow you to see without activating melanopsin and hence causes minimal disruption to circadian, sleep, and mood systems.
Final conclusion and future insights
At present, treatments for mood disorders are still limited and the development of more effective treatments for these disorders is required. A better understanding of the ipRGC-brain connections and their influence on complex behaviors such as depression and cognitive functions will be the first step towards understanding the role of light on these complex behaviors. The use of aberrant light schedules as a new model for induction of depression constitutes a new avenue to study the direct effect of light on depression, independently of circadian arrhythmicity or sleep disruption. Future studies using aberrant light cycles should be designed in order to provide a better understanding of the relationship between different brain areas involved in mood and learning. Understanding the interaction between light and complex behaviors will lead to more effective architectural designs for lighting environments in schools and work, leading to enhanced mood and better learning abilities.
Supplementary Material
References
- 1.Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287:1612–9. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 3.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 4.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 5.Altimus CM, et al. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai JW, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chellappa SL, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22:573–80. doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- 8.Cajochen C, Dijk DJ, Borbely AA. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 1992;15:337–43. [PubMed] [Google Scholar]
- 9.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–73. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 10.Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: Multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014;40C:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Underwood H, Groos G. Vertebrate circadian rhythms: retinal and extraretinal photoreception. Experientia. 1982;38:1013–21. doi: 10.1007/BF01955345. [DOI] [PubMed] [Google Scholar]
- 12.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–57. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–8. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 15.Czeisler CA, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 16.Freedman MS, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 17.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 19.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–7. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 21.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 22.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–70. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 23.Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–22. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–82. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu C, Hill DD, Wong KY. Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. J Neurophysiol. 2013;109:1876–89. doi: 10.1152/jn.00579.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–35. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol. 2011;519:1492–504. doi: 10.1002/cne.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–99. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–80. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou TC, et al. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–83. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 35.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–51. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 36.Nauta WJ. Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 37.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 38.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 39.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 41.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 42.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–91. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 43.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–18. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 44.Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci. 2009;29:748–60. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem. 1980;28:475–8. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- 46.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–52. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 47.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–29. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 48.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 49.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan WC, Jr, Pettigrew KD, Gillin JC. REM architecture changes in bipolar and unipolar depression. Am J Psychiatry. 1979;136:1424–7. doi: 10.1176/ajp.136.11.1424. [DOI] [PubMed] [Google Scholar]
- 51.Wehr TA, Goodwin FK, Wirz-Justice A, Breitmaier J, Craig C. 48-hour sleep-wake cycles in manic-depressive illness: naturalistic observations and sleep deprivation experiments. Arch Gen Psychiatry. 1982;39:559–65. doi: 10.1001/archpsyc.1982.04290050037008. [DOI] [PubMed] [Google Scholar]
- 52.Koenigsberg HW, et al. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J Psychiatr Res. 2004;38:503–11. doi: 10.1016/j.jpsychires.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Linkowski P, et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61:429–38. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan V, et al. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7:138–51. doi: 10.1080/15622970600571822. [DOI] [PubMed] [Google Scholar]
- 55.Rosenthal NE, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 56.Dijk DJ, Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms. 1997;12:627–35. doi: 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- 57.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 58.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6:149–56. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 59.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 60.Cajochen C, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 61.Danilenko KV, Putilov AA, Russkikh GS, Duffy LK, Ebbesson SO. Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arctic Med Res. 1994;53:137–45. [PubMed] [Google Scholar]
- 62.Putilov AA, Russkikh GS, Danilenko KV. Phase of melatonin rhythm in winter depression. Adv Exp Med Biol. 1999;460:441–58. doi: 10.1007/0-306-46814-x_53. [DOI] [PubMed] [Google Scholar]
- 63.Lavoie MP, et al. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biol Psychiatry. 2009;66:253–8. doi: 10.1016/j.biopsych.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Lewy AJ, Sack RL, Singer CM. Treating phase typed chronobiologic sleep and mood disorders using appropriately timed bright artificial light. Psychopharmacol Bull. 1985;21:368–72. [PubMed] [Google Scholar]
- 65.Lewy AJ, Sack RL, Singer CM, White DM, Hoban TM. Winter depression and the phase-shift hypothesis for bright light’s therapeutic effects: history, theory, and experimental evidence. J Biol Rhythms. 1988;3:121–34. doi: 10.1177/074873048800300203. [DOI] [PubMed] [Google Scholar]
- 66.Sack RL, et al. Morning vs evening light treatment for winter depression. Evidence that the therapeutic effects of light are mediated by circadian phase shifts. Arch Gen Psychiatry. 1990;47:343–51. doi: 10.1001/archpsyc.1990.01810160043008. [DOI] [PubMed] [Google Scholar]
- 67.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. doi: 10.1017/s1092852900019611. quiz 672. [DOI] [PubMed] [Google Scholar]
- 68.Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 69.Wirz-Justice A, et al. Morning or night-time melatonin is ineffective in seasonal affective disorder. J Psychiatr Res. 1990;24:129–37. doi: 10.1016/0022-3956(90)90053-s. [DOI] [PubMed] [Google Scholar]
- 70.Wirz-Justice A, et al. Light therapy in seasonal affective disorder is independent of time of day or circadian phase. Arch Gen Psychiatry. 1993;50:929–37. doi: 10.1001/archpsyc.1993.01820240013001. [DOI] [PubMed] [Google Scholar]
- 71.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–58. [PMC free article] [PubMed] [Google Scholar]
- 72.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford University Press; 2012. [Google Scholar]
- 74.Eagles JM. The relationship between mood and daily hours of sunlight in rapid cycling bipolar illness. Biol Psychiatry. 1994;36:422–4. doi: 10.1016/0006-3223(94)91216-5. [DOI] [PubMed] [Google Scholar]
- 75.Friedman E, et al. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta Psychiatr Scand. 2006;113:510–7. doi: 10.1111/j.1600-0447.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 76.Carney PA, Fitzgerald CT, Monaghan CE. Influence of climate on the prevalence of mania. Br J Psychiatry. 1988;152:820–3. doi: 10.1192/bjp.152.6.820. [DOI] [PubMed] [Google Scholar]
- 77.Faedda GL, et al. Seasonal mood disorders. Patterns of seasonal recurrence in mania and depression. Arch Gen Psychiatry. 1993;50:17–23. doi: 10.1001/archpsyc.1993.01820130019004. [DOI] [PubMed] [Google Scholar]
- 78.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11:497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Meesters Y, van Houwelingen CA. Rapid mood swings after unmonitored light exposure. Am J Psychiatry. 1998;155:306. doi: 10.1176/ajp.155.2.306. [DOI] [PubMed] [Google Scholar]
- 80.Wehr TA. Sleep loss: a preventable cause of mania and other excited states. J Clin Psychiatry. 1989;50(Suppl):8–16. discussion 45–7. [PubMed] [Google Scholar]
- 81.Tresguerres JA, et al. Circadian urinary 6-sulphatoxymelatonin, cortisol excretion and locomotor activity in airline pilots during transmeridian flights. J Pineal Res. 2001;31:16–22. doi: 10.1034/j.1600-079x.2001.310103.x. [DOI] [PubMed] [Google Scholar]
- 82.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120:2600–9. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doane LD, et al. Associations between jet lag and cortisol diurnal rhythms after domestic travel. Health Psychol. 2010;29:117–23. doi: 10.1037/a0017865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LeGates TA, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–8. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med (Lond) 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 87.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6:407–14. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 88.Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–67. [PMC free article] [PubMed] [Google Scholar]
- 89.Dallaspezia S, Benedetti F. Chronobiological therapy for mood disorders. Expert Rev Neurother. 2011;11:961–70. doi: 10.1586/ern.11.61. [DOI] [PubMed] [Google Scholar]
- 90.Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 91.Wirz-Justice A, Terman M. Chronotherapeutics (light and wake therapy) as a class of interventions for affective disorders. Handb Clin Neurol. 2012;106:697–713. doi: 10.1016/B978-0-444-52002-9.00042-5. [DOI] [PubMed] [Google Scholar]
- 92.Ashkenazy T, Einat H, Kronfeld-Schor N. Effects of bright light treatment on depression- and anxiety-like behaviors of diurnal rodents maintained on a short daylight schedule. Behav Brain Res. 2009;201:343–6. doi: 10.1016/j.bbr.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Einat H, Kronfeld-Schor N, Eilam D. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav Brain Res. 2006;173:153–7. doi: 10.1016/j.bbr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Krivisky K, Ashkenazy T, Kronfeld-Schor N, Einat H. Antidepressants reverse short-photoperiod-induced, forced swim test depression-like behavior in the diurnal fat sand rat: further support for the utilization of diurnal rodents for modeling affective disorders. Neuropsychobiology. 2011;63:191–6. doi: 10.1159/000321805. [DOI] [PubMed] [Google Scholar]
- 95.Walton JC, et al. Photoperiod-mediated impairment of long-term potention and learning and memory in male white-footed mice. Neuroscience. 2011;175:127–32. doi: 10.1016/j.neuroscience.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Workman JL, Manny N, Walton JC, Nelson RJ. Short day lengths alter stress and depressive-like responses, and hippocampal morphology in Siberian hamsters. Horm Behav. 2011;60:520–8. doi: 10.1016/j.yhbeh.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 97.LeGates TA, Altimus CM. Measuring circadian and acute light responses in mice using wheel running activity. J Vis Exp. 2011 doi: 10.3791/2463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Nagano M, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23:6141–51. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009;29:171–80. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- 100.Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004;19:1105–9. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Devan BD, et al. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem. 2001;75:51–62. doi: 10.1006/nlme.1999.3957. [DOI] [PubMed] [Google Scholar]
- 102.Fekete M, van Ree JM, Niesink RJ, de Wied D. Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol Behav. 1985;34:883–7. doi: 10.1016/0031-9384(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 103.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–8. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- 105.Loh DH, et al. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruby NF, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–62. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fonken LK, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–54. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Roybal K, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001:118–24. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 111.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–8. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 112.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 113.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 114.Perrin F, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Curr Biol. 2004;14:1842–6. doi: 10.1016/j.cub.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 115.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 116.Vandewalle G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vandewalle G, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–95. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 118.Vandewalle G, et al. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci U S A. 2010;107:19549–54. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vandewalle G, et al. Blue light stimulates cognitive brain activity in visually blind individuals. J Cogn Neurosci. 2013;25:2072–85. doi: 10.1162/jocn_a_00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gooley JJ, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012;32:14242–53. doi: 10.1523/JNEUROSCI.1321-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mure LS, et al. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS One. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vandewalle G, et al. Abnormal hypothalamic response to light in seasonal affective disorder. Biol Psychiatry. 2011;70:954–61. doi: 10.1016/j.biopsych.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- 124.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms. 2012;27:319–27. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- 125.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 2013;243:74–8. doi: 10.1016/j.bbr.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 126.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 127.Iyilikci O, Aydin E, Canbeyli R. Blue but not red light stimulation in the dark has antidepressant effect in behavioral despair. Behav Brain Res. 2009;203:65–8. doi: 10.1016/j.bbr.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 128.Roecklein KA, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–85. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Warthen DM, Wiltgen BJ, Provencio I. Light enhances learned fear. Proc Natl Acad Sci U S A. 2011;108:13788–93. doi: 10.1073/pnas.1103214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–49. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–5. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vandewalle G, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 135.Vandewalle G, et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms. 2011;26:249–59. doi: 10.1177/0748730411401736. [DOI] [PubMed] [Google Scholar]
- 136.Vimal RL, et al. Activation of suprachiasmatic nuclei and primary visual cortex depends upon time of day. Eur J Neurosci. 2009;29:399–410. doi: 10.1111/j.1460-9568.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 137.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–9. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009;120:203–12. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 139.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biol Psychiatry. 2006;59:502–7. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Benedetti F, et al. Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J Clin Psychiatry. 2003;64:648–53. doi: 10.4088/jcp.v64n0605. [DOI] [PubMed] [Google Scholar]
- 141.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–5. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bellingham J, et al. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 144.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–5. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 145.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–9. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 146.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–93. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 147.Viney TJ, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–8. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 148.Association, A.P. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 149.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–88. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 150.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chaudhury D, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tye KM, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 155.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 156.Akerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- 157.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav. 1996;54:229–34. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 158.Kant GJ, et al. Effects of controllable vs. uncontrollable stress on circadian temperature rhythms. Physiol Behav. 1991;49:625–30. doi: 10.1016/0031-9384(91)90289-z. [DOI] [PubMed] [Google Scholar]
- 159.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–9. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 161.Fujioka A, et al. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci Lett. 2011;488:41–4. doi: 10.1016/j.neulet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 162.Ma WP, et al. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci Res. 2007;59:224–30. doi: 10.1016/j.neures.2007.06.1474. [DOI] [PubMed] [Google Scholar]
- 163.Gonzalez MM, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci U S A. 2008;105:4898–903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J Neuroendocrinol. 2013;25:590–6. doi: 10.1111/jne.12036. [DOI] [PubMed] [Google Scholar]
- 165.Bedrosian TA, et al. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J Neurosci. 2013;33:13081–7. doi: 10.1523/JNEUROSCI.5734-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2013;18:930–6. doi: 10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- 167.Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods. 2010;186:165–70. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


