Abstract
Summary
Previous studies have shown an association between duration of bisphosphonate use and atypical femur fractures. This cohort study showed an increasingly higher risk of subtrochanteric and femoral shaft fractures among those who were more adherent to oral bisphosphonates.
Introduction
Long-term use of oral bisphosphonates has been implicated in an increased risk of atypical femur fractures located in subtrochanteric and femoral shaft regions. Another measure of drug exposure, medication adherence, however, has not been investigated.
Methods
Among all Medicare fee-for-service female beneficiaries from 2006–2010, we followed 522,287 new bisphosphonate users from their index prescription until being censored or having a primary diagnosis of closed subtrochanteric/ femoral shaft or intertrochanteric/femoral neck fractures. Data about radiographs of fracture site and features were not available. Adherence was classified according to the medication possession ratio (MPR) as the following: MPR<1/3 as less compliant, MPR≥1/3–<2/3 as compliant, and MPR≥2/3 as highly compliant. Alternative cutoff points at 50 and 80 % were also used. Survival analysis was used to determine the cumulative incidence and hazard of subtrochanteric/femoral shaft or intertrochanteric/femoral neck fractures.
Results
There was a graded increase in incidence of subtrochanteric/femoral shaft fractures as the level of adherence increased (Gray’s test, P<0.001). The adjusted hazard ratio (HR) for the highly compliant vs. the less compliant was 1.23 (95 % Confidence Interval [CI] 1.06–1.43) overall, became significant after 2 years of follow-up (HR=1.51, 95 % CI 1.06–2.15) and reached the highest risk in the fifth year (HR=4.06, 95 % CI 1.47–11.19). However, age-adjusted incidence rates of intertrochanteric/femoral neck fractures were significantly lower among highly compliant beneficiaries, compared to less compliant users (HR=0.69, 95 % CI 0.66–0.73). Similar results were obtained when the cutoff points for being compliant and highly compliant were set at 50 and 80 %, respectively.
Conclusions
Subtrochanteric/femoral shaft fractures, unlike intertrochanteric/femoral neck fractures, are positively associated with higher adherence to long-term (≥3 years) oral bisphosphonates in the elderly female Medicare population.
Keywords: Bisphosphonates, Fracture, Osteoporosis, Atypical femur fracture
Introduction
Bisphosphonates, as potent antiresorptive agents, have been widely prescribed in the prevention and treatment of osteoporosis. While effective at reducing hip fractures such as intertrochanteric and femoral neck fractures, long-term use of bisphosphonates has been implicated in increased risks of atypical femur fractures [1–3]. Atypical femur fractures occur in the subtrochanteric region or in the femoral shaft. They are distinct subgroup of fractures that are associated with minimal trauma, prodromal pain, and particular radiographic features such as a transverse fracture orientation [4].
Numerous studies point to the secular trend of increased subtrochanteric/femoral shaft (ST/FS) fractures during the years of increasing bisphosphonate use [5–7] and among long-term bisphosphonate users [8–10]. Without radiographic adjudication, these fractures were found to be associated with bisphosphonates in one study [11] but not others [12–16]. With radiographic adjudication, atypical femur fractures were found to be strongly associated with bisphosphonates, although the absolute incidence remained low [8, 10, 17–20]. Additional radiographic features of these rare fractures have been defined and updated recently, with the notion that a causal relationship between bisphosphonate and atypical femur fractures has not been established [3].
One important feature that would support a causal relationship between bisphosphonate use and atypical femur fractures is a graded-response relationship between the two. Several studies have noted that a longer duration of bisphosphonate therapy was associated with a higher relative risk of atypical femur fracture [10, 17, 20], while others have not found similar associations [12, 13, 15]. Adherence to bisphosphonates, on the other hand, was important for reducing the risk of the intertrochanteric/femoral neck (IT/FN) fractures [21, 22], but its role in the risk of atypical femur or ST/FS fractures has been questioned [23].
Furthermore, there have been no large scale studies to quantify the actual risk and benefits associated with bisphosphonate use. To address these questions, we sought to investigate if there is a graded relationship between the cumulative incidence of ST/FS fractures and bisphosphonate exposure based on patient adherence among female Medicare beneficiaries, who initiated bisphosphonate treatment following their enrollments in the medication benefit program after 2005.
Materials and methods
Study population
We conducted a retrospective longitudinal cohort study, using data on Medicare beneficiaries provided by the Centers for Medicare and Medicaid Services (CMS). We included all women with at least 24 months of continuous coverage in the Part D drug program between 1/1/2006 and 12/31/2010 and at least one filled prescription for oral bisphosphonates (alendronate, risedronate, or ibandronate) during the study period. We excluded those women who were dually eligible for Medicare and Medicaid, or received medication benefit program (aka, Part D) due to low-income status. To identify new bisphosphonate users, we selected only those with at least a 3-month window between their Part D enrollment date and the date for first prescription for oral bisphosphonates (the index date). We excluded those with less than 90 days follow-up (defined below), not covered by both inpatient care (aka, Part A) and outpatient (aka, Part B) from 365 days prior to index date until the end of follow-up, not covered by prescription drug plans (PDPs) during the study period, a diagnosis of any hip fracture within 12 months prior to 90 days after the index date, or a diagnosis of malignancy or Paget disease any time during the study period.
Ascertainment of fractures
Users were followed from the index date until a primary inpatient diagnosis of subtrochanteric/femoral shaft (ST/FS) or intertrochanteric/femoral neck (IT/FN) closed fracture, a switch to another bisphosphonate or other types of anti-osteoporosis medications (raloxifene, calcitonin, denosumab, teriparatide, and estrogen), disenrollment, death or end of the study, whichever came first. From the cohort of oral bisphosphonate users, we captured their first instances of inpatient claims for hip fractures 90 days or more after index date with the following ICD9 codes: (1) ‘82022’, ‘82101’ for ST/FS fractures and (2) ‘82000’, ‘82001’, ‘82002’, ‘82003’, ‘82009’, ‘8208’, ‘82021’ for IT/FN fractures [5]. We followed the same order as above for prioritization when more than one site was diagnosed as fractured. Data about radiographs of fracture site and features were not available.
Data analysis
Adherence to oral bisphosphonates was calculated as the medicine possession ratio (MPR), i.e., the proportion of the number of days from index date to a set date that had been covered by the (re)fills of prescription. If there was an overlap in the covered days for consecutive (re)fills of prescription, the medication usage was deemed continuous. Persistence was also used as covariate and measured as whether the patients were refilling prescriptions at the current quarter [23].
We stratified the final cohort into subpopulations according to the MPR compliance at the end of each year of follow-up, estimated the absolute incidence rates of fractures during each year of follow-up, and directly standardized these rates to the age distribution of the subpopulations at the index date using 5-year age categories. The 95 % confidence limits of the standardized rates were estimated using the method developed by Fay and Feuer [24].
To test the graded-response relationship between adherence to bisphosphonates and fracture outcomes, we used competing-risk analysis to estimate the cumulative incidence of ST/FS fractures, with IT/FN fractures and death as competing interests. Gray’s test was used to test the equality of cumulative incidence functions among groups with different levels of compliance [25].
MPR was categorized as <1/3 (less compliant or low compliance), 1/3–<2/3 (compliant or moderate compliance), or ≥2/ 3 (highly compliant or high compliance). In addition, we used 1/2 (50 %) and 4/5 (80 %) as the conventional cutoff points. We treated MPR as time-dependent variable based on daily values of the variable, and we used proportional hazard Cox model to estimate the hazard ratio for the fracture outcomes.
In the multivariate model, we included age, race, prior vertebral fractures, healthcare utilization (total numbers of bone mineral density [BMD] test, hospitalization, doctor visits, or medications), comorbidities (dementia, Parkinson disease, alcoholism, liver disease, chronic kidney disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, inflammatory arthritis, and inflammatory bowel disease), and other medication usage (antiepileptics, benzodiazepines, opioids, proton pump inhibitors, statins, selective serotonin reuptake inhibitors, corticosteroids, and warfarin). Age was determined at the index date, and race was classified as White, Asian, and others. All the non-demographic variables were determined during the 12 months prior to the index date. Comorbidity index was developed using Gagne et al. 2011 [26]. Linearity and proportionality of hazard functions were tested using Martingale and Schoenfeld residuals, respectively. Particularly, the proportionality of time-dependent covariates such as MPR was tested against the main outcomes of ST/FS fractures and deemed linear.
All analyses were conducted using SAS Enterprise Guide 4.3 with SAS 9.2 (SAS Institute, Inc., Cary, NC, USA) at α= 0.05 significance level.
This study was reviewed and approved by CMS and the Institutional Review Board of NIH.
Results
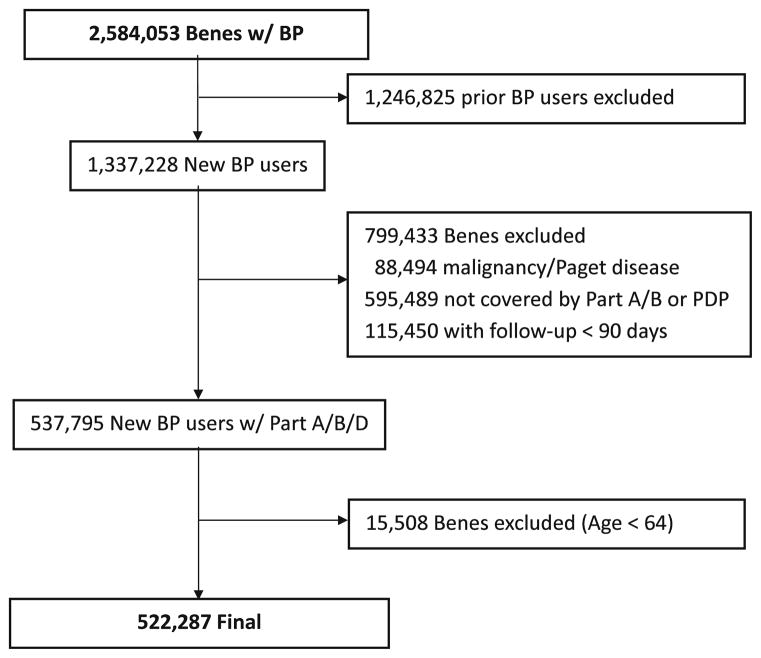
Since the inception of Part D in 2006 to the December 31, 2010, there were 2,584,053 female Medicare beneficiaries who participated in Part D for 24 consecutive months or more, and had at least one prescription of oral bisphosphonates (alendronate [60.3 %], risedronate [22.2 %], or ibandronate [17.5 %]) during the study period. In addition, after restricting to those with both Part A and B coverage during the study period (including the 12 months prior to index date) and prescription drug plan during the follow-up, 522,287 beneficiaries were eligible for data analysis (Fig. 1). Table 1 shows a description of the final cohort according to their compliance to oral bisphosphonates as MPR categories measured at the end of follow-up. It should be noted that at least 70 % of the beneficiaries reached the end of the study. There was no difference in the average age among beneficiaries with different levels of adherence, and there were minor differences in the race distribution.
Fig. 1.
Flow diagram of cohort selection. Benes beneficiaries, BP bisphosphonates, Part A inpatient care, Part B outpatient care, Part D drug benefit, PDP, prescription drug plan
Table 1.
Baseline Characteristics of the US Medicare female beneficiaries by medication possession ratio (MPR) at the end of follow-up
| MPR | Oral bisphosphonate users (N=522,287)
|
||
|---|---|---|---|
| <1/3 (N=214,615) | 1/3–<2/3 (N=112,556) | ≥2/3 (N=195,116) | |
| Demographic factors | |||
| Age, years, mean (SD) | 75.9 (7.2) | 75.7 (7.2) | 75.9 (7.2) |
| Race, White | 91.6 | 92.4 | 93.3 |
| Race, Asian | 1.4 | 1.6 | 1.8 |
| Health care utilization | |||
| No. of visits, mean (SD) | 21.4 (15.9) | 21.0 (15.8) | 20.1 (15.2) |
| Hospitalization | 7.0 | 6.9 | 8.3 |
| No. of prescriptions, mean (SD) | 7.9 (5.1) | 7.4 (5.0) | 7.1 (4.8) |
| Switched to other medications | 7.4 | 9.7 | 10.4 |
| Switched to different BIS | 10.0 | 15.8 | 15.6 |
| Reached the end of study | 78.6 | 70.7 | 70.7 |
| Comorbidities | |||
| Prior vertebral fractures | 3.1 | 3.0 | 3.1 |
| BMD test | 35.0 | 28.9 | 34.0 |
| Dementia | 1.8 | 2.2 | 2.3 |
| Parkinson disease | 2.2 | 2.2 | 2.0 |
| Alcoholism | 0.1 | 0.2 | 0.1 |
| Liver disease | 0.8 | 0.7 | 0.7 |
| Chronic kidney disease | 2.5 | 2.2 | 2.1 |
| Hypertension | 57.0 | 55.2 | 54.7 |
| Diabetes mellitus | 17.9 | 15.9 | 14.7 |
| Chronic obstructive pulmonary disease | 15.6 | 13.7 | 11.5 |
| Congestive heart failure | 5.2 | 4.5 | 4.2 |
| Inflammatory arthritis | 5.0 | 5.0 | 4.0 |
| Inflammatory bowel disease | 0.8 | 0.7 | 0.7 |
| Comorbidity index, mean (SD) | 0.6 (1.6) | 0.5 (1.6) | 0.5 (1.6) |
| Other medications | |||
| Antiepileptics | 3.0 | 2.8 | 2.8 |
| Benzodiazepines | 4.4 | 4.0 | 4.0 |
| Opioids | 4.2 | 3.6 | 3.0 |
| Proton pump inhibitors | 21.3 | 19.3 | 17.9 |
| Statin | 41.3 | 41.4 | 42.8 |
| Selective serotonin reuptake inhibitors | 16.1 | 15.2 | 13.3 |
| Steroid | 28.8 | 26.3 | 24.8 |
| Wafarin | 7.5 | 6.9 | 7.2 |
Based on the primary diagnosis, a total of 10,486 fractures were observed during a total of 1,240,435 person-years of follow-up: 948 subtrochanteric/femoral shaft (9.0 %) fractures; 9,521 (91.0 %) IT/FN fractures.
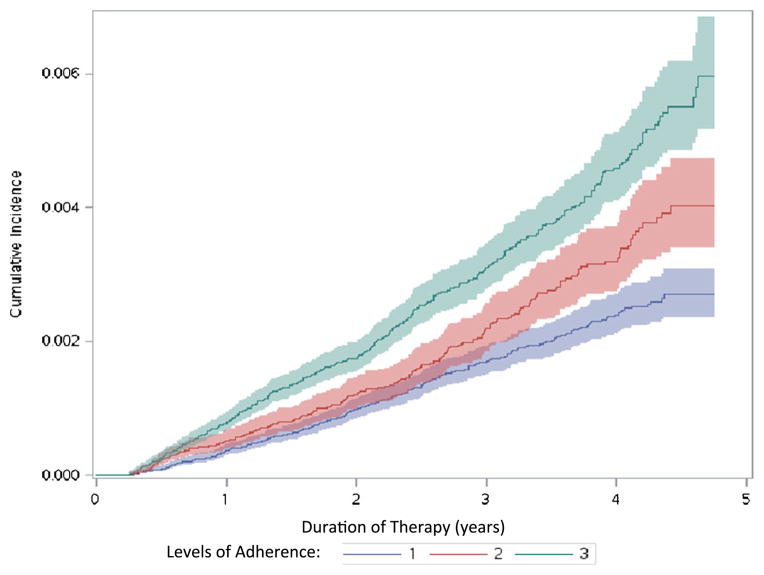
Figure 2 shows the cumulative incidence and number at risk of ST/FS fractures according to the adherence level of beneficiaries. There were significant graded differences between adherence levels to oral bisphosphonates and cumulative incidence functions of ST/FS fractures (Gray’s test, p<0.001). Specifically, the more adherent beneficiaries were, the more ST/FS fractures accumulated.
Fig. 2.
Cumulative incidence and numbers at risk of subtrochanteric/femoral shaft fractures according to the levels of adherence. 1 less compliant, 2 compliant, 3 highly compliant
Similar results were obtained when the cutoff points for being compliant and highly compliant were set at 50 and 80 %, respectively. However, the graded differences between there groups were not as linear as the original cutoff (data not shown).
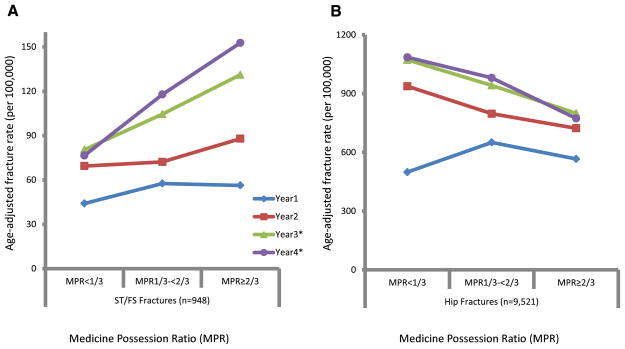
As shown in Fig. 3, the annual age-standardized incidence rates of ST/FS fractures (A) or IT/FN fractures (B) were plotted according the three categories of MPR measured at the end of each year of follow-up, or the end of follow-up if the beneficiary was censored during that year. For ST/FS fractures, no significant increases in the age-adjusted rates with higher level of compliance compared to those with lower levels of compliance were seen the first 2 years of treatment. However, in the third and fourth year of treatment, significant higher incidence rates of ST/FS fractures were detected for those with higher compliance (also see Table 2). Specifically, within the highly compliant group, the age-adjusted rate of ST/FS fractures increased from 56.3 per 100,000 person years in the first year to 152.7 in the fourth year, compared to an increase from 44.1 to 76.6 for the less compliant group during the same period. In contrast, for IT/FN fractures, the significant reductions in the age-adjusted rates with increasing levels of adherence were seen after just one year of exposure, with both the baseline rates and the magnitudes of reduction much larger than those of ST/FS fractures (Table 2).
Fig. 3.
Age adjusted incidence rate of subtrochanteric/femoral shaft fractures (a) and regular hip fractures (b) according to the categories of MPR (medication possession ratio) for each year (year 1–year 4) of bisphosphonate treatment. * indicates that tests were significance. The fifth year was absent due to empty cell counts during age standardization
Table 2.
Age-standardized fracture incidence rates (per 100,000 person-years) since the initiation of bisphosphonate treatment according to medication possession ratio (MPR)
| Intertrochanteric/femoral neck fractures
|
Subtrochanteric/femoral Shaft fractures
|
|||||
|---|---|---|---|---|---|---|
| MPR<1/3 | MPR 1/3–<2/3 | MPR ≥2/3 | MPR<1/3 | MPR 1/3–<2/3 | MPR ≥2/3 | |
| #Cases | ||||||
| Year 1 | 813 | 598 | 1307 | 72 | 53 | 130 |
| Year 2 | 1341 | 592 | 1016 | 99 | 54 | 123 |
| Year 3 | 1122 | 466 | 645 | 84 | 52 | 106 |
| Year 4 | 671 | 293 | 325 | 47 | 36 | 64 |
| Year 5 | 162 | 80 | 90 | <11 | 14 | <11 |
| Rate(95 % CI) | ||||||
| Year 1 | 498.8(465.1–534.4) | 650.6(599.5–704.9) | 566.2(535.9–597.8) | 44.1(34.5–55.5) | 57.6(43.2–75.4) | 56.3(47.0–66.9) |
| Year 2 | 937.5(888.0–989.1) | 797.1(734.2–864.0) | 722.8(679.0–768.7) | 69.4(56.4–84.5) | 72.2(54.2–94.2) | 87.9(73.1–105.0) |
| Year 3 | 1072.2(1010.4–1136.9) | 941.9(858.2–1031.7) | 799.4(738.8–864.7) | 80.5(64.2–99.7) | 104.5(78.0–137.1) | 131.1(107.3–158.7) |
| Year 4 | 1085.2(1004.5–1170.8) | 980.2(870.6–1099.9) | 773.3(691.1–862.8) | 76.5(56.1–101.8) | 117.8(82.4–163.5) | 152.7(117.5–195.4) |
| Year 5 | 1166.0(992.3–1361.6) | 1020.7(807.7–1273.1) | 892.4(715.8–1100.3) | 73.1(34.8–135.2) | 172.3(93.7–291.8) | na |
| P value | ||||||
| Year 1 | ref | <0.001 | 0.0046 | ref | 0.14 | 0.09 |
| Year 2 | ref | 0.001 | <0.001 | ref | 0.82 | 0.08 |
| Year 3 | ref | 0.02 | <0.001 | ref | 0.14 | 0.0009 |
| Year 4 | ref | 0.15 | <0.001 | ref | 0.05 | 0.0003 |
| Year 5 | ref | 0.33 | 0.04 | ref | 0.04 | na |
| P values | ||||||
| Year 1 | ref | 0.0049 | ref | 0.88 | ||
| Year 2 | ref | 0.06 | ref | 0.22 | ||
| Year 3 | ref | 0.007 | ref | 0.18 | ||
| Year 4 | ref | 0.003 | ref | 0.21 | ||
| Year 5 | ref | 0.39 | ref | na | ||
Table 3 shows multivariate analysis of common risk factors for ST/FS and for IT/FN fractures. Age and comorbidity were significantly associated with higher hazards of both ST/FS and IT/FN fractures. Other risk factors included prior vertebrate fracture, diabetes, and inflammatory arthritis. As for the ST/FS fractures among bisphosphonate users, the adjusted hazard ratio for the highly compliant vs. less compliant group was 1.23 (95 % confidence interval [CI] 1.06–1.43), whereas the hazard ratio comparing the moderate compliant group and less compliant group was not significant. As for the IT/FN fractures among these users, the adjusted hazard ratio for the highly compliant vs. less compliant group was 0.69 (95 % CI 0.66–0.73), whereas that for the moderate compliant group vs. the less compliant was 0.86 (95 % CI 0.81–0.90). Among all the other medications included for this study, statin use was associated with reduced risk of ST/FS fractures (HR=0.82, 95 % CI 0.71–0.94), and IT/FN fractures (HR=0.86, 95 % CI 0.82–0.90).
Table 3.
Multivariable-adjusted hazard ratios for subtrochanteric/femoral shaft (ST/FS) and intertrochanteric/femoral neck (IT/FN) fractures
| Covariate | Hazard ratio for ST/FS Fx (95 % CI) | P value | Hazard ratio for IT/FN Fx (95 % CI) | P value |
|---|---|---|---|---|
| Age | 1.36(1.30–1.42) | <0.001 | 1.59(1.57–1.62) | <0.001 |
| Less compliant (MPR: <1/3) | 1.00 | ref | 1.00 | ref |
| Compliant (MPR:1/3–<2/3) | 1.15(0.97–1.38) | 0.07 | 0.86(0.81–0.90) | <0.001 |
| Highly compliant (MPR:≥2/3) | 1.23(1.06–1.43) | 0.007 | 0.69(0.66–0.73) | <0.001 |
| Prior vertebral fractures | 1.84(1.41–2.41) | <0.001 | 1.88(1.74–2.04) | <0.001 |
| Alcoholism | 2.58(0.82–8.16) | 0.10 | 2.28(1.58–3.30) | <0.001 |
| Diabetes mellitus | 1.33(1.13–1.57) | <0.001 | 1.12(1.07–1.19) | <0.001 |
| Inflammatory arthritis | 1.78(1.39–2.29) | <0.001 | 1.35(1.23–1.48) | <0.001 |
| Statin | 0.82(0.71–0.94) | 0.004 | 0.86(0.82–0.90) | <0.001 |
| Comorbid index | 1.09(1.03–1.15) | 0.004 | 1.08 (1.06, 1.10) | <0.001 |
ST/FS subtrochanteric/femoral shaft, IT/FN intertrochanteric/femoral neck, Fx, fractures, CI confidence intervals, MPR medication possession ratio
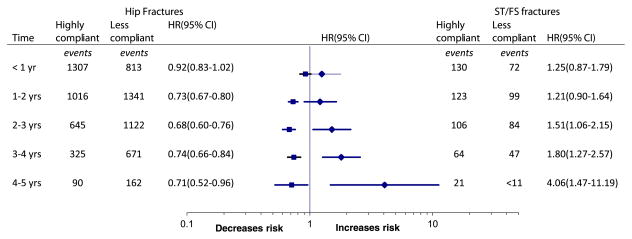
Figure 4 shows the adjusted hazard ratios of IT/FN and ST/ FS fractures comparing highly compliant vs. less compliant group, based on the number of years of treatment. After 1 year of treatment, the risk of IT/FN fractures in high compliance group became significantly lower than that in low compliant group and remained so for the rest of treatment duration. In contrast, the hazard for ST/FS fractures turn significantly higher after 2 years of treatment, and reached the highest risk at 4.06 (95 % CI 1.47–11.19) in the fifth year.
Fig. 4.
Multivariate-adjusted hazard ratios (HR) for regular hip fractures (left side) and subtrochanteric/femoral shaft fractures (right side) by treatment duration. For numbers at risk, please see Fig. 2
Discussion
In this study of over half-a-million female Medicare fee-for-service beneficiaries, we showed that higher exposure to bisphosphonates was associated with increased risk of subtrochanteric/femoral shaft fractures, in contrast to significant reductions of IT/FN fractures. To our knowledge, this is the first cohort study that demonstrates these inverse relationships in a large nationwide cohort. In addition, the positive association tended to depend on bisphosphonate treatment in both dose-response and duration-of-use manners, supporting the hypothesis that cumulative exposure to oral bisphosphonates may be causally related to these rare ST/FS fractures.
Our results showed that both age- and multivariate-adjusted rates of ST/FS fractures were significantly higher after 2 years of treatment among patients in the high compliance group as compared to less compliant group, consistent with findings from other studies[5, 10, 20]. Similarly, recent studies indicated that there were modest increases in age-adjusted incidence rates of atypical hip fractures within 2 years of bisphosphonate treatment, but significantly more after 2 years [10, 20]. This implies that less than 5 years of treatment may be sufficient for the risks of these fractures to become significantly higher, especially for those with high adherence. Thus, the determination of drug exposure before drug holiday should take adherence into consideration, not just the duration of exposure.
In this study, we selected long-term users with at least 24 months of continuous Part D coverage. More than 70 % of our subjects reached the end of study, providing a stable population to study long-term effect of medications. In contrast, less than one-third of enrollees remained in cohorts generated through commercial insurance databases [23]. This may account for some of the differences in results from previous studies using commercial databases [23]. Additionally, we used principal diagnosis of fracture as the primary outcomes. Interestingly, significant results were obtained from Parzianas et al. when they used principal diagnoses for sensitivity analysis (see Table 3, column 6) to study the hazard associated with each additional year of therapy with oral bisphosphonates. When they used any diagnosis as the fracture outcomes, the results were not significant, perhaps due to less specificity associated with non-primary diagnosis.
Based on our age-adjusted rates from the highly and less compliant groups, we estimated that, during 4th year of treatment, there was a decrease of 312 per 100,000 in the rate of IT/FN fractures, but an increase of 76 per 100,000 in the rate of ST/FS fractures. So, the number needed to treat with high adherence to prevent an IT/FN fracture was 320, compared to 1,316 for one additional ST/FS fracture as the number needed to harm. Clearly, the number needed to harm (NNH) is higher that the number needed to treat (NNT), even without considering preventive effects from bisphosphonate treatment on vertebral and other non-vertebral fractures. However, it should be pointed out that our study compared bisphosphonate users with different adherence, whereas other studies compared users with non-users. It is expected that the ratio of NNT/NNH should be different when comparing users against non-users. However, the overall benefit of bisphosphonate therapy within 5 years is reassuring [10].
Our study has several advantages over prior studies. First, our sample size was one of the largest, allowing us to study rare events such as ST/FS fractures. Second, we reduced the bias of confounding-by-indication associated with comparison between users with non-users [12, 13, 20], by comparing users with similar clinical indication but with varying degrees of compliance. Third, we used adjustment techniques including competing-risk analysis, direct age standardization, and multivariate survival analysis, all of which reached similar conclusions.
Despite these advantages, our study has certain limitations. First, while we do have a very large cohort, their experience may not represent that of the entire female Medicare beneficiary population as we excluded those who had shorter term of Part D coverage or in low-income status. Additional biases may have been introduced as we had no information about the bisphosphonate use prior to beneficiaries’ enrollment into part D and used a minimal gap of 3 months between enrollment date and index date to define new bisphosphonate users. Furthermore, although we managed to reduce the confounding by indication, there are still some fundamental differences between patients with different levels of adherence, such as their motivation to adhere to medication based on the strength of the indication. And finally, the non-specificity of our outcomes from claims data [27] without X-ray adjudication could elevate our type II error rate, reducing the likelihood that we would detect significant associations and limit our ability to define additional risk factors that may help identify those susceptible to these fractures.
In conclusion, we found that increased adherence to bisphosphonate medications was associated with an elevated risk of subtrochanteric/femoral shaft fracture as well as a decrease in the incidence of IT/FN fracture. The progressive increase in subtrochanteric/femoral shaft fractures following each additional year of treatment is in contrast to a steady reduction in the IT/FN fractures. Postmenopausal women who benefited from the initiation of bisphosphonate treatment far outnumber those who might suffer subtrochanteric or femoral shaft fractures following 2 years or more of treatment with high adherence. The clinical implication of this study is that adherence to oral bisphosphonates after 2 years of treatment remains beneficial for overall fracture reduction but needs to be monitored for possible increased risk of atypical femur fractures.
Acknowledgments
This study was funded by the Intramural Research Program at NIAMS/NIH. We want to thank Shannon Pietzsch from General Dynamic Information Technology, Inc., Drs. Elizabeth Rasch and Alex Constantin from Department of Rehabilitation Medicine of NIH Clinical Center, and Dr. Seo Young Kim from Brigham and Woman’s hospital for their supports.
Footnotes
Author’s roles: ZW, MW, and TB had full access to the study details and output but limited access to the data due to the CMS ENCLAVE data user agreement, and will take responsibility for the integrity of the data and the accuracy of data analysis. Conception and design: ZW, MW, TB and LC. Data acquisition: ZW and LC. Analysis and interpretation: ZW, MW, and TB. Drafting of manuscript: ZW. Critical revision of the manuscript: ZW, MW, LC, and TB. Statistical analysis: ZW and MW.
Conflicts of interest: None.
Contributor Information
Z. Wang, Intramural Research Program, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA
M. M. Ward, Intramural Research Program, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA
L. Chan, Department of Rehabilitation Medicine, the Clinical Center, National Institutes of Health, Bethesda, MD, USA
T. Bhattacharyya, Email: timothy.bhattacharyya@nih.gov, Intramural Research Program, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA. Bldg 10 CRC 4-1350, 10 Center Drive, Bethesda 20892, USA
References
- 1.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 3.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American society for bone and mineral research. J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. Epub 2013/05/29. [DOI] [PubMed] [Google Scholar]
- 4.Schilcher J, Koeppen V, Ranstam J, Skripitz R, Michaelsson K, Aspenberg P. Atypical femoral fractures are a separate entity, characterized by highly specific radiographic features. A comparison of 59 cases and 218 controls. Bone. 2013;52(1):389–392. doi: 10.1016/j.bone.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Bhattacharyya T. Trends in incidence of subtrochanteric fragility fractures and bisphosphonate use among the US elderly, 1996–2007. J Bone Miner Res. 2011;26(3):553–560. doi: 10.1002/jbmr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng AC, Drake MT, Clarke BL, Sems SA, Atkinson EJ, Achenbach SJ, et al. Trends in subtrochanteric, diaphyseal, and distal femur fractures, 1984–2007. Osteoporos Int. 2012;23(6):1721–1726. doi: 10.1007/s00198-011-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon BH, Lee YK, Kim SC, Kim SH, Ha YC, Koo KH. Epidemiology of proximal femoral fractures in South Korea. Arch Osteoporos. 2013;8(1–2):157. doi: 10.1007/s11657-013-0157-9. [DOI] [PubMed] [Google Scholar]
- 8.Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med. 2012;172(12):930–936. doi: 10.1001/archinternmed.2012.1796. [DOI] [PubMed] [Google Scholar]
- 9.Feldstein AC, Black D, Perrin N, Rosales AG, Friess D, Boardman D, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res. 2012;27(5):977–986. doi: 10.1002/jbmr.1550. [DOI] [PubMed] [Google Scholar]
- 10.Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 11.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. Jama. 2011;305(8):783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab. 2010;95(12):5258–5265. doi: 10.1210/jc.2010-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–1102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao FY, Huang WF, Chen YM, Wen YW, Kao YH, Chen LK, et al. Hip and subtrochanteric or diaphyseal femoral fractures in alendronate users: a 10-year, nationwide retrospective cohort study in Taiwanese women. Clin Ther. 2011;33(11):1659–1667. doi: 10.1016/j.clinthera.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res. 2011;26(5):993–1001. doi: 10.1002/jbmr.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vestergaard P, Schwartz F, Rejnmark L, Mosekilde L. Risk of femoral shaft and subtrochanteric fractures among users of bisphosphonates and raloxifene. Osteoporos Int. 2011;22(3):993–1001. doi: 10.1007/s00198-010-1512-y. [DOI] [PubMed] [Google Scholar]
- 17.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20(8):1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med. 2010;362(19):1848–1849. doi: 10.1056/NEJMc0910389. [DOI] [PubMed] [Google Scholar]
- 19.Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone. 2011;48(5):966–971. doi: 10.1016/j.bone.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–1441. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 23.Pazianas M, Abrahamsen B, Wang Y, Russell RG. Incidence of fractures of the femur, including subtrochanteric, up to 8 years since initiation of oral bisphosphonate therapy: a register-based cohort study using the US MarketScan claims databases. Osteoporos Int. 2012;23(12):2873–2884. doi: 10.1007/s00198-012-1952-7. [DOI] [PubMed] [Google Scholar]
- 24.Fay MP, Feuer EJ. A semi-parametric estimate of extra-Poisson variation for vital rates. Stat Med. 1997;16(21):2389–2401. doi: 10.1002/(sici)1097-0258(19971115)16:21<2389::aid-sim683>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 26.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narongroeknawin P, Patkar NM, Shakoory B, Jain A, Curtis JR, Delzell E, et al. Validation of diagnostic codes for subtrochanteric, diaphyseal, and atypical femoral fractures using administrative claims data. J Clin Densitom. 2012;15(1):92–102. doi: 10.1016/j.jocd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]