Summary
The role of novel tests for TB in reducing morbidity and mortality depends on the system in which they are implemented.
To the Editors
At least three million people with active tuberculosis (TB) are missed by national systems every year. Reaching these individuals is a critical priority [1]. Novel molecular diagnostics, notably Xpert MTB/RIF (“Xpert,” Cepheid, Inc.; Sunnyvale, CA, USA) [2,3], are important tools in this effort. Over six million Xpert cartridges have been procured worldwide since late 2010 [4], but two recent randomized trials in Southern Africa [5,6] suggest that Xpert – despite high sensitivity – may not significantly reduce morbidity and mortality. It is therefore useful to demonstrate how TB diagnostics function not in isolation but rather as part of a “diagnostic cascade.”
We therefore adapted a transmission model of diagnostic testing among adults with active TB in Southeast Asia [7]. This model categorizes a high-burden population into subpopulations characterized by TB status, HIV status, and access to TB care. Parameter values, available in the original publication, are consistent with other published models of TB [8,9]. For transparency in this analysis, we do not consider multidrug-resistant TB, public/private sector differences, or urban/rural differences.
We modeled TB diagnosis as a series of care-seeking attempts occurring after a “pre-diagnostic” delay. Depending on the diagnostic test sensitivity, probability of empiric treatment, and efficacy of therapy, each attempt ends either in recovery or return to the active infectious state. Our primary outcomes were ten-year reductions in TB incidence and mortality if TB were diagnosed using Xpert (implemented immediately and fully throughout the population) versus sputum smear microscopy.
We fit model parameters at steady-state to epidemiological data in India, including life expectancy (66 years [10]), adult HIV prevalence (0.3% [11]), TB incidence (176 cases per 100,000/year [1]), mortality (14–32 deaths per 100,000/year [1]), proportion of incident cases that were previously treated (19% [1]), and case detection proportion (59% [1]). We assumed that all active TB starts as smear-negative and progresses over time such that 25% of all prevalent TB is smear-positive [12].
We constructed six sequential scenarios, with each scenario incorporating one additional step in the diagnostic cascade. Each scenario was independently calibrated to the epidemiological characteristics above, after which a 2% annual decline in TB incidence was initiated, representing current trends. The scenarios are:
Baseline: No pre-diagnostic period, population without access to care, Xpert machine failure, pre-treatment loss to follow-up, or empiric treatment.
Pre-Diagnostic Period: Addition of an initial 4.5-month “pre-diagnostic” delay [7] during which individuals are infectious, but symptoms are insufficiently severe to prompt care-seeking.
Reduced Access: Further consideration that 15% of active TB patients may never access the system of “passive” TB diagnosis and treatment.
Mechanical Difficulty: Further consideration that 10% of Xpert machines might be nonfunctional due to mechanical failure, outstanding calibration, or inconsistent electricity, thus requiring diagnosis with sputum smear.
Pre-Treatment Loss: Further consideration that 15% of diagnosed patients are lost to follow-up before starting treatment [13].
Empiric Treatment: Further consideration that a percentage of TB patients with negative smear or Xpert results start treatment without microbiological diagnosis (e.g., based on chest X-ray or response to broad-spectrum antibiotics), but after a one-month delay [5].
Without Xpert, TB incidence was projected to fall at 2% annually, from 176 to 144 per 100,000/year over ten years. In the baseline scenario, Xpert reduced TB incidence to 69.5 per 100,000/year (51% reduction relative to diagnosis with smear) and mortality to 5 per 100,000/year (82% reduction). Sequential incorporation of steps in the diagnostic cascade reduced the impact of Xpert (Figure), with projected ten-year reductions in incidence of 42% after including a 4.5-month pre-diagnostic period, 33% after also accounting for people without access to care, 32% after incorporating mechanical difficulty, and 27% after including pre-treatment loss to follow-up (Figure, dark bars). Corresponding reductions in mortality were 76%, 60%, 58%, and 52% (Figure, light bars).
Figure. The Diagnostic Cascade in Tuberculosis.
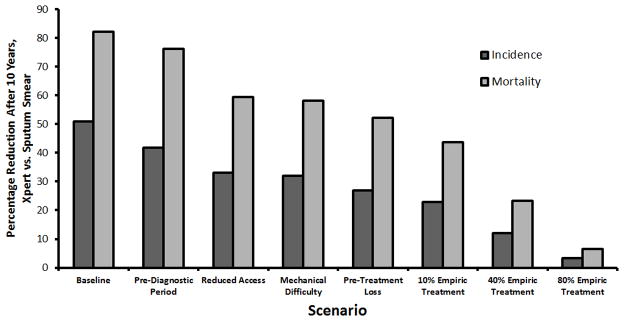
Shown are the expected reductions in tuberculosis incidence (dark bars) and mortality (light bars) ten years after full replacement of sputum smear microscopy with Xpert MTB/RIF. Each sequential step to the right of the graph incorporates an additional element of tuberculosis diagnosis that may reduce the number of individuals benefiting from a more sensitive diagnostic test.
Empiric diagnosis dramatically affected projected impact of Xpert. Assuming 40% empiric treatment in the idealized baseline scenario blunted projected Xpert-associated reductions in incidence and mortality from 51% to 36% and 82% to 58%, respectively. When added to the other elements of the “diagnostic cascade” above, empiric treatment for 10%, 40%, and 80% (as seen in the TB-NEAT trial [5]) of smear-negative TB cases attenuated the projected impact of Xpert from a 27% reduction in incidence to 23%, 12%, and 3%, respectively, with impact on mortality following a similar trajectory (7% reduction at 80% empiric treatment).
This model of TB diagnosis and transmission demonstrates that novel diagnostic tests including Xpert function as part of a “diagnostic cascade.” As such, the population-level impact of the same test, in the same population, can vary by over an order of magnitude. The impact of any new TB diagnostic test depends ultimately not on sensitivity, but on the effectiveness of a cascade containing the novel test compared to a cascade incorporating standard diagnostics and empiric therapy. Realistic diagnostic cascades include elements such as pre-diagnostic delay, reduced access, mechanical difficulties, pre-treatment loss to follow-up, and empiric treatment; each step sequentially reduces Xpert’s impact on TB morbidity and mortality.
These findings demonstrate that understanding the “diagnostic cascade” in any given locality is critical to identifying the appropriate role of novel diagnostics. Notably, this model represents a low-HIV-prevalence population, suggesting that empiric findings in HIV-endemic countries [5,6] may not be restricted to areas with high HIV. In some settings (e.g., rural health outposts in low-income countries), empiric diagnosis is rare, such that novel diagnostics may have substantial impact; however, in these settings, earlier steps of the cascade (e.g., pre-diagnostic delays, mechanical difficulties, and losses to follow-up) are more constraining. By contrast, other settings (e.g., urban middle-income countries) may have few losses in early steps of the cascade but have high empiric treatment levels (e.g. due to use of chest X-ray and other supportive diagnostics), rendering novel diagnostic tests less effective. This analysis shows that high-quality data on all steps of the cascade – particularly empiric treatment practices – are necessary for accurate projections of local impact. Conversely, it also demonstrates the importance of quality assurance, linkage to care, and provider confidence in a negative diagnostic test.
As with any transmission model, this model has limitations, including the assumption of homogeneous mixing. We do not account for public/private sector differences, HIV-induced immunosuppression, or drug-resistant TB. Our depiction of the TB diagnostic cascade is incomplete; other steps (e.g., patient referral, communication of test results to providers/patients) may further limit diagnostic tests’ impact. Ultimately, however, we aimed to demonstrate the importance of the diagnostic cascade, not to precisely project future trajectories in specific settings or any single step’s role in that cascade.
In conclusion, TB diagnostic tests function as part of a larger system of diagnosis and treatment that we term the “diagnostic cascade.” Their impact in any given community therefore depends on the steps in that local cascade; the same test, deployed in different cascades, can spur a reduction in mortality ranging from nearly imperceptible to >80%. An understanding of these diagnostic cascades, and Xpert’s role within them, will be critical to the successful implementation of the World Health Organization’s new TB Elimination framework [14,15], now a priority in over 30 countries. In scaling up Xpert and other novel tests, greater attention must be paid to describing local TB diagnostic cascades and addressing factors that might limit their population-level impact.
Acknowledgments
This work is supported in part by the U.S. National Institutes of Health, 1R21AI106031 and 5R21AI101152. CMD is supported by a fellowship of the Burroughs –Wellcome Fund from the American Society of Tropical Medicine and Hygiene. The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
C.M.D. is employed by FIND, a non-profit organization that collaborates with industry partners, including Cepheid Inc. amongst others, for the development, evaluation and demonstration of new diagnostic tests for poverty-related diseases. A.Y.S. and D.W.D. have no conflict of interest to declare.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva: WHO; 2013. [Google Scholar]
- 2.Pantoja A, Fitzpatrick C, Vassall A, Weyer K, Floyd K. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J. 2013;42:708–720. doi: 10.1183/09031936.00147912. [DOI] [PubMed] [Google Scholar]
- 3.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D’Ambrosio L, Zignol M, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. [Accessed 18 July 2014];WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://who.int/tb/laboratory/mtbrifrollout/en/
- 5.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 6.Churchyard G, McCarthy K, Fielding KL, Stevens W, Chihota V, Nicol M, et al. Effect of Xpert MTB/RIF on early mortality in adults with suspected TB: a pragmatic randomized trial. Oral Abstract 95. Conference on Retroviruses and Opportunistic Infections 2014; Boston, MA. March 2014.. [Google Scholar]
- 7.Sun AY, Pai M, Salje H, Satyanarayana S, Deo S, Dowdy DW. Modeling the impact of alternative strategies for rapid molecular diagnosis of tuberculosis in Southeast Asia. Am J Epidemiol. 2013;178:1740–1749. doi: 10.1093/aje/kwt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menzies NA, Cohen T, Lin HL, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLOS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 10.World Bank. [Accessed 9 April 2014];Life expectancy at birth, total (years) Available at: http://data.worldbank.org/indicator/SP.DYN.LE00.IN.
- 11.UNAIDS. UNAIDS report on the global AIDS epidemic 2012. Geneva: UNAIDS; 2012. [Google Scholar]
- 12.Gothi GD, Chakraborty AK, Nair SS, Ganapathy KT, Banerjee GC. Prevalence of tuberculosis in a south Indian district - twelve years after initial survey. Indian J Tuberc. 1979;26:121–135. [Google Scholar]
- 13.Macpherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92:126–138. doi: 10.2471/BLT.13.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diel R, Loddenkemper R, Zellweger JP, Sotgiu G, D’Ambrosio L, Centis R, et al. Old ideas to innovate tuberculosis control: preventive treatment to achieve elimination. Eur Respir J. 2013;42:785–801. doi: 10.1183/09031936.00205512. [DOI] [PubMed] [Google Scholar]
- 15.D’Ambrosio L, Dara M, Tadolini M, Centis R, Sotgiu G, van der Werf MJ, et al. Tuberculosis elimination: theory and practice in Europe. Eur Respir J. 2014;43:1410–1420. doi: 10.1183/09031936.00198813. [DOI] [PubMed] [Google Scholar]


