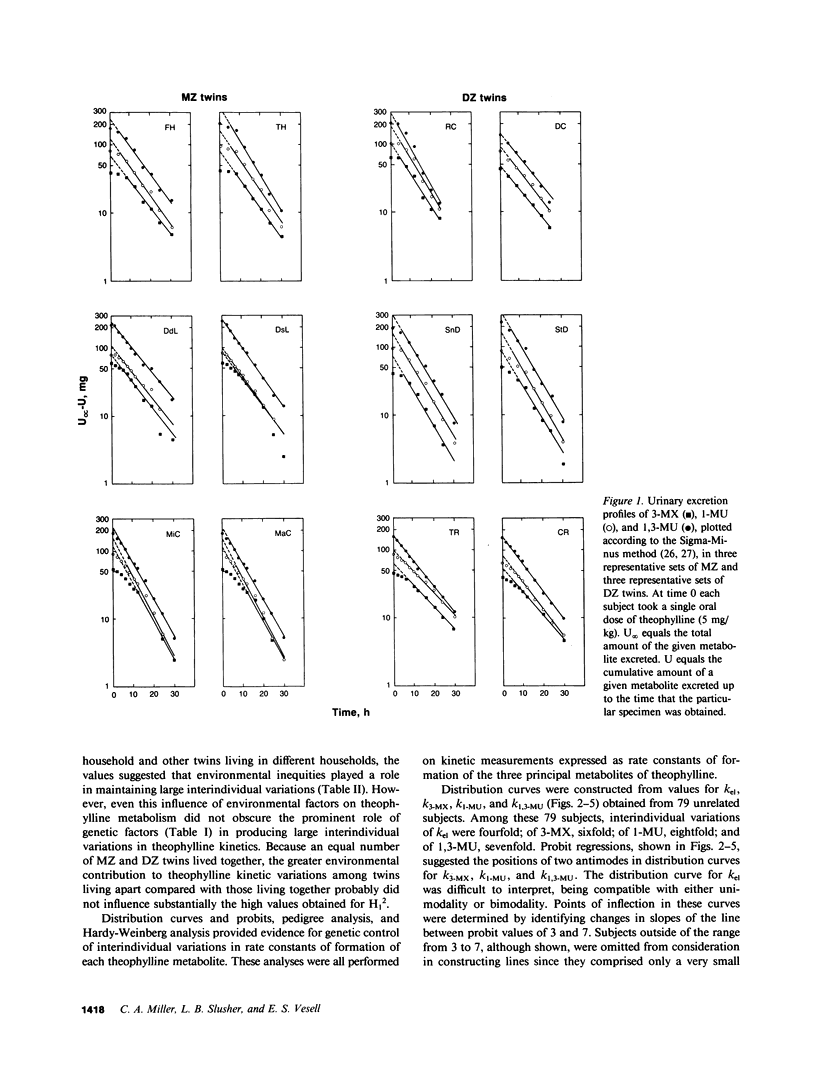
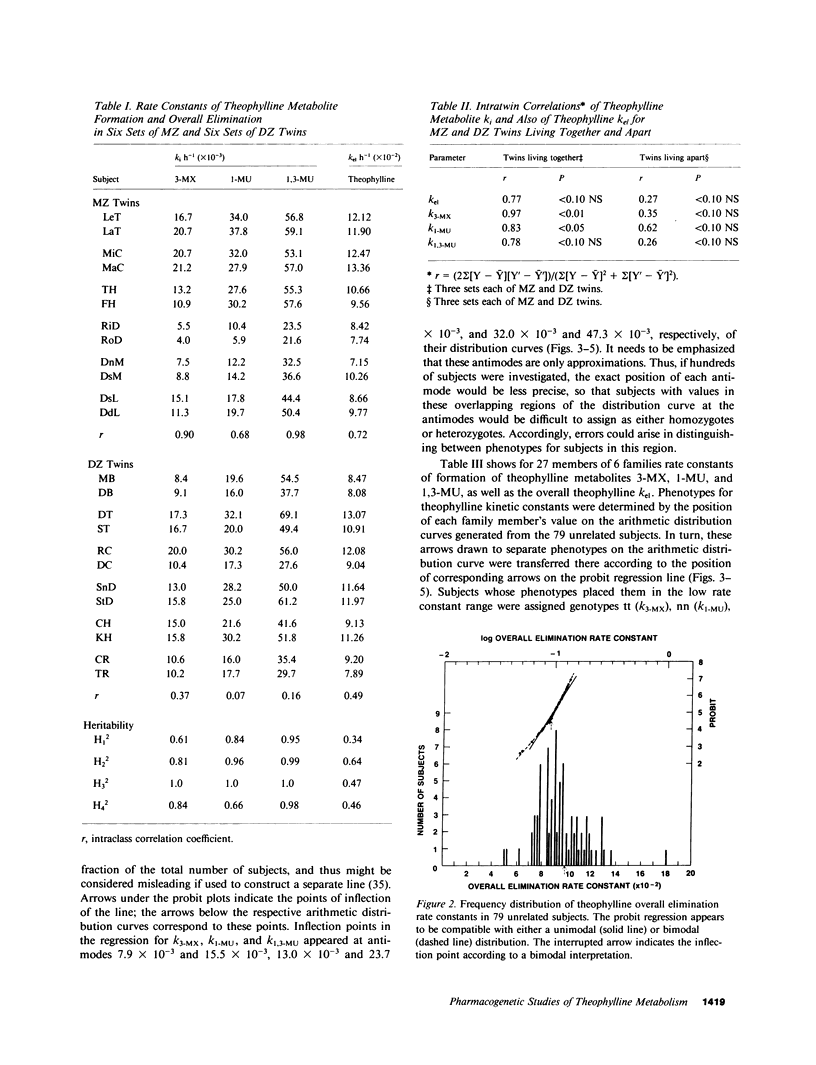
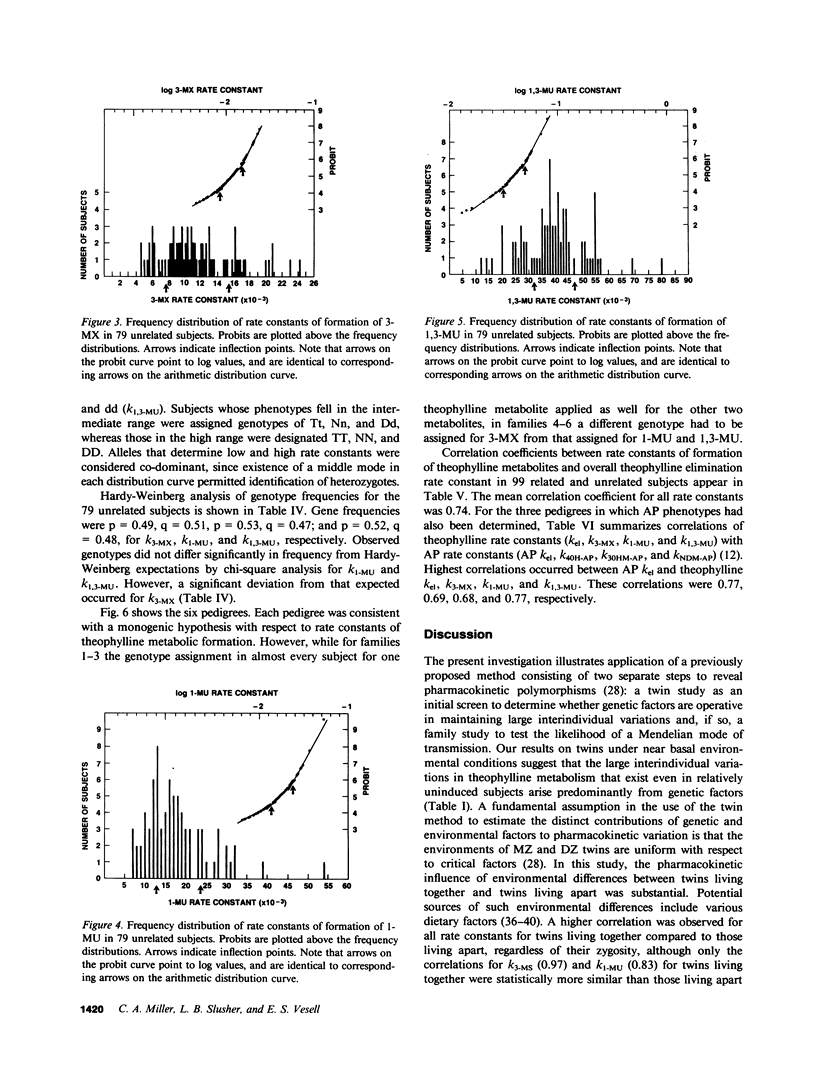
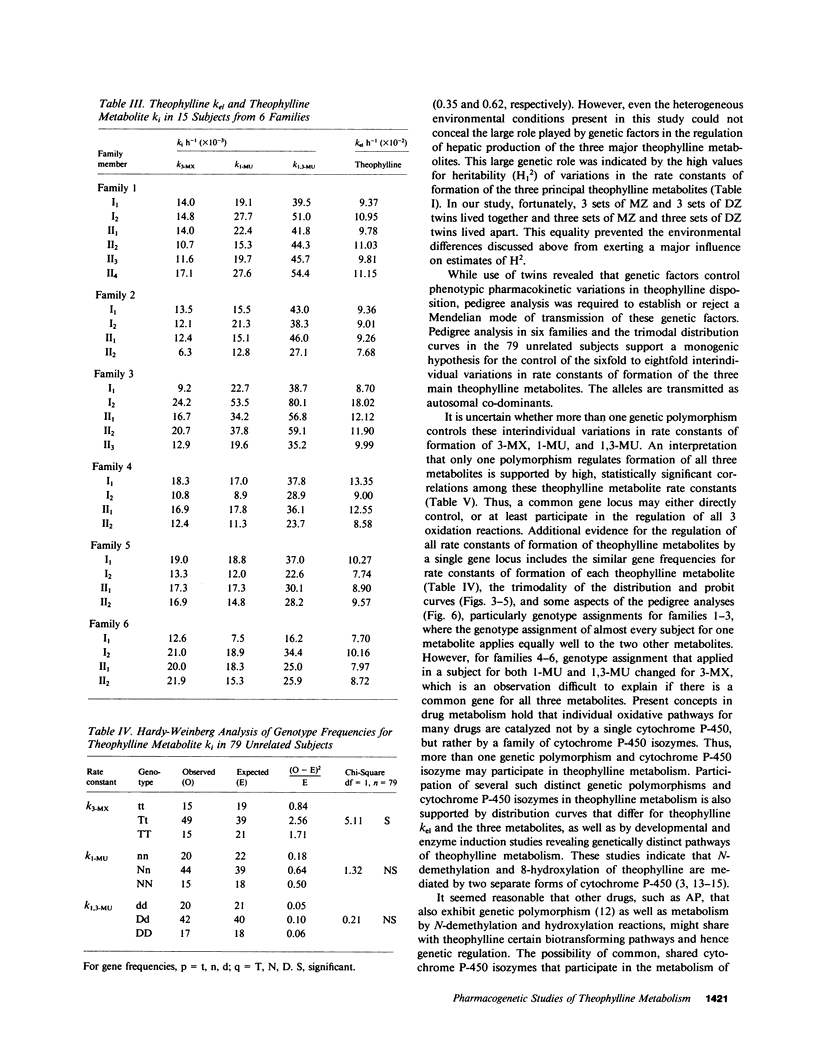
Abstract
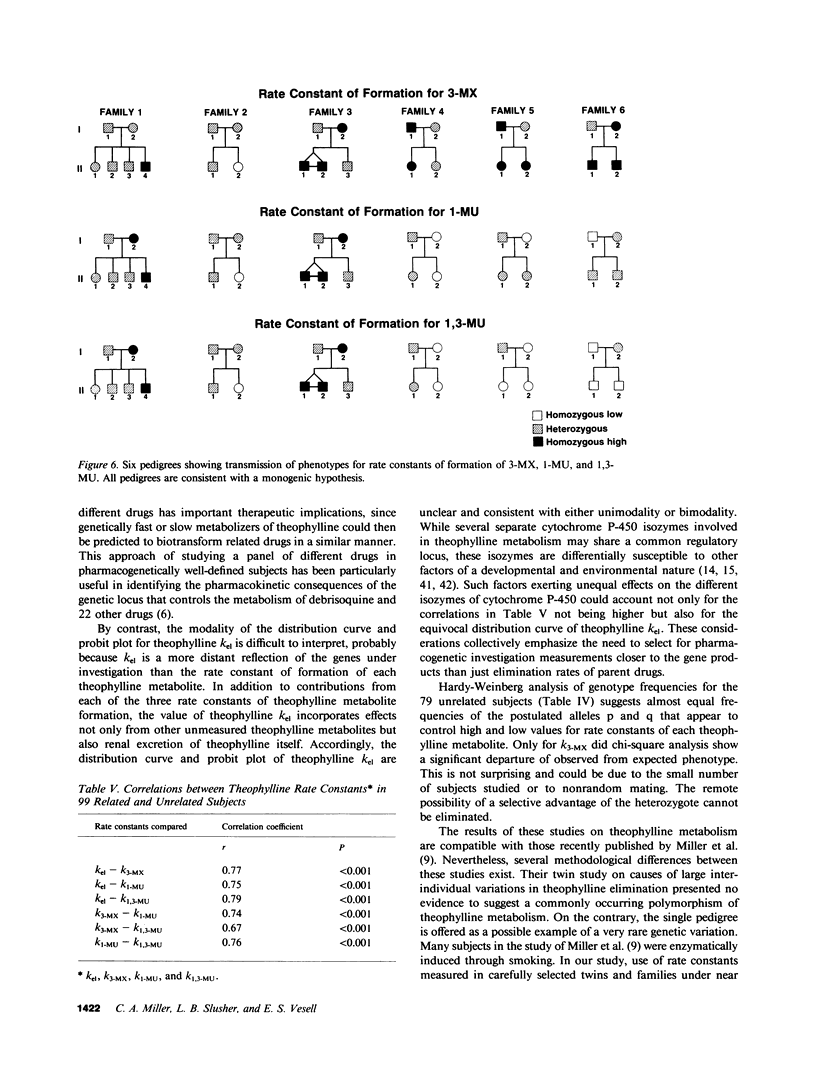
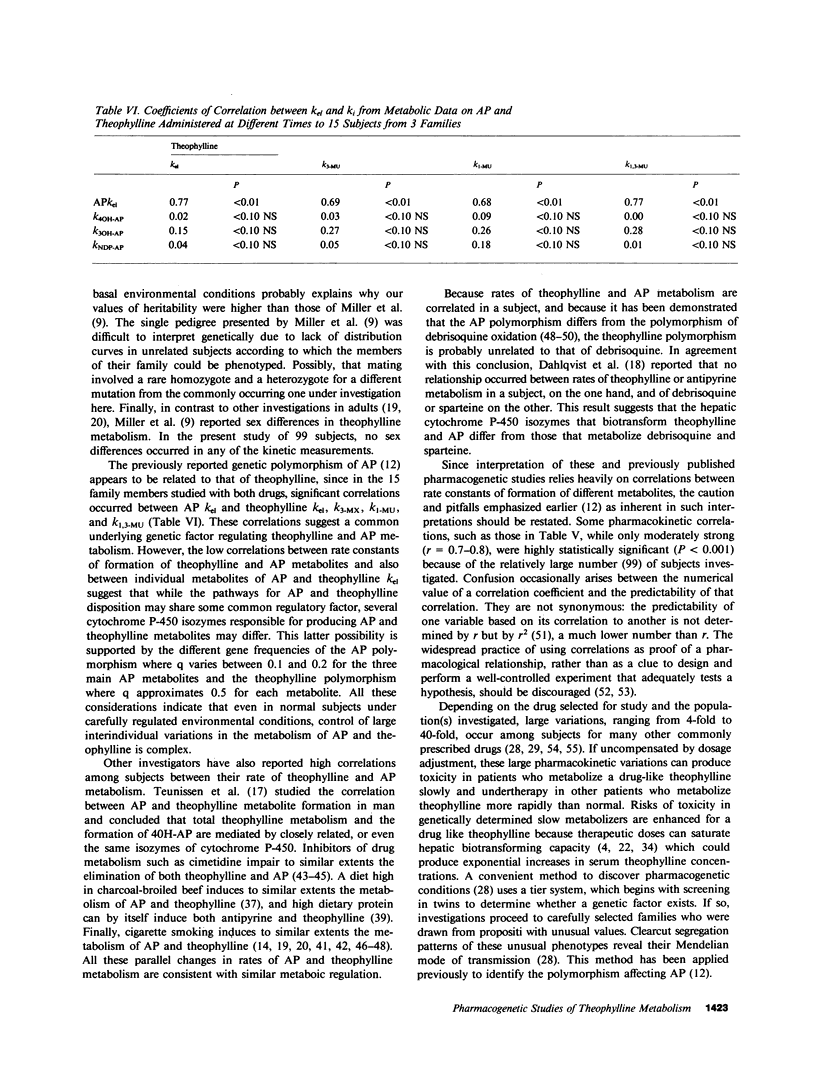
To determine whether genetic mechanisms control large interindividual variations in theophylline elimination in normal uninduced human subjects, and, if so, to test the possibility that these genetic factors are transmitted as a simple Mendelian trait, theophylline was administered to 79 unrelated adults, six sets of monozygotic twins, six sets of dizygotic twins, and six two-generation families. Thereafter, in urine collected from each subject at regular intervals for 48 h, concentrations of theophylline and its three principal metabolites were measured and rate constants of formation of these metabolites calculated. The twin study, designed to determine the relative contributions of genetic and environmental factors to large interindividual variation in theophylline elimination, revealed predominantly genetic control. Values for this genetic component, designated heritability (H1(2)), of interindividual variation in rate constants of metabolite formation were 0.61, 0.84, and 0.95 for 3-methylxanthine, 1-methyluric acid, and 1,3-dimethyluric acid, respectively. H1(2) for the overall theophylline elimination rate constant (kel) was lower (0.34). In the 79 unrelated adults, each distribution curve for rate constants of formation of each theophylline metabolite appeared to be trimodal. By contrast, the distribution curve for the overall theophylline elimination rate constant appeared to be either unimodal or bimodal. The extent of interindividual variation was fourfold for theophylline kel and 6-8-fold for the three principal metabolites. High correlations among the three rate constants in individual subjects suggested their regulation by a single shared factor. In six families carefully selected to be under near basal environmental conditions so that hepatic theophylline metabolism of each family member would be neither markedly induced nor inhibited, phenotypes for theophylline metabolite rate constants were assigned. This assignment of phenotype was made by the position of each family member's rate constant on the three distribution curves that were generated from the 79 unrelated subjects. In each family, pedigree analysis of the three phenotypes for each rate constant was consistent with their control by two alleles at a single genetic locus and with autosomal codominant transmission. Frequencies of the two alleles at each genetic locus controlling rate constants of formation of theophylline metabolites were similar (p = 0.49, 0.53, and 0.52). In the three families studied with antipyrine (AP) as well as with theophylline, AP k(el) correlated (r approximately 0.7) with each rate constant of theophylline metabolite formation, as well as with theophylline k(el). While these results are compatible with a common regulatory element in the AP and theophylline polymorphisms, other evidence suggests more than a single genetic polymorphism. This additional evidence includes different gene frequencies for the AP (p approximately 0.1) and theophylline (p approximately 0.5) polymorphisms, different genotype assignments in several families for some theophylline metabolites, different distribution curves for theophylline k(el) from those for the three theophylline metabolites in 79 unrelated subjects, and finally low correlations between AP metabolite rate constants and theophylline metabolite rate constants in the three families receiving both drugs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Conney A. H., Kappas A. Nutritional influences on chemical biotransformations in humans. Nutr Rev. 1982 Jun;40(6):161–171. doi: 10.1111/j.1753-4887.1982.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Atlas S. A., Vesell E. S., Nebert D. W. Genetic control of interindividual variations in the inducibility of aryl hydrocarbon hydroxylase in cultured human lymphocytes. Cancer Res. 1976 Dec;36(12):4619–4630. [PubMed] [Google Scholar]
- Bertilsson L., Eichelbaum M., Mellström B., Säwe J., Schulz H. U., Sjöqvist F. Nortriptyline and antipyrine clearance in relation to debrisoquine hydroxylation in man. Life Sci. 1980 Nov 3;27(18):1673–1677. doi: 10.1016/0024-3205(80)90642-6. [DOI] [PubMed] [Google Scholar]
- Bonati M., Latini R., Marra G., Assael B. M., Parini R. Theophylline metabolism during the first month of life and development. Pediatr Res. 1981 Apr;15(4 Pt 1):304–308. doi: 10.1203/00006450-198104000-00003. [DOI] [PubMed] [Google Scholar]
- Dahlqvist R., Bertilsson L., Birkett D. J., Eichelbaum M., Säwe J., Sjöqvist F. Theophylline metabolism in relation to antipyrine, debrisoquine, and sparteine metabolism. Clin Pharmacol Ther. 1984 Jun;35(6):815–821. doi: 10.1038/clpt.1984.118. [DOI] [PubMed] [Google Scholar]
- Dvorchik B. H., Vesell E. S. Pharmacokinetic interpretation of data gathered during therapeutic drug monitoring. Clin Chem. 1976 Jun;22(6):868–878. [PubMed] [Google Scholar]
- Dvorchik B. H., Vessell E. S. Significance of error associated with use of the one-compartment formula to calculate clearance of thirty-eight drugs. Clin Pharmacol Ther. 1978 Jun;23(6):617–623. doi: 10.1002/cpt1978236617. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Bertilsson L., Säwe J. Antipyrine metabolism in relation to polymorphic oxidations of sparteine and debrisoquine. Br J Clin Pharmacol. 1983 Mar;15(3):317–321. doi: 10.1111/j.1365-2125.1983.tb01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E. F., Koysooko R., Levy G. Pharmacokinetics of theophylline in children with asthma. Pediatrics. 1976 Oct;58(4):542–547. [PubMed] [Google Scholar]
- Feldman C. H., Hutchinson V. E., Pippenger C. E., Blumenfeld T. A., Feldman B. R., Davis W. J. Effect of dietary protein and carbohydrate on theophylline metabolism in children. Pediatrics. 1980 Dec;66(6):956–962. [PubMed] [Google Scholar]
- Gardner M. J., Tornatore K. M., Jusko W. J., Kanarkowski R. Effects of tobacco smoking and oral contraceptive use on theophylline disposition. Br J Clin Pharmacol. 1983 Sep;16(3):271–280. doi: 10.1111/j.1365-2125.1983.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginchansky E., Weinberger M. Relationship of theophylline clearance to oral dosage in children with chronic asthma. J Pediatr. 1977 Oct;91(4):655–660. doi: 10.1016/s0022-3476(77)80527-1. [DOI] [PubMed] [Google Scholar]
- Grygiel J. J., Birkett D. J. Cigarette smoking and theophylline clearance and metabolism. Clin Pharmacol Ther. 1981 Oct;30(4):491–496. doi: 10.1038/clpt.1981.193. [DOI] [PubMed] [Google Scholar]
- Grygiel J. J., Birkett D. J. Effect of age on patterns of theophylline metabolism. Clin Pharmacol Ther. 1980 Oct;28(4):456–462. doi: 10.1038/clpt.1980.188. [DOI] [PubMed] [Google Scholar]
- Gundert-Remy U., Hildebrandt R., Hengen N., Weber E. Non-linear elimination processes of theophylline. Eur J Clin Pharmacol. 1983;24(1):71–78. doi: 10.1007/BF00613930. [DOI] [PubMed] [Google Scholar]
- Hendeles L., Weinberger M. Theophylline. A "state of the art" review. Pharmacotherapy. 1983 Jan-Feb;3(1):2–44. doi: 10.1002/j.1875-9114.1983.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Idle J. R., Smith R. L. Polymorphisms of oxidation at carbon centers of drugs and their clinical significance. Drug Metab Rev. 1979;9(2):301–317. doi: 10.3109/03602537908993896. [DOI] [PubMed] [Google Scholar]
- Jenne J. W., Wyze M. S., Rood F. S., MacDonald F. M. Pharmacokinetics of theophylline. Application to adjustment of the clinical dose of aminophylline. Clin Pharmacol Ther. 1972 May-Jun;13(3):349–360. doi: 10.1002/cpt1972133349. [DOI] [PubMed] [Google Scholar]
- Jusko W. J., Gardner M. J., Mangione A., Schentag J. J., Koup J. R., Vance J. W. Factors affecting theophylline clearances: age, tobacco, marijuana, cirrhosis, congestive heart failure, obesity, oral contraceptives, benzodiazepines, barbiturates, and ethanol. J Pharm Sci. 1979 Nov;68(11):1358–1366. doi: 10.1002/jps.2600681106. [DOI] [PubMed] [Google Scholar]
- Kappas A., Alvares A. P., Anderson K. E., Pantuck E. J., Pantuck C. B., Chang R., Conney A. H. Effect of charcoal-broiled beef on antipyrine and theophylline metabolism. Clin Pharmacol Ther. 1978 Apr;23(4):445–450. doi: 10.1002/cpt1978234445. [DOI] [PubMed] [Google Scholar]
- Kappas A., Anderson K. E., Conney A. H., Alvares A. P. Influence of dietary protein and carbohydrate on antipyrine and theophylline metabolism in man. Clin Pharmacol Ther. 1976 Dec;20(6):643–653. doi: 10.1002/cpt1976206643. [DOI] [PubMed] [Google Scholar]
- Lalonde R. L., Koob R. A., McLean W. M., Balsys A. J. The effects of cimetidine on theophylline pharmacokinetics at steady state. Chest. 1983 Feb;83(2):221–224. doi: 10.1378/chest.83.2.221. [DOI] [PubMed] [Google Scholar]
- Levy G., Koysooko R. Renal clearance of theophylline in man. J Clin Pharmacol. 1976 Jul;16(7):329–332. doi: 10.1002/j.1552-4604.1976.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Miech R. P. Theophylline metabolism by the rat liver microsomal system. J Pharmacol Exp Ther. 1976 Jan;196(1):213–225. [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Miller M., Opheim K. E., Raisys V. A., Motulsky A. G. Theophylline metabolism: variation and genetics. Clin Pharmacol Ther. 1984 Feb;35(2):170–182. doi: 10.1038/clpt.1984.23. [DOI] [PubMed] [Google Scholar]
- Monks T. J., Caldwell J., Smith R. L. Influence of methylxanthine-containing foods on theophylline metabolism and kinetics. Clin Pharmacol Ther. 1979 Oct;26(4):513–524. doi: 10.1002/cpt1979264513. [DOI] [PubMed] [Google Scholar]
- Muir K. T., Jonkman J. H., Tang D. S., Kunitani M., Riegelman S. Simultaneous determinations by theophylline and its major metabolites in urine by reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr. 1980 Nov 14;221(1):85–95. doi: 10.1016/s0378-4347(00)81010-5. [DOI] [PubMed] [Google Scholar]
- Ogilvie R. I. Clinical pharmacokinetics of theophylline. Clin Pharmacokinet. 1978 Jul-Aug;3(4):267–293. doi: 10.2165/00003088-197803040-00002. [DOI] [PubMed] [Google Scholar]
- Pantuck E. J., Pantuck C. B., Garland W. A., Min B. H., Wattenberg L. W., Anderson K. E., Kappas A., Conney A. H. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979 Jan;25(1):88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- Penno M. B., Dvorchik B. H., Vesell E. S. Genetic variation in rates of antipyrine metabolite formation: a study in uninduced twins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5193–5196. doi: 10.1073/pnas.78.8.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno M. B., Vesell E. S. Monogenic control of variations in antipyrine metabolite formation. New polymorphism of hepatic drug oxidation. J Clin Invest. 1983 Jun;71(6):1698–1709. doi: 10.1172/JCI110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. R., Thiercelin J. F., Vozeh S., Sansom L., Riegelman S. The influence of cigarette smoking and sex on theophylline disposition. Am Rev Respir Dis. 1977 Jul;116(1):17–23. doi: 10.1164/arrd.1977.116.1.17. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Muir K., Upton R. Factors affecting the pharmacokinetics of theophylline. Eur J Respir Dis Suppl. 1980;109:67–82. [PubMed] [Google Scholar]
- Roberts R. K., Grice J., Wood L., Petroff V., McGuffie C. Cimetidine impairs the elimination of theophylline and antipyrine. Gastroenterology. 1981 Jul;81(1):19–21. [PubMed] [Google Scholar]
- Rovei V., Chanoine F., Strolin Benedetti M. Pharmacokinetics of theophylline: a dose-range study. Br J Clin Pharmacol. 1982 Dec;14(6):769–778. doi: 10.1111/j.1365-2125.1982.tb02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher L. B., Vesell E. S. Cimetidine-induced reduction in gastrointestinal absorption of antipyrine and rate constants for formation of its metabolites. Clin Pharmacol Ther. 1984 May;35(5):568–575. doi: 10.1038/clpt.1984.79. [DOI] [PubMed] [Google Scholar]
- Tang-Liu D. D., Williams R. L., Riegelman S. Nonlinear theophylline elimination. Clin Pharmacol Ther. 1982 Mar;31(3):358–369. doi: 10.1038/clpt.1982.46. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., King K. C., Takieddine F. N. Theophylline metabolism in premature infants. Clin Pharmacol Ther. 1981 May;29(5):594–600. doi: 10.1038/clpt.1981.83. [DOI] [PubMed] [Google Scholar]
- Upton R. A., Sansom L., Guentert T. W., Powell J. R., Thiercelin J. F., Shah V. P., Coates P. E., Riegelman S. Evaluation of the absorption from 15 commercial theophylline products indicating deficiencies in currently applied bioavailability criteria. J Pharmacokinet Biopharm. 1980 Jun;8(3):229–242. doi: 10.1007/BF01059644. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Penno M. B. Assessment of methods to identify sources of interindividual pharmacokinetic variations. Clin Pharmacokinet. 1983 Sep-Oct;8(5):378–409. doi: 10.2165/00003088-198308050-00002. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979 Sep;26(3):275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. Twin studies in pharmacogenetics. Hum Genet Suppl. 1978;(1):19–30. doi: 10.1007/978-3-642-67179-1_4. [DOI] [PubMed] [Google Scholar]
- Vestal R. E., Norris A. H., Tobin J. D., Cohen B. H., Shock N. W., Andres R. Antipyrine metabolism in man: influence of age, alcohol, caffeine, and smoking. Clin Pharmacol Ther. 1975 Oct;18(4):425–432. doi: 10.1002/cpt1975184425. [DOI] [PubMed] [Google Scholar]
- Weinberger M., Ginchansky E. Dose-dependent kinetics of theophylline disposition in asthmatic children. J Pediatr. 1977 Nov;91(5):820–824. doi: 10.1016/s0022-3476(77)81051-2. [DOI] [PubMed] [Google Scholar]



