Abstract
Diabetes Mellitus exerts a strong effect on atherosclerotic cardiovascular disease risk into older age (beyond ages 70 to 74 years). This effect is particularly noticeable with regard to coronary artery disease and cerebral microvascular disease. Thus Diabetes Mellitus in older age deserves the same careful medical attention as it does in middle age.
Keywords: diabetes, subclinical vascular disease, risk, cardiovascular disease, mortality, older age
INTRODUCTION
In this review article we focus on four specific questions relevant to the practicing physician regarding the impact of diabetes mellitus (DM) on the risk of atherosclerotic cardiovascular disease (CVD) in older adults:
Is diabetes mellitus a significant risk factor for total and CVD mortality in older people? Does diabetes have the same strength of association with total and atherosclerotic CVD mortality in people 75 years or older as it does in younger people? Is diabetes a coronary heart disease (CHD) equivalent in older age?
What impact does newly discovered DM have on CVD risk?
What are the prime risk factors for CVD in older adults with diabetes?
How common is subclinical cerebrovascular disease in older adults with diabetes?
The data for this review draws primarily from our work with the Cardiovascular Health Study (CHS), an ongoing observational study of cardiovascular risk factors in adults age 65 years of age and older. CHS enrolled nearly 6000 older adults in four field centers across the U.S. between 1989 and 1993 and has followed them continuously for the occurrence of cardiovascular events, including myocardial infarction and stroke. Of particular interest, CHS administered a 2-hour oral glucose tolerance test at the initial examination for the first 5,201 participants, offering the unique opportunity to address fasting and post-load glucose values as risk factors for atherosclerotic vascular disease. In addition, we also review studies of other older population cohorts.
ARE MORTALITY AND ATHEROSCLEROTIC CVD INCREASED IN OLDER ADULTS WITH DM?
It may be argued that the impact of DM as a risk factor for vascular disease wanes with increasing age, just as happens with some traditional CVD risk factors, such as hypercholesterolemia (1). The “wear and tear” of growing old and the high prevalence of subclinical vascular disease might attenuate the effect of DM on total and CVD mortality risk.
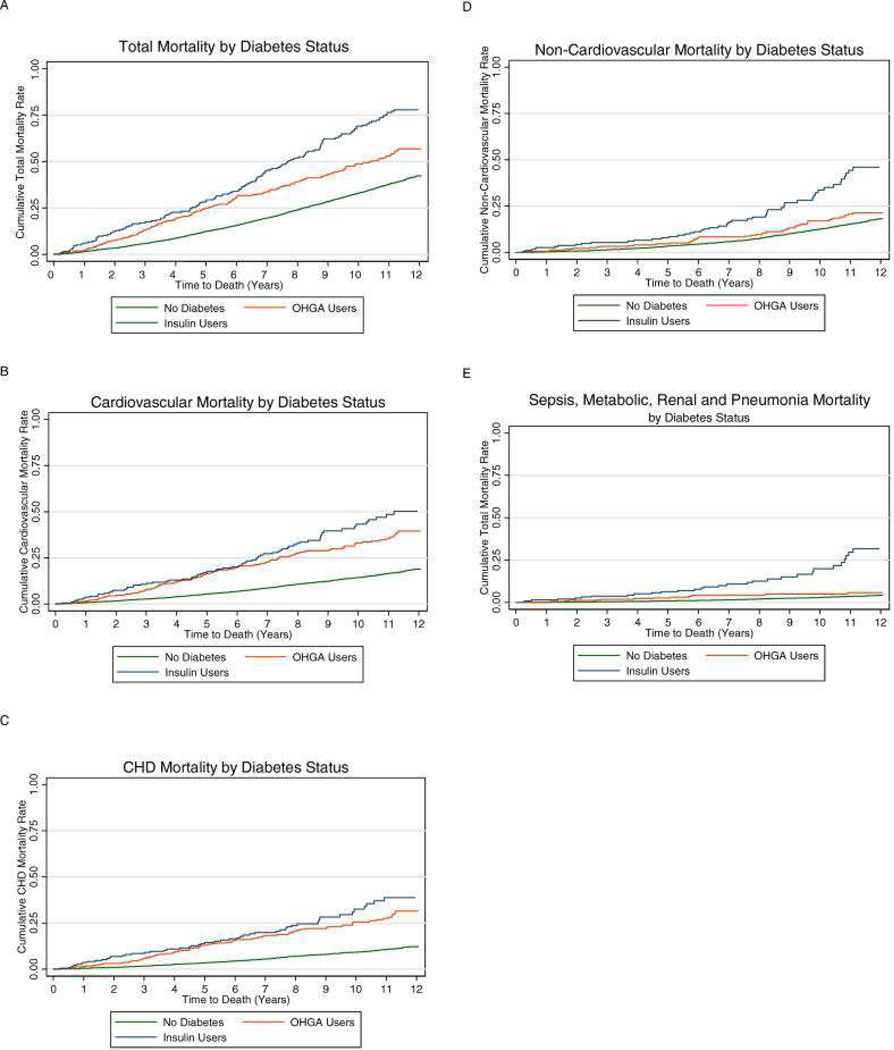
To examine this issue, we performed an analysis of the CHS data set from 1989 – 2001 (2). Similar to other population-based diabetes studies (3), we adjusted for traditional risk factors such as age, sex, hypertension, and smoking status. We also included other “non-traditional” factors associated with DM. These included low levels of attained education, high rates of disability, depression, frailty, subclinical CVD, and elevated levels of inflammation factors. We found that the adjusted relative risk of total mortality for participants treated with oral hypoglycemic agents and insulin, relative to those without diabetes, was 1.33 (95% CI, 1.10, 1.62) and 2.04 (95% CI, 1.62, 2.57), respectively. The total mortality risk estimate for oral hypoglycemic agent users was somewhat lower than that of prior studies, while the estimate for insulin users was in line with prior studies (4). For CVD mortality, the adjusted relative risks were 1.99 (1.54, 2.57) and 2.16 (1.54, 3.03), and for CHD estimates were 2.47 (1.89, 3.24) and 2.75 (1.95, 3.87), respectively. These estimates were similar to those from studies of diabetes from predominantly middle aged cohorts from prior decades (4), which adjusted only for traditional CVD risk factors. From these results we conclude that diabetes continues to confer a substantial increase in risk of total and CVD mortality into older age, and the addition of non-traditional risk factors does not modify these relationships in a meaningful manner (Figure 1).
Figure 1.
Cumulative mortality curves for cardiovascular Health participants with or without diabetes. Those with diabetes are further categorized as oral hypoglycemic agent users (OHGA) and insulin users. From Kronmal RA, Barzilay JI, Smith NL, Psaty BM, Kuller LH, Burke GL, Furberg C. Mortality in Pharmacologically Treated Older Adults with Diabetes: The Cardiovascular Health Study, 1989–2001. PLoS Med. 2006 October; 3(10): e400; with permission.
Next, we examined the effect of age on mortality risk. We included an interaction term in our models (65–74 years versus >74 years of age). The term was not statistically significant, suggesting that the effect of diabetes on total and CVD mortality outcomes between those with and without diabetes was the same whether one was less than or greater than 74 years of age. A similar conclusion was reached in an analysis of a Medicare claims dataset, which demonstrated excess mortality risk from diabetes in all age groups, including the elderly (5). This issue is of clinical importance since there is a tendency to be less “aggressive” in risk factor management the older the patient. In contrast to our finding, the Emerging Risk Factor Collaboration reported, in a meta-analysis of 102 trials with individual data on people with diabetes, that the risks of CHD and stroke events, including mortality, were lower in people with diabetes versus people without diabetes in those older than 70 years of age as compared to those 60–69 and 40–59 years of age (6). Taken together, available evidence indicates significantly increased risks of mortality and atherosclerotic CVD among elders with diabetes, although the heightened incidence of these outcomes appears less pronounced than in middle-aged cohorts.
Finally, we examined the impact gender on total and CVD mortality in older adults with diabetes. In middle-aged cohort studies, women exhibit a higher relative risk of CVD (especially CHD) mortality compared to men (7). In CHS, we observed a similar finding (2). Women with diabetes had a relative total mortality risk of 2.28 [95% CI, 1.90 to 2.72]) compared to non-diabetic women, while diabetic men had a relative risk of 1.80 [95% CI, 1.53 to 2.11]. When this risk was categorized by treatment type an interesting finding emerged. Women treated with oral hypoglycemic agents had a total mortality risk similar to that of men (HR 1.62 [95% CI, 1.33, 2.09] versus HR 1.63 [95% CI, 1.33, 2.00], respectively). On the other hand, the relative mortality risk associated with insulin therapy was much higher in women than men (approximately three times higher in women compared with approximately 50% higher in men). The increased relative risk with insulin therapy for women with DM was due mainly to the lower risk of death in women without diabetes, since women and men with diabetes treated with insulin had similarly high absolute cumulative mortality (>75% at 12 year follow-up). Thus, the loss of the mortality advantage that women have over men in the setting of diabetes is driven mainly in those who use insulin. It is in this group of women that extra diligence in management is called for.
NEWLY DIAGNOSED DIABETES – WHAT IS THE MORTALITY RISK?
Clinicians who treat older adults often come across the situation where an older person’s blood glucose is elevated on a routine physical exam. The patient feels well and has no history of cardiovascular disease. The question arises whether newly diagnosed diabetes poses a risk of near-term CVD. Should the physician be aggressive in managing the risk factors associated with diabetes in this older person? It may be argued that the effects of elevated glucose levels take many years to result in clinical atherosclerosis, so urgency is not necessary.
Using data from CHS (8), we found that new-onset diabetes, defined by the initiation of anti-diabetes medication or by a fasting plasma glucose >125 mg/dl, was associated with a 90% increase in risk of all-cause mortality and a 120% increase in risk of cardiovascular mortality compared with study participants without diabetes. There was a large increase in cardiovascular mortality in the first 2 years of follow-up that diminished over time, whereas all-cause mortality risk remained consistently elevated throughout the 8 years of follow-up. There are two explanations for this short-term excess mortality risk. First, the limited duration of exposure to hyperglycemia may reflect more sustained exposure to associated oxidative stress, inflammation, and non-glycemic atherosclerosis risk factors (e.g., hyperinsulinemia, dyslipidemia, hypertension), which increase susceptibility to atherothrombotic events. Second, newly elevated fasting glucose levels in the diabetic range reflect prolonged glucose exposure to non-diabetic hyperglycemia, which increases risk as well. Whatever is the explanation of our finding, it is important to know that newly diagnosed diabetes poses a serious and near-term risk to the elderly person.
IS DIABETES A “CVD EQUIVALENT” IN OLDER ADULTS?
In the late 1990s Haffner et al (9) reported that diabetic patients without previous myocardial infarction had as high a risk of myocardial infarction as non-diabetic patients with previous myocardial infarction. The cohort for this study was approximately 58 years of age. Do the same findings hold true into older age?
We examined the CHS data set after a mean of 12 years follow-up and asked whether cardiovascular and all-cause mortality rates were similar between participants with prevalent CHD (confirmed history of myocardial infarction, angina, or coronary revascularization) versus participants with diabetes only (10). We found that CHD mortality risk was virtually identical between participants with CHD alone versus diabetes alone (hazard ratio [HR] 1.04, 95% confidence interval [CI], 0.83–1.30). The proportion of mortality attributable to prevalent diabetes (population-attributable risk percent 8.4%) and prevalent CHD (6.7%) were similar in women. On the other hand, the proportion of mortality attributable to atherosclerotic heart disease (16.5%) as compared to diabetes (6.4%) was higher in men. Similar patterns were found for cardiovascular disease mortality. Notably, however, the adjusted hazard ratio for total mortality was significantly lower among participants with atherosclerotic heart disease alone (HR 0.85, 95% CI, 0.75–0.96) compared to participants with DM alone.
In contrast to the above, the British Regional Heart Study (BRHS) arrived at different conclusions (11). In that study the risk of CVD outcomes was compared among participants with: (1) early diagnosis (age<60 years) and longer duration (mean 16.7 years) of diabetes; (2) those with late diagnosis (age>=60 years) and shorter duration (mean 1.9 years) of diabetes; (3) those with a myocardial infarction but no diabetes; and, (4) individuals without either condition (the reference group). The researchers found that risks of major CHD events and mortality were increased in participants with late onset diabetes (RR 1.54 [1.07, 2.21] but less than in those with early onset diabetes (RR 2.39 [1.41, 4.05]) and those with prior MI (RR 2.51 [1.88, 3.36]). In other words, both early and late onset of diabetes were associated with increased risk of major CHD events and mortality, but only early onset of diabetes (with >10 years' duration) appears to be a CHD equivalent. Differences in study characteristics (CHS participants were 5 years older than BRHS participants; the BRHS included only men, while CHS had both men and women) may account for these discordant findings.
With regard to the risk of CVD and mortality, the physician may ask what is the likelihood of a recurrent event. In a national study of diabetic patients from Italy, mean age 68 years, 6.1% of the patients with a prior CVD event developed a new major atherosclerotic complication annually, including mortality (12). A higher rate of 7.6% per year was reported in the Drugs and Evidence-Based Medicine in the Elderly (DEBATE) study (13), in subjects with a mean age of 80 years.
RISK FACTORS FOR ATHEROSCLEROTIC CVD IN THE ELDELRY
Subclinical CVD in Older Adults with Diabetes
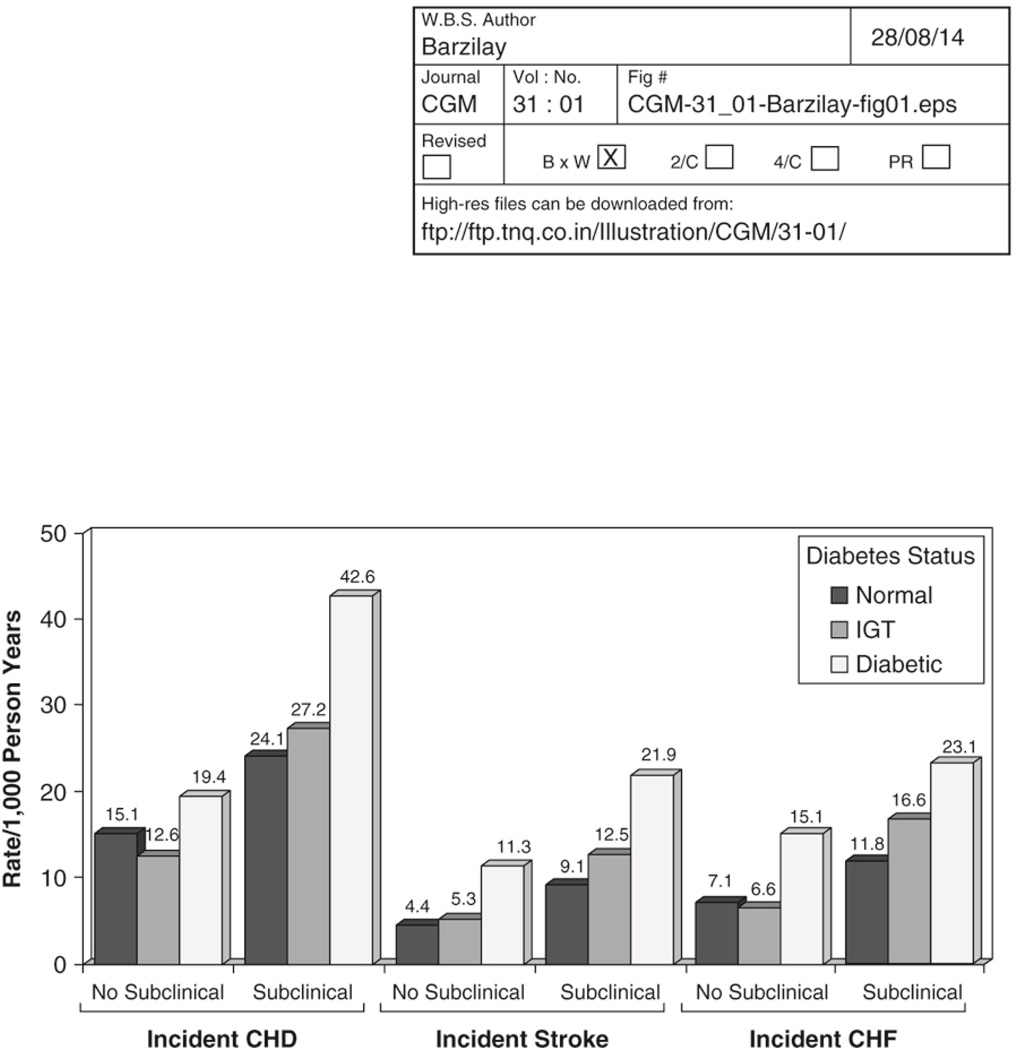
The relative importance of traditional CVD risk factors tends to wane with increasing age (14). What then is the main determinant of CVD in older diabetic adults? To answer this question, we analyzed the CHS data set (15) after a mean follow-up of 6.4 years adjusting for traditional CVD risk factors, as well as for the presence of subclinical CVD. The two risk factors that were consistently associated with CVD outcomes were age and the presence of subclinical CVD. The latter increased the risk of clinical outcomes approximately two-fold. The increase in risk of clinical CVD in the diabetic population of CHS was largely confined to those with prevalent subclinical CVD at baseline. The risk for the combination of diabetes and subclinical disease was more than twofold (HR 2.5, 95% CI 1.9 to 3.4), while that for diabetes with no subclinical disease was not significantly increased (HR 1.3, 95% CI 0.8 to 2.0), as compared to participants with neither diabetes nor subclinical disease (Figure 2). Of note, participants with impaired glucose tolerance (IGT) (defined as a 2-hour glucose between 140–199 mg/dl), a condition that is associated with a high risk of subsequent diabetes, generally had an intermediate risk.
Figure 2.
Incidence of several cardiovascular end points in the Cardiovascular Health Study according to glucose status on a 2 hour oral glucose tolerance test (normal glycemia, impaired glucose tolerance [IGT], diabetes) categorized by the presence or absence of subclinical vascular disease. From Kuller LH, Velentgas P, Barzilay J, Beauchamp NJ, O'Leary DH, Savage PJ. Diabetes mellitus: Subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol 2000; 20: 823 – 829.
We have previously demonstrated that elevated LDL cholesterol, lower HDL cholesterol, and elevated triglyceride levels, along with cigarette smoking and elevated systolic blood pressure, are related to subclinical CVD (16). Our findings suggest that once subclinical CVD has developed, these risk factors (and especially lipid levels) may have little association with clinical disease. However, modifications of these risk factors may still reduce the risk of clinical disease earlier in life by modifying the extent or characteristics of subclinical disease and particularly of plaque stability and susceptibility to thrombosis (17).
Distribution of Subclinical and Clinical CVD in Older Adults with Diabetes
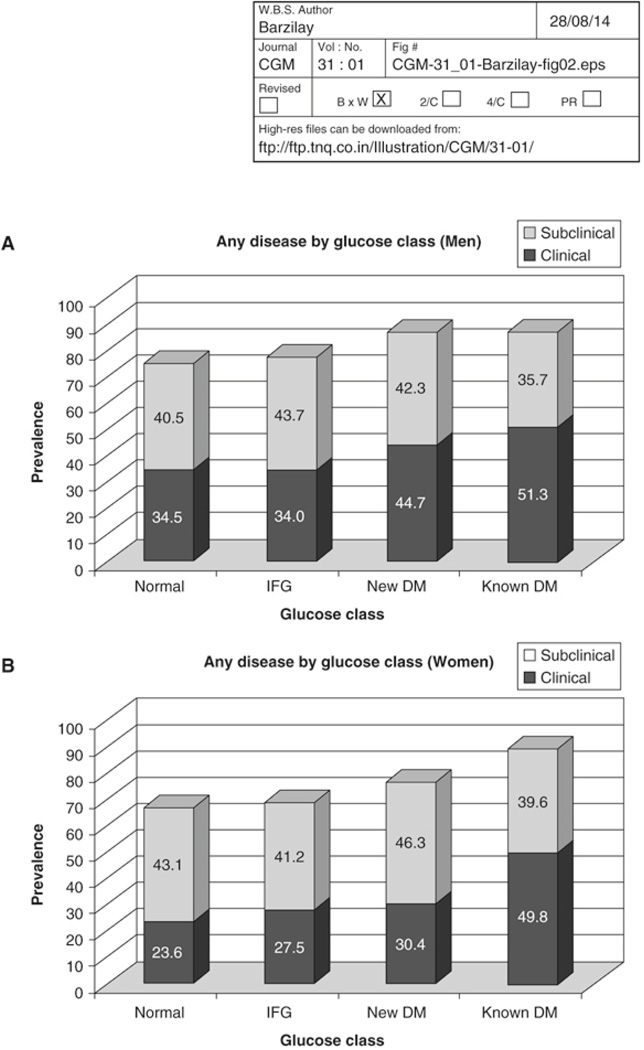
To clarify what subclinical CVD consists of and to better characterize the overall prevalence of CVD in older adults, we summarized the results of several complementary vascular tests that were conducted in CHS (18). These tests included an echocardiogram (for left ventricular hypertrophy, wall motion abnormalities, low ejection fraction), electrocardiography (for major ST and T wave changes), carotid ultrasound (for intimal-medial thickness and plaque), cranial magnetic resonance imaging (for strokes and lacunar infarcts), and peripheral blood pressure measurement (for ankle arm index (AAI)). Clinical disease consisted of previous myocardial infarction, coronary revascularization, angina, heart failure, stroke, and claudication. We further categorized the cohort as having normal glucose tolerance, impaired fasting glucose (IFG), newly diagnosed DM, or known DM. IFG, defined as a fasting glucose of 100–125 mg/dl, is associated with an increased risk of subsequent diabetes.
We found that ~30% of the cohort had clinical CVD of at least one form (Figure 3). The most common form was coronary heart disease. Of those without clinical disease, ~60% had some form of isolated subclinical disease. The most common form of such isolated subclinical disease was cerebrovascular disease (see below). The prevalence of either clinical or isolated subclinical CVD increased with severity of the glucose disorder. The increased risk was more pronounced in women than in men, as we previously noted. Last, clinical CVD composed a larger proportion of the total CVD burden among those with glucose disorders than among those with normal fasting glucose status (i.e., CVD was relatively more likely to be clinical than subclinical in those with abnormal glucose regulation). Taken together, these results support the hypothesis that glucose disorders (and associated pathophysiologic processes and metabolic changes) promote atherosclerosis and its progression from subclinical to clinical disease.
Figure 3.
The prevalence of clinical and subclinical cardiovascular disease among participants from the Cardiovascular Health Study according to glucose status on a 2-hour oral glucose tolerance test (normal glycemia, impaired glucose tolerance [IGT], diabetes). Data from Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E; Diabetes and Informatics Study Group. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care 2008; 31:2154–2159.
Fasting versus Post-Prandial Hyperglycemia
Aging tends to have a stronger effect on post-prandial than on fasting glucose levels (19). Given this fact, one might wonder if post-prandial glucose is as strong a risk factor for CVD and mortality as fasting glucose in older adults. Our work suggests that it is strongly related to risk (20). When we compared the American Diabetes Association criteria for diabetes based on fasting glucose levels with World Health Organization criteria that incorporate oral glucose tolerance testing (and 2 hour glucose levels), the number of cases of CVD attributable to abnormal fasting glucose states was a third that attributable to abnormal glucose tolerance. The sensitivity of fasting criteria for incident CVD was only 28%, compared with 54% using WHO criteria. In similar analyses that simultaneously evaluated fasting and two-hour glucose among CHS participants who were not receiving hypoglycemic treatment, only 2-hour glucose level was associated with risk of CVD (21).
These findings are consistent with those reported from the Rancho Bernardo Study (22), where isolated postprandial hyperglycemia was associated with increased risks of coronary heart disease and CVD mortality in older women, though not men. They are also compatible with the findings of the DECODE study, which likewise reported that 2-hour glucose was associated with incident CVD and mortality independent of fasting glucose, but not vice versa, across cohorts spanning the adult age range (23).
CEREBROVASCULAR DISEASE
Diabetes is a risk factor for clinical stroke. Population-based registries of stroke report a prevalence of DM ranging from 9.5% to 20% (24). In addition, 16% to 24% of non-diabetic patients at the time of admission for acute stroke have DM according to an oral glucose tolerance test performed 12 weeks after the stroke (25). In the Framingham Heart Study, the proportion of cardiovascular disease, including stroke, attributable to diabetes increased from 5.4% to 8.7% between 1952 and 1998 (26). In CHS (15), we found that the risk of stroke mortality in people with diabetes was 4.1 (95% CI, 2.6, 6.7) and 2.5 (95% CI, 1.3 to 4.8) times higher than that of non-diabetic participants depending on whether subclinical vascular disease was present or not, respectively.
Aside from large-vessel cerebrovascular disease, it is now recognized that small vessel disease of the brain is much more common than large vessel disease. CT and MRI scans have made it apparent that a majority of cases of cerebral infarction are "silent" and not recognized clinically. Reports conflict about whether DM is a risk factor for these silent strokes. In CHS (27), 3660 participants underwent brain MRI scanning. Of these subjects, 2529 (69%) were free of infarcts of any kind. Another 841 (23%) had 1 or more lacunar infarcts without other stroke types present. For most of the 841 subjects, the lacunar infarcts were single (66%) and silent (89%), i.e., without a history of transient ischemic attack or stroke. In multivariate analyses, people with DM had a ~33% higher prevalence of lacunes than people without DM. In a follow-up study (28) of 1433 CHS participants who underwent 2 MRI scans separated by 5 years and who had no infarcts on the initial MRI, 254 participants (17.7%) had 1 or more infarcts. DM was not associated with these incident MRI-defined infarcts late in life. In the Rotterdam Scan Study (29), a population-based cohort study of 1077 participants 60 to 90 years of age, participants also underwent cerebral MRI. For 259 participants (24%), 1 or more infarcts on MRI were seen; 217 persons had only silent and 42 had symptomatic infarcts. Diabetes was not related to silent strokes in this study.
A recent report may explain why there is inconsistency regarding diabetes as a risk factor for lacunar infarcts. Theories pertaining to the etiology of small brain infarcts have varied but two possible mechanisms have been emphasized: (1) development of microatheroma obstructing flow of small penetrating arteries, and (2) the occurrence of fibrinoid necrosis in these penetrating arteries. Using data from the Atherosclerosis Risk in Communities (ARIC) study (30), investigators studied which risk factors were associated with infarct–like lesions (ILLs) of <20 mm in maximum dimension. ILL’s that were ≤7 mm in diameter were hypothesized to be due to lipohyalinosis. Those that were 8–20 mm were hypothesized to reflect microatheromatous disease. If their hypothesis was correct, different risk factors associated with each size of lesion would be expected. The authors found that very small lesions were associated with diabetes or elevated HbA1c level. Metabolic factors are known to affect the function of small blood vessels (endothelial dysfunction), as well as lumen size. The larger lesions were more strongly associated with LDL cholesterol. The latter is a risk factor linked to large-vessel atherosclerosis. Both types of lesions shared the common risk factors of increasing age, hypertension and smoking. This distinction between the two forms of small-vessel disease may explain why studies of lacunar infarcts differ with regard to whether diabetes is an underlying risk factor.
CONCLUSION
In this brief review of atherosclerotic cardiovascular disease in older diabetic adults, we have emphasized four points. First, although the relative risk for cardiovascular events and mortality appears to be less pronounced in older than middle-aged adults, diabetes remains an important risk factor for these outcomes, particularly mortality, well into old age (i.e., beyond age 70 to 74 years). Second, the presence of subclinical vascular disease is an important determinant of who develops and who does not develop clinical CVD. Third, newly detected diabetes poses a near-term risk of CVD. Finally, diabetes affects not only the large arteries of the brain but the small arteries and arterioles as well.
We hope that those reading this review will come away with a better appreciation of the importance of established or newly diagnosed diabetes as a determinant of cardiovascular disease and mortality in older age.
Acknowledgments
Funding Support: This work was funded in part by R01 HL094555 from the National Heart, Lung, and Blood Institute (to Kenneth J. Mukamal, MD, MPH, and Jorge R. Kizer, MD, MSc).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has a conflict of interest regarding the contents of this article.
REFERENCES
- 1.Psaty BM, Furberg CD, Kuller LH, Bild DE, Rautaharju PM, et al. Traditional risk factors and subclinical disease measures as predictors of first myocardial infarction in older adults: the Cardiovascular Health Study. Arch Intern Med. 1999;159:1339–1347. doi: 10.1001/archinte.159.12.1339. [DOI] [PubMed] [Google Scholar]
- 2.Kronmal RA, Barzilay JI, Smith NL, Psaty BM, Kuller LH, Burke GL, Furberg C. Mortality in Pharmacologically Treated Older Adults with Diabetes: The Cardiovascular Health Study, 1989–2001. PLoS Med. 2006 Oct;3(10):e400. doi: 10.1371/journal.pmed.0030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard DL, Barrett-Connor E. Heart Disease and Diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, et al., editors. Diabetes in America. 2nd edition. Washington (D. C.): NIH publication 95–1468; 1995. pp. 429–448. Available at http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter19.pdf. [Google Scholar]
- 4.Geiss LS, Herman WH, Smith PJ. Mortality in Non-insulin-Dependent Diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, et al., editors. Diabetes in America. 2nd edition. Washington (D. C.): NIH publication 95–1468; 1995. pp. 233–258. Available at http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter11.pdf. [Google Scholar]
- 5.Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25:471–475. doi: 10.2337/diacare.25.3.471. [DOI] [PubMed] [Google Scholar]
- 6.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- 8.Smith NL, Barzilay JI, Kronmal R, Lumley T, Enquobahrie D, Psaty BM. New-onset diabetes and risk of all-cause and cardiovascular mortality: the Cardiovascular Health Study. Diabetes Care. 2006;29:2012–2017. doi: 10.2337/dc06-0574. [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 10.Carnethon MR, Biggs ML, Barzilay J, Kuller LH, Mozaffarian D, Mukamal K, Smith NL, Siscovick D. Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med. 2010;123:556.e1–556.e9. doi: 10.1016/j.amjmed.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men. Arch Intern Med. 2011;171:404–410. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- 12.Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E Diabetes and Informatics Study Group. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31:2154–2159. doi: 10.2337/dc08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strandberg TE, Pitkala KH, Berglind S, Nieminen MS, Tilvis RS. Multifactorial intervention to prevent recurrent cardiovascular events in patients 75 years or older: the Drugs and Evidence-Based Medicine in the Elderly (DEBATE) study: a randomized, controlled trial. Am Heart J. 2006;152:585–592. doi: 10.1016/j.ahj.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Koller MT1, Leening MJ, Wolbers M, Steyerberg EW, Hunink MG, Schoop R, Hofman A, Bucher HC, Psaty BM, Lloyd-Jones DM, Witteman JC. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389–397. doi: 10.7326/0003-4819-157-6-201209180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuller LH, Velentgas P, Barzilay J, Beauchamp NJ, O'Leary DH, Savage PJ. Diabetes mellitus: Subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol. 2000;20:823–829. doi: 10.1161/01.atv.20.3.823. [DOI] [PubMed] [Google Scholar]
- 16.Kuller L, Borhani N, Furberg C, Gardin J, Manolio T, O’Leary D, Psaty B, Robbins J. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139:1164–1179. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, Davis BR, Cole TG, Pfeffer MA, Braunwald E for the CARE investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events. Circulation. 1998;98:2513–2519. doi: 10.1161/01.cir.98.23.2513. [DOI] [PubMed] [Google Scholar]
- 18.Barzilay JI, Spiekerman CF, Kuller LH, Burke G, Bittner V, Gottdiener JS, Brancati FL, Orchard TJ, O’Leary DH, Savage PJ. Prevalence of clinical and isolated subclinical cardiovascular disease in older adults with glucose disorders. The Cardiovascular Health Study. Diabetes Care. 2001;24:1233–1239. doi: 10.2337/diacare.24.7.1233. [DOI] [PubMed] [Google Scholar]
- 19.Metter EJ1, Windham BG, Maggio M, Simonsick EM, Ling SM, Egan JM, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and mortality prediction. Diabetes Care. 2008;31:1026–1030. doi: 10.2337/dc07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzilay JI, Spiekerman CF, Wahl PW, Kuller LH, Cushman M, Furberg CD, Dobs A, Polak JF, Savage PJ. Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet. 1999;354:622–625. doi: 10.1016/s0140-6736(98)12030-5. [DOI] [PubMed] [Google Scholar]
- 21.Smith NL, Barzilay JI, Shaffer D, Savage PJ, Heckbert SR, Kuller LH, Kronmal RA, Resnick HE, Psaty BM. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Arch Intern Med. 2002;162:209–216. doi: 10.1001/archinte.162.2.209. [DOI] [PubMed] [Google Scholar]
- 22.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo Study. Diabetes Care. 1998;21:1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 23.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617–621. [PubMed] [Google Scholar]
- 24.Kuller LH. Stroke and Diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, et al., editors. Diabetes in America. 2nd edition. Washington (D.C.): NIH publication 95–1468; 1995. pp. 449–456. Available at http://diabetes.niddk.nih.gov/dm/pubs/america/pdf/chapter20.pdf. [Google Scholar]
- 25.Gray CS, Scott JF, French JM, Alberti KG, O'Connell JE. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing. 2004;33:71–77. doi: 10.1093/ageing/afh026. [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, Coady S, Sorlie PD, D'Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O'Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 29.Vermeer SE, Deb Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Rotterdam Scan Study. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 30.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH, Jr, Coresh J, Szklo M, Carvalho MS, Selvin E. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78:102–108. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]