Abstract
Diabetic peripheral neuropathy is a prevalent, disabling condition. The most common manifestation is a distal symmetric polyneuropathy (DSP), but many patterns of nerve injury can occur. Currently, the only effective treatments are glucose control and pain management. While glucose control dramatically decreases the development of neuropathy in those with type 1 diabetes, the effect is likely much smaller in those with type 2 diabetes. High levels of evidence support the use of certain anticonvulsants and antidepressants for pain management in diabetic peripheral neuropathy. However, the lack of disease modifying therapies for diabetic DSP makes the identification of new modifiable risk factors essential. Intriguingly, growing evidence supports an association between metabolic syndrome components, including pre-diabetes, and neuropathy. Future studies are needed to further explore this relationship with implications for new treatments for this common disease.
Introduction
Neuropathy, or damage to the nerves of the peripheral nervous system, is a debilitating yet surprisingly common and complex condition. Its prevalence is greater than 2% in the general population1, 2 and approximately 15% in those over the age of 403. By far, the most common cause of neuropathy is diabetes4. In fact, the prevalence of neuropathy in patients with diabetes is approximately 30%, and up to 50% will eventually develop neuropathy during the course of their disease5. Diabetes can damage the peripheral nervous system in a variety of ways, but the most common presentation is a distal symmetric polyneuropathy (DSP). Other patterns of injury include small fiber predominant neuropathy, radiculoplexopathy, and autonomic neuropathy, amongst others. Since DSP is the most common neuropathy subtype and is the best studied, this will be the main focus of this review. Currently, the only treatments available to patients with diabetic DSP are improved glucose control and pain management. Both of these topics will be covered in depth.
Given the limitations in current clinical care, it is essential to identify new modifiable risk factors for the development of neuropathy. Top candidates include metabolic syndrome components such as hypertriglyceridemia, obesity, hyperglycemia, hypertension, and dyslipidemia. Establishing whether there is a causal relationship between these components, including pre-diabetes, has the potential to lead to new disease modifying therapies.
Diabetic neuropathy: clinical manifestations
Diabetes can impact the peripheral nervous system in a multitude of ways. DSP accounts for such a large proportion of all peripheral nerve manifestations attributed to diabetes that some use the terms diabetic DSP and diabetic neuropathy interchangeably. Patients with DSP typically have numbness, tingling, pain, and/or weakness that begin in the feet and spread proximally in a length-dependent fashion (stocking and glove distribution). The symptoms are symmetric with sensory symptoms more prominent than motor involvement. Many patients with neuropathy experience a sensation of their socks being bunched up or their shoes not fitting correctly. They even have the apparent paradox of numbness and exquisite sensitivity at the same time. Interestingly, which symptom predominates varies dramatically from patient to patient.
The constellation of symptoms from DSP creates many down-stream effects that can affect patients’ quality of life, both physical and mental6. DSP associated numbness often causes balance problems which can lead to falls. DSP is one of three main risk factors for falls in patients with diabetes, along with retinopathy and vestibular dysfunction7. In fact, patients with diabetic DSP are 2–3 times more likely to fall than those with diabetes and no neuropathy7. Additionally, patients with severe DSP are at risk for ulcerations and lower extremity amputations, with 15% developing an ulcer during the course of their disease8. Diabetes is the leading cause of lower extremity amputations, with a 15-fold increase in the likelihood of this life-changing complication9. Moreover, 80,000 lower extremity amputations are performed each year in patients with diabetes9. Overall, diabetic DSP can severely affect quality of life, particularly in those with pain6.
This common, disabling disease also profoundly impacts the health care system. Costs associated with diabetic neuropathy are estimated to be between 4.6 and 13.7 billion dollars, with most of the cost attributed to those with type 2 diabetes8. Therefore, neuropathy is associated with one fourth of the total costs of diabetes care in the United States.
Neuropathic pain is one of the major disabling symptoms of patients with DSP. It is a difficult condition to treat and therefore causes significant patient suffering and societal burden10. It is estimated that diabetic neuropathic pain (DNP) develops in 10% to 20% of the diabetic population overall, and can be found in 40% to 60% with documented neuropathy11–13. However, these numbers are likely to be underestimates, as one study showed that approximately 12% of patients with DNP had never mentioned this condition to their doctors11. Like other types of neuropathic pain, DNP is characterized by burning, electric, and stabbing sensations with or without numbness. Frequently, patients develop allodynia (painful sensations to innocuous stimuli) and hyperalgesia (increased sensitivity to painful stimuli). However, less than half are treated for pain, despite many available effective therapies11. Fortunately, there are multiple neuropathic pain screening instruments available to aid the clinician in identifying those who would benefit from treatment14.
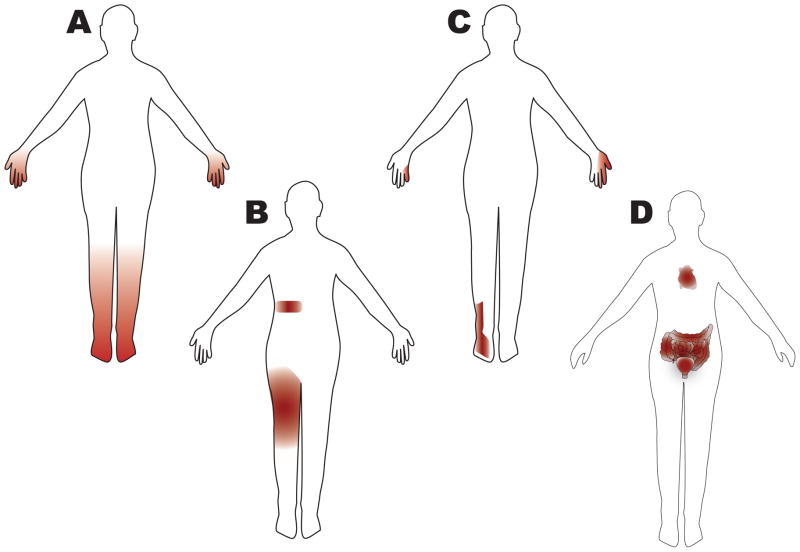
Other types of peripheral nerve injury that can occur in patients with diabetes includes small fiber predominant neuropathy, autonomic neuropathy, radiculoplexopathy (diabetic amyotrophy), radiculopathy, mononeuritis multiplex, mononeuropathy, and treatment-induced neuropathy (Figure 1). Small fiber-predominant neuropathy is an increasingly recognized pattern of involvement and typically is an early manifestation (Figure 2). In fact, patients often progress from a small fiber-predominant neuropathy to a DSP. Small fiber-predominant neuropathy can be difficult to diagnose because the examination (decreased reflexes, impaired vibration, weakness) and electrodiagnostic testing can be normal. Autonomic neuropathy (a type of small fiber neuropathy) is also common in patients with diabetes. Symptoms include gastroparesis, constipation, urinary retention, erectile dysfunction, and cardiac arrhythmias. Importantly, patients with autonomic neuropathy are at a greater than 2-fold increased risk of death15. Diabetic radiculoplexopathy can involve either the lumbosacral (more common) or the cervical plexus. Patients present with pain and weight loss followed by weakness in the distribution of the involved plexus. Pathology reveals evidence of ischemic injury and a microvasculitis, but whether immunosuppressive medications are effective is unclear16, 17. To date, only one randomized controlled trial has been completed for diabetic lumbar radiculoplexopathy and no significant effect was found on the primary outcome, although secondary outcome measures did show improvement with intravenous methylprednisolone17. Diabetes is also one of the few causes of non-compressive radiculopathy. Patients with diabetes can also present with mononeuritis multiplex without an underlying rheumatologic cause. Furthermore, patients are at increased risk of mononeuropathy which can be secondary to compressive or ischemic mechanisms. The most commonly involved nerves are the oculomotor and median nerves. Whether the mechanism of these four peripheral nerve manifestations (radiculoplexopathy, radiculopathy, mononeuritis multiplex, mononeuropathy) is the same is unclear. Though poor glucose control is associated with increased risk for neuropathy, the treatment of diabetes can also cause neuropathy. Treatment-induced neuropathy presents as acute pain and/or autonomic involvement, usually after the institution of insulin but can happen after any quick establishment of glucose control18. The pain and autonomic features can improve significantly with time, and this pattern of nerve injury underscores the fact that even quick improvements in glucose control can lead to neuropathy. Of note, there are also a substantial number of patients with diabetes that have asymptomatic neuropathy19. Thus, while DSP accounts for the vast majority of neuropathic manifestations in patients with diabetes, there are other important conditions for physicians to consider.
Figure 1. Patterns of nerve injury in diabetic neuropathy.
Many patterns of nerve injury are observed in patients with diabetes. By far the most common neuropathy subtype is distal symmetric polyneuropathy (DSP), which is the focus of this review. However, clinicians should be aware of all potential patterns as they have implications for the evaluation and treatment of these patients. For example, patients with diabetes can develop a radiculopathy without a disc herniation or degenerative changes in the spine. This knowledge could prevent a patient from spine surgery in the case where imaging results are equivocal. Furthermore, patients with diabetes can have more than one pattern of nerve injury, and the clinician needs to ask patients about specific symptoms such as autonomic involvement, which is often overlooked. The following patterns are shown in the figure: (A) DSP, small fiber predominant neuropathy, treatment induced neuropathy (B) radiculoplexopathy, radiculopathy (C) mononeuropathy, mononeuritis multiplex (D) autonomic neuropathy, treatment induced neuropathy. Note that small fiber predominant neuropathy has the same pattern as DSP but that the neurologic examination and electrodiagnostic studies are quite different, which has the potential to lead the clinician astray. Diabetic radiculoplexopathy may be responsive to immunotherapy and in contrast to most nerve injury in patients with diabetes, usually improves with time16, 17. Treatment induced neuropathy is an under-recognized phenomenon18. Unlike the other peripheral manifestations of diabetes, this condition is caused by overaggressive control of glucose levels.
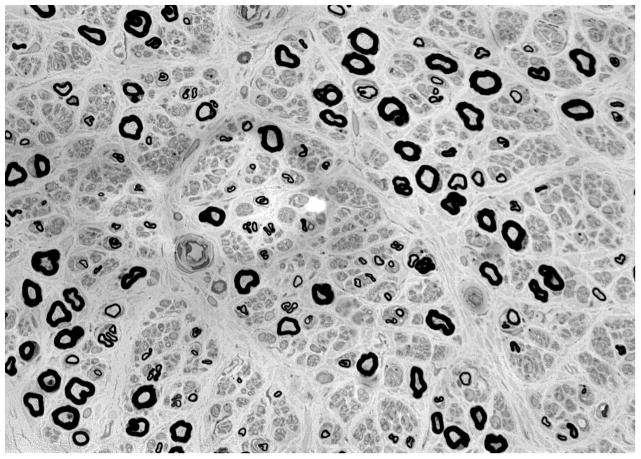
Figure 2. Small fiber predominant neuropathy on skin biopsy.
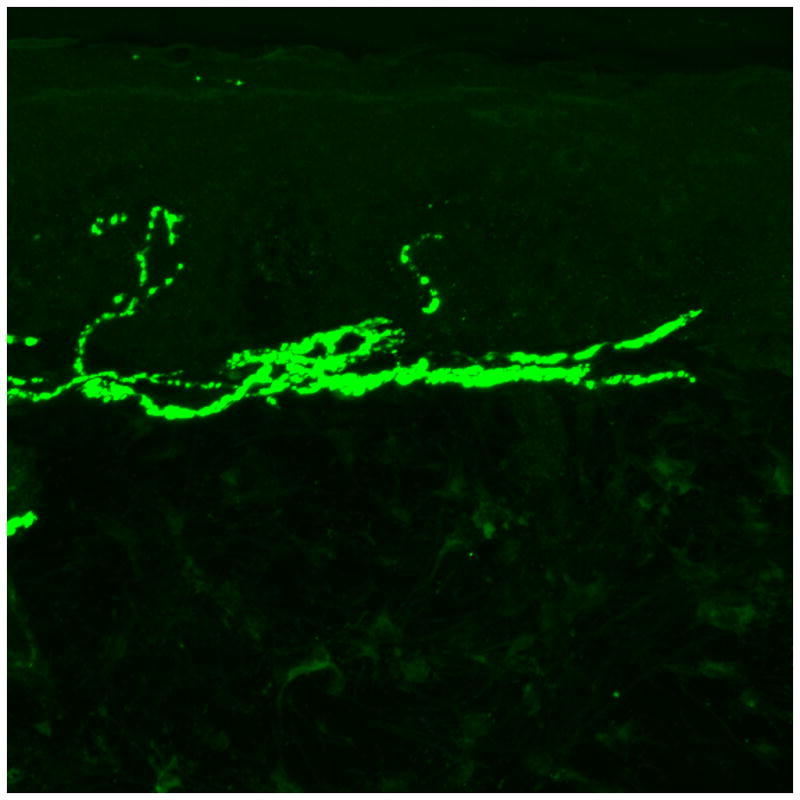
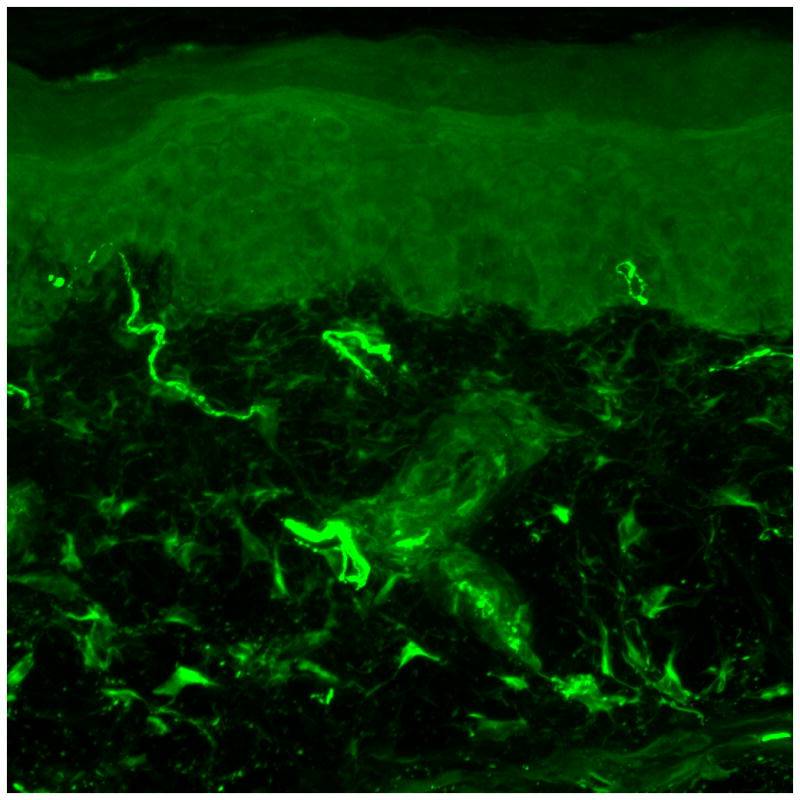
(A) Skin biopsy evaluating intra-epidermal nerve fiber density (stained with protein gene product 9.5, 50 micrometer sections) from a 41 year old male without neuropathy. Two nerves are seen crossing the dermal-epidermal junction. (B) Skin biopsy evaluating intra-epidermal nerve fiber density from a 50 year old male with diabetic neuropathy. No nerves are seen crossing the dermal-epidermal junction. (C) Sural nerve biopsy from a 44 year old male with diabetic neuropathy (40X magnification). Biopsy reveals axonal loss of small and large diameter nerves.
Glucose control in type 1 and type 2 diabetes
A body of research conducted over the past 20 years has added to our knowledge of glucose control as a modifiable risk factor for the development of neuropathy in patients with diabetes (Table 1). Seventeen randomized, controlled clinical trials have studied the effects of enhanced glucose control over at least a 12 month period on neuropathy20–37. Seven of these studies focused on patients with type 1 diabetes, but only two of these reported on outcomes related to clinical impairment20, 23, 26, 29, 31, 32, 34. In 1993, the DCCT study group followed over 1400 subjects for five years and found a 60% reduction in the development of neuropathy in those receiving more frequent insulin dosing20. Similarly, Linn et al in 1996 followed 49 subjects for 5 years and reported an approximately 70% reduction in the development of neuropathy in those with enhanced glucose control32. Both of these groups revealed a large, statistically significant reduction in the development of neuropathy with tighter glucose control. Furthermore, only one of the seven studies did not show a statistically significant benefit of tighter glucose control.
Table 1.
Clinical trials investigating the role of enhanced glucose control on neuropathy
| Investigator | Trial Size | Length of study | Clinical outcome | Other outcomes | Enhanced glycemic control superior? |
|---|---|---|---|---|---|
| Type 1 diabetes | |||||
| Holman 1983 | 74 | 2 years | No | QST | Yes |
| Lauritzen 1985 | 30 | 2 years | No | QST | No |
| Dahl-Jorgensen 1986 | 45 | 2 years | No | NCS | Yes |
| Jakobsen 1988 | 24 | 2 years | No | QST | Yes |
| DCCT 1993 | 1,441 | 5 years | Yes | NCS | Yes |
| Reichard 1993 | 102 | 7.5 years | No | NCS, QST | Yes |
| Linn 1996 | 49 | 5 years | Yes | None | Yes |
| Type 2 diabetes | |||||
| Kawamori 1991 | 50 | 4 years | No | NCS | Yes |
| UKPDS 1998 | 3,867 | 10 years | No | QST | Yes |
| Tovi 1998 | 38 | 1 year | Yes | None | No |
| Azad 1999 | 153 | 2 years | Yes | None | No |
| Shichiri 2000 | 110 | 8 years | No | NCS, QST | Yes |
| Gaede 2003 | 160 | 8 years | No | QST | No |
| Duckworth 2009 | 1,791 | 5.6 years | Yes | None | No |
| ACCORD 2010 | 10,251 | 3.7 years | Yes | None | No |
In contrast to the robust results seen in subjects with type 1 diabetes, the 8 randomized, controlled trials in type 2 diabetes have produced less definitive results21, 22, 24, 25, 28, 30, 33, 36, 37. Only four of these studies investigated the effects of glucose control on clinical impairment secondary to neuropathy. In 2010, the ACCORD study group compared the effectiveness of a lower hemoglobin A1C goal (less than 6 compared to 7–7.9) on the Michigan Neuropathy Screening instrument28. In the more than 5500 subjects followed for a median of 3.7 years, they discovered a 7% reduction in the development of neuropathy, which was not statistically significant. In 2009, Duckworth et al followed 1791 military veterans for a median of 5.6 years and found a non-significant 5% reduction in the development of neuropathy24. Studies by Azad et al and Tovi et al followed much smaller numbers of patients and their results produced relative risks (RR) with large confidence intervals (no statistically significant differences)22, 37. However, three of the four studies that investigated nerve conduction studies and/or quantitative sensory testing revealed statistically significant results in favor of glucose control. One such study, performed by the UKPDS study group in 1998, is the second largest and longest randomized, controlled trial in patients with type 2 diabetes21. The main neuropathy outcome measure in this study was vibration threshold using a biothesiometer. This group followed 3867 subjects for 15 years and reported a RR of 0.95 (95%CI 0.76–1.18) at 3 years, 0.88 (95%CI 0.72–1.08) at 6 years, 0.84 (95%CI 0.68–1.04) at 9 years, 0.92 (95%CI 0.70–1.20) at 12 years, and 0.60 (95%CI 0.39–0.94) at 15 years (the only statistically significant result) in those receiving enhanced glucose control. Overall, these eight studies support only a modest reduction in the development of neuropathy in patients with type 2 diabetes receiving enhanced glucose control, which is in stark contrast to the substantial effect in those with type 1 diabetes. Possible explanations for this difference include the different outcome measures used, the different treatment regimens, the higher incidence of neuropathy in control subjects with type 2 diabetes, and the difference in baseline glucose control in these clinical trials. However, despite the similarities between type 1 and type 2 diabetes, these trials highlight the significant differences which exist in the disease mechanisms and complications of the two.
Pathophysiology of type 1 and type 2 diabetic neuropathy
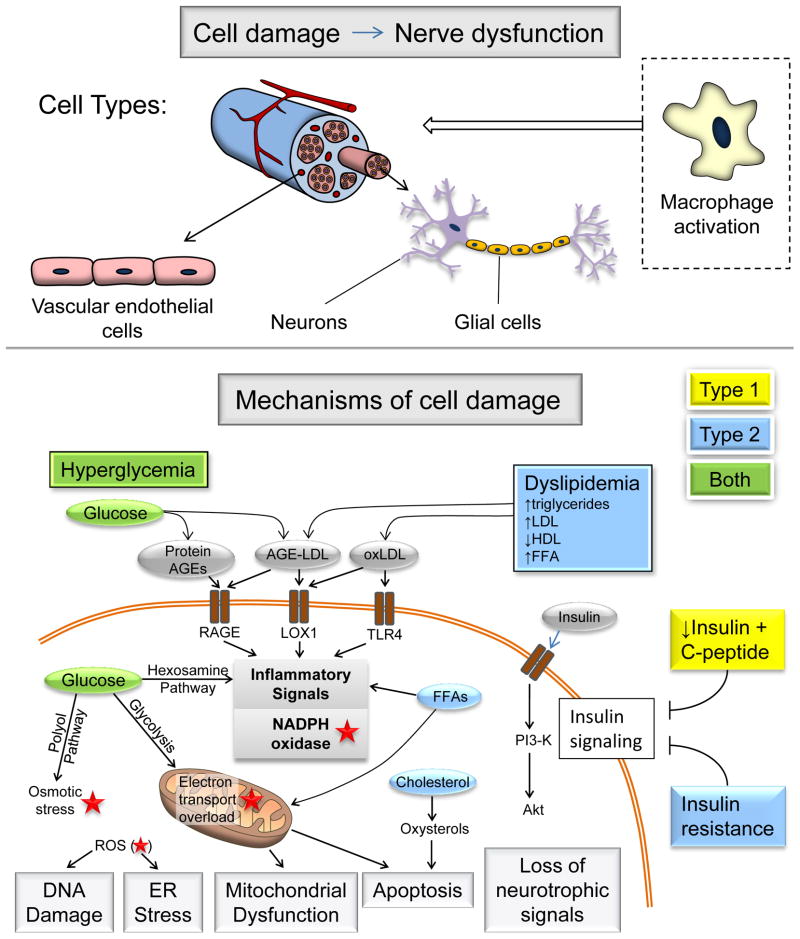
Hyperglycemia is a major factor underlying diabetic neuropathy, but other changes also contribute. In type 2 diabetes, dyslipidemia is thought to play a major role38. Changes in insulin signaling are also key; in type 1 diabetes levels of both insulin and C-peptide are reduced, whereas in type 2 diabetes there is thought to be reduced neuronal insulin sensitivity39, 40. Several recent reviews discuss the mechanisms of diabetic neuropathy in depth38, 41–43. Therefore, we will briefly outline the major mechanisms (Figure 3) and consider how the disease states in type 1 and type 2 diabetes are different, and why this may impact treatment efficacy.
Figure 3. Mechanisms of diabetic neuropathy.
Factors linked to type 1 diabetes (yellow), type 2 diabetes (blue) and both (green) cause DNA damage, ER stress, mitochondrial dysfunction, apoptosis and loss of neurotrophic signaling. This cell damage can occur in neurons, glial cells and vascular endothelial cells, as well as triggering macrophage activation, all of which can lead to nerve dysfunction and neuropathy. The relative importance of the pathways in this network will vary with cell type, disease profile and time. Abbreviations: AGE, advanced glycation end-products; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FFA, free fatty acids; ROS, reactive oxygen species (red star); ER, endoplasmic reticulum; PI3K, phosphatidylinositol 3-kinase.
Hyperglycemia
Excess intracellular glucose is processed by increased flux through one or more glucose metabolism pathways, and prolonged hyperglycemia can lead to cellular damage in several ways. First, excess glycolysis may lead to overload of the mitochondrial electron transport chain and generation of reactive oxygen species (ROS)43. Second, increased flux through the polyol pathway can increase cellular osmolarity, reduce NADPH levels and lead to oxidative stress44. Finally, increased flux through the hexosamine pathway is associated with inflammatory injury41.
Another consequence of hyperglycemia is the generation of advanced glycation end products (AGEs)45, via attachment of reactive carbohydrate groups to proteins, lipids or nucleic acids. This tends to impair the biological function of protein AGEs, thus impacting cellular function46. Extracellular AGEs also bind to the receptor for AGE (RAGE), initiating inflammatory signaling cascades, activating NADPH oxidases and generating oxidative stress47. Long-term inflammatory responses are also triggered, including the upregulation of RAGE and activation of NFκB48.
Dyslipidemia
There is a high incidence of dyslipidemia in type 2 diabetic patients49. Dyslipidemia is linked to diabetic neuropathy50, and several underlying mechanisms have been identified. Free fatty acids (FFAs) have been shown to directly cause injury to Schwann cells in vitro51, but also have systemic effects such as promoting inflammatory cytokine release from adipocytes and macrophages52. Plasma lipoproteins, particularly low-density lipoproteins (LDLs), can be modified by oxidation (oxLDL) and/or glycation, and these modified LDLs can bind to extracellular receptors (including the oxLDL receptor LOX153, Toll-like receptor 454 and RAGE47), triggering signaling cascades that activate NADPH oxidase and subsequent oxidative stress53. Additionally, cholesterol may be oxidized to oxysterols, which have been shown to cause apoptosis in neurons41, 55.
Impaired insulin signaling
While insulin is not involved in glucose uptake into neurons, it has been shown to have neurotrophic effects, promoting neuronal growth and survival56, 57. Reduction of this neurotrophic signaling due to insulin deficiency (type 1 diabetes) or insulin resistance (IR; type 2 diabetes) is thought to contribute to the pathogenesis of diabetic neuropathy39. In neurons, IR occurs by inhibition of the PI3K/Akt signaling pathway, similarly to IR in muscle and adipose tissues42. Disruption of this pathway may also lead to mitochondrial dysfunction and oxidative stress, further promoting neuropathy39.
In type 1 diabetes, reduction in C-peptide may lead to nerve dysfunction in a number of ways, including reduction in Na/K ATPase activity, endothelial nitric oxide synthase (eNOS) activity, and endoneurial blood flow40. Treatment with C-peptide may slow progression of neuropathy in type 1 diabetic patients58.
The mechanisms outlined above lead to multiple cellular disturbances, including mitochondrial dysfunction, endoplasmic reticulum (ER) stress, DNA damage and apoptosis. Another layer of complexity is added when you consider that these processes of cell stress and/or damage occur in several different cell types within the nerves, including neurons (in axons and at nerve terminals), glial cells, and endothelial cells of the microvasculature. Furthermore, many of these changes will trigger activation and recruitment of macrophages59, feeding back into inflammatory mechanisms of cell stress and death. Ultimately, these different forms of cellular stress cause dysfunction and/or death of the nerve, which manifests as clinical neuropathy.
As discussed above, tight glucose control can reduce neuropathy in type 1 diabetic patients but is not as efficacious in type 2 patients20, 21. This is likely to be related to differences in the underlying mechanisms: hyperglycemia and reduction in insulin signaling in type 1 diabetes, compared with a combination of hyperglycemia, dyslipidemia and IR in type 2 diabetes. Differences in the duration of pro-neuropathic changes prior to the onset/diagnosis of diabetes may also contribute to the differences in neuropathy progression between the two diseases. A patient does not typically develop type 2 diabetes rapidly; it occurs after many years of obesity and other aspects of the metabolic syndrome (see below). Tight glucose control will not necessarily reduce the dyslipidemia, systemic inflammation and IR, and following years of these insults it is not entirely surprising that neuropathy is difficult to halt/reverse. Although hyperglycemia contributes to the vicious cycles of oxidative stress, inflammation and cellular damage in type 2 diabetes, reducing hyperglycemia alone may not be enough to stop the cycle from continuing.
Pre-diabetes and neuropathy
Whereas the link between diabetes and neuropathy is well-established, there remains scientific uncertainty regarding the effects of pre-diabetes (impaired fasting glucose and/or impaired glucose tolerance (IGT)) on neuropathy. Two separate groups have shown that there is an increased prevalence of IGT in subjects with idiopathic neuropathy compared to literature-based controls60, 61. A third group identified an increased prevalence of neuropathy in patients with IGT compared with controls62. In addition, Smith et al followed a cohort of subjects with IGT and neuropathy that underwent an extensive diet and exercise regimen. They found that these subjects had an increase in nerve fiber density (NFD) over time, which is in stark contrast to historical controls63. The implication of this study is that treatment of IGT may improve neuropathy outcomes, although this study lacked a control group for comparison. In contrast, Hughes et al did not find a statistically significant association of IGT with neuropathy in a small case-control study64. Similarly, Dyck et al found no difference in the prevalence of neuropathy in patients with IGT compared to matched controls in a population based study in Olmsted County (abstract only)65.
Since there are conflicting data linking pre-diabetes with neuropathy, there is a need for a comprehensive study investigating pre-diabetes to understand if it is one of the metabolic “drivers” underlying the onset and progression of neuropathy. The answer has direct implications for potential therapies for many patients with neuropathy. Currently, one third of adult Americans meet criteria for pre-diabetes66. Since less than 5% have received a formal diagnosis from their providers and only a small percentage are being treated for this condition, establishing a causal relationship between pre-diabetes and neuropathy would change the clinical management of a substantial number of patients66.
Pain management in DNP
While glucose control is the only disease modifying therapy for diabetic neuropathy, pain management is the other mainstay of treatment that can dramatically improve the quality of life of these patients. Over the last two decades, tremendous effort has been made to improve the treatment of DNP using randomized placebo controlled clinical trials. Data from these trials have provided support for the use of certain pharmacological treatments for DNP, as outlined below. Taking into consideration the efficacy of these interventions, several guidelines have been generated. The 2006 and 2010 guidelines from the European Federation of Neurological Societies task Force (EFNS) 67,68 and the 2011 guidelines from the American Academy of Neurology (AAN), the American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation69 are the most thorough and recent guidelines on this topic. According to these guidelines, several classes of drugs are considered to be effective for the treatment of DNP (Table 2). Here we discuss and compare the EFNS and AAN guidelines (Table 3). The consensus from these guidelines provides information for the best evidence-based practice for the treatment of DNP.
Table 2.
Published class I and class II evidence of pharmacological treatment for DNP
| Drugs | Trial size | Dosage | Study design | Result | NNT | EFNS | AAN |
|---|---|---|---|---|---|---|---|
| Pregabalin | 146 | 300 mg | Parallel, 8 weeks | Pregabalin > placebo103 | 3.9 | I | I |
| 338 | 75, 300, 600 mg | Parallel, 5 weeks | Pregabalin (300, 600 mg) > placebo104 | 300 mg: 3.6 600 mg: 3.3 |
I | I | |
| 246 | 150, 600 mg | Parallel, 6 weeks | Pregabalin (600 mg) > placebo105 | 600 mg: 4.2 | I | I | |
| 338 | Fixed or Flexible: 150 – 600 mg | Parallel, 12 weeks | Fixed and flexible > placebo106 | 3.6 | II | ||
| 167 | 600 mg | Parallel, 13 weeks | Pregabalin (600 mg) > placebo107 | I | I | ||
| 396 | 150, 300, 600 mg | Parallel, 12 weeks | Pregabalin (600 mg) > placebo108 | 600 mg: 6.3 | |||
| Gabapentin | 165 | < 3600 mg | Parallel, 8 weeks | Gabapentin > placebo109 | 4 | I | I |
| 40 | 900 mg | Crossover, 2×6 weeks | Gabapentin = placebo70 | II | |||
| Lamotrigine | 59 | < 400 mg | Parallel, 8 weeks | Lamotrigine > placebo72 | 4 | I | II |
| 360 | 200, 300, 400 mg | Parallel, 19 weeks | Lamotrigine = placebo71 | I | I | ||
| 53 | 200 mg vs amitriptyline 75 mg | Parallel, 6 weeks | Lamotrigine = amitriptyline110 | II | |||
| Valproate | 52 | 600–>1200 mg | Parallel, 4 weeks | Valproate > placebo73 | II | II | |
| 39 | 500 mg | Parallel, 16 weeks | Valproate > placebo74 | II | II | ||
| 31 | 1500 mg | Parallel, 4 weeks | Valproate = placebo75 | I | |||
| Amitriptyline | 29 | ≤ 150mg | Crossover, 2×6 weeks | Amitriptyline > placebo78 | 2.1 | II | |
| 24 | 25–75 mg | Crossover, 2×6 weeks | Amitriptyline > placebo111 | II | |||
| 19 | 75 mg | 3-phase, crossover amitriptyline and maprotiline | Amitriptyline > Maprotiline > placebo80 | I | |||
| Desipramine | 20 | Average 201 mg | Crossover, 2×6 weeks | Desipramine > placebo112 | 2.2 | II | |
| Venlafaxine ER | 244 | 150–225 mg | Parallel, 6 weeks | Venlafaxine > placebo113 | 4.5 | I | I |
| 60 | 75–150 mg | Parallel, 8 weeks | Venlafaxine > placebo114 | II | |||
| Venlafaxine + gabapentin | 11 and 42 | Parallel, 2×8 weeks | Venlafaxine+gabapentin > placebo in gabapentin unresponsive patients115 | II and III | |||
| Duloxetine | 457 | 20, 60, 120 mg | Parallel, 12 weeks | Duloxetine (60mg, 120 mg) > placebo116 | 60 mg: 4.3 120 mg: 3.8 |
I | II |
| 348 | 60 mg, 120 mg | Parallel, 12 weeks | Duloxetine (60mg, 120 mg) > placebo117 | 60 mg: 11 120 mg: 5 |
I | I | |
| 334 | 60 mg, 120 mg | Parallel, 12 weeks | Duloxetine (60mg, 120 mg) > placebo118 | 60 mg: 6.3 120 mg: 3.8 |
I | II | |
| Oxycodone CR | 159 | 10–100 mg | Parallel, 6 weeks | Oxycodone > placebo119 | N/A | I | II |
| 338 | 10–80 mg with gabapentin (100–3600 mg) | Parallel, 12 weeks | Oxycodone + gabapentin > placebo + gabapentin120 | I | I | ||
| Morphine sulfate | 57 | 120 morphine, 60 mg morphine +2400 mg gabapentin, 3600 mg gabapentin | Crossover, 4×4 weeks | Morphine + gabapentin>morphine>gabapentin>placebo81 | I | II | |
| Tramadol | 127 | 100–400 mg (mean 210 mg) | Parallel, 6 weeks | Tramadol > placebo121 | 3.1 | II | |
| 45 | 200–400 mg | Crossover, 2×6 weeks | Tramadol > placebo122 | 4.3 | II | ||
| 311 | 37.5 mg + 325 mg acetaminophen | Parallel, 8 weeks | Tramadol/APAP > placebo123 | I | |||
| Dextromethorphan | 19 | 400 mg | Crossover, 2×9 weeks | Dextromethorphan > placebo124 | 3.2 | I | I |
| 14 | Mean 381 mg | Crossover, 2×6 weeks | Dextromethorphan > placebo125 | 4 | I | II | |
| Capsaicin 0.075% | 252 | 0.075% qid | Parallel, 8 weeks | Capsaicin > placebo126 | NA | ||
| 22 | 0.075% qid | Parallel, 8 weeks | Capsaicin > placebo82 | I | |||
| Isosorbide dinitrate | 22 | 30 mg | Crossover, 2×4 weeks | Isosorbide dinitrate > placebo84 | I | ||
| Glyceryl trinitrate | 48 | Crossover, 2×4 weeks | Glyceryl trinitrate> placebo85 | II | |||
| ABT-594 | 266 | 150, 225, 300 mg bid | Parallel, 7 weeks | ABT-594>placebo86 | I | ||
| Botulinum toxin | 18 | Fifty units of subtype A in 1.2 mL 0.9% saline intradermally into each foot, each injection 4 U subtype A | Crossover, 12×12 weeks | Botulinum toxin > placebo127 | II | ||
| Levodopa | 25 | 100 mg levodopa plus benserazide 25 mg tid | Parallel, 28 days | Levodopa + benserazide > placebo128 | II |
DNP = Diabetic neuropathic pain
Table 3.
Comparison of the EFNS and AAN guidelines
| Drug | EFNS67 | AAN 69 |
|---|---|---|
| Pregabalin (300–600 mg/d) | A | A |
| Gabapentin | A | B |
| Lamotrigine | A/B ineffective/discrepant | B against |
| Oxcarbazepine | A/B ineffective/discrepant | B against |
| Lacosamide | A/B ineffective/discrepant | B against |
| Sodium Valproate | A/B ineffective/discrepant | B |
| TCA | A | B (amitriptyline) |
| SNRI | A | B (venlafaxine, Duloxetine) |
| Opioids | A (oxycodone) | B (morphine sulfate, oxycodone) |
| Tramadol | A | B |
| Dextromethorphan | B | B |
| Topical capsaicin | A/B ineffective/discrepant | B |
| Isosorbide dinitrite spray | A | B |
| ABT-594 | A | |
| Botulinum toxin | B | |
| Levodopa | B | |
| Lidocaine patch | C |
EFNS = European Federation of Neurological Societies task Force, AAN = American Academy of Neurology
A=Established as effective, B=Probably effective, C=Possibly effective
Anticonvulsants
Pregabalin is classified as effective with Level A evidence by both the EFNS and AAN guidelines. This recommendation is based on three to four class I studies that all revealed superiority of pregabalin compared with placebo. Interestingly, the effect size was small in the highest quality studies. The recommended dosage for pregabalin is 300–600 mg/d.
Gabapentin is also classified as effective with Level A evidence by the EFNS, though the AAN (Level B) did not consider it to be a Level A drug based on the fact that only one class I study showed benefit and that one negative class II study has been published70. In contrast, the EFNS guidelines are based on a meta-analysis of 7 trials with class I evidence for a systematic review. The recommended dose is 900–3600 mg/d.
Lamotrigine is classified as ineffective or with discrepant results with Level A/B evidence by the EFNS because of one negative class I study and one class II study that showed comparable efficacy to amitryptiline71. Lamotrigine is also not recommended by the AAN based on two class I studies that failed to show benefit compared with placebo72. Similarly, both guidelines state that oxcarbazepine and lacosamide are not effective with Level A/B (EFNS) or Level B (AAN) evidence.
Sodium valproate is classified as ineffective or with discrepant results with Level A/B evidence by EFNS. In contrast, this medication is classified as effective with Level B evidence by the AAN for doses of 500–1200 mg/d. The EFNS justified its decision to classify it as ineffective or with discrepant results because both positive studies were published from the same group73,74 and one negative study has been published75. The negative study was not discussed in the AAN guideline. Of note, the two positive studies did not report a significant placebo effect or significant side effects that are usually attributed to this medication. The two guidelines disagree on whether the current evidence supports or refutes the effectiveness of sodium valproate for the treatment of DNP.
Antidepressants
Tricyclic antidepressants (TCAs) are classified as effective with Level A evidence by the EFNS based on two class I meta-analyses76, 77; however the EFNS guidelines do not provide a recommendation of a specific drug within the TCA class. In contrast, the AAN states that amitriptyline (25–100 mg/d) is supported by Level B evidence based on one class I and two class II studies78,79, 80. The AAN guidelines state that there is insufficient evidence in regards to other TCAs because only class III evidence is available for these drugs (Level U evidence).
Serotonin norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine and duloxetine are supported by both the EFNS (Level A) and the AAN (Level B) guidelines. The reason for the discrepancy in the level of evidence is that the EFNS describes three class I studies for duloxetine whereas the AAN classifies only one of these studies as class I. Similarly, the AAN classifies only one of the two studies of venlafaxine as class I. The recommended dosages are 75–225 mg/d for venlafaxine and 60–120 mg/d for duloxetine.
Opioids
Controlled release oxycodone is recommended by EFNS as effective with Level A evidence based on two class I studies. In contrast, the AAN recommends both controlled release oxycodone (mean 37 mg/d and up to 120 mg/day) based on three class II trials and morphine sulfate (up to 120 mg/d) based on one class II trial 81 as effective with Level B evidence.
Tramadol 200–400 mg/d and 37.5 mg + acetaminophen were listed by the EFNS as Level A effective treatments based on two class I studies. In contrast, only tramadol 210 mg/d was recommended by the AAN as effective with Level B evidence for DNP (two class II studies).
Other medications
Dextromethorphan (400 mg/d) is classified as effective with Level B evidence in both EFNS and AAN guidelines based on one class I and one class II study.
Topical capsaicin treatment (0.075% QID) is supported with Level B evidence in the AAN guidelines based on one class I 82 and one class II study 83. However, EFNS classified capsaicin as Level A/B for inefficacy or discrepant results based on a systematic review of 5 class I–II studies.
Isosorbide dinitrate spray is backed by Level B evidence in the AAN guideline based on one class I trial 84. Similarly, the EFNS cited the same study but also reported a study that used glyceryl trinitrate spray (class I) and determined treatment with nitrate derivatives is supported with Level A evidence based on these two studies85.
Nicotine derivative ABT-594 is listed by the EFNS as effective with Level A evidence based on one class I study86. This treatment is not discussed in the AAN guidelines.
Botulinum toxin and levodopa are classified as effective with Level B evidence by the EFNS based on one class II study for each medication; however, neither is discussed in the AAN guidelines.
The lidocaine patch is classified as effective with Level C evidence by the AAN based on two class III studies, but it was not discussed in the EFNS guidelines.
Overall, these two guidelines are in close agreement for the vast majority of medications evaluated. However, the data for sodium valproate and capsaicin cream is conflicting with one guideline providing evidence for and one revealing evidence against their use. The more subtle differences in Levels of evidence are likely due to the fact that the AAN guidelines required a completion rate of greater than 80%, which was not required by the EFNS. Therefore, many trials were downgraded from class I to class II because of this stringent criteria with a resulting effect that only pregabalin was shown to have Level A evidence in the AAN guidelines. Of note, with increasing knowledge of the proper conduct and reporting of clinical trials through the years, there is likely a bias in favor of newer medications. Furthermore, the levels of evidence do not take into account the number needed to treat or the number needed to harm. Rather, the levels of evidence are based on the number of high quality studies that show benefit. Unfortunately, few studies compare medications head to head or evaluate for effect on quality of life. Future studies are needed to further clarify the role of these medications in the treatment of DNP.
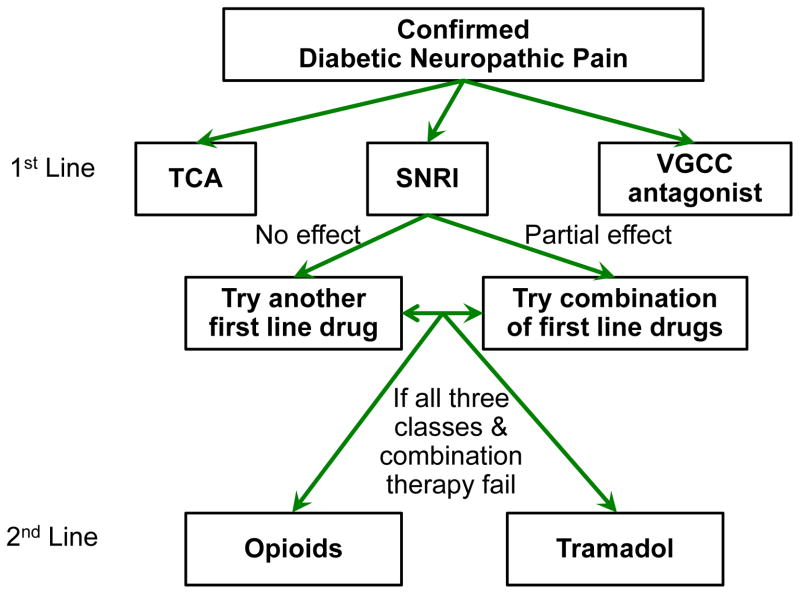
Treatment algorithm
Several review articles recommended treatment algorithms for DNP based on their efficacy and safety (Figure 4). We reviewed algorithms from Jensen et al.87 and EFNS68 for treating DNP and Dworkin et al for treating neuropathic pain88, 89. Importantly, there is no evidence to support one treatment algorithm versus another.
Figure 4. Algorithm for the treatment of diabetic painful neuropathy.
First and seconds line treatments for diabetic painful neuropathy.
1st line treatment
All three algorithms recommend gabapentin, pregabalin, TCAs, venlafaxine, and duloxetine as first line medications. Which agent to choose is largely determined by co-morbidities of the patient and side effect profiles of the medications. This is especially important in treating DNP because none of the drugs were designed specifically for neuropathic pain and therefore they each have other indications such as the treatment of seizures and depression. Dworkin et al also recommends topical lidocaine for those with localized neuropathic pain and in those with concern for central nervous system side effects. All three sources recommend titrating a first line medication to a maximum tolerated dose before switching to a second first line medication or combination therapy. Only once all these options fail is a second line agent recommended. All of these medications are supported by Level A evidence in the EFNS guidelines and by Level A or B evidence in the AAN guidelines.
2nd line
All three algorithms also support opioid analgesics and tramadol as second line medications. While these medications are also backed by Level A evidence in the EFNS and Level B evidence in the AAN guidelines, concern exists over their long term use given their addiction potential, side effect profile, and waning effectiveness over time.
None of the recommendations incorporate cost into the decision, but this is also an important consideration for not only the patients, but also the health system. TCAs are the most affordable of the first line agents. Gabapentin and venlafaxine are cheaper than pregabalin and duloxetine, respectively.
Metabolic syndrome: implications for future treatments
Currently, glucose control and pain management are the backbones of treatment for diabetic neuropathy. However, glucose control is not the sole answer as patients with adequate glucose control continue to develop neuropathy or their neuropathy worsens over time. Furthermore, pain management is not a disease modifying therapy. Therefore, discovery of modifiable risk factors for neuropathy is essential, with metabolic syndrome (MetS) components representing one possibility. Over the last 10 years, there has been an increased interest in the possible role of MetS in the development of neuropathy. In 2001, Isomaa et al compared 85 subjects with MetS and type 2 diabetes to subjects without MetS controlled for age, gender, glycemic control, and duration of diabetes90. They found that subjects with MetS had a higher prevalence of neuropathy, but that in multiple logistic regression models, MetS and its components were not associated with neuropathy. Later, Costa et al and the Metascreen investigators used cross-sectional designs to independently show that MetS was associated with neuropathy in subjects with diabetes91, 92. In 2007, Cull et al utilized the UKPDS cohort of 5102 subjects with type 2 diabetes followed for 10.3 years to assess for the association of MetS with neuropathy by using four different definitions of MetS93. They found that MetS was associated with a combined macrovascular endpoint, but not with a combined microvascular endpoint. Recently in 2008, Smith et al compared subjects with idiopathic neuropathy and normoglycemia to those with IGT and discovered that each group had the same prevalence of the separate components of the MetS94. This result suggests that the other components of the MetS besides IGT may have a role in the development of neuropathy. However, these studies have almost all been carried out on subjects with diabetes, have used cross-sectional designs, and have utilized inconsistent definitions of neuropathy.
Complementing the studies investigating the role of the MetS on neuropathy, many groups evaluated the effect of the individual MetS components on neuropathy. In 1994, Straub et al conducted a cross-sectional study of 91 subjects with type 2 diabetes, and stratified them based on Body Mass Index (BMI)95. Those subjects with a BMI > 26.5 had a worse clinical neuropathy score than those with a lower BMI. However, this study did not account for any confounding factors to this association. In 2005, Tesfaye et al followed 1172 patients with type 1 diabetes for a median of 7.3 years and found that BMI and smoking were independent risk factors for the development of neuropathy96. They also found associations with hypertension and LDL in minimally adjusted models. In the same year, De Block and colleagues performed a cross-sectional study in 592 subjects with type 1 diabetes97. Their study revealed no association between BMI, lipid abnormalities, triglycerides, or hypertension and neuropathy. More recently in 2009, Van Acker et al investigated 1111 subjects with diabetes in a cross-sectional design6. They discovered that obesity, HDL, and triglyceride levels were all independently associated with neuropathy. Moreover, Wiggins et al revealed that diabetic subjects with progressive neuropathy had higher triglyceride levels compared to non-progressors.50 While most studies have shown an association between some MetS components and neuropathy, all of these studies have been performed in subjects with frank diabetes, most have used a cross-sectional design, and the definition of neuropathy has differed between studies. Given the conflicting results reported to date, further studies are needed to adequately define the role of MetS in the development and progression of neuropathy. There is also a need to better understand the underlying mechanisms by which MetS components cause neuropathy.
Pathophysiology of the Metabolic Syndrome on neuropathy
Our knowledge of how MetS components damage nerves is rapidly evolving. We have already outlined the involvement of dyslipidemia and IR and their contribution to neuropathy in patients with type 2 diabetes (see “Pathophysiology of type 1 and type 2 diabetic neuropathy”). Another central MetS component, visceral adiposity, may be particularly detrimental as it causes increased plasma FFAs and also induces a pro-inflammatory state by secretion of adipokines (also contributing to development of IR)98. Hypertension, another aspect of the MetS, may also be connected to neuropathy, though the link is less well-established. The renin-angiotensin system (RAS), which controls blood pressure, is upregulated in obesity, and may also contribute to development of type 2 diabetes (in part through promotion of IR and pro-inflammatory cytokine secretion from adipose tissue)99. Angiotensin-converting enzyme (ACE) inhibitors have been shown to improve diabetic neuropathy in animal studies100, 101, but the mechanism is unclear. Microvascular dysfunction in the nerve and decreased endoneurial perfusion are also thought to contribute to neuropathy102. While this may be regulated by metabolic factors, upregulation of RAS might also contribute102.
These mechanisms are likely to be linked at multiple levels. Indeed, in terms of the mechanisms linking MetS and type 2 diabetes to neuropathy, it may be more accurate to describe these pathways as a network in which hyperglycemia, IR, dyslipidemia, systemic inflammation and RAS activation all feed into a self-perpetuating cycle of oxidative stress, inflammatory signals and disruption of normal cellular function. Thus, even in the absence of overt diabetes, other aspects of the MetS may be sufficient to cause neuropathy. One of the major challenges for research scientists is to determine which aspects of this network of mechanisms can be blocked at which times to effectively limit/prevent progression of the neuropathy.
Conclusions and future directions
Diabetes can injure peripheral nerves in a variety of distributions. The most common pattern is DSP, which is characterized by numbness, tingling, pain, and/or weakness that affect the nerves in a “stocking and glove” pattern beginning in the distal extremities. DSP leads to substantial pain, morbidity, and impaired quality of life. Societal, personal, and healthcare costs associated with diabetic neuropathy are high. Unfortunately, few interventions are currently available for the remediation of non-painful symptoms, and glucose control is the only proven disease-modifying intervention for these patients. While pain is a common feature, it is often under-reported and undertreated. However, many effective therapies exist for DNP including medicines designed to treat seizures and depression. Evidence-based consensus guidelines have been created to guide the use of these pain interventions.
There are many areas of research that are yet to be fully explored in regards to diabetic neuropathy, which could lead to improved prevention and treatment of the condition. The magnitude of the effect of glucose control on neuropathy is much smaller in patients with type 2 diabetes as compared to patients with type 1 diabetes. Given this small effect size and that many patients with type 2 diabetes continue to develop neuropathy despite adequate glucose control, discovery of modifiable risk factors for neuropathy is essential. MetS components, including pre-diabetes, are potential risk factors for neuropathy, and future studies are needed to define whether they are causally related to neuropathy with direct implications for new treatments.
Acknowledgments
Funding is provided by the A. Alfred Taubman Medical Research Institute, the Program for Neurology Research and Discovery, and the Kathy Rayner Program. B.C. is supported by an American Diabetes Association Junior Faculty Award and H.C. is supported by the NIH (KO8 NS061039-01A2A). C.S is supported by a JDRF fellowship. E.F. is supported by NIH 1 DP3 DK094292 and NIH 1 R24 DK082841. We thank J. Boldt and G. Walker for excellent secretarial support during the preparation of the manuscript. B.C. had full access to all the data and had final responsibility for the decision to submit for publication.
Footnotes
Contributors
All authors contributed equally to the concept and design of the manuscript. B. C. had full access to all the data in the study. B. C., H. C., and C.S. analyzed the literature and wrote the manuscript. A. S. and E. F. provided critical review of the manuscript. All authors have approved the final manuscript.
Conflicts of Interest
There are no conflicts of interest to declare.
Contributor Information
Hsinlin Cheng, Email: chengt@med.umich.edu.
Catherine L. Stables, Email: cstables@med.umich.edu.
Andrea L. Smith, Email: anlsmith@med.umich.edu.
Eva L. Feldman, Email: efeldman@med.umich.edu.
References
- 1.Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology. 1991;41(8):1315–7. doi: 10.1212/wnl.41.8.1315. [DOI] [PubMed] [Google Scholar]
- 2.Savettieri G, Rocca WA, Salemi G, Meneghini F, Grigoletto F, Morgante L, et al. Prevalence of diabetic neuropathy with somatic symptoms: a door-to-door survey in two Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Neurology. 1993;43(6):1115–20. doi: 10.1212/wnl.43.6.1115. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 4.Johannsen L, Smith T, Havsager AM, Madsen C, Kjeldsen MJ, Dalsgaard NJ, et al. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J Clin Neuromuscul Dis. 2001;3(2):47–52. doi: 10.1097/00131402-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989;38(11):1456–61. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 6.Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–13. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010;31(9):1445–50. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- 8.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DJ, Malay DS, Hoffstad OJ, Leonard CE, MaCurdy T, de Nava KL, et al. Incidence of Diabetic Foot Ulcer and Lower Extremity Amputation Among Medicare Beneficiaries, 2006 to 2008: Data Points #2. 2011 [PubMed] [Google Scholar]
- 10.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8 (Suppl 2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 11.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–82. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 12.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47(2):123–8. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 13.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–4. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127(3):199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS) Diabetes Care. 2008;31(7):1360–6. doi: 10.2337/dc08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–91. doi: 10.1002/mus.10080. [DOI] [PubMed] [Google Scholar]
- 17.Thaisetthawatkul P, Dyck PJ. Treatment of diabetic and nondiabetic lumbosacral radiculoplexus neuropathy. Curr Treat Options Neurol. 2010;12(2):95–9. doi: 10.1007/s11940-010-0059-8. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67(4):534–41. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miralles-Garcia JM, de Pablos-Velasco P, Cabrerizo L, Perez M, Lopez-Gomez V. Prevalence of distal diabetic polyneuropathy using quantitative sensory methods in a population with diabetes of more than 10 years’ disease duration. Endocrinol Nutr. 2010;57(9):414–20. doi: 10.1016/j.endonu.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 20.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 21.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 22.Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, et al. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) J Diabetes Complications. 1999;13(5–6):307–13. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 23.Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J (Clin Res Ed) 1986;293(6556):1195–9. doi: 10.1136/bmj.293.6556.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 25.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR, Dornan TL, Mayon-White V, Howard-Williams J, Orde-Peckar C, Jenkins L, et al. Prevention of deterioration of renal and sensory-nerve function by more intensive management of insulin-dependent diabetic patients. A two-year randomised prospective study. Lancet. 1983;1(8318):204–8. doi: 10.1016/s0140-6736(83)92586-2. [DOI] [PubMed] [Google Scholar]
- 27.Hotta N, Kawamori R, Sano T, Kakuta H, Kamada T, Sakamoto N. Diabetic neuropathy: effects of intensified glycaemic control with multiple insulin injections. Diabet Med. 1993;10 (Suppl 2):91S–4S. doi: 10.1111/j.1464-5491.1993.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 28.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakobsen J, Christiansen JS, Kristoffersen I, Christensen CK, Hermansen K, Schmitz A, et al. Autonomic and somatosensory nerve function after 2 years of continuous subcutaneous insulin infusion in type I diabetes. Diabetes. 1988;37(4):452–5. doi: 10.2337/diab.37.4.452. [DOI] [PubMed] [Google Scholar]
- 30.Kawamori R, Kamada T. Determination of the glycemic threshold for the regression or prevention of diabetic microangiopathies, and the insulin injection regimen to establish strict glycemic control in NIDDM. Jpn J Med. 1991;30(6):618–21. doi: 10.2169/internalmedicine1962.30.618. [DOI] [PubMed] [Google Scholar]
- 31.Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes. 1985;34 (Suppl 3):74–9. doi: 10.2337/diab.34.3.s74. [DOI] [PubMed] [Google Scholar]
- 32.Linn T, Ortac K, Laube H, Federlin K. Intensive therapy in adult insulin-dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism. 1996;45(12):1508–13. doi: 10.1016/s0026-0495(96)90180-8. [DOI] [PubMed] [Google Scholar]
- 33.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 34.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329(5):304–9. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 35.Service FJ, Daube JR, O’Brien PC, Zimmerman BR, Swanson CJ, Brennan MD, et al. Effect of blood glucose control on peripheral nerve function in diabetic patients. Mayo Clin Proc. 1983;58(5):283–9. [PubMed] [Google Scholar]
- 36.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23 (Suppl 2):B21–9. [PubMed] [Google Scholar]
- 37.Tovi J, Svanborg E, Nilsson BY, Engfeldt P. Diabetic neuropathy in elderly Type 2 diabetic patients: effects of insulin treatment. Acta Neurol Scand. 1998;98(5):346–53. doi: 10.1111/j.1600-0404.1998.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 38.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14(4):257–67. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012 doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sima AA, Zhang W, Grunberger G. Type 1 diabetic neuropathy and C-peptide. Exp Diabesity Res. 2004;5(1):65–77. doi: 10.1080/15438600490424541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–83. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 42.Kim B, McLean LL, Philip SS, Feldman EL. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011;152(10):3638–47. doi: 10.1210/en.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–28. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 44.Obrosova IG. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal. 2005;7(11–12):1543–52. doi: 10.1089/ars.2005.7.1543. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14(10):953–61. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 46.Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, et al. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes. 2009;58(12):2893–903. doi: 10.2337/db09-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148(2):548–58. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 48.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52(11):2251–63. doi: 10.1007/s00125-009-1458-9. [DOI] [PubMed] [Google Scholar]
- 49.Clemens A, Siegel E, Gallwitz B. Global risk management in type 2 diabetes: blood glucose, blood pressure, and lipids--update on the background of the current guidelines. Exp Clin Endocrinol Diabetes. 2004;112(9):493–503. doi: 10.1055/s-2004-821306. [DOI] [PubMed] [Google Scholar]
- 50.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–40. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padilla A, Descorbeth M, Almeyda AL, Payne K, De Leon M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 2011;1370:64–79. doi: 10.1016/j.brainres.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCall KD, Holliday D, Dickerson E, Wallace B, Schwartz AL, Schwartz C, et al. Phenylmethimazole blocks palmitate-mediated induction of inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7 macrophages. J Endocrinol. 2010;207(3):343–53. doi: 10.1677/JOE-09-0370. [DOI] [PubMed] [Google Scholar]
- 53.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58(10):2376–85. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowicki M, Muller K, Serke H, Kosacka J, Vilser C, Ricken A, et al. Oxidized low-density lipoprotein (oxLDL)-induced cell death in dorsal root ganglion cell cultures depends not on the lectin-like oxLDL receptor-1 but on the toll-like receptor-4. J Neurosci Res. 2010;88(2):403–12. doi: 10.1002/jnr.22205. [DOI] [PubMed] [Google Scholar]
- 55.Jang ER, Lee CS. 7-ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-kappaB and Akt pathways. Neurochem Int. 2011;58(1):52–9. doi: 10.1016/j.neuint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience. 2006;139(2):429–49. doi: 10.1016/j.neuroscience.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 57.Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol. 2004;188(1):43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Ekberg K, Johansson BL. Effect of C-peptide on diabetic neuropathy in patients with type 1 diabetes. Exp Diabetes Res. 2008;2008:457912. doi: 10.1155/2008/457912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tesch GH. Role of macrophages in complications of type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34(10):1016–9. doi: 10.1111/j.1440-1681.2007.04729.x. [DOI] [PubMed] [Google Scholar]
- 60.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001;24(9):1229–31. doi: 10.1002/mus.1137. [DOI] [PubMed] [Google Scholar]
- 61.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24(8):1448–53. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 62.Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131(4):633–43. doi: 10.1093/oxfordjournals.aje.a115547. [DOI] [PubMed] [Google Scholar]
- 63.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 64.Hughes RA, Umapathi T, Gray IA, Gregson NA, Noori M, Pannala AS, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain. 2004;127(Pt 8):1723–30. doi: 10.1093/brain/awh192. [DOI] [PubMed] [Google Scholar]
- 65.Dyck PJCV, Overland CJ, Davies JL, Zafft AJ, Pach JM, Dyck PJB, Klein CJ, Rizza RA, Weigland SD, Melton LJ, Carter R, Klein R, Litchy WJ. Does Impaired Glycemia Cause Polyneuropathy and Other Diabetic Complications? Journal of the Peripheral Nervous Society. 2011;16(Supplement s3) [Google Scholar]
- 66.Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care. 2010;33(11):2355–9. doi: 10.2337/dc09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153–69. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 68.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 69.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–65. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorson KC, Schott C, Herman R, Ropper AH, Rand WM. Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. J Neurol Neurosurg Psychiatry. 1999;66(2):251–2. doi: 10.1136/jnnp.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Wilks K, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128(1–2):169–79. doi: 10.1016/j.pain.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 72.Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology. 2001;57(3):505–9. doi: 10.1212/wnl.57.3.505. [DOI] [PubMed] [Google Scholar]
- 73.Kochar DK, Jain N, Agarwal RP, Srivastava T, Agarwal P, Gupta S. Sodium valproate in the management of painful neuropathy in type 2 diabetes - a randomized placebo controlled study. Acta Neurol Scand. 2002;106(5):248–52. doi: 10.1034/j.1600-0404.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- 74.Kochar DK, Rawat N, Agrawal RP, Vyas A, Beniwal R, Kochar SK, et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM. 2004;97(1):33–8. doi: 10.1093/qjmed/hch007. [DOI] [PubMed] [Google Scholar]
- 75.Otto M, Bach FW, Jensen TS, Sindrup SH. Valproic acid has no effect on pain in polyneuropathy: a randomized, controlled trial. Neurology. 2004;62(2):285–8. doi: 10.1212/wnl.62.2.285. [DOI] [PubMed] [Google Scholar]
- 76.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2005;(3):CD005454. doi: 10.1002/14651858.CD005454. [DOI] [PubMed] [Google Scholar]
- 77.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399–409. doi: 10.1111/j.1742-7843.2005.pto_96696601.x. [DOI] [PubMed] [Google Scholar]
- 78.Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37(4):589–96. doi: 10.1212/wnl.37.4.589. [DOI] [PubMed] [Google Scholar]
- 79.Hanna FW, Peters JR, Harlow J, Jones PW. Gestational diabetes screening and glycaemic management; national survey on behalf of the Association of British Clinical Diabetologists. QJM. 2008;101(10):777–84. doi: 10.1093/qjmed/hcn069. [DOI] [PubMed] [Google Scholar]
- 80.Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T, Thorell LH. A comparison a amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clin J Pain. 1997;13(4):313–23. doi: 10.1097/00002508-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 82.Tandan R, Lewis GA, Krusinski PB, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care. 1992;15(1):8–14. doi: 10.2337/diacare.15.1.8. [DOI] [PubMed] [Google Scholar]
- 83.The Capsaicin Study G. Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Diabetes Care. 1992;15:159–65. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- 84.Yuen KC, Baker NR, Rayman G. Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: a double-blind placebo-controlled cross-over study. Diabetes Care. 2002;25(10):1699–703. doi: 10.2337/diacare.25.10.1699. [DOI] [PubMed] [Google Scholar]
- 85.Agrawal RP, Choudhary R, Sharma P, Sharma S, Beniwal R, Kaswan K, et al. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pract. 2007;77(2):161–7. doi: 10.1016/j.diabres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146(3):245–52. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 87.Jensen TS, Backonja MM, Hernandez Jimenez S, Tesfaye S, Valensi P, Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res. 2006;3(2):108–19. doi: 10.3132/dvdr.2006.013. [DOI] [PubMed] [Google Scholar]
- 88.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 90.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44(9):1148–54. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 91.Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care. 2006;29(12):2701–7. doi: 10.2337/dc06-0942. [DOI] [PubMed] [Google Scholar]
- 92.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med. 2004;21(3):252–5. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 93.Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation. 2007;116(19):2119–26. doi: 10.1161/CIRCULATIONAHA.107.733428. [DOI] [PubMed] [Google Scholar]
- 94.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008;273(1–2):25–8. doi: 10.1016/j.jns.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Straub RH, Elbracht R, Kramer BK, Roth M, Palitzsch KD, Scholmerich J. Influence of digoxin-like immunoreactive factor on late complications in patients with diabetes mellitus. Eur J Clin Invest. 1994;24(7):482–7. doi: 10.1111/j.1365-2362.1994.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 96.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 97.De Block CE, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005;28(7):1649–55. doi: 10.2337/diacare.28.7.1649. [DOI] [PubMed] [Google Scholar]
- 98.Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10(2):156–64. doi: 10.1007/s11906-008-0029-7. [DOI] [PubMed] [Google Scholar]
- 99.Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13(2):136–49. doi: 10.1111/j.1467-789X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 100.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Effect of inhibition of angiotensin converting enzyme and/or neutral endopeptidase on vascular and neural complications in high fat fed/low dose streptozotocin-diabetic rats. Eur J Pharmacol. 2012;677(1–3):180–7. doi: 10.1016/j.ejphar.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Dake B, Yorek MA. Role of the effect of inhibition of neutral endopeptidase on vascular and neural complications in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2011;650(2–3):556–62. doi: 10.1016/j.ejphar.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973–88. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 103.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–38. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–10. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 105.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–60. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 106.McGovern MP, Williams DJ, Hannaford PC, Taylor MW, Lefevre KE, Boroujerdi MA, et al. Introduction of a new incentive and target-based contract for family physicians in the UK: good for older patients with diabetes but less good for women? Diabet Med. 2008;25(9):1083–9. doi: 10.1111/j.1464-5491.2008.02544.x. [DOI] [PubMed] [Google Scholar]
- 107.Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. doi: 10.1186/1471-2377-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12(2):203–13. doi: 10.1016/j.ejpain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831–6. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 110.Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabet Med. 2007;24(4):377–83. doi: 10.1111/j.1464-5491.2007.02093.x. [DOI] [PubMed] [Google Scholar]
- 111.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159(16):1931–7. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 112.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 113.Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697–706. doi: 10.1016/j.pain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 114.Kadiroglu AK, Sit D, Kayabasi H, Tuzcu AK, Tasdemir N, Yilmaz ME. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. J Diabetes Complications. 2008;22(4):241–5. doi: 10.1016/j.jdiacomp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis. 2001;3(2):53–62. doi: 10.1097/00131402-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 116.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 117.Raskin J, Pritchett YL, Wang F, D’Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346–56. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 118.Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–20. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 119.Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology. 2003;60(6):927–34. doi: 10.1212/01.wnl.0000057720.36503.2c. [DOI] [PubMed] [Google Scholar]
- 120.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12(6):804–13. doi: 10.1016/j.ejpain.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50(6):1842–6. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 122.Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain. 1999;83(1):85–90. doi: 10.1016/s0304-3959(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 123.Freeman R, Raskin P, Hewitt DJ, Vorsanger GJ, Jordan DM, Xiang J, et al. Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Curr Med Res Opin. 2007;23(1):147–61. doi: 10.1185/030079906X162674. [DOI] [PubMed] [Google Scholar]
- 124.Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology. 2002;96(5):1053–61. doi: 10.1097/00000542-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 125.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48(5):1212–8. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 126.Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Capsaicin Study Group. Diabetes Care. 1992;15(2):159–65. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- 127.Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473–8. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- 128.Ertas M, Sagduyu A, Arac N, Uludag B, Ertekin C. Use of levodopa to relieve pain from painful symmetrical diabetic polyneuropathy. Pain. 1998;75(2–3):257–9. doi: 10.1016/s0304-3959(98)00003-7. [DOI] [PubMed] [Google Scholar]