Abstract
Background
The incidence and determinants of hepatic decompensation have been incompletely examined among HIV/hepatitis C virus (HCV)-coinfected patients in the antiretroviral therapy (ART) era, and few studies have compared rates of outcomes to those of patients with chronic HCV alone.
Objectives
To compare the incidence of hepatic decompensation between antiretroviral-treated HIV/HCV-coinfected and HCV-monoinfected patients, and evaluate factors associated with decompensation among coinfected patients on ART.
Design
Retrospective cohort study.
Setting
Veterans Health Administration.
Patients
4,280 HIV/HCV-coinfected patients who initiated ART and 6,079 HCV-monoinfected patients receiving care between 1997 and 2010. All patients had detectable HCV RNA and were HCV treatment-naïve.
Measurements
Incident hepatic decompensation, determined by diagnoses of ascites, spontaneous bacterial peritonitis, or esophageal variceal hemorrhage.
Results
The incidence of hepatic decompensation was greater among coinfected than monoinfected patients (at 10 years: 7.4% versus 4.8%; p<0.001). Compared to HCV-monoinfected patients, antiretroviral-treated HIV/HCV-coinfected patients had a higher rate of hepatic decompensation (hazard ratio [HR] accounting for competing risks, 1.56 [95% confidence interval (CI), 1.31–1.86]). Coinfected patients who maintained HIV RNA levels <1,000 copies/mL still had higher rates of decompensation than HCV-monoinfected patients (HR, 1.44 [95% CI, 1.05–1.99]). Baseline advanced hepatic fibrosis (FIB-4 >3.25; HR, 5.45 [95% CI, 3.79–7.84]), baseline hemoglobin <10 g/dL (HR, 2.24 [CI, 1.20–4.20]), diabetes mellitus (HR, 1.88[95% CI, 1.38–2.56]), and non-black race (HR, 2.12 [95% CI, 1.65–2.72]) were each associated with higher rates of decompensation among coinfected patients on ART.
Limitations
Observational study of predominantly male patients.
Conclusions
Despite ART, HIV/HCV-coinfected patients had higher rates of hepatic decompensation than HCV-monoinfected individuals. Rates of decompensation were higher for coinfected patients with advanced liver fibrosis, severe anemia, diabetes, and non-black race.
Keywords: hepatic decompensation, end-stage liver disease, HIV/HCV coinfection, HIV, hepatitis C
INTRODUCTION
Coinfection with chronic hepatitis C virus (HCV) occurs in 10–30% of patients with human immunodeficiency virus (HIV) infection (1–4). The course of chronic HCV is accelerated in HIV/HCV-coinfected patients, with more rapid progression of liver fibrosis compared to HCV-monoinfected patients (5–7). Consequently, HCV-related liver complications, particularly hepatic decompensation (defined by the presence of ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy (8)), have emerged as important causes of morbidity in coinfected patients (9, 10).
Despite the importance of HCV-related end-stage liver disease, few longitudinal studies have evaluated the incidence and determinants of hepatic decompensation among HIV/HCV-coinfected persons during the antiretroviral therapy (ART) era. Previous studies suggest that ART slows progression of HCV-associated liver fibrosis, possibly by reducing HIV-related inflammation and immune dysfunction and inhibiting the ability of HIV to directly infect hepatocytes (10–13). However, it remains unclear if rates of hepatic decompensation and other severe liver events (e.g., hepatocellular carcinoma [HCC], liver-related death) in coinfected patients on ART are similar to those of HCV-monoinfected patients. Further, the determinants of hepatic decompensation among coinfected patients on ART remain unknown. Determination of these factors could help define the mechanisms of decompensation in coinfected patients and suggest interventions to reduce the risk of end-stage liver disease in this population.
We first compared the incidence of hepatic decompensation between antiretroviral-treated HIV/HCV-coinfected and HCV-monoinfected patients. We hypothesized that rates of decompensation would remain higher in coinfected patients despite ART. We then evaluated host and viral factors associated with decompensation among coinfected patients.
METHODS
Study Design and Data Source
We conducted a retrospective cohort study among antiretroviral-treated HIV/HCV-coinfected and HCV-monoinfected patients in the Veterans Aging Cohort Study Virtual Cohort (VACS-VC) between January 1, 1997 and September 30, 2010 (14). The VACS-VC consists of electronic medical record data from HIV-infected patients receiving care at Veterans Affairs (VA) medical facilities across the US. Each HIV-infected patient is matched on age, sex, race/ethnicity, and site to two HIV-uninfected patients. Data include hospital and outpatient diagnoses (recorded using International Classification of Diseases, Ninth Revision [ICD-9] codes), procedures (recorded using Current Procedural Terminology [CPT] codes), laboratory results, and pharmacy data. Clinically confirmed cancer diagnoses are available from the VA Central Cancer Registry. Deaths are identified from the VA Vital Status file, which determines mortality using the Social Security Death Master File, Medicare Vital Status Files, and VA Beneficiary Identification and Records Locator Subsystem. For patients who died, principal cause of death can be determined by linkage with the National Death Index (15). Additionally, U.S. Medicare and Medicaid claims data are available for veterans also enrolled in these programs and have been merged with VACS-VC data.
Study Patients
Coinfected patients were included if they had: 1) detectable HCV RNA, 2) newly initiated ART (defined as use of at least three antiretrovirals from two different classes (16) or ≥3 nucleoside analogues [a previously accepted ART regimen (17)]) within the VA system, 3) HIV RNA >500 copies/mL within 180 days prior to initiating ART (to identify new ART initiators (18)), and 4) at least 12 months of observation in the VACS-VC after starting ART. Monoinfected patients had: 1) detectable HCV RNA, 2) no recorded HIV ICD-9 diagnosis or antiretroviral prescriptions, and 3) at least 12 months of observation in the VACS-VC. Patients were excluded if during the baseline period (defined below) they had: 1) hepatic decompensation, 2) HCC, 3) liver transplantation, or 4) received interferon-based HCV therapy (since treatment reduces the risk of hepatic decompensation (19, 20)).
Main Study Outcomes
The primary outcome was incident hepatic decompensation, which was defined by one hospital discharge ICD-9 diagnosis or two or more outpatient ICD-9 diagnoses of ascites, spontaneous bacterial peritonitis, or esophageal variceal hemorrhage in the VACS-VC (Appendix A). A prior study validated this determination, with 91% of events confirmed by medical records (21). The requirement of two outpatient diagnoses aimed to exclude events that were suspected but not subsequently confirmed at follow-up visits. Based on the results of the prior validation study (21), we did not include ICD-9 diagnoses for hepatic encephalopathy and jaundice, which could indicate decompensation, because these diagnoses frequently were linked to unrelated conditions (e.g., narcotic overuse, stroke recorded as encephalopathy; biliary obstruction, atazanavir-associated hyperbilirubinemia recorded as jaundice). Date of decompensation was defined as the hospital discharge date (if identified by hospital diagnosis) or initial outpatient diagnosis date (if identified by outpatient diagnoses).
Secondary outcomes included: 1) incident hepatic decompensation (determined by the above ICD-9-based definition) within VACS-VC, Medicare, or Medicaid data (to capture outcomes occurring at non-VA hospitals that did not result in transfer to a VA facility; this outcome was secondary because non-VA events have not been validated), 2) HCC, and 3) severe liver events, a composite outcome of hepatic decompensation within the VACS-VC, HCC, or liver-related death. HCC was determined using the VA Central Cancer Registry, which confirmed diagnoses by histology, cytology, or consistent radiography. Deaths were classified as liver-related if the underlying cause of death from the National Death Index was recorded as hepatic decompensation, liver cancer, alcoholic liver disease, viral hepatitis, or non-alcoholic liver disease (Appendix A) (15).
Data Collection
Baseline data (Table 1) included: age; sex; race/ethnicity; VA center patient volume; body mass index (BMI); diabetes mellitus; alcohol dependence/abuse; injection/non-injection drug use; hepatitis B surface antigen status; HCV genotype; HCV RNA; pre-ART CD4 count; pre-ART plasma HIV RNA; and baseline antiretroviral regimen. Diabetes was defined by random glucose level ≥200 mg/dL or anti-diabetic medication use (22, 23). Alcohol dependence/abuse (24) and injection/non-injection drug use (24, 25) were defined by previously validated ICD-9 diagnoses (Appendix A). Baseline serum creatinine, hemoglobin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and platelet count were collected from dates closest to but prior to start of follow-up. Baseline FIB-4, a noninvasive measure of advanced hepatic fibrosis, was determined by: (age [years] × AST [U/L])/((platelet count [109/L]) × (ALT [U/L])1/2) (26). Because liver fibrosis can progress by one stage as early as within 4 years for antiretroviral-treated HIV/HCV-coinfected persons (7) and within 5 years for HCV-monoinfected persons (27), we determined baseline FIB -4 scores using ALT, AST, and platelet counts within a 2-year period around the start of follow-up. FIB-4 scores <1.45 identify no/minimal fibrosis and scores >3.25 indicate advanced hepatic fibrosis/cirrhosis in coinfected (26) and HCV-monoinfected patients (28).
Table 1.
Characteristics of the study cohorts.
| Characteristic | Antiretroviral-Treated HIV/HCV-Coinfected (n=4,280) |
HCV-Monoinfected (n=6,079) |
|---|---|---|
| Median baseline age (years, IQR) | 48 (44–52) | 47 (43–50) |
| Male sex (n, %) | 4,214 (98.5) | 6,022 (99.1) |
| Race/ethnicity (n, %) | ||
| Black | 2,788 (65.1) | 3,733 (61.4) |
| Caucasian | 975 (22.8) | 1,775 (29.2) |
| Hispanic | 414 (9.7) | 477 (7.8) |
| Other/Unknown | 103 (2.4) | 94 (1.5) |
| Baseline body mass index (n, %) | ||
| <18.5 kg/m2 | 135 (3.2) | 107 (1.8) |
| 18.5–24.9 kg/m2 | 2,184 (51.0) | 2,006 (33.0) |
| 25–29.9 kg/m2 | 1,368 (32.0) | 2,242 (36.9) |
| ≥30 kg/m2 | 473 (11.1) | 1,669 (27.5) |
| Missing weight and/or height | 120 (2.8) | 55 (0.9) |
| Baseline diabetes mellitus (n, %) | 318 (7.4) | 453 (7.5) |
| Baseline history of alcohol dependence/abuse (n, %) | 1,130 (26.4) | 1,866 (30.7) |
| Baseline history of injection/non-injection drug use (n, %) | 1,501 (35.1) | 2,025 (33.3) |
| Baseline history of tobacco use (n, %) | ||
| Current | 2,956 (69.1) | 4,457 (73.3) |
| Former | 482 (11.3) | 776 (12.8) |
| Never | 486 (11.4) | 650 (10.7) |
| Unknown | 356 (8.2) | 196 (3.2) |
| Median follow-up (years, IQR) | 6.8 (3.6–10.1) | 9.9 (6.0–12.4) |
| Total follow-up time (person-years) | 29,005 | 54,411 |
| HCV genotype (n, %) | ||
| 1 or 4 | 2,513 (58.7) | 3,648 (60.0) |
| 2 or 3 | 315 (7.4) | 513 (8.4) |
| Other | 7 (0.2) | 11 (0.2) |
| Missing | 1,445 (33.8) | 1,907 (31.4) |
| Baseline HCV RNA (n, %)* | ||
| ≥400,000 IU/mL and/or ≥1,000,000 copies/mL | 3,186 (74.5) | 2,309 (38.0) |
| <400,000 IU/mL and/or <1,000,000 copies/mL | 450 (10.5) | 2,006 (33.0) |
| Qualitative HCV RNA result only | 644 (15.0) | 1,764 (29.0) |
| Hepatitis B surface antigen (n, %) | ||
| Positive (ever) | 241 (5.6) | 78 (1.3) |
| Negative | 3,992 (93.3) | 5,701 (93.8) |
| Never tested | 47 (1.1) | 300 (4.9) |
| Median pre-ART HIV RNA (log copies/mL, IQR) | 4.7 (4.0–5.1) | – |
| Pre-ART CD4 cell count (n, %) | ||
| ≥500 cells/mm3 | 391 (9.1) | – |
| 350–499 cells/mm3 | 588 (13.7) | – |
| 200–349 cells/mm3 | 1,279 (29.9) | – |
| <200 cells/mm3 | 1,921 (44.9) | – |
| Not tested at baseline | 101 (2.4) | – |
| Most common baseline antiretroviral regimens (n, %) | ||
| Nelfinavir/zidovudine/lamivudine | 553 (12.9) | – |
| Efavirenz/zidovudine/lamivudine | 551 (12.9) | – |
| Indinavir/zidovudine/lamivudine | 545 (12.7) | – |
| Indinavir/stavudine/lamivudine | 391 (9.1) | – |
| Efavirenz/tenofovir/lamivudine | 268 (6.3) | – |
| Nevirapine/zidovudine/lamivudine | 237 (5.5) | – |
Abbreviations: HCV=hepatitis C virus; HIV=human immunodeficiency virus; IQR=interquartile range; RNA=ribonucleic acid
Based on highest baseline HCV RNA result
Longitudinal data included hepatitis B surface antigen, plasma HIV RNA, diabetes, and liver transplantation (determined by diagnosis and procedural codes; Appendix A).
Statistical Analysis
The 12 months prior to the start of follow-up represented the baseline period for both cohorts. Follow-up for coinfected patients began 12 months after ART initiation and for monoinfected patients after 12 months in the VACS-VC. The rationale for defining the baseline period as the first year on ART for coinfected patients was due to the fact that many of these patients initially entered into care at the time of ART initiation, which was shortly after their HIV diagnosis. Follow-up continued until a study endpoint, death, initiation of HCV therapy, or last visit before September 30, 2010.
For descriptive purposes, we estimated incidence rates (events/1,000 person-years) of endpoints with 95% confidence intervals (CIs), standardized by the age and race/ethnicity distribution of coinfected patients (29). We then used Cox models to estimate adjusted hazard ratios (HRs) of outcomes in coinfected compared to monoinfected patients (30). We controlled for all available clinically relevant variables in Table 1. The proportionality of hazards was evaluated by plots of Schoenfeld residuals (31). As a sensitivity analysis, we addressed the potential for informative censoring by use of inverse probability of censoring weights and Cox regression (Appendix B) (32).
Since mortality rates were higher for coinfected than monoinfected patients and because death could be a competing risk for decompensation, we determined HRs of decompensation accounting for death as a competing risk (33). We estimated the incidence of decompensation for both cohorts, standardized to the characteristics of patients in the overall sample based on the covariates in the Cox model (Table 2) and assuming that death was a competing risk.
Table 2.
Incidence rates, standardized for age and race/ethnicity, and adjusted relative hazards of specified liver-related outcomes in antiretroviral-treated HIV/hepatitis C-coinfected compared to hepatitis C-monoinfected patients.
| Incidence Rate (95% Confidence Interval [CI]), Events/1,000 Person-Years | ||||
|---|---|---|---|---|
| Outcome | Antiretroviral-Treated HIV/HCV-Coinfected (n=4,280) |
HCV-Monoinfected (n=6,079) |
Adjusted HR with 95% CI* (Death is Censored) |
Adjusted HR with 95% CI* (Death is a Competing Risk) |
| Hepatic decompensation | ||||
| All patients (n=10,359) | 9.5 (7.6–11.9) | 5.7 (4.4–7.4) | 1.83 (1.54–2.18) | 1.56 (1.31–1.86) |
| HIV/HCV patients with: | ||||
| HIV RNA <1,000 copies/mL during follow-up (n=966†) | 9.4 (5.4–16.2) | ** | 1.65 (1.20–2.27) | 1.44 (1.05–1.99) |
| HIV RNA ≥1,000 copies/mL during follow-up (n=3,180†) | 9.6 (7.5–12.2) | ** | 1.87 (1.56–2.25) | 1.59 (1.33–1.91) |
| Pre-ART CD4 cell count ≥500 cells/mm3 (n=391†) | 8.8 (4.4–17.8) | ** | 1.56 (1.04–2.33)‡ | 1.44 (0.96–2.17)‡ |
| Pre-ART CD4 cell count 350–499 cells/mm3 (n=588†) | 8.1 (4.4–14.8) | ** | 1.61 (1.13–2.28)‡ | 1.44 (1.02–2.05)‡ |
| Pre-ART CD4 cell count 200–349 cells/mm3 (n=1,279†) | 8.6 (5.6–13.1) | ** | 1.63 (1.26–2.11)‡ | 1.45 (1.12–1.88)‡ |
| Pre-ART CD4 cell count <200 cells/mm3 (n=1,921†) | 10.9 (7.9–15.0) | ** | 2.03 (1.65–2.51) | 1.71 (1.38–2.13)‡ |
| Hepatocellular carcinoma (n=10,359) | 2.4 (1.7–3.4) | 1.9 (1.3–2.6) | 1.60 (1.16–2.21) | 1.19 (0.88–1.61) |
| Severe liver events (n=10,359) | 12.9 (10.8–15.5) | 8.1 (6.7–9.9) | 1.77 (1.52–2.05) | 1.47 (1.27–1.70) |
Abbreviations: ART=antiretroviral therapy; CI=confidence interval; HIV=human immunodeficiency virus; HR=hazard ratio
Hazard ratios adjusted for age, race, diabetes mellitus, body mass index, history of alcohol dependence/abuse, history of injection/non-injection drug use, and VA center patient volume. The proportion of hepatitis C-monoinfected patients who had a hemoglobin and hepatitis B surface antigen result determined during the baseline period was very small. As a result, these variables were not included in multivariable models comparing incidence rates of hepatic decompensation between antiretroviral-treated HIV/HCV-coinfected and HCV-monoinfected patients.
Incidence rates unchanged from overall HCV-monoinfected result of 5.7 (CI, 4.4–7.4) events/1,000 person-years.
Represents number of patients included in antiretroviral-treated HIV/hepatitis C virus-coinfected subgroup for specified analysis.
Because the HRs for strata ≥200 cells/mm3 were indistinguishable, they are displayed as a single plot in Figure 2c.
We also performed three subgroup analyses. First, since coinfected patients may have had varying antiretroviral adherence during follow-up or might have interrupted or discontinued ART leading to virologic failure, we compared decompensation rates in the monoinfected cohort to subgroups of coinfected patients stratified by HIV RNA level during follow-up (<1,000 copies/mL on all HIV RNA results during follow-up; ≥1,000 copies/mL on any result during follow-up). This threshold was chosen because intermittent low-level HIV viremia <1,000 copies/mL may occur among patients on ART and is not necessarily indicative of HIV virologic failure (34). As a sensitivity analysis, we compared decompensation rates in the much smaller subgroup of coinfected patients who maintained HIV RNA <400 copies/mL throughout follow-up (n=386) compared to monoinfected patients. Second, we compared decompensation rates in the monoinfected cohort to coinfected patients stratified by pre-ART CD4 count (<200; 200–349; 350–499; ≥500 cells/mm3). Third, we evaluated the risk of decompensation between coinfected and monoinfected patients at the same baseline stage of liver fibrosis. This analysis focused on patients with no/minimal hepatic fibrosis (determined by FIB-4 <1.45) to be able to fully evaluate the association between coinfection and decompensation.
Among coinfected patients, we used Cox and competing risk regression analyses to estimate HRs of decompensation for risk factors of interest. Hypothesized risk factors included: baseline advanced hepatic fibrosis (FIB-4 >3.25), baseline obesity (BMI ≥30 kg/m2), baseline severe anemia (hemoglobin <10 g/dL), diabetes mellitus, active hepatitis B infection, non-black race, pre-ART CD4 count, and HIV viremia copy-years, defined as the log copies of plasma HIV RNA/mL over time, which is a measure of cumulative plasma HIV burden (35). Viremia copy-years were estimated by the cumulative annual HIV viral load based on the median value of each year of follow-up. Details are presented in Appendix C. Diabetes, hepatitis B, and HIV viremia copy-years were evaluated as time-varying covariates.
In a subgroup analysis among 3,314 (77.4%) coinfected patients with baseline HCV RNA results reported in international units (IU)/mL, we evaluated the association between high baseline HCV RNA level (≥400,000 IU/mL) and decompensation. If multiple baseline HCV RNA loads were available, the highest result was analyzed.
We performed a simulation-based sensitivity analysis to examine the effect of unmeasured confounders on the HR of decompensation between coinfected and monoinfected patients (36). Details appear in Appendix D.
We implemented multiple imputation to address the potential bias of missing data, by means of 10 imputations using all variables in Table 1 and outcomes (37). Results across the 10 datasets were combined to arrive at CIs that accounted for within- and across-dataset variances (38). Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC) and Stata 12.1 (Stata Corporation, College Station, TX).
Regulatory Approvals
This study was approved by the Institutional Review Boards of the University of Pennsylvania and Philadelphia VA Medical Center.
Role of the Funding Sources
The study was funded by the National Institutes of Health. The funding source had no role in data collection, analysis, or interpretation.
RESULTS
Patient characteristics
Between 1997 and 2010, 10,359 patients (4,280 antiretroviral-treated HIV/HCV-coinfected; 6,079 HCV-monoinfected) met our inclusion criteria (Figure 1). Absence of HCV RNA assessment was the most common reason for exclusion from both cohorts. There were no clinically relevant differences in the characteristics between included patients (Table 1) and those excluded due to lack of HCV RNA for both cohorts.
Figure 1.
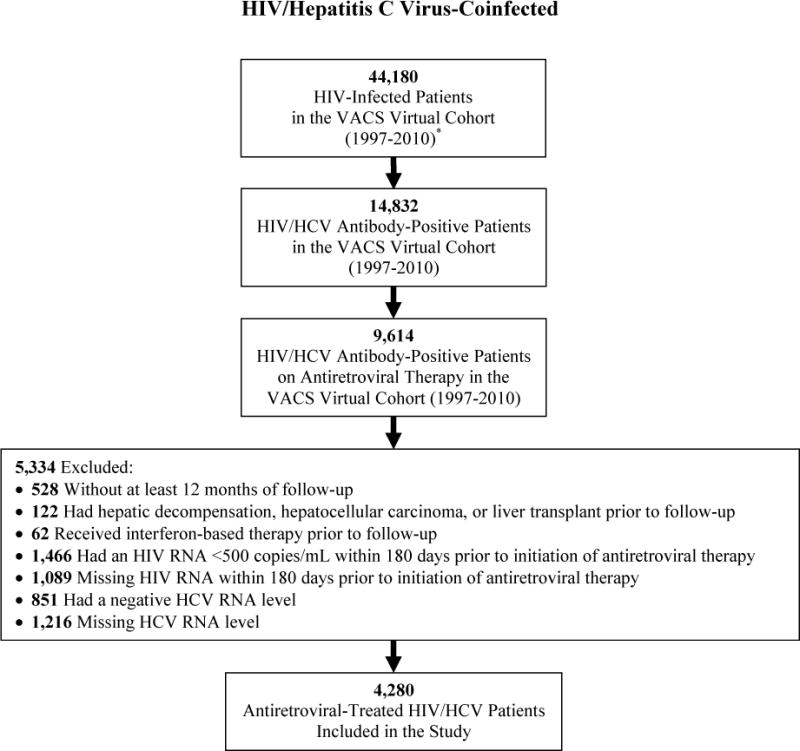
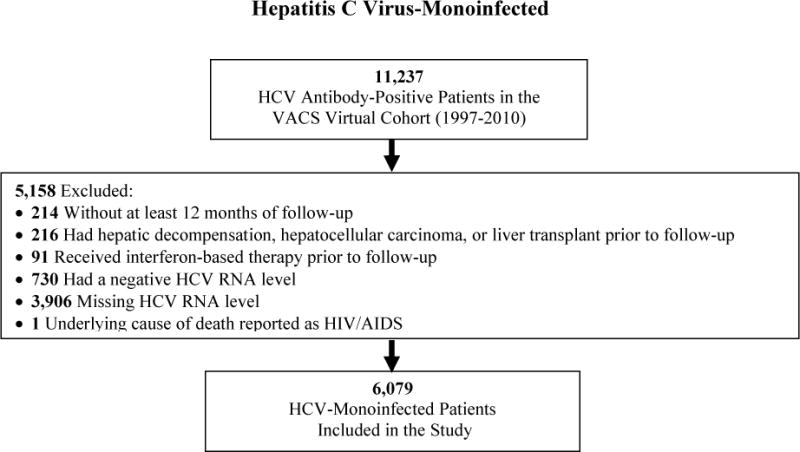
Selection of antiretroviral-treated HIV/hepatitis C virus-coinfected and hepatitis C virus-monoinfected patients for inclusion in the study.
* HIV infection determined by International Classification of Diseases, Ninth Revision diagnosis codes.
Table 1 summarizes the baseline cohort characteristics. Coinfected and monoinfected patients were similar in age, race/ethnicity, diabetes, history of alcohol dependence/abuse, and history of injection/non-injection drug use. The majority of both cohorts were infected with HCV genotype 1. Coinfected patients more commonly had a high HCV RNA level. Follow-up was shorter for coinfected than monoinfected patients (6.8 versus 9.9 years; p<0.001). During follow-up, 330 (7.7%) coinfected and 505 (8.3%) monoinfected patients initiated HCV therapy and were censored.
Baseline ART regimens prescribed to HIV/HCV-coinfected patients reflected the antiretroviral drugs used at the time of VACS-VC entry (Table 1). Only very few (162 [3.8%]) were prescribed abacavir/lamivudine/zidovudine as their baseline regimen. Among the 241 hepatitis B surface antigen-positive HIV/HCV-coinfected patients, 207 (85.9%) received lamivudine alone, tenofovir plus emtricitabine, or tenofovir plus lamivudine as part of their baseline regimen. HIV/HCV-coinfected patients had a median of 2.8 (interquartile range, 1.8–3.8) HIV RNA results measured each year during follow-up to assess HIV virologic response.
Hepatic decompensation
Hepatic decompensation occurred more frequently in antiretroviral-treated coinfected than monoinfected patients (271 [6.3%] versus 305 [5.0%]; p=0.004). At the time of initial decompensation, variceal hemorrhage was less common among coinfected patients (71 [26.2%] versus 168 [55.1%]; p<0.001). However, similar proportions of coinfected and monoinfected patients presented with ascites (226 [83.4%] versus 236 [77.4%]; p=0.070) and spontaneous bacterial peritonitis (48 [17.7%] versus 68 [22.3%]; p=0.171). Among those who presented with ascites, similar proportions of coinfected and monoinfected patients had the diagnosis recorded in the inpatient setting (145 [64.2%] versus 133 (56.4%); p=0.087).
After adjustment for age, race/ethnicity, diabetes, BMI, history of alcohol abuse and injection/non-injection drug use, and VA center volume, coinfected patients on ART had a higher rate of decompensation than monoinfected patients (HR, 1.83 [95% CI, 1.54–2.18]). This association was almost identical when we expanded our outcome to include VA, Medicare, or Medicaid events (HR, 1.85 [95% CI, 1.59–2.18]) and remained when death was treated as a competing risk (HR, 1.56 [95% CI, 1.31–1.86]). HRs adjusted for informative censoring differed little (data not shown). The standardized cumulative incidence of decompensation was higher among coinfected than monoinfected patients at 10 years (7.4% versus 4.8%; p<0.001; Figure 2a).
Figure 2.
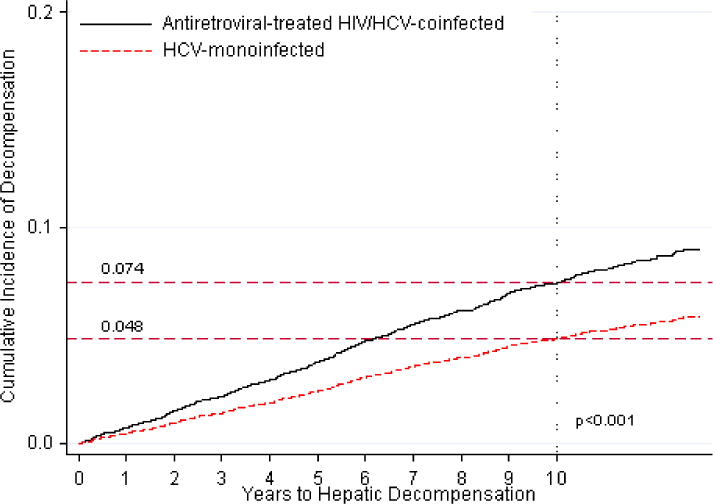
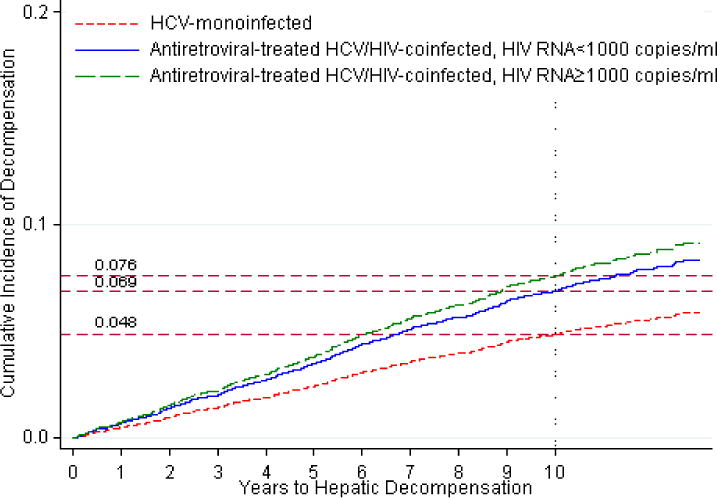
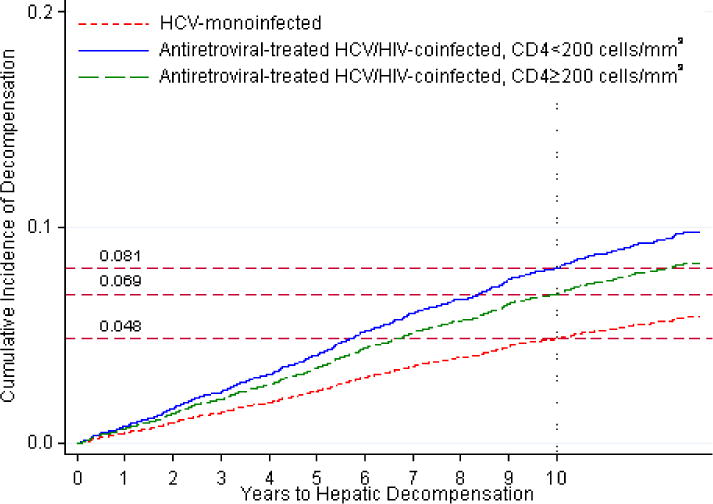
Standardized cumulative incidences of hepatic decompensation, based on competing risk regression analyses. Estimates of the cumulative incidence of hepatic decompensation are reported for each group.
a. Standardized cumulative incidences of hepatic decompensation between antiretroviral-treated HIV/hepatitis C virus-coinfected (denoted by solid line) and hepatitis C virus-monoinfected patients (denoted by dotted line).
b. Standardized cumulative incidences of hepatic decompensation between antiretroviral-treated HIV/hepatitis C virus-coinfected patients with HIV RNA levels ≥1,000 copies/mL on any HIV RNA test result during follow-up (denoted by dashed line), antiretroviral-treated HIV/hepatitis C virus-coinfected patients with HIV RNA <1,000 copies/mL on all HIV RNA test results throughout follow-up (denoted by solid line), and hepatitis C virus-monoinfected patients (denoted by dotted line).
c. Standardized cumulative incidences of hepatic decompensation between antiretroviral-treated HIV/hepatitis C virus-coinfected patients with pre-antiretroviral therapy CD4 T lymphocyte counts <200 cells/mm3 (denoted by solid line), antiretroviral-treated HIV/hepatitis C virus-coinfected patients with pre-antiretroviral therapy CD4 T lymphocyte counts ≥200 cells/mm3 (denoted by dashed line), and hepatitis C virus-monoinfected patients (denoted by dotted line). Strata for CD4 cell counts ≥200 cells/mm3 were collapsed into a single plot because these HRs were indistinguishable (see Table 2).
In subgroup analyses, rates of decompensation remained higher in coinfected patients who maintained HIV RNA levels <1,000 copies/mL throughout follow-up compared to monoinfected persons (Table 2; Figure 2b). Similar results were observed when coinfected patients with HIV RNA <400 copies/mL throughout follow-up were compared to HCV-monoinfected patients (data not shown). Across strata of pre-ART CD4 count, the hazard ratio of decompensation was highest for coinfected patients with a pre-ART CD4 count of <200 cells/mm3 compared to monoinfected persons (Table 2; Figure 2c). Among those with a baseline FIB-4 <1.45, the risk of decompensation remained higher in coinfected than monoinfected patients (adjusted HR, 1.98 [95% CI, 1.23–3.18]).
Hepatocellular carcinoma
The proportion of coinfected and monoinfected patients who developed HCC was similar between the cohorts (74 [1.7%] versus 100 [1.6%]; p=0.74). After adjustment for age, race, diabetes, BMI, alcohol abuse, injection/non-injection drug use, and center volume, rates of HCC were higher for coinfected patients (HR, 1.60 [95% CI, 1.16–2.21]). This association did not remain significant in competing risk analysis (Table 2). Median survival after HCC diagnosis was shorter for coinfected patients (8.7 [IQR, 2.3–27.3] versus 14.2 [IQR, 3.8–101.8] months), but this difference was not significant (p=0.22). There were no important differences in age at diagnosis, race, BMI, diabetes, or alcohol abuse between coinfected and monoinfected patients with HCC (Appendix E).
Severe liver events and death
Severe liver events were more common in coinfected than monoinfected patients (373 [8.7%] versus 433 [7.1%]; p=0.003). Further, coinfected patients had a higher risk of this endpoint (Table 2). Coinfected patients less commonly underwent liver transplantation (5 [0.1%] vs. 26 [0.4%]; p=0.004).
Mortality occurred more frequently among coinfected patients (1,407 [32.9%] vs. 934 [15.4%]; p<0.001). HIV/AIDS was most commonly recorded as the underlying cause of death in this cohort (46.3%). Liver disease was the most common cause of death in monoinfected patients (20.1%; Appendix F).
Factors associated with decompensation among coinfected patients
Baseline advanced hepatic fibrosis, baseline hemoglobin <10 g/dL, diabetes, and non-black race were risk factors for decompensation (Table 3). The hazard for HIV viremia copy-years >6 log copy-years/mL was approximately twice that of the reference group (<2 log copy-years/mL), but these results did not approach statistical significance.
Table 3.
Factors associated with hepatic decompensation among 4,280 antiretroviral-treated HIV/hepatitis C virus-coinfected patients.
| Characteristic | Adjusted HR with 95% CI* (Death is Censored) |
Adjusted HR with 95% CI* (Death is a Competing Risk) |
|---|---|---|
| Body mass index (kg/m2) | ||
| <18.5 | 0.58 (0.22–1.52) | 0.46 (0.17–1.23) |
| 18.5–24.9 | Ref | Ref |
| 25–29.9 | 1.09 (0.83–1.42) | 1.13 (0.86–1.47) |
| ≥30 | 1.04 (0.69–1.56) | 1.07 (0.71–1.62) |
|
| ||
| Diabetes mellitus† | ||
| Absent | Ref | Ref |
| Present | 1.88 (1.39–2.53) | 1.88 (1.38–2.56) |
|
| ||
| FIB-4 | ||
| <1.45 | Ref | Ref |
| 1.45–3.25 | 1.99 (1.39–2.85) | 1.91 (1.33–2.73) |
| >3.25 | 6.54 (4.56–9.39) | 5.45 (3.79–7.84) |
|
| ||
| Hemoglobin (g/dL) | ||
| ≥10 | Ref | Ref |
| <10 | 3.50 (1.97–6.24) | 2.24 (1.20–4.20) |
|
| ||
| Hepatitis B surface antigen-positive† | ||
| Absent | Ref | Ref |
| Present | 1.04 (0.61–1.77) | 0.98 (0.57–1.69) |
|
| ||
| Race | ||
| Black | Ref | Ref |
| Non-black | 2.14 (1.67–2.75) | 2.12 (1.65–2.72) |
|
| ||
| HIV viremia copy-years (log copy-years/mL)† | ||
| <2 | Ref | Ref |
| 2–6 | 1.37 (0.67–2.77) | 1.64 (0.84–3.23) |
| ≥6 | 2.01 (0.66–6.12) | 2.06 (0.69–6.10) |
|
| ||
| Pre-antiretroviral therapy CD4 count (cells/mm3)‡ | ||
| ≥200 | Ref | Ref |
| <200 | 1.22 (0.95–1.56) | 1.17 (CI, 0.91–1.49) |
Abbreviations: CI=confidence interval; HCV= hepatitis C virus; HIV=human immunodeficiency virus; HR=hazard ratio
Hazard ratios adjusted for all other risk factors as well as history of alcohol dependence/abuse, history of injection/non-injection drug use, age, serum creatinine, and VA center patient volume.
Diabetes mellitus, hepatitis B surface antigen status, and HIV RNA level were evaluated as time-varying covariates.
CD4 T lymphocyte count measured within 180 days prior to initiation of antiretroviral therapy.
The incidence of decompensation was similar between coinfected patients with baseline HCV RNA ≥400,000 IU/mL compared to <400,000 IU/mL (5.8% [169/2,936] versus 6.9% [26/378]; HR, 0.78 [95% CI, 0.52–1.18]). Results were similar for competing risk regression analyses (data not shown).
DISCUSSION
In this study, antiretroviral-treated HIV/HCV-coinfected patients had higher rates of hepatic decompensation and severe liver events compared to HCV-monoinfected patients. Coinfected patients who maintained HIV RNA levels <1,000 copies/mL or <400 copies/mL over the median 6.8 years of follow-up still had higher rates of decompensation, suggesting that HIV viral suppression below these thresholds with ART is not sufficient to reduce rates of end-stage liver disease to those of HCV-monoinfected patients. Further, among patients with minimal/no fibrosis at baseline, the risk of decompensation remained higher for coinfected patients. Rates of decompensation were highest for patients with pre-ART CD4 counts <200 cells/mm3 compared to monoinfected patients. Finally, among coinfected patients on ART, baseline advanced liver fibrosis, severe anemia, diabetes, and non-black race were each associated with higher rates of decompensation.
Our analyses examining the risk of decompensation between coinfected and monoinfected patients did not control for baseline stage of hepatic fibrosis because the development of liver fibrosis is in the causal pathway to hepatic decompensation. Thus, controlling for baseline hepatic fibrosis could potentially adjust away an association between treated HIV and decompensated cirrhosis. Therefore, we conducted a secondary analysis restricted to patients with minimal/no liver fibrosis (FIB-4 <1.45) at baseline. Antiretroviral-treated coinfected patients continued to have an increased risk of hepatic decompensation compared to monoinfected patients.
The mechanisms for the higher rates of hepatic decompensation in coinfected patients on ART remain unclear. HIV-related immune dysregulation, HIV-mediated depletion of CD4 cells in the gastrointestinal tract with resultant microbial translocation, HIV/HCV-related oxidative stress, and HIV-induced hepatocyte apoptosis have been implicated in the pathogenesis of progressive hepatic disease in HIV/HCV-coinfected patients (13). Other cofactors, such as hepatic steatosis and cumulative exposure to potentially hepatotoxic medications, particularly select antiretroviral drugs (39–41), might also accelerate liver disease progression in coinfected patients.
We observed that variceal hemorrhage was less commonly an initial presenting decompensation event among coinfected patients. A similar finding was reported in a cohort study of HIV/HCV-coinfected patients from Spain (42). Prior studies have suggested that HIV infection is associated with protein C deficiency (43–45) and protein S deficiency (46), which can promote hypercoagulability and reduce the likelihood of variceal bleeding among coinfected patients.
We observed a higher rate of HCC in coinfected patients on ART compared to HCV-monoinfected patients, but this difference was not statistically significant after adjustment for competing risk. A prior cohort study conducted between 1992 and 2000 showed that HIV/HCV coinfection was not associated with an increased risk of HCC either in the pre-ART or ART eras compared to HCV-monoinfection (47). As life expectancy continues to extend among HIV/HCV-coinfected patients, clinically important differences in rates of HCC may become apparent in the future.
We identified several important factors associated with hepatic decompensation in coinfected patients. Diabetes is associated with hepatic steatosis (48), which could promote hepatic inflammation and accelerate liver fibrosis progression (49). Diabetes might also induce hepatic fibrosis independent of steatosis via stimulation of hepatic stellate cells by insulin (50, 51). Severe anemia might be a marker of advanced HCV-associated liver disease, potentially indicating blood loss from variceal bleeding. Alternatively, anemia may be a marker for systemic inflammation (52), which may promote hepatic fibrosis. Non-black HCV-infected patients are reported to have stronger HCV-specific immunity than those of black race (53), which could result in increased immune-mediated hepatic inflammation and accelerated liver fibrosis progression.
Our results suggest that the highest level of HIV viremia copy-years (>6 log copy-years/mL of HIV RNA) might increase the risk of hepatic decompensation. However, plasma viral loads were measured infrequently in many patients, with variable numbers and timing. Given our additional finding of a higher risk of decompensation for coinfected patients with pre-ART CD4 counts <200 cell/mm3 compared to monoinfected persons, suppression of HIV on ART with resultant immune reconstitution should remain a key goal for HIV/HCV patients to possibly reduce the risk of end-stage liver disease.
Neither hepatitis B infection nor HCV RNA level was associated with hepatic decompensation among coinfected patients. The majority (85.9%) of coinfected patients who were hepatitis B surface antigen-positive received hepatitis B-active ART, and this might have mitigated the association between hepatitis B and decompensation. Future analyses should test this hypothesis. Moreover, while several studies in HCV-monoinfected patients have suggested that higher HCV RNA levels are associated with more advanced liver fibrosis (54–56) or death from end-stage liver disease (57), the majority have found no association between HCV RNA level and liver disease severity (58–62). Our results are consistent with these findings.
Our results have important clinical implications. The finding that antiretroviral-treated coinfected patients who maintained an HIV RNA <1,000 copies/mL had lower rates of hepatic decompensation compared to those who did not achieve HIV suppression below this level suggests that suppression of HIV RNA with ART is an important factor in slowing progression of HCV-related liver fibrosis. This observation supports current management guidelines that recommend initiation of ART among HIV/HCV-coinfected patients, regardless of CD4 cell count (16, 63). Further, the increased rate of hepatic decompensation among coinfected patients should prompt earlier consideration for initiating HCV therapy to try to reduce the risk of liver complications (19, 20). Recent clinical trials evaluating boceprevir- and telaprevir-based antiviral therapy among HIV/HCV genotype 1-coinfected patients demonstrate sustained HCV virologic response rates that are comparable to those of HCV-monoinfected patients (64, 65). Providers might also address modifiable risk factors (e.g., control diabetes) to try to decrease the risk of end-stage liver disease.
This study has several potential limitations. Decompensation outcomes could have been misclassified. However, hepatic decompensation was identified using a validated definition (21). We might have underestimated the number of decompensation events since our definition did not include hepatic encephalopathy. However, the negative predictive value of our validated definition exceeded 99% (21). Further, the incidence rates of decompensation among coinfected (9.5/1,000 person-years) and monoinfected (5.7/1,000 person-years) patients in this study were similar to those reported in other cohorts of coinfected (11.6/1,000 person-years (10)) and monoinfected (3.4/1,000 person-years (62)) patients. There is the potential for differential ascertainment of outcomes if patients in one of the cohorts more frequently presented to non-VA hospitals for care. To address this issue, we evaluated: 1) incident decompensation events within the VA system as well as in the U.S. Medicare and Medicaid programs, and 2) liver-related deaths within a composite severe liver event outcome. The association between antiretroviral-treated coinfection and these composite endpoints remained in both analyses. Second, residual confounding by unmeasured factors (e.g., duration of HCV infection; alcohol dependence/drug use during follow-up) is possible. However, sensitivity analyses (Appendix D) suggested that our results were robust to the potential bias from unobserved confounders. Third, the median follow-up among the monoinfected cohort was longer than that for the coinfected cohort. However, both cohorts included patients in all age groups, produced risk sets of adequate size for all combinations of decompensation risk factors across age groups, and therefore covered the spectrum of risks experienced by young, middle-aged, and older HCV-infected patients. Finally, our study sample was predominantly comprised of male U.S. veterans, and so results may not be generalizable to women. Since HCV-related liver fibrosis progression differs by sex (60, 66, 67), future epidemiologic analyses should evaluate end-stage liver disease events among HIV/HCV-coinfected and HCV-monoinfected women.
This study had a number of strengths. It is the largest study evaluating liver-related events among antiretroviral-treated coinfected patients and compared outcomes to HCV-monoinfected persons. It had a long duration of follow-up, evaluated validated endpoints (21), accounted for time-varying covariates, and identified decompensations and liver-related deaths outside the VA system, ensuring that few outcomes were missed.
In conclusion, we observed that HIV/HCV-coinfected patients receiving ART had higher rates of hepatic decompensation and severe liver events than HCV-monoinfected individuals. Among coinfected patients on ART, baseline advanced liver fibrosis, severe anemia, diabetes, and non-black race were associated with higher rates of decompensation. Clinicians should address modifiable risk factors and consider treatment of HCV infection in coinfected patients to reduce rates of hepatic decompensation.
Supplementary Material
Acknowledgments
We are grateful to the VA Central Cancer Registry for permitting us to link the VACS Virtual Cohort database with the VA Central Cancer Registry database and for providing us with a dataset containing the linked records.
Grant Support: National Institute of Allergy and Infectious Diseases research grant K01 AI070001 [Dr. Lo Re], National Institute on Alcohol Abuse and Alcoholism research grant U01 AA13566 [Dr. Justice], and National Institute of Mental Health research grant T32 MH020031 [Ms. Park].
Abbreviations
- ART
antiretroviral therapy
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus infection
- VA
Veterans Affairs
Footnotes
Potential conflicts of interest: None to report.
Publisher's Disclaimer: Disclaimer: The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Presented in part at the 19th International AIDS Conference, Washington, DC, 22–27 July 2012, and the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia, 3–6 March 2013.
References
- 1.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 2.Frederick T, Burian P, Terrault N, Cohen M, Augenbraun M, Young M, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23(11):915–23. doi: 10.1089/apc.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx, NY. J Urban Health. 2011;88(3):507–16. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond HF, Hughes A, O’Keefe K, Stall RD, McFarland W. Hepatitis C prevalence among HIV-positive MSM in San Francisco: 2004 and 2008. Sex Transm Dis. 2011;38(3):219–20. doi: 10.1097/OLQ.0b013e3181f68ed4. [DOI] [PubMed] [Google Scholar]
- 5.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 6.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21(16):2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51(4):1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 10.Pineda JA, Garcia-Garcia JA, Aguilar-Guisado M, Rios-Villegas MJ, Ruiz-Morales J, Rivero A, et al. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46(3):622–30. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 11.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362(9397):1708–13. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 13.Lin W, Weinberg EM, Chung RT. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J Infect Dis. 2013;207(Suppl 1):S13–8. doi: 10.1093/infdis/jis926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 15.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40(9):808–13. doi: 10.1097/00043764-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 17.Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2002;288(2):222–35. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi NR, Tate JP, Rodriguez-Barradas MC, Rimland D, Goetz MB, Gibert C, et al. Validation of an algorithm to identify antiretroviral-naive status at time of entry into a large, observational cohort of HIV-infected patients. Pharmacoepidemiol Drug Saf. 2013;22(9):1019–25. doi: 10.1002/pds.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 20.Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308(4):370–8. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Re V, 3rd, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20(7):689–99. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40(1):115–9. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 23.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice AC, McGinnis KA, Atkinson JH, Heaton RK, Young C, Sadek J, et al. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS. 2004;18(Suppl 1):S49–59. [PubMed] [Google Scholar]
- 25.Kazis LE, Miller DR, Clark J, Skinner K, Lee A, Rogers W, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626–32. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 26.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 27.Zarski JP, Mc Hutchison J, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38(3):307–14. doi: 10.1016/s0168-8278(02)00387-2. [DOI] [PubMed] [Google Scholar]
- 28.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA. Direct standardization: A tool for teaching linear models for unbalanced data. Am Stat. 1982;36:38–43. [Google Scholar]
- 30.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23(16):2191–8. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. New York, NY: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 32.Cain LE, Cole SR. Inverse probability-of-censoring weights for the correction of time-varying noncompliance in the effect of randomized highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2009;28(12):1725–38. doi: 10.1002/sim.3585. [DOI] [PubMed] [Google Scholar]
- 33.Fine J, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34.van Sighem A, Zhang S, Reiss P, Gras L, van der Ende M, Kroon F, et al. Immunologic, virologic, and clinical consequences of episodes of transient viremia during suppressive combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48(1):104–8. doi: 10.1097/QAI.0b013e31816a1d4f. [DOI] [PubMed] [Google Scholar]
- 35.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171(2):198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–63. [PubMed] [Google Scholar]
- 37.Royston P. Multiple imputation of missing values: update. The STATA Journal. 2005;5(2):188–201. [Google Scholar]
- 38.Freedman VA, Wolf DA. A case study on the use of multiple imputation. Demography. 1995;32(3):459–70. [PubMed] [Google Scholar]
- 39.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283(1):74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 40.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18(17):2277–84. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 41.Blanco F, Barreiro P, Ryan P, Vispo E, Martin-Carbonero L, Tuma P, et al. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18(1):11–6. doi: 10.1111/j.1365-2893.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 42.Pineda JA, Romero-Gomez M, Diaz-Garcia F, Giron-Gonzalez JA, Montero JL, Torre-Cisneros J, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41(4):779–89. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 43.Bissuel F, Berruyer M, Causse X, Dechavanne M, Trepo C. Acquired protein S deficiency: correlation with advanced disease in HIV-1-infected patients. J Acquir Immune Defic Syndr. 1992;5(5):484–9. [PubMed] [Google Scholar]
- 44.Sorice M, Griggi T, Arcieri P, Circella A, d’Agostino F, Ranieri M, et al. Protein S and HIV infection. The role of anticardiolipin and anti-protein S antibodies. Thromb Res. 1994;73(3–4):165–75. doi: 10.1016/0049-3848(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 45.Sugerman RW, Church JA, Goldsmith JC, Ens GE. Acquired protein S deficiency in children infected with human immunodeficiency virus. Pediatr Infect Dis J. 1996;15(2):106–11. doi: 10.1097/00006454-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Feffer SE, Fox RL, Orsen MM, Harjai KJ, Glatt AE. Thrombotic tendencies and correlation with clinical status in patients infected with HIV. South Med J. 1995;88(11):1126–30. doi: 10.1097/00007611-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 48.Youssef W, McCullough AJ. Diabetes mellitus, obesity, and hepatic steatosis. Semin Gastrointest Dis. 2002;13(1):17–30. [PubMed] [Google Scholar]
- 49.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 50.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125(6):1695–704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 51.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34(4 Pt 1):738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 52.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–94. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freiberg MS, Chang CC, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gretch D, Corey L, Wilson J, dela Rosa C, Willson R, Carithers R, Jr, et al. Assessment of hepatitis C virus RNA levels by quantitative competitive RNA polymerase chain reaction: high-titer viremia correlates with advanced stage of disease. J Infect Dis. 1994;169(6):1219–25. doi: 10.1093/infdis/169.6.1219. [DOI] [PubMed] [Google Scholar]
- 55.Kato N, Yokosuka O, Hosoda K, Ito Y, Ohto M, Omata M. Quantification of hepatitis C virus by competitive reverse transcription-polymerase chain reaction: increase of the virus in advanced liver disease. Hepatology. 1993;18(1):16–20. [PubMed] [Google Scholar]
- 56.Mita E, Hayashi N, Kanazawa Y, Hagiwara H, Ueda K, Kasahara A, et al. Hepatitis C virus genotype and RNA titer in the progression of type C chronic liver disease. J Hepatol. 1994;21(3):468–73. doi: 10.1016/s0168-8278(05)80330-7. [DOI] [PubMed] [Google Scholar]
- 57.Hisada M, Chatterjee N, Kalaylioglu Z, Battjes RJ, Goedert JJ. Hepatitis C virus load and survival among injection drug users in the United States. Hepatology. 2005;42(6):1446–52. doi: 10.1002/hep.20938. [DOI] [PubMed] [Google Scholar]
- 58.Fanning L, Kenny E, Sheehan M, Cannon B, Whelton M, O’Connell J, et al. Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatology. 1999;29(3):904–7. doi: 10.1002/hep.510290310. [DOI] [PubMed] [Google Scholar]
- 59.Lau JY, Davis GL, Kniffen J, Qian KP, Urdea MS, Chan CS, et al. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993;341(8859):1501–4. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- 60.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34(5):730–9. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 61.Zeuzem S, Franke A, Lee JH, Herrmann G, Ruster B, Roth WK. Phylogenetic analysis of hepatitis C virus isolates and their correlation to viremia, liver function tests, and histology. Hepatology. 1996;24(5):1003–9. doi: 10.1002/hep.510240505. [DOI] [PubMed] [Google Scholar]
- 62.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 63.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 10, 2011. pp. 1–166. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 16, 2013. [Google Scholar]
- 64.Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13(7):597–605. doi: 10.1016/S1473-3099(13)70149-X. [DOI] [PubMed] [Google Scholar]
- 65.Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 66.Bissell DM. Sex and hepatic fibrosis. Hepatology. 1999;29(3):988–9. doi: 10.1002/hep.510290351. [DOI] [PubMed] [Google Scholar]
- 67.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340(16):1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


