Abstract
Improvements in digital amplification, cochlear implants, and other innovations have extended the potential for improving hearing function; yet, there remains a need for further hearing improvement in challenging listening situations, such as when trying to understand speech in noise or when listening to music. Here, we review evidence from animal and human models of plasticity in the brain’s ability to process speech and other meaningful stimuli. We considered studies targeting populations of younger through older adults, emphasizing studies that have employed randomized controlled designs and have made connections between neural and behavioral changes. Overall results indicate that the brain remains malleable through older adulthood, provided that treatment algorithms have been modified to allow for changes in learning with age. Improvements in speech-in-noise perception and cognition function accompany neural changes in auditory processing. The training-related improvements noted across studies support the need to consider auditory training strategies in the management of individuals who express concerns about hearing in difficult listening situations. Given evidence from studies engaging the brain’s reward centers, future research should consider how these centers can be naturally activated during training.
Introduction
Hearing aid satisfaction has significantly improved in the last decade, largely because of improvements in hearing aid algorithms, which now provide better signal-to-noise ratios (SNRs) and better comfort (Hawkins & Yacullo, 1984; Kochkin, 2010; Valente, Fabry, & Potts, 1995). Nevertheless, even with the best technology, hearing aid users encounter difficulty when trying to understand a conversation in noise. In fact, some hearing aid wearers will remove one hearing aid to “to hear better in noise,” despite the demonstrated advantages of binaural hearing for listening in noise (as reviewed in Holmes, 2003). Similarly, although many individuals achieve excellent open-set word recognition through cochlear implants in quiet environments, they continue to struggle in difficult listening environments. There has been greater recognition of the roles of central processing and cognition in the ability to communicate with others and awareness that amplification and implantation provide audibility but may not improve sound processing ability. For these reasons, increased attention has focused on the efficacy of providing auditory training to individuals with hearing difficulties.
In this review, we first summarize effects of aging and hearing loss on physiological central processing of speech in both animal and human models. These biological mechanisms may underlie speech-in-noise perception difficulties in older adults, and they support the need to consider auditory training when developing treatment plans. We then consider studies that have demonstrated plasticity through both long- and short-term training in animal and human models. Examples of plasticity from long-term life experiences include language exposure and musical training. Short-term laboratory-based experiments in humans have demonstrated principles of auditory plasticity, such as stimulus-specific learning and generalized effects of training. We then review software-based auditory training strategies that have demonstrated benefits for real-world communication in both young and older adults. Finally, the implications of these studies are discussed in terms of optimal training strategies and future research questions.
Effects of Aging
Older adults experience greater difficulty hearing in background noise than do younger adults (Gordon-Salant, 2005; Souza, Boike, Witherell, & Tremblay, 2007), and these differences cannot be explained solely by reduced audibility (Hargus & Gordon-Salant, 1995; Souza et al., 2007). Animal and human models of the aging auditory system support the theory that slower neural processing accompanies aging. Several mechanisms may contribute to this slowing, including prolonged neural refractory times (Parthasarathy & Bartlett, 2011; Recanzone, Engle, & Juarez-Salinas, 2011; Walton, Frisina, & O’Neill, 1998), loss of myelin integrity (Lu et al., 2011), decreased brain connectivity (Forstmann et al., 2011), and increased variability in neural firing (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2012; Clinard & Tremblay, in press; MacDonald, Nyberg, & Bäckman, 2006; Turner, Hughes, & Caspary, 2005; Wang, Wu, Li, & Schneider, 2011; Yang, Liang, Li, Wang, & Zhou, 2009). Precise subcortical timing is an important factor in the ability to understand speech in noise (Anderson, Skoe, Chandrasekaran, & Kraus, 2010), and accurate encoding of both temporal and spectral aspects of speech is crucial for identification of the target speaker in a background of other talkers (as reviewed in Shinn-Cunningham & Best, 2008). Therefore, deficits in spectrotemporal processing may cause the older adult to experience difficulties when listening to speech in noise (Harkrider, Plyler, & Hedrick, 2005; Tremblay, Piskosz, & Souza, 2003).
Because peripheral hearing loss often accompanies advanced age, especially in the higher frequencies, it is difficult to rule out the influence of hearing loss on perceptual or physiological central processing, even in well-controlled studies (Anderson et al., 2012). A recent meta-analysis undertaken by the American Academy of Audiology Task Force on Central Presbycusis concluded that there is little evidence to support the existence of perceptual declines in central auditory function that are independent of hearing loss or cognitive function (Humes et al., 2012). Moreover, cognitive deficits can exacerbate slight declines in hearing sensitivity, thus further contributing to the older adult’s speech-in-noise perception deficits (Pichora-Fuller, 2003). Despite these findings, there is evidence supporting the remediation of central auditory processing and cognitive deficits; these studies will be addressed below.
Effects of Hearing Loss
Although technology has improved the ability to provide increased audibility and better SNRs (Hawkins & Yacullo, 1984; Valente et al., 1995), individuals with hearing loss continue to struggle when listening in difficult environments. These difficulties may arise from consequences of hearing loss on physiological central processing, including increased excitability (Kotak et al., 2005), decreased inhibition (Dong, Rodger, Mulders, & Robertson, 2010), tonotopic reorganization (Thai-Van, Veuillet, Norena, Guiraud, & Collet, 2010), and a disrupted balance of speech cues (Henry & Heinz, 2012; Kale & Heinz, 2010). These neural changes may have repercussions for speech understanding.
Two components of speech—the envelope and temporal fine structure—may provide a framework for understanding the hearing impaired individual’s perceptual difficulties. The envelope represents the slowly modulating aspect of speech, and the temporal fine structure (TFS) refers to the rapidly modulating aspect of the signal that carries the envelope. Envelope cues are adequate for hearing in quiet situations, but TFS cues appear to be important for understanding speech in noise (Lorenzi, Gilbert, Carn, Garnier, & Moore, 2006; Rubinstein, 2004; Shannon, 2007). In an animal model, Heinz and colleagues have determined that VIII nerve coding of the envelope is enhanced with hearing loss (Kale & Heinz, 2010), while the coding of the TFS is unchanged in quiet but decreased in noise (Henry & Heinz, 2012). These effects suggest that a disruption in the balance of envelope and TFS may contribute to the hearing-impaired listener’s hearing difficulties—increased envelope encoding may result from “turning up the energy of the auditory signal,” possibly from efferent activation of a central gain mechanism (Munro & Blount, 2009). However, this increased envelope gain may make the TFS less salient, especially in noise. Hearing aids increase volume; even with compression, they will likely increase envelope coding, which may be counterproductive for the individual with hearing loss—especially in louder environments, such as crowded restaurants or large family gatherings.
Combined Effects of Aging and Hearing Loss
Both younger and older individuals with hearing loss experience pronounced decreases in understanding of syntactically complex sentences, but older hearing-impaired adults experience the greatest losses in understanding (Wingfield, McCoy, Peelle, Tun, & Cox, 2006). Furthermore, the effects of speech rate are greater for participants with hearing impairment than for participants with normal hearing, suggesting that effortful hearing drains them of needed additional resources as the processing load is increased. Younger and older listeners with hearing loss experience only small decreases in understanding of syntactically easy sentences, and only at higher speech rates. For both complex and simple sentence structures, aging has a smaller effect than hearing loss and can be seen only at the higher speech rates, consistent with previously noted temporal processing declines in older adults (Anderson et al., 2012; Gordon-Salant, Fitzgibbons, & Friedman, 2007; Tremblay et al., 2003).
Therefore, management of older individuals’ hearing loss must consider strategies to compensate for deficits that emerge with high processing or cognitive loads. Waning cognitive ability can exacerbate slight declines in hearing sensitivity and contribute to the older adult’s speech-in-noise perception deficits (Pichora-Fuller, 2003), suggesting that cognitive training may also benefit speech-in-noise perception. These aspects of training will be considered below.
Auditory Training
Long-Term Plasticity
Language Experience
The plasticity of the auditory system (i.e., changes in brainstem or cortical processing) is evident in studies demonstrating the modulating influences of life experiences, specifically language and musical experience. For example, tonal-language speakers have enhanced brainstem encoding of linguistically relevant pitch contours relative to non-native tonal-language speakers who have not been exposed to these contours in a linguistic context (Krishnan, Xu, Gandour, & Cariani, 2005). This tonal-language speaker advantage was also found for pitch representation in severely degraded stimuli, with greater pitch strength for rapid changes of pitch (Krishnan, Bidelman, & Gandour, 2010). Krishnan and colleagues suggest that language experiences sharpen the tuning properties of neurons engaged in processing pitch contours, even in noise, enabling the listener to extract meaning from communication in suboptimal environments. It has also been demonstrated that this enhanced pitch processing develops over time; in a comparison of pitch processing in infant and adult English and Mandarin Chinese speakers, only the adult Chinese speakers demonstrated pitch enhancement—it was not present in the brainstem responses of infant Chinese speakers or English speakers (Jeng et al., 2011).
Similarly, bilingual language speakers have greater brainstem encoding of the fundamental frequency (F0) compared to monolingual speakers for stimuli presented both in quiet and in noise, with this advantage present as early as adolescence (Krizman, Marian, Shook, Skoe, & Kraus, 2012; Figure 1). The strength of F0 encoding in this study was related to a measure of auditory attention in the bilingual children, suggesting that the enhancement may be driven by afferent enhancements from immersion in a linguistically rich environment, combined with top-down executive function control as the bilingual speaker attends to specific features of the two languages. Evidence of enhanced neural function can also be found in older bilingual adults (Luk, Bialystok, Craik, & Grady, 2011), for whom diffusion tensor imaging revealed higher white matter integrity than in older monolingual adults. The enhanced white matter integrity, measured by fractional anisotropy, was found in the corpus callosum extending to the inferior longitudinal fasciculi. Furthermore, a resting-state functional connectivity analysis showed that bilinguals had stronger anterior to posterior functional activity compared to monolinguals.
Figure 1.
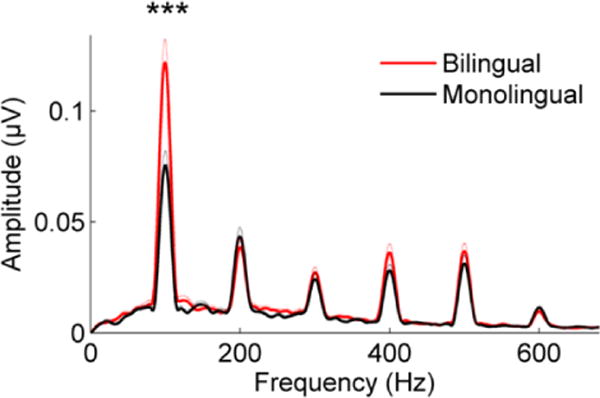
Bilingual adolescent children (red) have greater representation of the fundamental frequency than age-matched monolinguals (black) in brainstem responses to the speech syllable [da] presented in noise. Adapted from Krizman et al., 2012. Thin dotted lines: 1 S.E., ***p < 0.001.
Musical Experience
Musical experience appears to have a powerful influence on auditory skill development. Musicians are not only better tuned to music, but they also appear to have an advantage for processing speech. The benefits of musical training for speech processing might be understood from a framework proposed by Ani Patel (2011), termed the OPERA hypothesis. Patel suggests that music is an important contributor to auditory learning for the following reasons: there is overlap in the anatomical pathways or brain networks that process speech and music components, the precision required for music processing is greater than that of speech, the emotion elicited in performing music induces plasticity, the repetition involved in extensive practice of a musical instrument tunes the auditory system, and attention is necessary for focusing on the details of musical sounds.
The factors listed in the OPERA hypothesis may lead to enhanced neural encoding of speech and music. This enhancement can occur even in infancy: infants who participated in “active engagement” music classes for 6 months had larger and earlier cortical responses to a piano note compared to infants who participated in “passive” music classes (Trainor, Marie, Gerry, Whiskin, & Unrau, 2012). School-age children who have consistently practiced a musical instrument for at least 5 years not only have better scores on the Hearing in Noise Test (HINT; Nilsson, Soli, & Sullivan, 1994), but also have brainstem responses to speech that are more resistant to the degradative effects of noise than nonmusicians (Strait, Parbery-Clark, Hittner, & Kraus, 2012). This resistance to noise degradation in the brainstem has also been found in young adult musicians (Bidelman & Krishnan, 2010; Parbery-Clark, Skoe, & Kraus, 2009). Older normal-hearing musicians have faster brainstem timing and greater representation of the harmonics of a speech syllable compared to age-matched peers (Parbery-Clark, Anderson, Hittner, & Kraus, 2012b), and the expected age-related delays in subcortical timing (Anderson et al., 2012) are offset in middle-aged musicians (Parbery-Clark, Anderson, Hittner, & Kraus, 2012a; Figure 2). Musicians’ enhanced neural processing has also been found in cortical responses to speech: young adult musicians have earlier cortical latencies than nonmusicians, and these measures, along with brainstem onset timing and harmonic representation, are correlated with the age of onset of musical training and years of practice (Musacchia, Strait, & Kraus, 2008). A neural correlate of superior concurrent stream segregation (a necessary component of speech-in-noise perception) is found in cortical responses of musicians compared to nonmusicians (Zendel & Alain, 2012). Musicians also demonstrate cross-domain effects—that is, musicians who are non–tonal-language speakers experience similar enhancements in brainstem pitch tracking as those observed in native tonal-language speakers in comparison to nonmusician, non–tonal-language speakers (Bidelman, Gandour, & Krishnan, 2009; Wong, Skoe, Russo, Dees, & Kraus, 2007).
Figure 2.
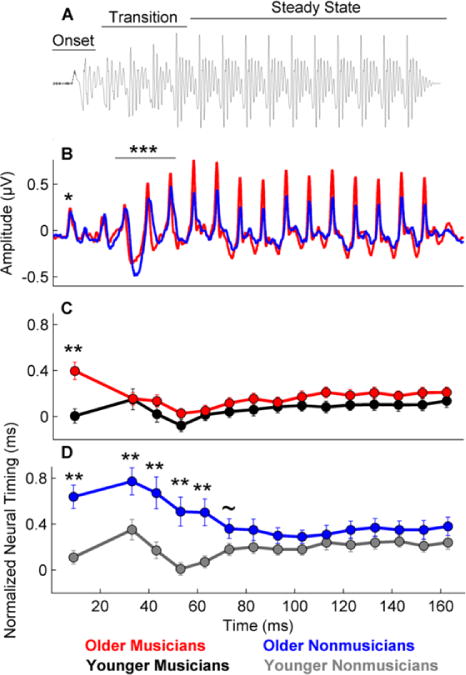
Older musicians have earlier brainstem timing than older nonmusicians. A. The stimulus waveform for the speech syllable [da] with 3 time regions marked: onset, transition (20–60 ms) and steady state (160–170 ms). B. Brainstem waveforms in response to the speech syllable [da] presented in quiet. Older musicians (red) have earlier responses and greater amplitudes than older nonmusicians (blue). C. No age-related latency delays are noted in older musicians (red) compared to younger musicians (black), except for the onset. D. Compared to younger nonmusicians, older nonmusicians (blue) have significantly delayed brainstem latencies for the onset and transition, but not for the steady-state region of the response. Adapted from Parbery-Clark et al., Neurobiology of Aging, 2012 and Parbery-Clark et al., 2012b. ~p < 0.10, **p < 0.01. Error bars equal 1 S.E.
Importantly, musicians also exhibit superior listening skills in noise and in related cognitive skills. Given the nature of musical training, and the complexity of listening in noise, this makes sense. After all, memory ability has an important role in speech-in-noise perception: as perception becomes degraded, individuals rely on cognitive resources such as memory to fill in gaps of missing information (Pichora-Fuller, Schneider, & Daneman, 1995). Musical training appears to offset age-related declines both in speech-in-noise perception and in working memory in middle-aged adults, and working memory also relates to speech-in-noise perception in middle-aged to older adults (Parbery-Clark, Strait, Anderson, Hittner, & Kraus, 2011), children (Strait et al., 2012), and young adults (Parbery-Clark, Skoe, Lam, & Kraus, 2009). These benefits appear to be specific to music training and are not seen in other forms of training such as art instruction (Moreno et al., 2011).
To understand the neural mechanisms underlying better memory in musicians, Gaab and Shlaug (2003) compared cortical activation in musicians and nonmusicians, who were matched on pitch memory ability, using a sparse temporal sampling fMRI method to ensure that the clustered volume MR acquisition time (2.75 ms) was separated from the actual auditory task, with high-resolution scanning (1 mm3). They found that musicians had greater activation of auditory storage sites (i.e., superior parietal lobe, supramarginal gyrus, and inferior frontal gyrus) than nonmusicians, who more heavily recruited sensory areas in the primary and secondary auditory cortices. Nonmusicians appear to rely on brain regions that are important for pitch discrimination, focusing on the perceptual component of the task, whereas musicians use brain regions specialized for short-term memory or recall, presumably because the perceptual component does not require as much effort as it does in nonmusicians.
These results demonstrate that consistent musical practice results in auditory advantages for normal-hearing individuals across all age groups, but there is also evidence for benefits from the type of less-intense musical training that many children and teenagers typically participate in during elementary and high school years, even after music training stops. The brainstem frequency following response (FFR) is smaller in amplitude for normal-hearing young adults who have never had musical training compared to those who have had even a few years of training in childhood (Skoe & Kraus, 2012), consistent with animal data showing the impact of childhood experiences of enriched or restricted environments on the adult’s brain (Engineer et al., 2004; Yu, Sanes, Aristizabal, Wadghiri, & Turnbull, 2007). Furthermore, the robustness of brainstem encoding in the musical training groups related to the number of years since music lessons were discontinued (Figure 3). There is also evidence from structural equation modeling that suggests that though cognitive resources (memory, attention) contribute significantly to speech-in-noise perception in all older adults (hearing levels ranging from normal to moderate hearing loss), cognition accounts for more of the variance in older adults who took music lessons in childhood. On the other hand, life experiences (based on socioeconomic status and physical activity) account for more of the variance in those with no musical training (Anderson, White-Schwoch, Parbery-Clark, & Kraus, 2013).
Figure 3.
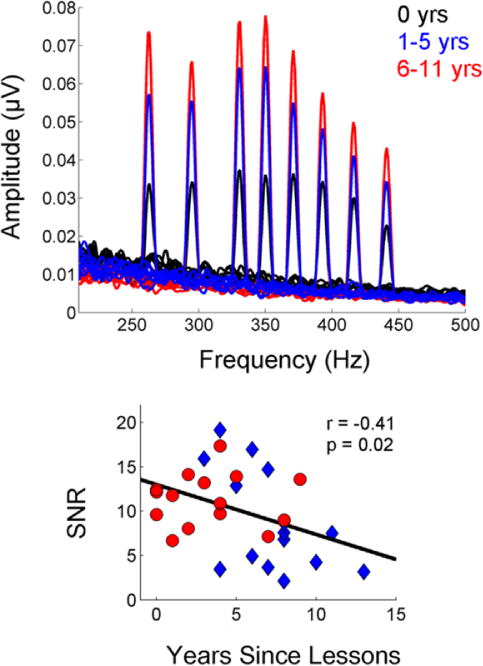
A history of taking music lessons is associated with more robust brainstem responses. Top. Young adults who have had either 1 to 5 (blue) or 6 to 11 (red) years of musical training have higher amplitude brainstem responses than young adults with no musical experience (black) in response to 8 sounds of varying frequency. Bottom: The number of years since musical lessons is negatively correlated with the SNR of the brainstem response. Adapted from Skoe and Kraus, 2012.
Short-Term Plasticity
Musical Training for Rehabilitation
Though there is robust evidence that long-term plasticity from linguistic or musical experience engenders auditory benefits, long-term training is not a viable option in the clinic. Therefore, short-term training strategies must be developed. There may be benefits of starting musical training later in life. Older adults, ages 60–85 years, with no previous musical experience who participated in a music education program involving 30-minute weekly piano lessons and at least 3 weekly hours of practice had better speed of processing and memory after just 3 months, while a control group showed no changes (Bugos, Perlstein, McCrae, Brophy, & Bedenbaugh, 2007). In another study, recent musical experience predicted visuospatial skills, even in individuals with low education, suggesting that musical training may serve as a buffer against impoverished early education influences on cognitive function (Hanna-Pladdy & Gajewski, 2012).
Future work should consider musical training as a remediation tool for older adults and individuals of all ages with hearing loss. There has been increased interest in using music as a training tool for improving music perception in cochlear implant users (Driscoll, Oleson, Jiang, & Gfeller, 2009; Fu & Galvin, 2007; Galvin, Fu, & Shannon, 2009; Gfeller et al., 2007; Limb & Rubinstein, 2012). Given the converging results of musician studies, one might expect that benefits of musical training would transfer to speech perception in this population.
Animal Models
Several investigators have used animals as models of the neural mechanisms underlying auditory plasticity. For example, a ferret model has been used to demonstrate auditory plasticity in primary auditory cortex using a task requiring signal detection in noise (Atiani, Elhilali, David, Fritz, & Shamma, 2009). Spectrotemporal receptive fields (STRFs) in a passive state were compared to STRFs in an active task (trained to lick water only when the target tone was presented to avoid a mild shock). Greater STRF gain was noted in cells close to the target tone’s best frequency at high SNRs, but at low SNRs, there was suppression of cells both near and far from the target tone on the tonotopic map. Importantly, the ferrets’ behavioral performance correlated with the task, in that STRF changes were greatest when the ferrets’ performance was the highest. A functional connection between the prefrontal cortex and the auditory cortex appears to mediate this plasticity (Fritz, Elhilali, David, & Shamma, 2007). This kind of top-down modulation has also been established in a model of auditory learning. In an auditory localization study, ferrets were able to relearn to localize sound when a temporary earplug was placed in one ear to alter spatial cues. This ability to adapt to altered inputs was lost, however, when the corticocollicular (i.e., auditory cortex to inferior colliculus) connection was eliminated (Bajo, Nodal, Moore, & King, 2010), demonstrating that the corticollicular pathway is critical for the ability to relocalize sound inputs.
Given that older adults represent a large percentage of people in need of hearing rehabilitative services, it is important to demonstrate that auditory plasticity can be achieved in older animals and humans. In another study of auditory relocalization, in young owls, the neurophysiological map for interaural time differences (ITD) adjusted to accommodate a distorted visual input from the use of prisms (Brainard & Knudsen, 1998). In contrast, the ITD maps in older owls did not change, suggesting a sensitivity period for ITD map plasticity. A follow-up study, however, determined that older owls were able to recalibrate their ITD maps when the visual field was displaced in increments, and they retained this learning for 60 days (Linkenhoker & Knudsen, 2002). Another demonstration of plasticity in an animal model was performed with older rats who underwent frequency discrimination (de Villers-Sidani et al., 2010). Before the training, neural firing in the older rat auditory cortex was asynchronous, especially at higher rates of stimulation, compared to neural firing in the younger rat. After training, however, neural synchrony in the auditory cortex increased significantly in the older rats, although they did not achieve the behavioral performance of younger rats. The improvements in neural synchrony were accompanied by an increase in the levels of inhibitory neurotransmitters, suggesting that a training-induced increase in inhibition led to better temporal resolution. A role for inhibitory neurotransmission in temporal resolution has also been suggested by Caspary Ling, Turner, and Hughes (2008), who have posited that age-related decreases in inhibitory neurotransmission are responsible for reduced precision in neural timing in older animals.
Animal models have also been used to assess plasticity for clinical impairments. For example, vagus nerve stimulation has been paired with tone presentation to treat tinnitus in noise-exposed rats (Engineer et al., 2011). Vagus nerve stimulation promotes plasticity by increasing the release of neurotransmitters (Dorr & Debonnel, 2006). The rats were evaluated behaviorally on a gap-detection paradigm to test for the presence of subjective tinnitus prior to and after noise exposure and after the vagus nerve–tone stimulation treatment. These rats were simultaneously tested neurophysiologically by calculating the percentage of auditory cortex neurons firing at different frequencies and intensities to generate tonotopic maps. This treatment not only resulted in a reduction or elimination of the behavioral percept of tinnitus, but also tonotopic maps depicted more normal neural firing in the auditory cortex, so that there was no longer excessive neural firing in the high-frequency range corresponding to the tinnitus. Kilgard and colleagues suggest that these findings have ramifications for treatment of other forms of pathological plasticity, such as chronic pain or phantom limb syndrome (as reviewed in Kilgard, 2012).
Human Laboratory Models
In humans, there is evidence that perceptual learning is accompanied by neural plasticity in the auditory brainstem and cortex. In another example of the inferior colliculus’s role in auditory learning, young adult non–tonal-language speakers who were trained to recognize vocabulary items containing a linguistically relevant dipping vowel contour had better brainstem tracking of these vowel contours after training (Song, Banai, & Kraus, 2008; Figure 4). Furthermore, changes in training-induced behavioral performance relate to changes in F0 representation in the FFR, suggesting that changes in FFR strength, arising largely from the inferior colliculus, may be a mechanism for perceptual learning of pitch discrimination (Carcagno & Plack, 2011). This mechanism is also supported by the results of Chandrasekaran, Kraus, and Wong (2012), who trained participants to distinguish words in a novel language based on pitch differences. They found that that increased inferior colliculus representation of pitch patterns (demonstrated through fMRI and FFR data) was associated with more successful word learning.
Figure 4.
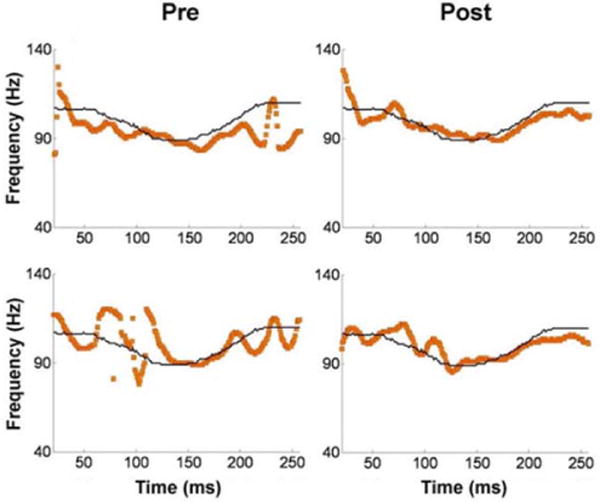
Short-term training on a linguistically relevant dipping vowel counter leads to better brainstem processing of this contour. The top and bottom panels show pre- and post-training brainstem tracking of a dipping vowel in 2 representative subjects. The thin black lines represent the stimulus F0 contour and the heavy orange lines represent brainstem pitch tracking of this contour. Adapted from Song et al., 2008.
Training effects can also be found in cortical evoked responses. After training on novel consonant perception, changes can be found in the mismatch negativity response (Kraus et al., 1995); moreover, training on one novel consonant generalizes to a similar consonant (Tremblay, Kraus, Carrell, & McGee, 1997). An important aspect of training is that cortical changes can precede behavioral changes (Tremblay, Kraus, & McGee, 1998); therefore, evaluation of neural plasticity might be used to assess the efficacy of a particular training strategy. The cortical response (the N1-P2 complex) has also been used to demonstrate stimulus-specific and general effects of training (Tremblay, Shahin, Picton, & Ross, 2009).
The influence of training is seen even at the level of the cochlea. Young adults were trained to discriminate between consonant-vowel (CV) syllables embedded in a continuous broad-band noise at a +10 dB SNR (de Boer & Thornton, 2008). Activation of the medial olivocochlear bundle (MOCB) was monitored during the 5 days of training through the use of contralateral suppression of evoked otoacoustic emissions. Training improved performance on the CV discrimination task, with the greatest improvement occurring over the first 3 training days. A significant increase in MOCB activation was found, but only in the participants who showed robust improvement (“learners”). The learners showed weaker suppression than the nonlearners on the first day; in fact, the level of MOCB activation was predictive of learning. This last finding would be particularly important for clinical purposes—a measure predicting benefit would be useful for determining treatment candidacy.
These human studies demonstrate plasticity in the young adult auditory system, but it is important to demonstrate plasticity in the older adult auditory system, particularly changes associated with improved communication in challenging listening situations. In addition to a large body of work investigating speech perception difficulties in older adults (Busey, Craig, Clark, & Humes, 2010; Fogerty, Humes, & Kewley-Port, 2010; Humes, 2007; Humes, Burk, Coughlin, Busey, & Strauser, 2007; Humes, Kewley-Port, Fogerty, & Kinney, 2010; Lee & Humes, 2012), Humes and colleagues have extensively studied the effects of word-based auditory training on speech perception in older adults (Burk & Humes, 2007, 2008; Burk, Humes, Amos, & Strauser, 2006), and have demonstrated that training of words in noise generalizes to unfamiliar talkers and persists for at least 6 months (Burk & Humes, 2008).
Home-Based Software Training
Despite the evidence for its benefits in older individuals with hearing loss (Kricos, Holmes, & Doyle, 1992; Rubinstein & Boothroyd, 1987), auditory training is not typically considered in the management of adults with hearing loss. Based on his personal experience of aural rehabilitation following World War II, Ross (1997) makes a case for its inclusion as part of the hearing aid selection process. In the last decade, hearing aid companies have begun to supply auditory training software with the purchase of instruments, presumably in response to the observation that hearing aid users continue to struggle when trying to hear in difficult listening situations, even when wearing the most advanced hearing aid technology. The use of home-based software training programs may eliminate some of the barriers to implementing an in-office training program, such as insufficient time or lack of reimbursement for treatment services.
Sweetow and Sabes conceived of the first in-home training program that achieved widespread use: Listening and Communication Enhancement (LACE™; Neurotone, Inc., Redwood City, CA). LACE combines listening exercises for challenging situations, such as noisy restaurants, competing speakers, and rapid speakers, with top-down approaches to aid understanding—use of context, memory, and strategies to improve communication. Sweetow and Sabes (2006) evaluated the efficacy of this program in a group of older adults with hearing loss who used the training for 30 minutes per day, 5 days a week, for 4 weeks. Older adults who underwent this program experienced improved performance on two clinical tests of speech-in-noise perception, the HINT and the Quick Speech-in-Noise test (QuickSIN; Etymotic Research, Elk Grove Village, IL; Killion, Niquette, Gudmundsen, Revit, & Banerjee, 2004), and on two self-assessments of performance, the Hearing Handicap Inventory for the Elderly (HHIE; Ventry & Weinstein, 1982) and the Communication Scale for Older Adults (COSA; Kaplan, Bally, Brandt, Busacco, & Pray, 1997).
The neural mechanisms underlying improved speech-in-noise perception in young adults who underwent LACE training were assessed in brainstem responses to a speech syllable presented in quiet and in two- and six-talker babble (Song, Skoe, Banai, & Kraus, 2012). The results indicated stronger representation of the F0 in brainstem responses in noise (2- and 6-talker babble) but not in quiet, suggesting that the training increased the robustness of subcortical speech representation, making it more resistant to the degradative effects of noise (Figure 5).
Figure 5.
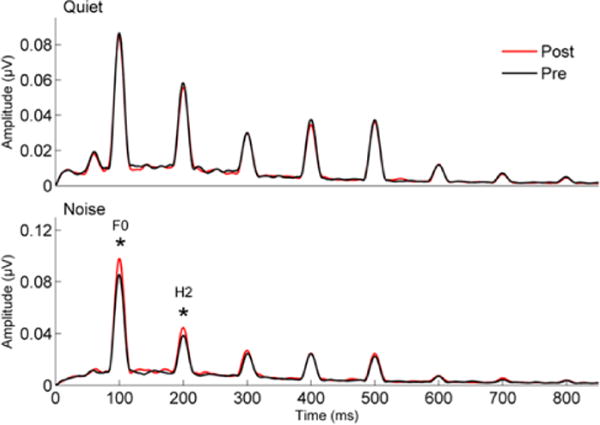
Short-term training on speech-in-noise perception leads to greater representation of pitch (F0 and H2) to the [da] syllable presented in noise (bottom) but not in quiet (top) in young adults. Adapted from Song et al., 2012.
Auditory-Based Cognitive Training
The majority of patients seeking help for hearing in noise, however, are older adults. A large-scale clinical trial was conducted to evaluate the efficacy of an auditory-based cognitive software training program (Brain Fitness Program, Posit Science, San Francisco, CA) in 487 older adults (Smith et al., 2009). This training is based on the assumption that reductions in sensory input can lead to cognitive decline, but that performance of sensory systems can be improved with practice. Participants underwent this training 60 minutes per day, 5 days a week, for 8 weeks. The auditory component of the training focuses the listener’s attention on the CV transition, adaptively expanding and contracting this transition as performance improves or worsens. This perceptual training is combined with increased memory demands. The primary outcome measure in this study was the Repeatable Battery for the Assessment of Neurophysiological Status (Psychological Corp., San Antonio, TX), which demonstrated improvements in memory and attention. The participants also noted subjective improvements in cognitive performance on a cognitive self-report questionnaire (Spina, Ruff, & Mahncke, 2006).
Smith et al. (2009) focused on cognitive outcomes in older adults. Given that the Brain Fitness™ program provides perceptual training, it may also serve as an auditory training tool to improve speech-in-noise perception. The CV transition is perceptually vulnerable (Miller & Nicely, 1955) and older adults have delayed subcortical timing in this transition compared to younger adults (Anderson et al., 2012). Therefore, training that attempts to improve speed of processing of this transition may result in faster neural timing. Faster neural response timing, in turn, may lead to better speech-in-noise perception, given the known relationship between brainstem timing and hearing in noise in children (Anderson et al., 2010; Hornickel, Skoe, Nicol, Zecker, & Kraus, 2009) and older adults (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2013).
We assessed the use of Brain Fitness auditory-cognitive training in a group of older adults (ages 55–65 years) randomly assigned to complete training or an active control program and found improvements in brainstem timing in response to the consonant-vowel transition (Anderson, White-Schwoch, et al., 2013), the region that is most affected by aging (Anderson et al., 2012; Parbery-Clark et al., 2012a). In addition to this neurophysiological improvement, participants demonstrated gains in speech-in-noise performance, memory, and speed of processing (Figure 6). No changes were found in the active control group, who watched educational DVDs and answered content questions, and whose training was matched to the auditory/cognitive training group for computer usage and training time. This study provides the first evidence for the possibility of auditory training reversing effects of aging on subcortical timing and perceptual and cognitive function.
Figure 6.
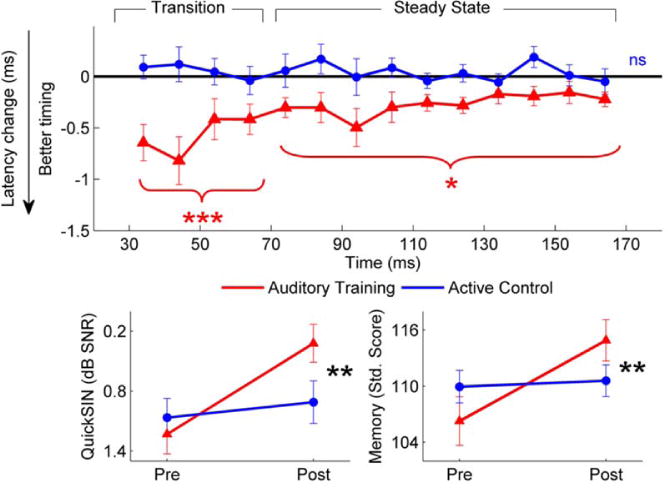
Auditory-based cognitive training leads to better brainstem timing, speech-in-noise perception, and memory in older adults (55 to 65). Top: Reduced latencies were found in brainstem responses to the speech syllable [da] presented in noise in the auditory training group (red) but not the active control group (blue). Bottom: Auditory training participants had better QuickSIN scores (left) and short-term memory standard scores (right) after training, whereas no changes were noted in the active control group. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars equal 1 S.E. Adapted from Anderson et al., White-Schwoch, et al., 2013.
Training for Individuals With Cochlear Implants
The aforementioned studies were carried out with individuals who have sufficient hearing to benefit from traditional hearing aid amplification. But, individuals who have hearing loss to the extent that they benefit from cochlear implantation have an even greater need for auditory training. Cochlear implants have performed exceptionally well for these individuals when listening to speech in quiet situations, but performance varies from auditory-only open-set word recognition to limited closed-set recognition with visual cues. In addition, cochlear implant design, which delivers the temporal speech envelope but not the fine structure, does not provide sufficient cues for good speech-in-noise or music perception (Ekelid, Kamath, Shannon, Wygonski, & Zeng, 1995; Galvin et al., 2009; Gfeller et al., 2007). Much of the variability in cochlear implant performance is due to the function of the central auditory nervous system. The same cochlear implant will provide different outcomes for individuals who have experienced deprivation-induced changes in auditory mapping, such that the auditory cortex is responding to nonauditory stimuli, compared to individuals who have not experienced great changes in their auditory maps. For example, in most children who have been implanted before 3 years of age, P1 latencies to a CV syllable (representing time of syllable detection in the auditory cortex) gradually decrease until they are equivalent to latencies obtained in age-matched normal hearing children (Gilley, Sharma, & Dorman, 2008). In children who are implanted later, however, P1 latencies may never decrease to levels typical for normal hearing children of the same age.
In an animal model, pairing a tone with food reinforcement leads to increased strength of neural firing to the tone in the rabbit auditory association cortex (i.e., a change in the brain’s response; Kraus & Disterhoft, 1982), but can auditory training change neural responses in individuals with cochlear implants? Ten CI users (ages 25–60 years, three post-lingual onset of deafness) underwent speech-recognition training and experienced gains in vowel, consonant, and sentence identification (Fu, Galvin, Wang, & Nogaki, 2004). They did not, however, improve in gender identification, an ability that would require pitch perception to a degree that is not provided by cochlear implants. Melodic contour identification was used to improve music perception in nine CI users, who demonstrated improvements in both melodic contour identification and in familiar melody identification, suggesting that this kind of training improved the listener’s ability to perceive pitch cues. These studies demonstrate that plasticity is possible despite limitations imposed by cochlear implant technology. The exercises used in the above studies and other training are available to the CI user or any interested party on the website www.tigerspeech.com. Further work is needed to document neural plasticity in older cochlear implant recipients.
Ingredients for Successful Learning
Animal models of learning may inform us of the important ingredients for learning. For example, several studies have demonstrated that pairing auditory stimulation with nucleus basalis activation leads to cortical reorganization and enhanced perception (Bakin & Weinberger, 1996; Kilgard & Merzenich, 1998; Puckett, Pandya, Moucha, Dai, & Kilgard, 2007). The nucleus basalis is the primary source of acetylcholine in the cerebral cortex, and its stimulation can increase cortical excitability and the transmission of action potentials (Metherate & Ashe, 1993). The release of acetylcholine is required for certain types of learning, such as recognition memory (Warburton et al., 2003), and is activated through mechanisms that include enhanced attention (Herrero et al., 2008); therefore, an auditory training paradigm that induces acetylcholine release may have increased effectiveness. Nucleus basalis stimulation is highly invasive and cannot therefore be considered a treatment tool for learning or rehabilitation in humans. Because of the limitations of nucleus basalis stimulation in the treatment of humans, Kilgard and colleagues have successfully applied vagus stimulation, which is less invasive, as a tool for treating tinnitus in rats (Engineer et al., 2011) and are currently investigating applications in humans.
Bavelier and colleagues have investigated another model of learning: the effects of videogame playing on both perceptual and cognitive function. They have found that videogame players have enhanced attention (Dye, Green, & Bavelier, 2009a), speed of processing (Dye, Green, & Bavelier, 2009b), and visual perception (Green & Bavelier, 2007; Li, Polat, Scalzo, & Bavelier, 2010). Importantly, these experiments have demonstrated causal relationships: non-videogame players can improve on these skills after a period of training on videogames. These effects generalized to real-world skills and maintained over a period of several months (as reviewed in Bavelier, Green, Pouget, & Schrater, 2012). Although a direct link has not been established between neuromodulatory release and these gains, one might expect that videogame playing taps into neural reward centers with a subsequent increase in neuromodulators such as acetylcholine.
The abovementioned experiments demonstrate that specific training platforms can substantially increase training effects; however, not every computerized training program shows benefits. A large (N = 11,340) 6-week online study failed to find any improvement in general cognitive function (Owen et al., 2010). There are possible reasons for the lack of benefit found in this study. The participants did the training tasks 10 minutes per day, 3 days a week, for 6 weeks, resulting in a total training time of 3 hours, in contrast with the 40 hours of training supplied by the Brain Fitness program or thousands of hours engaged in by musicians. The age range in the study was large, 18–60 years, and we expect that the older participants may have required more training or a different training strategy than one that is effective for young adults. Given the evidence of Bavelier’s group, effective training may need to provide adequate rewards to be effective. As we seek to improve auditory training programs, we must consider ways in which to increase intrinsic rewards and to make the training more engaging. Collaborations with developers of popular video games may be beneficial in this respect.
Outstanding Questions
To provide the most effective treatment, a number of questions should be explored in future studies. Comparisons of auditory-only training versus auditory plus cognitive training or hearing aids only versus hearing aids plus training should be performed to determine the most efficacious course of treatment. Along the same lines, it would be important to identify the features of each training program that provide the greatest benefit for specific deficits. The clinician will also benefit from a measure that predicts benefits from treatment, such as level of medial olivary cochlear bundle activation identified in the de Boer and Thornton (2008) study or brainstem F0 amplitude in the Song et al. (2012) study. The LACE and Brain Fitness programs greatly differ in total treatment time (20 vs. 40 hours). Future work should identify how much training is needed for different age groups and levels of hearing loss, and the persistence of training effects. Finally, the degree to which training needs differ for increased levels of hearing loss should be ascertained.
Summary
Given evidence of perceptual and physiological central processing deficits associated with aging and/or hearing loss, auditory training must be considered an essential component of management for individuals with hearing loss. Older adults rely on cognitive resources to enhance perception; therefore, training for older adults that focuses on both perceptual and cognitive function may provide greater benefits than training that does not include cognitive demands. Both lifelong experiences and short-term training affect speech-in-noise perception and neural speech processing. Auditory evoked potentials, especially the auditory brainstem response to complex sounds, provide an objective assessment of treatment efficacy. Developers of auditory training programs should consider platforms that naturally engage neural reward centers; both musical training and video game playing may inform the principles that lead to enhanced learning.
Acknowledgments
The authors thank Travis White-Schwoch for his comments and suggestions on the manuscript. This work was supported by NSF BCS-1057556, by NIH (T32 DC009399-01A10 & RO1 DC10016), and the Knowles Hearing Center.
Footnotes
Disclosures: Samira Anderson and Nina Kraus have no financial or nonfinancial relationships related to the content of this article.
Contributor Information
Samira Anderson, Auditory Neuroscience Laboratory, Department of Communication Sciences, Northwestern University, Evanston, IL; Department of Hearing and Speech Sciences, University of Maryland, College Park, MD.
Nina Kraus, Auditory Neuroscience Laboratory, Department of Communication Sciences, Neurobiology and Physiology, Otolaryngology, Institute for Neuroscience, Northwestern University, Evanston, IL.
References
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. The Journal of Neuroscience. 2012;32(41):14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Auditory brainstem response to complex sounds predicts self-reported speech-in-noise performance. Journal of Speech, Language & Hearing Research. 2013;56(1):31–43. doi: 10.1044/1092-4388(2012/12-0043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. The Journal of Neuroscience. 2010;30(14):4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proceedings of the National Academy of Sciences of the USA. 2013;110(11):4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61(3):467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nature Neuroscience. 2010;13(2):253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the USA. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: Learning to learn and action video games. Annual Review of Neuroscience. 2012;35(1):391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Gandour JT, Krishnan A. Cross-domain effects of music and language experience on the representation of pitch in the human auditory brainstem. Journal of Cognitive Neuroscience. 2009;23(2):425–434. doi: 10.1162/jocn.2009.21362. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Krishnan A. Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Research. 2010;1355:112–125. doi: 10.1016/j.brainres.2010.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum. Journal of Neuroscience. 1998;18(10):3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugos J, Perlstein W, McCrae C, Brophy T, Bedenbaugh P. Individualized piano instruction enhances executive functioning and working memory in older adults. Aging and Mental Health. 2007;11(4):464–471. doi: 10.1080/13607860601086504. [DOI] [PubMed] [Google Scholar]
- Burk MH, Humes LE. Effects of training on speech recognition performance in noise using lexically hard words. Journal of Speech, Language and Hearing Research. 2007;50(1):25. doi: 10.1044/1092-4388(2007/003). [DOI] [PubMed] [Google Scholar]
- Burk MH, Humes LE. Effects of long-term training on aided speech-recognition performance in noise in older adults. Journal of Speech, Language & Hearing Research. 2008;51(3):759–771. doi: 10.1044/1092-4388(2008/054). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk MH, Humes LE, Amos NE, Strauser LE. Effect of training on word-recognition performance in noise for young normal-hearing and older hearing-impaired listeners. Ear and Hearing. 2006;27(3):263–278. doi: 10.1097/01.aud.0000215980.21158.a2. [DOI] [PubMed] [Google Scholar]
- Busey T, Craig J, Clark C, Humes L. Age-related changes in visual temporal order judgment performance: Relation to sensory and cognitive capacities. Vision Research. 2010;50(17):1628–1640. doi: 10.1016/j.visres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcagno S, Plack C. Subcortical plasticity following perceptual learning in a pitch discrimination task. Journal of the Association for Research in Otolaryngology. 2011;12(1):89–100. doi: 10.1007/s10162-010-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology. 2008;211(11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N, Wong PCM. Human inferior colliculus activity relates to individual differences in spoken language learning. Journal of Neurophysiology. 2012;107(5):1325–1336. doi: 10.1152/jn.00923.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard C, Tremblay K. Aging degrades the neural encoding of simple and complex sounds. Journal of the American Academy of Audiology. doi: 10.3766/jaaa.24.7.7. (in press) [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton ARD. Neural correlates of perceptual learning in the auditory brainstem: Efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. The Journal of Neuroscience. 2008;28(19):4929–4937. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proceedings of the National Academy of Sciences of the USA. 2010;107(31):13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Research. 2010;1342:24–32. doi: 10.1016/j.brainres.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. Journal of Pharmacology and Experimental Therapeutics. 2006;318(2):890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Driscoll VD, Oleson J, Jiang D, Gfeller K. Effects of training on recognition of musical instruments presented through cochlear implant simulations. Journal of the American Academy of Audiology. 2009;20(1):71. doi: 10.3766/jaaa.20.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye M, Green C, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009a;47(8–9):1780. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MWG, Green CS, Bavelier D. Increasing speed of processing with action video games. Current Directions in Psychological Science. 2009b;18(6):321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelid M, Kamath V, Shannon RV, Wygonski J, Zeng FG. Speech recognition with primarily temporal clues. Science. 1995;270(5234):303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. Journal of Neurophysiology. 2004;92(1):73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty D, Humes LE, Kewley-Port D. Auditory temporal-order processing of vowel sequences by young and elderly listeners. Journal of the Acoustical Society of America. 2010;127(4):2509–2520. doi: 10.1121/1.3316291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Tittgemeyer M, Wagenmakers EJ, Derrfuss J, Imperati D, Brown S. The speed-accuracy tradeoff in the elderly brain: A structural model-based approach. The Journal of Neuroscience. 2011;31(47):17242–17249. doi: 10.1523/JNEUROSCI.0309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hearing Research. 2007;229(1–2):186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ. Perceptual learning and auditory training in cochlear implant recipients. Trends in Amplification. 2007;11(3):193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin J, Wang X, Nogaki G. Effects of auditory training on adult cochlear implant patients: A preliminary report. Cochlear Implants International. 2004;5(S1):84–90. doi: 10.1179/cim.2004.5.Supplement-1.84. [DOI] [PubMed] [Google Scholar]
- Gaab N, Schlaug G. The effect of musicianship on pitch memory in performance matched groups. Neuro Report. 2003;14(18):2291–2295. doi: 10.1097/00001756-200312190-00001. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, Fu QJ, Shannon RV. Melodic contour identification and music perception by cochlear implant users. Annals of the New York Academy of Sciences. 2009;1169(1):518–533. doi: 10.1111/j.1749-6632.2009.04551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller K, Turner C, Oleson J, Zhang X, Gantz B, Froman R, Olszewski C. Accuracy of cochlear implant recipients on pitch perception, melody recognition, and speech reception in noise. Ear and Hearing. 2007;28(3):412–423. doi: 10.1097/AUD.0b013e3180479318. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Research. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S. Hearing loss and aging: New research findings and clinical implications. Journal of Rehabilitation Research & Development. 2005;42:9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA. Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. Journal of Speech, Language & Hearing Research. 2007;50(5):1181–1193. doi: 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action-video-game experience alters the spatial resolution of vision. Psychological Science. 2007;18(1):88–94. doi: 10.1111/j.1467-9280.2007.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Gajewski B. Recent and past musical activity predicts cognitive aging variability: Direct comparison with general lifestyle activities. Frontiers in Human Neuroscience. 2012;6:1–11. doi: 10.3389/fnhum.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus SE, Gordon-Salant S. Accuracy of speech intelligibility index predictions for noise-masked young listeners with normal hearing and for elderly listeners with hearing impairment. Journal of Speech & Hearing Research. 1995;38(1):234–243. doi: 10.1044/jshr.3801.234. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Plyler PN, Hedrick MS. Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clinical Neurophysiology. 2005;116(9):2153–2164. doi: 10.1016/j.clinph.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Hawkins DB, Yacullo WS. Signal-to-noise ratio advantage of binaural hearing aids and directional microphones under different levels of reverberation. Journal of Speech & Hearing Disorders. 1984;49(3):278–286. doi: 10.1044/jshd.4903.278. [DOI] [PubMed] [Google Scholar]
- Henry KS, Heinz MG. Diminished temporal coding with sensorineural hearing loss emerges in background noise. Nature Neuroscience. 2012;15:1362–1364. doi: 10.1038/nn.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero J, Roberts M, Delicato L, Gieselmann M, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AE. Bilateral amplification for the elderly: Are two aids better than one? International Journal of Audiology. 2003;42:2–2. [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proceedings of the National Academy of Sciences of the USA. 2009;106(31):13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE. The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults. Journal of the American Academy of Audiology. 2007;18:590–603. doi: 10.3766/jaaa.18.7.6. [DOI] [PubMed] [Google Scholar]
- Humes LE, Burk MH, Coughlin MP, Busey TA, Strauser LE. Auditory speech recognition and visual text recognition in younger and older adults: Similarities and differences between modalities and the effects of presentation rate. Journal of Speech, Language, and Hearing Research. 2007;50(2):283–303. doi: 10.1044/1092-4388(2007/021). [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Wingfield A. Central presbycusis: A review and evaluation of the evidence. Journal of the American Academy of Audiology. 2012;23(8):635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Kewley-Port D, Fogerty D, Kinney D. Measures of hearing threshold and temporal processing across the adult lifespan. Hearing Research. 2010;264(1–2):30–40. doi: 10.1016/j.heares.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L, Lee J, Coughlin M. Auditory measures of selective and divided attention in young and older adults using single-talker competition. Journal of the Acoustical Society of America. 2006;120:2927–2937. doi: 10.1121/1.2354070. [DOI] [PubMed] [Google Scholar]
- Jeng FC, Hu J, Dickman B, Montgomery-Reagan K, Tong M, Wu G, Lin CD. Cross-linguistic comparison of frequency-following responses to voice pitch in American and Chinese neonates and adults. Ear and Hearing. 2011;32(6):699. doi: 10.1097/AUD.0b013e31821cc0df. [DOI] [PubMed] [Google Scholar]
- Kale S, Heinz M. Envelope coding in auditory nerve fibers following noise-induced hearing loss. Journal of the Association for Research in Otolaryngology. 2010;11(4):657–673. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Bally S, Brandt F, Busacco D, Pray J. Communication scale for older adults (CSOA) Journal of the American Academy of Audiology. 1997;8(3):203–217. [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends in Neurosciences. 2012;35(12):715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nature Neuroscience. 1998;1(8):727. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killion M, Niquette P, Gudmundsen G, Revit L, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. Journal of the Acoustical Society of America. 2004;116:2935–2405. doi: 10.1121/1.1784440. [DOI] [PubMed] [Google Scholar]
- Kochkin S. MarkeTrak VIII: Consumer satisfaction with hearing aids is slowly increasing. Hearing Journal. 2010;63(1):19–32. [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. The Journal of Neuroscience. 2005;25(15):3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Disterhoft J. Response plasticity in single neurons in rabbit auditory association cortex during tone signalled learning. Brain Research. 1982;246:205–215. doi: 10.1016/0006-8993(82)91168-4. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Carrell TD, King C, Tremblay K, Nicol T. Central auditory system plasticity associated with speech discrimination training. Journal of Cognitive Neuroscience. 1995;7(1):25–32. doi: 10.1162/jocn.1995.7.1.25. [DOI] [PubMed] [Google Scholar]
- Kricos P, Holmes AE, Doyle D. Efficacy of a communication training program for hearing-impaired elderly adults. Journal of the Academy of Rehabilitative Audiology. 1992;25:69–80. [Google Scholar]
- Krishnan A, Bidelman G, Gandour JT. Neural representation of pitch salience in the human brainstem revealed by psychophysical and electrophysiological indices. Hearing Research. 2010;268(1–2):60–66. doi: 10.1016/j.heares.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour J, Cariani P. Encoding of pitch in the human brainstem is sensitive to language experience. Journal of Cognitive Neuroscience. 2005;25(1):161–168. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Krizman J, Marian V, Shook A, Skoe E, Kraus N. Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proceedings of the National Academy of Sciences of the USA. 2012;109(20):7877–7881. doi: 10.1073/pnas.1201575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Humes LE. Effect of fundamental-frequency and sentence-onset differences on speech-identification performance of young and older adults in a competing-talker background. Journal of the Acoustical Society of America. 2012;132(3):1700–1717. doi: 10.1121/1.4740482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Polat U, Scalzo F, Bavelier D. Reducing backward masking through action game training. Journal of Vision. 2010;10(14) doi: 10.1167/10.14.33. [DOI] [PubMed] [Google Scholar]
- Limb CJ, Rubinstein JT. Current research on music perception in cochlear implant users. Otolaryngologic Clinics of North America. 2012;45:129–140. doi: 10.1016/j.otc.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419(6904):293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BCJ. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proceedings of the National Academy of Sciences of the USA. 2006;103(49):18866–18869. doi: 10.1073/pnas.0607364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, Bartzokis G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. Journal of Clinical and Experimental Neuropsychology. 2011;33(10):1059–1068. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. The Journal of Neuroscience. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences. 2006;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Miller GA, Nicely PE. An analysis of perceptual confusions among some English consonants. Journal of the Acoustical Society of America. 1955;27(2):338–352. [Google Scholar]
- Moreno S, Bialystok E, Barac R, Schellenberg EG, Cepeda NJ, Chau T. Short-term music training enhances verbal intelligence and executive function. Psychological Science. 2011;22(11):1425–1433. doi: 10.1177/0956797611416999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro KJ, Blount J. Adaptive plasticity in brainstem of adult listeners following earplug-induced deprivation. Journal of the Acoustical Society of America. 2009;126(2):568–571. doi: 10.1121/1.3161829. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Strait D, Kraus N. Relationships between behavior, brainstem and cortical encoding of seen and heard speech in musicians and non-musicians. Hearing Research. 2008;241(1–2):34. doi: 10.1016/j.heares.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Soli S, Sullivan J. Development of the Hearing In Noise Test for the measurement of speech reception thresholds in quiet and in noise. Journal of the Acoustical Society of America. 1994;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Ballard CG. Putting brain training to the test. Nature. 2010;465(7299):775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiology of Aging. 2012a doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience strengthens the neural representation of sounds important for communication in middle-aged adults. Frontiers in Aging Neuroscience. 2012b;4 doi: 10.3389/fnagi.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. Journal of Neuroscience. 2009;29(45):14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Lam C, Kraus N. Musician enhancement for speech-in-noise. Ear and Hearing. 2009;30(6):653–661. doi: 10.1097/AUD.0b013e3181b412e9. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Strait DL, Anderson S, Hittner E, Kraus N. Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLOS ONE. 2011;6(5):e18082. doi: 10.1371/journal.pone.0018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Patel AD. Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Frontiers in Psychology. 2011;2:142. doi: 10.3389/fpsyg.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK. Cognitive aging and auditory information processing. International Journal of Audiology. 2003;42:26–32. [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. Journal of the Acoustical Society of America. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai WW, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. Journal of Neurophysiology. 2007;98(1):253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Engle JR, Juarez-Salinas DL. Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hearing Research. 2011;271(1–2):115–122. doi: 10.1016/j.heares.2010.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. A retrospective look at the future of aural rehabilitation. Journal of the Academy of Rehabilitative Audiology. 1997;30:11–28. [Google Scholar]
- Rubinstein A, Boothroyd A. Effect of two approaches to auditory training on speech recognition by hearing-impaired adults. Journal of Speech, Language and Hearing Research. 1987;30(2):153. doi: 10.1044/jshr.3002.153. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT. How cochlear implants encode speech. Current Opinion in Otolaryngology & Head and Neck Surgery. 2004;12(5):444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Understanding hearing through deafness. Proceedings of the National Academy of Sciences of the USA. 2007;104(17):6883–6884. doi: 10.1073/pnas.0702220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Cunningham BG, Best V. Selective attention in normal and impaired hearing. Trends in Amplification. 2008;12(4):283–299. doi: 10.1177/1084713808325306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Kraus N. A little goes a long way: How the adult brain is shaped by musical training in childhood. Journal of Neuroscience. 2012;32(34):11507–11510. doi: 10.1523/JNEUROSCI.1949-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: Results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Banai K, Kraus N. Brainstem timing deficits in children with learning impairment may result from corticofugal origins. Audiology and Neurotology. 2008;13(5):335–344. doi: 10.1159/000132689. [DOI] [PubMed] [Google Scholar]
- Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: Biological mechanisms. Cerebral Cortex. 2012;22:1180–1190. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza P, Boike K, Witherell K, Tremblay K. Prediction of speech recognition from audibility in older listeners with hearing loss: Effects of age, amplification, and background noise. Journal of the American Academy of Audiology. 2007;18:54–65. doi: 10.3766/jaaa.18.1.5. [DOI] [PubMed] [Google Scholar]
- Spina LM, Ruff R, Mahncke H. Cognitive Self-Report Questionnaire (CSRQ) manual. San Francisco, CA: Posit Science; 2006. [Google Scholar]
- Strait DL, Parbery-Clark A, Hittner E, Kraus N. Musical training during early childhood enhances the neural encoding of speech in noise. Brain and Language. 2012;123(5) doi: 10.1016/j.bandl.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetow RW, Sabes JH. The need for and development of an adaptive Listening and Communication Enhancement (LACE) program. Journal of the American Academy of Audiology. 2006;17:538–558. doi: 10.3766/jaaa.17.8.2. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Veuillet E, Norena A, Guiraud J, Collet L. Plasticity of tonotopic maps in humans: Influence of hearing loss, hearing aids and cochlear implants. Acta Oto-laryngologica. 2010;130(3):333–337. doi: 10.3109/00016480903258024. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Marie C, Gerry D, Whiskin E, Unrau A. Becoming musically enculturated: Effects of music classes for infants on brain and behavior. Annals of the New York Academy of Sciences. 2012;1252(1):129–138. doi: 10.1111/j.1749-6632.2012.06462.x. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, Carrell TD, McGee T. Central auditory system plasticity: Generalization to novel stimuli following listening training. Journal of the Acoustical Society of America. 1997;102:3762. doi: 10.1121/1.420139. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, McGee T. The time course of auditory perceptual learning: neurophysiological changes during speech-sound training. Neuro Report. 1998;9(16):3556–3560. doi: 10.1097/00001756-199811160-00003. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Shahin AJ, Picton T, Ross B. Auditory training alters the physiological detection of stimulus-specific cues in humans. Clinical Neurophysiology. 2009;120(1):128–135. doi: 10.1016/j.clinph.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Hughes LF, Caspary DM. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. Journal of Neurophysiology. 2005;94(4):2738–2747. doi: 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- Valente M, Fabry D, Potts LG. Recognition of speech in noise with hearing aids using dual microphones. Journal of the American Academy of Audiology. 1995;6(6):440–449. [PubMed] [Google Scholar]
- Ventry I, Weinstein B. The Hearing Handicap Inventory for the Elderly: A new tool. Ear and Hearing. 1982;3(3):128–134. doi: 10.1097/00003446-198205000-00006. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. Journal of Neuroscience. 1998;18(7):2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wu X, Li L, Schneider BA. The effects of age and interaural delay on detecting a change in interaural correlation: The role of temporal jitter. Hearing Research. 2011;275(1–2):139–149. doi: 10.1016/j.heares.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Warburton E, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38(6):987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wingfield A, McCoy SL, Peelle JE, Tun PA, Cox CL. Effects of adult aging and hearing loss on comprehension of rapid speech varying in syntactic complexity. Journal of the American Academy of Audiology. 2006;17(7):487–497. doi: 10.3766/jaaa.17.7.4. [DOI] [PubMed] [Google Scholar]
- Wong PCM, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nature Neuroscience. 2007;10(4):420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Li G, Wang Y, Zhou Y. Aging affects response variability of V1 and MT neurons in rhesus monkeys. Brain Research. 2009;1274:21–27. doi: 10.1016/j.brainres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proceedings of the National Academy of Sciences of the USA. 2007;104(29):12193–12198. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendel BR, Alain C. Musicians experience less age-related decline in central auditory processing. Psychological Aging. 2012;27:410–417. doi: 10.1037/a0024816. [DOI] [PubMed] [Google Scholar]


