Abstract
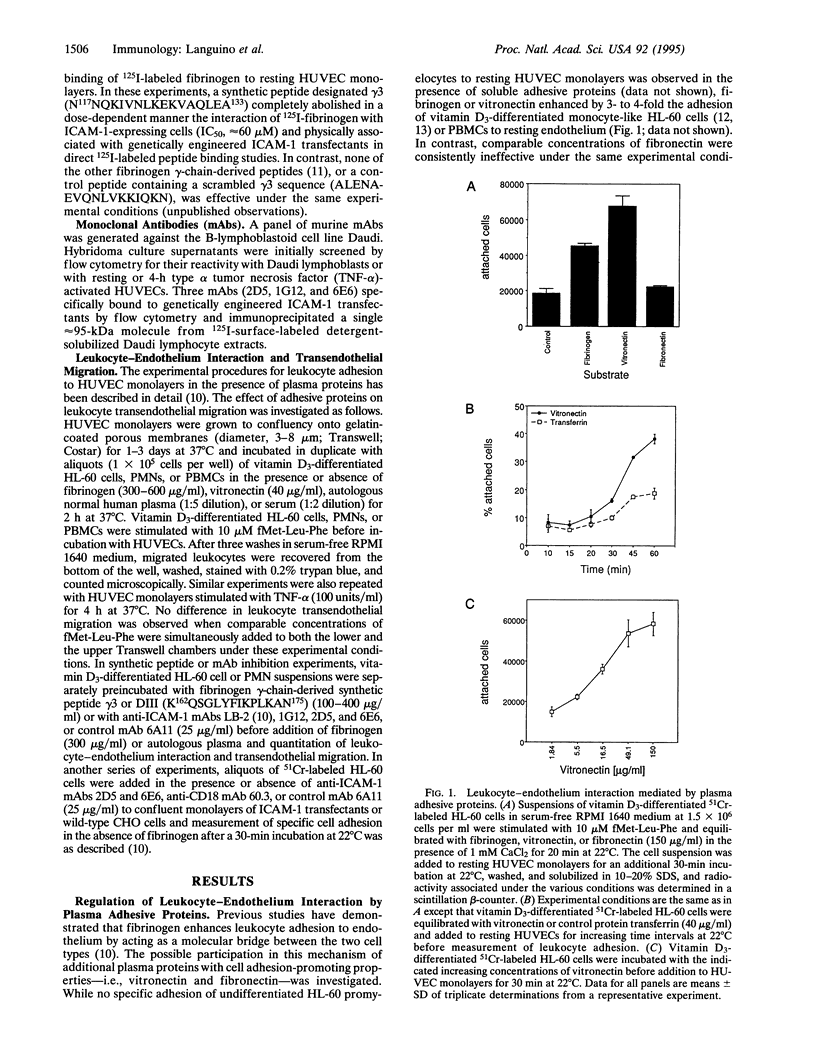
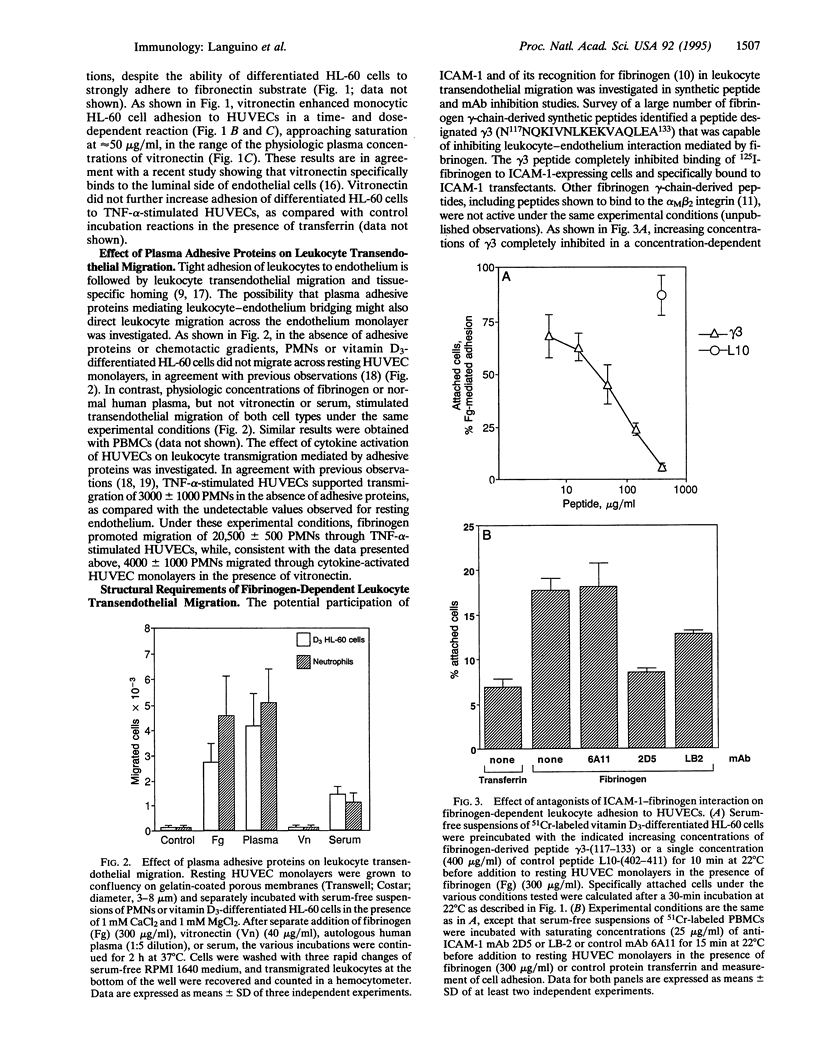
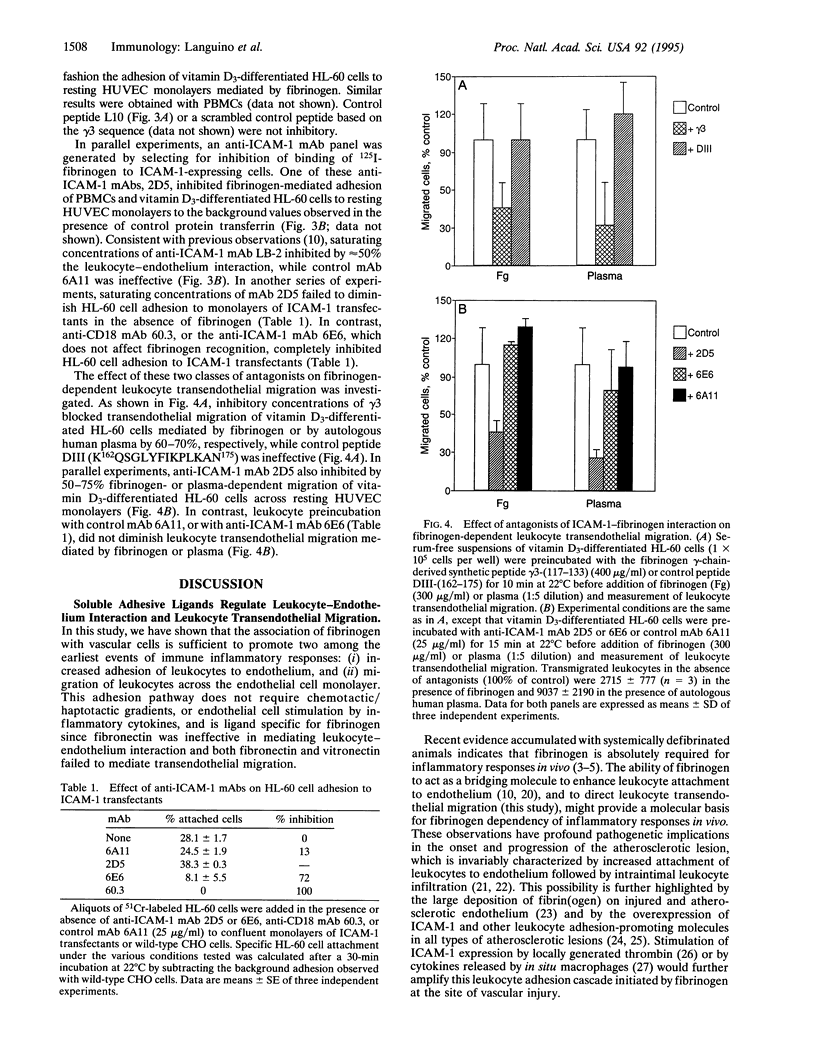
Although primarily recognized for its role in hemostasis, fibrinogen is also required for competent inflammatory reactions in vivo. It is now shown that fibrinogen promotes adhesion to and migration across an endothelial monolayer of terminally differentiated myelomonocytic cells. This process does not require chemotactic/haptotactic gradients or cytokine stimulation of the endothelium and is specific for the association of fibrinogen with intercellular adhesion molecule 1 (ICAM-1) on endothelium. Among other adhesive plasma proteins, fibronectin fails to increase the binding of leukocytes to endothelium, or transendothelial migration, whereas vitronectin promotes the binding but not the migration. The fibrinogen-mediated leukocyte adhesion and transendothelial migration could be inhibited by a peptide from the fibrinogen gamma-chain sequence N117NQKIVNL-KEKVAQLEA133, which blocks the binding of fibrinogen to ICAM-1. This interaction could also be inhibited by new anti-ICAM-1 monoclonal antibodies that did not affect the ICAM-1-CD11a/CD18 recognition, thus suggesting that the fibrinogen binding site on ICAM-1 may be structurally distinct from regions previously implicated in leukocyte-endothelium interaction. Therefore, binding of fibrinogen to vascular cell receptors is sufficient to initiate (i) increased leukocyte adhesion to endothelium and (ii) leukocyte transendothelial migration. These two processes are the earliest events of immune inflammatory responses and may also contribute to atherosclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Plescia J., Plow E. F. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (alpha M beta 2, Mac-1) and promotes leukocyte adhesion. J Biol Chem. 1993 Jan 25;268(3):1847–1853. [PubMed] [Google Scholar]
- Bini A., Fenoglio J. J., Jr, Mesa-Tejada R., Kudryk B., Kaplan K. L. Identification and distribution of fibrinogen, fibrin, and fibrin(ogen) degradation products in atherosclerosis. Use of monoclonal antibodies. Arteriosclerosis. 1989 Jan-Feb;9(1):109–121. doi: 10.1161/01.atv.9.1.109. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemer G. S., Edgington T. S. Monoclonal antibody to an activation neoepitope of alpha M beta 2 inhibits multiple alpha M beta 2 functions. J Immunol. 1994 Jun 15;152(12):5836–5844. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Furie M. B., McHugh D. D. Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with interleukin-1 or tumor necrosis factor-alpha. J Immunol. 1989 Nov 15;143(10):3309–3317. [PubMed] [Google Scholar]
- Hickstein D. D., Smith A., Fisher W., Beatty P. G., Schwartz B. R., Harlan J. M., Root R. K., Locksley R. M. Expression of leukocyte adherence-related glycoproteins during differentiation of HL-60 promyelocytic leukemia cells. J Immunol. 1987 Jan 15;138(2):513–519. [PubMed] [Google Scholar]
- Kuijpers T. W., Harlan J. M. Monocyte-endothelial interactions: insights and questions. J Lab Clin Med. 1993 Dec;122(6):641–651. [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino L. R., Plescia J., Duperray A., Brian A. A., Plow E. F., Geltosky J. E., Altieri D. C. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993 Jul 2;73(7):1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- McRitchie D. I., Girotti M. J., Glynn M. F., Goldberg J. M., Rotstein O. D. Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J Lab Clin Med. 1991 Jul;118(1):48–55. [PubMed] [Google Scholar]
- Moser R., Schleiffenbaum B., Groscurth P., Fehr J. Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest. 1989 Feb;83(2):444–455. doi: 10.1172/JCI113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Lymphocyte suppressive peptides from fibrinogen are derived predominantly from the A alpha chain. J Immunol. 1986 Sep 15;137(6):1910–1915. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Skogen W. F., Senior R. M., Griffin G. L., Wilner G. D. Fibrinogen-derived peptide B beta 1-42 is a multidomained neutrophil chemoattractant. Blood. 1988 May;71(5):1475–1479. [PubMed] [Google Scholar]
- Smith C. W. Transendothelial migration. Curr Top Microbiol Immunol. 1993;184:201–212. doi: 10.1007/978-3-642-78253-4_16. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Sugama Y., Tiruppathi C., offakidevi K., Andersen T. T., Fenton J. W., 2nd, Malik A. B. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J Cell Biol. 1992 Nov;119(4):935–944. doi: 10.1083/jcb.119.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993 Dec 1;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Hancock W. W. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- Ugarova T. P., Budzynski A. Z., Shattil S. J., Ruggeri Z. M., Ginsberg M. H., Plow E. F. Conformational changes in fibrinogen elicited by its interaction with platelet membrane glycoprotein GPIIb-IIIa. J Biol Chem. 1993 Oct 5;268(28):21080–21087. [PubMed] [Google Scholar]
- Wu X., Helfrich M. H., Horton M. A., Feigen L. P., Lefkowith J. B. Fibrinogen mediates platelet-polymorphonuclear leukocyte cooperation during immune-complex glomerulonephritis in rats. J Clin Invest. 1994 Sep;94(3):928–936. doi: 10.1172/JCI117459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti A., Conforti G., Hess S., Martìn-Padura I., Ghibaudi E., Preissner K. T., Dejana E. Clustering of vitronectin and RGD peptides on microspheres leads to engagement of integrins on the luminal aspect of endothelial cell membrane. Blood. 1994 Aug 15;84(4):1116–1123. [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Tigges A. J., Becker A. E. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992 Dec;141(6):1427–1433. [PMC free article] [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Tigges A. J., Becker A. E. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992 Dec;141(6):1427–1433. [PMC free article] [PubMed] [Google Scholar]


