Abstract
Background and Purpose
Cryptogenic stroke is common in patients with cancer. Autopsy studies suggest that many of these cases may be due to marantic endocarditis, which is closely linked to cancer activity. We, therefore, hypothesized that among patients with cancer and ischemic stroke, those with cryptogenic stroke would have shorter survival.
Methods
We retrospectively analyzed all adult patients with active systemic cancer diagnosed with acute ischemic stroke at a tertiary-care cancer center from 2005 through 2009. Two neurologists determined stroke mechanisms by consensus. Patients were diagnosed with cryptogenic stroke if no specific mechanism could be determined. The diagnosis of marantic endocarditis was restricted to patients with cardiac vegetations on echocardiography or autopsy and negative blood cultures. Patients were followed until July 31, 2012 for the primary outcome of death. Kaplan-Meier statistics and the log-rank test were used to compare survival between patients with cryptogenic stroke and patients with known stroke mechanisms. Multivariate Cox proportional hazard analysis evaluated the association between cryptogenic stroke and death after adjusting for potential confounders.
Results
Among 263 patients with cancer and ischemic stroke, 133 (51%) were cryptogenic. Median survival in patients with cryptogenic stroke was 55 days (interquartile range [IQR] 21-240) versus 147 days (IQR 33-735) in patients with known stroke mechanisms (p<0.01). Cryptogenic stroke was independently associated with death (hazard ratio 1.64, 95% confidence interval 1.25-2.14) after adjusting for age, systemic metastases, adenocarcinoma histology, and functional status.
Conclusions
Cryptogenic stroke is independently associated with reduced survival in patients with active cancer and ischemic stroke.
Keywords: endocarditis, non-infective, survival, mortality
Introduction
Patients with cancer often develop hypercoagulability through alterations of the clotting cascade, platelet function, and endothelial integrity.1-3 These patients, therefore, face a highrisk of venous and arterial thromboembolism, including ischemic stroke.1-5 Approximately 7% of patients with cancer will develop a symptomatic stroke during their lifetime, and another 8% will have evidence of cerebrovascular disease at autopsy.6 Stroke is especially significant in patients with cancer because neurological disability from stroke can preclude or change cancer treatments and therefore may affect the prognosis of the underlying cancer and patient survival.
Stroke mechanisms in patients with cancer may differ from those that occur in the general population.5, 7 Cancer-specific mechanisms include marantic endocarditis, intravascular coagulation, and tumor embolism. Marantic endocarditis is characterized by sterile platelet-fibrin vegetations on normal cardiac valves.8, 9 These lesions are generally small and friable and, as such, are very difficult to diagnose definitively in vivo, including with transesophageal echocardiography.10 However, a large autopsy study reported that marantic endocarditis is the most common cause of symptomatic ischemic stroke in patients with cancer.6 Therefore, it is possible that a sizeable proportion of cryptogenic strokes in patients with active cancer are from undiagnosed marantic endocarditis.11 Since marantic endocarditis is likely a direct manifestation of cancer-mediated hypercoagulability and therefore closely linked to the activity of the underlying malignancy,8 wehypothesized that survival would be shorter in cancer patients with cryptogenic stroke as compared to cancer patients with stroke from other mechanisms.
Methods
Study Design
We conducted a retrospective cohort study of all patients over the age of 17with active systemic cancer diagnosed with acute ischemic stroke at Memorial Sloan Kettering Cancer Center (MSKCC) from January 1, 2005 through December 31, 2009. Active systemic cancer was defined in the standard fashion as the diagnosis of, or treatment for, any systemic cancer besides local basal cell or squamous cell carcinoma of the skin within the prior six months, or known recurrent or metastatic disease; patients with primary brain tumors were excluded.5, 12 Acute ischemic stroke was defined as a new neurological deficit with corresponding evidence of acute ischemia on brain MRI and no radiological or clinical indication of a non-cerebrovascular mimic.
Study Subjects
As previously described,13 patients were identified through an administrative database search for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for ischemic stroke (codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91), and by reviewing the MSKCC Department of Neurology's prospective clinical database for diagnoses of “ischemic stroke”, “stroke”, or “transient ischemic attack”. We excluded patients if: (1) their stroke was diagnosed solely by CT (i.e., no brain MRI), as brain metastases can mimic stroke in patients with cancer and thus differentiating stroke from metastases by CT alone can be difficult, (2) their stroke was diagnosed as an outpatient, as these patients were less likely to have complete diagnostic evaluations. After these initial screens, a board-certified neurologist reviewed all identified charts to determine final eligibility. Our institutional review board approved this study; the requirement of informed consent was waived due to minimal risk to patients.
Study Setting
MSKCC is an academic, urban, tertiary-care hospital dedicated to the care of patients with suspected or confirmed cancer. While it is not certified as a stroke center, patients who are diagnosed with stroke at outside facilities are often transferred to MSKCC for completion of their diagnostic evaluation; therefore, a substantial number of cancer patients (∼120) are treated annually at MSKCC for ischemic or hemorrhagic stroke.13, 14 Furthermore, outside hospital records are rigorously collected and scanned into the MSKCC electronic health records, providing a comprehensive system for data capture.
Measurements
In compliance with recommended guidelines for cohort studies, relevant patient data were collected using a standardized data abstraction form that had undergone multiple revisions prior to official use based on pilot abstractions and investigator meetings.15, 16 Using this standardized abstraction form and a data dictionary that contained prespecified definitions for all variables of interest, a neurologist abstracted data about patients' demographics, vascular risk factors, cancer type and histology, presence of metastases, prior cancer therapies, diagnostic stroke evaluations, use of antithrombotic agents, and functional status as determined by the modified Rankin Scale (mRS) score at the time of discharge from index stroke hospitalization.17 All abstracting neurologists were trained and certified in calculating the mRS score. Medical records were reviewed from the time of index stroke hospitalization to the time of death or July 31, 2012.
In addition, after reviewing all clinical, laboratory, radiological, and pathological data, including independent review of all neuroimaging and cardiac studies, two board-certified neurologists determined by consensus each patient's specific stroke mechanism. Of note, marantic endocarditis was diagnosed only if there was echocardiographic or pathological evidence of cardiac valve vegetation(s) in the absence of positive blood cultures or other clear explanations. In addition, the adjudicating neurologists also determined patients' stroke subtype according to the modified TOAST (Trial of ORG 10172 in Acute Stroke Treatment Study) classification.18 In line with TOAST, patients were determined to have cryptogenic stroke if the cause of stroke could not be determined with any degree of confidence, or there were two or more potential causes of stroke preventing the adjudicators from making a specific final diagnosis. For the purposes of this study, patients diagnosed with cryptogenic stroke were further subcategorized by whether their radiographic infarction pattern suggested cardioembolism (i.e., wedge-shaped or cortical infarctions in multiple vascular territories) or not.
Our clinical predictor of interest was cryptogenic stroke. Our outcome of interest was death, which is systematically evaluated at MSKCC through patient contact, electronic medical record and central research database review, and linkage to cancer registries and the Social Security Death Index.19
Statistical Analysis
Descriptive statistics were used to characterize patients' baseline characteristics, rates of stroke mechanisms, and crude rates of death. Kaplan-Meier survival statistics and the log-rank test were used to compare survival in patients with cryptogenic stroke to those with stroke from other mechanisms. Patients were censored at the time of death, last available follow-up visit, or July 31, 2012. Additional survival analyses were performed for patients with cryptogenic stroke and radiographic cardioembolic infarction patterns and for those diagnosed with marantic endocarditis.
Multivariate Cox proportional hazard analysis was performed to evaluate for an independent association between stroke mechanisms and death while accounting for potential clinical confounders. After considering the results of previous studies and expert opinion, and in an effort to prevent model overfitting, we selected a priori the following clinical factors for our model: age, known metastases to other organs (i.e., stage 4 cancer), functional status at discharge from index stroke hospitalization, and adenocarcinoma histology.4, 8, 20 Functional status was entered as a dichotomous variable with Rankin scores of 0 to 2 (normal or minimal disability) signifying a good outcome and scores of 3 to 6 (moderate disability or worse) signifying a poor outcome.17 Clinical factors were entered into the model regardless of significance at the univariate level. A p-value of <0.05 was considered significant for the multivariate analysis.
Since recurrent thromboembolic events may affect stroke mortality risk in patients with cancer,13 we performed a post hoc, exploratory analysis that included recurrent thromboembolic events (i.e., composite of recurrent ischemic stroke, myocardial infarction, systemic embolism, transient ischemic attack, or venous thromboembolism) in the final multivariate model. Recurrent thromboembolism was entered into the model as a time-varying covariate. All statistical analyses were performed using Stata MP (Version 13, College Station, Texas).
Results
Patient Characteristics
The final cohort comprised 263 patients. Mean patient age was 66 years (standard deviation [SD] 12) and 51% were men (Table 1). Most underlying cancers were solid in origin and originated from the lung (32%), gastrointestinal tract (25%), or genitourinary system (12%). Adenocarcinoma cancer histology was common (60%). Most patients (56%) had received cancer treatment within the month prior to stroke. Median time from cancer diagnosis to ischemic stroke was 9.6 months (interquartile range [IQR] 2.4-32.2) for those with known stroke mechanisms and 9.7 months (IQR 2.2-27.1) for those with cryptogenic stroke (p=0.88). At the time of stroke, disseminated cancer was typical, with 69% having known metastases to other organs; the dissemination of cancer was similarly widespread for those in the cryptogenic group (73%) compared to the remainder of the cohort (65%, p=0.19). Patients with cryptogenic stroke tended to have fewer vascular risk factors than patients with known stroke mechanisms, particularly diabetes mellitus, coronary artery disease, atrial fibrillation, and prior stroke.
Table 1. Baseline Characteristics of Patients, Stratified by Cryptogenic Stroke.
| Cryptogenic Stroke (n=133) * | Stroke from Known Mechanisms (n=130) | P-Value | |
|---|---|---|---|
|
| |||
| Age, mean years (SD) | 66 (12) | 66 (13) | 0.70 |
|
| |||
| Men, n (%) | 71 (53) | 64 (49) | 0.50 |
|
| |||
| Race, n (%) | |||
| White | 109 (82) | 102 (79) | 0.56 |
| Black | 12 (9) | 15 (12) | 0.49 |
| Asian | 8 (6) | 5 (4) | 0.43 |
| Hispanic | 4 (3) | 6 (5) | 0.49 |
| Other | 0 (0) | 2 (2) | 0.15 |
|
| |||
| Cancer type, n (%) | |||
| Lung | 44 (33) | 39 (30) | 0.59 |
| Gastrointestinal | 36 (27) | 30 (23) | 0.46 |
| Genitourinary | 14 (10) | 17 (13) | 0.52 |
| Other solid | 6 (5) | 14 (11) | 0.06 |
| Breast | 9 (7) | 9 (7) | 0.96 |
| Gynecological | 7 (5) | 10 (8) | 0.42 |
| Lymphoma | 6 (5) | 6 (5) | 0.97 |
| Leukemia | 5 (4) | 3 (2) | 0.49 |
| Other hematological | 3 (2) | 2 (2) | 0.67 |
| Melanoma | 3 (3) | 0 (0) | 0.09 |
|
| |||
| Cancer status, n (%) | |||
| Metastases to other organs | 97 (73) | 85 (65) | 0.19 |
| Recent treatment† | 74 (56) | 74 (57) | 0.83 |
| Chemotherapy | 58 (44) | 55 (42) | 0.83 |
| Biological‡ | 8 (6) | 11 (8) | 0.44 |
| Radiotherapy | 7 (5) | 9 (7) | 0.57 |
| Anti-angiogenesis§ | 9 (7) | 5 (4) | 0.29 |
|
| |||
| Vascular risk factors, n (%) | |||
| Hypertension | 70 (53) | 77 (59) | 0.28 |
| Hyperlipidemia | 49 (37) | 55 (42) | 0.37 |
| Diabetes mellitus | 17 (13) | 35 (27) | <0.01 |
| Coronary artery disease | 14 (11) | 28 (22) | 0.02 |
| Atrial fibrillation | 1 (1) | 22 (17) | <0.01 |
| Prior stroke | 4 (3) | 15 (12) | <0.01 |
| Current smoking | 5 (4) | 9 (7) | 0.25 |
|
| |||
| Discharge Antithrombotic, n (%)¶ | 87 (65) | 85 (65) | 0.99 |
| Antiplatelet | 54 (41) | 48 (37) | 0.54 |
| Aspirin | 50 (38) | 42 (32) | 0.37 |
| Anticoagulants | 43 (32) | 47 (36) | 0.51 |
| Low-molecular weight heparin | 40 (30) | 39 (30) | 0.99 |
Percentages have been rounded up, thus total values may not equal 100
Some patients received multiple types of treatment (i.e., chemotherapy and radiotherapy); recent treatment is defined as treatment within 30 days of index stroke.
Biological refers to cancer treatments made from a living organism or its product. These mostly consisted of monoclonal antibody medicines such as rituximab and imitinib that target specific tumor cell receptors or proteins.
Anti-angiogenesis refers to treatment with medicines that inhibit the growth of new blood vessels, including bevacizumab, sunitinib, and sorafenib.
Some patients were treated with both antiplatelet and anticoagulant agents.
Despite a very sick patient population, diagnostic stroke evaluations were generally comprehensive, as 100% had inpatient cardiac rhythm analysis, 84% had echocardiography (82% transthoracic, 14% transesophageal, and 13% both), and 76% had cranial and neck vessel imaging. Among patients who lived past 30 days, 90% had echocardiography and 85% had vessel imaging; there was no difference in the completeness of diagnostic stroke evaluations between those with known versus cryptogenic etiologies (p=0.36). At hospital discharge, the median mRS score for those with cryptogenic stroke was similar to those with known stroke mechanisms: 3 (IQR, 2-5) versus 3 (IQR, 2-5)(p=0.48). Most patients (56%) were discharged home.
Stroke Classification
Based on the TOAST classification, stroke subtypes for the entire cohort comprised 22% cardioembolism, 15% large artery atherosclerosis, 8% small vessel occlusion, 5% other determined causes, and 51% cryptogenic. Among the cryptogenic cohort (n=133), 76 (57%) had a radiographic cardioembolic infarction pattern.
Specific stroke mechanisms varied and included typical mechanisms seen in the general stroke population (e.g., atrial fibrillation) and more atypical mechanisms unique to patients with cancer (e.g., extrinsic compression of cerebral artery by tumor). Following our pre-specified definition, marantic endocarditis was diagnosed in only 4% (n=10) of our cohort. Among those with determined stroke mechanisms, the most common specific mechanisms were atrial fibrillation (26%), extracranial large artery atherosclerosis (17%), small vessel disease (15%), and intracranial large artery atherosclerosis (14%); infective endocarditis was diagnosed in two patients (2%) (Table 2).
Table 2. Specific Mechanisms of Stroke in Determined Cases.
| Specific Stroke Mechanism | Number (%)*, (n=130) |
|---|---|
|
| |
| Atrial fibrillation | 34 (26%) |
| Extracranial large artery atherosclerosis | 22 (17%) |
| Small vessel disease | 20 (15%) |
| Intracranial large artery atherosclerosis | 18 (14%) |
| Marantic endocarditis | 10 (8%) |
| Paradoxical embolism | 7 (5%) |
| Other† | 5 (4%) |
| Cardiomyopathy | 4 (3%) |
| Cardiac thrombus | 3 (2%) |
| Extrinsic compression of artery by tumor | 3 (2%) |
| Infectious endocarditis | 2 (2%) |
| Disseminated intravascular coagulation | 2 (2%) |
Percentages have been rounded up, thus the total value may not equal 100
Other diagnoses consisted of aortic arch atheroma, vasculitis, arterial dissection, cerebral vein thrombosis, and cardiac tumor
Survival
The in-hospital mortality rate was 10% for patients with cryptogenic stroke versus 17% for patients with known stroke mechanisms (p=0.09). However, median survival was significantly shorter in patients with cryptogenic stroke (55 days, IQR 21-240) than in those with known stroke mechanisms (147 days, IQR 33-735; log rank test, p<0.01; Figure 1). At 3 months, only 39% of the cryptogenic cohort was alive, as compared to 56% of the non-cryptogenic cohort. Median survival was even shorter for cryptogenic stroke patients with radiographic cardioembolic infarction patterns (31 days, IQR 16-112; log rank test for comparison with known stroke mechanisms, p<0.01; Figure 2), which closely mirrored survival of patients with diagnosed marantic endocarditis (median 31 days, IQR 3-51; log rank test for comparison with known stroke mechanisms, p<0.01).
Figure 1.
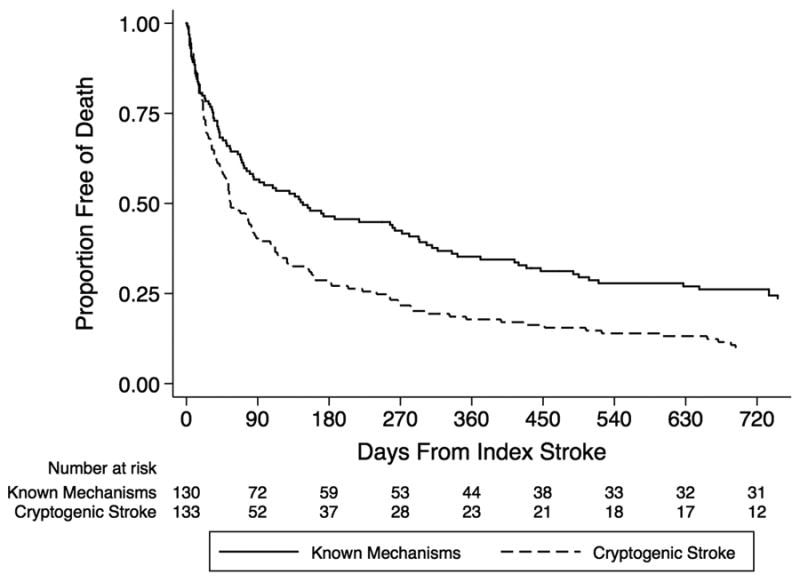
Kaplan-Meier plot of survival free of death, stratified by cryptogenic stroke.
Figure 2.
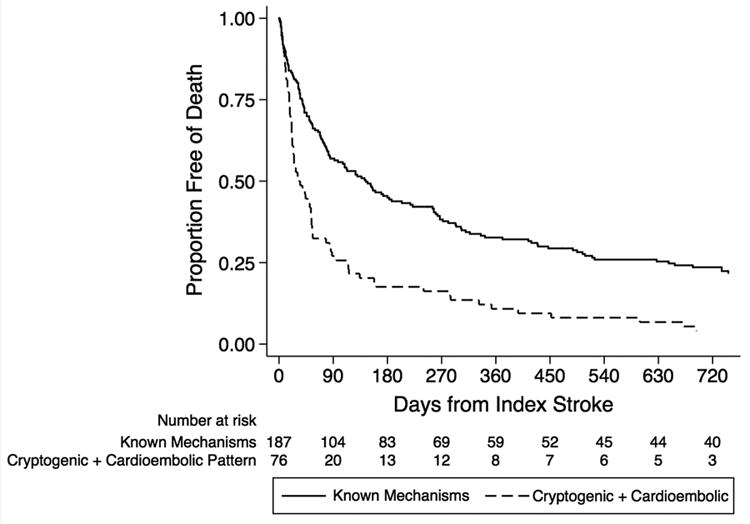
Kaplan-Meier plot of survival free of death, stratified by cryptogenic stroke with a radiographic cardioembolic infarction pattern.
In a multivariate analysis, cryptogenic stroke remained a significant predictor of death (hazard ratio [HR] 1.64, 95% confidence interval [CI] 1.25-2.14) after accounting for age, adenocarcinoma histology, known metastases to other organs, and functional status (Table 3). This association was even stronger when only cryptogenic stroke patients with radiographic cardioembolic infarction patterns were entered into the model (HR 2.01, 95% CI 1.50-2.70). This association appeared similar to the association between diagnosed marantic endocarditis and death(HR 1.92, 95% CI 1.00-3.71).
Table 3. Multivariate Cox Hazard Analysis of Prespecified Clinical Factors and Death.
| Hazard Ratio (95% CI) | P-value | |
|---|---|---|
|
| ||
| Metastases to other organs | 2.55 (1.84 – 3.53) | <0.01 |
| Cryptogenic stroke | 1.64 (1.25 – 2.14) | <0.01 |
| Adenocarcinoma | 1.08 (0.81 – 1.44) | 0.60 |
| Age, years | 1.00 (0.99 – 1.01) | 0.42 |
| Modified Rankin Scale score ≤2 | 0.48 (0.36 – 0.63) | <0.01 |
CI indicates confidence interval.
In a post hocexploratory analysis where recurrent thromboembolism was added to the final multivariate model, cryptogenic stroke remained independently associated with death (HR 1.57, 95% CI 1.20-2.06).
Discussion
In this cohort study, we demonstrate that survival is very short in patients with active systemic cancer who suffer a cryptogenic ischemic stroke and that cryptogenic mechanisms are associated with death independent of several potential confounders. The association of cryptogenic stroke with death is even stronger in patients with radiographic cardioembolic infarction patterns. In fact, only 17% of patients with cryptogenic stroke and 9% of those with radiographic cardioembolic infarction patterns survived to 1 year. This is clinically relevant because cryptogenic mechanisms accounted for 51% of all strokes in our cohort.
In the general stroke population, cryptogenic strokes are typically associated with lower mortality and recurrence than strokes from known mechanisms, particularly as compared to those from large artery atherosclerosis or cardioembolism.21, 22 One potential explanation for this phenomenon is that many patients with cryptogenic stroke are young and otherwise healthy and therefore their strokes probably arise from low-risk conditions such as patent foramen ovale. However, in our active cancer population, survival was considerably shorter in patients with cryptogenic stroke than in those with known stroke mechanisms. Additionally, the survival of patients with cryptogenic stroke and a radiographic cardioembolic infarction pattern closely mirrored that of patients with confirmed marantic endocarditis, a condition linked to cancer activity and hypercoagulability. Therefore, the worse overall survival in patients with cryptogenic stroke in our cohort may be because some of these patients harbored undiagnosed marantic endocarditis. This hypothesis is supported by a large autopsy study of 3,426 patients with active systemic cancer, which found marantic endocarditis to be the most common cause of symptomatic cerebral infarction.6 However, other stroke mechanisms such as intravascular coagulation, paradoxical embolism, and occult atrial fibrillation could also cause cryptogenic stroke in cancer patients. Further research will be needed to confirm the mechanistic basis of these cryptogenic strokes and their resultant poor survival in patients with cancer.
Our study has several important limitations. First, this study was conducted at a single, tertiary-care, academic hospital that is dedicated to the care of cancer patients, and thus its results may not generalize to cancer patients with stroke treated in the community. Second, diagnostic stroke evaluations were not standardized or complete, and a small proportion of patients had transesophageal echocardiography; therefore, some patients with cryptogenic stroke may have had undiagnosed atrial fibrillation or other stroke mechanisms associated with a high-risk of death. In addition, the cryptogenic stroke rate in our cohort (51%) is higher than typically reported cryptogenic stroke rates in the general population.21, 22 However, this rate is similar to previously reported rates of cryptogenic stroke in patients with cancer, including from prospective studies.5, 7, 23 Furthermore, the completeness of diagnostic evaluations between patients with cryptogenic and known stroke mechanisms was not significantly different. We also excluded outpatient strokes, so our cohort may have been enriched with sicker patients who were more likely to have cryptogenic stroke or marantic endocarditis. Third, in order to prevent overfitting of our multivariate Cox model, we only evaluated five clinically relevant variables; it is possible that the association between cryptogenic stroke and survival is confounded or mediated by another variable that we did not account for. For instance, we did not account for initial stroke severity (i.e., NIH Stroke Scale), which has been shown previously to correlate with stroke mortality.24 Fourth, our study was retrospective and purely epidemiological in nature and therefore we were unable to explore the pathophysiology or mechanistic implications of cryptogenic stroke in patients with cancer. In addition, our nonrandomized study design prevents us from determining whether a self-fulfilling prophecy contributed to decreased survival in these patients (i.e., those with cryptogenic stroke or suspected marantic endocarditis were assumed to do worse and therefore were treated less aggressively), although this is unlikely given the lack of previous data about the association between cryptogenic stroke and reduced survival in patients with cancer. Furthermore, the in-hospital mortality rate was actually lower in patients with cryptogenic stroke (10%) as compared to those with known stroke mechanisms (17%).
In summary, cryptogenic stroke is associated with decreased survival in patients with active systemic cancer and acute ischemic stroke, independent of age, functional status, presence of systemic metastases, and adenocarcinoma histology. Although marantic endocarditis is a possibility, further studies are needed to determine the factors underlying this decreased survival. Furthermore, it remains to be established whether aggressive treatments aimed at preventing stroke and suppressing cancer activity and hypercoagulability can improve patient outcomes. The previously unappreciated association between cryptogenic stroke and reduced survival should be considered in the evaluation and treatment of cancer patients with stroke.
Acknowledgments
None.
Funding: The Florence Gould Endowment for Discovery in Stroke and an NIH award administered to Babak Navi (KL2TR000458-06) through the Weill Cornell Clinical and Translational Science Center supported this study.
Footnotes
Conflict of Interest/Disclosures: None.
References
- 1.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dammacco F, Vacca A, Procaccio P, Ria R, Marech I, Racanelli V. Cancer-related coagulopathy (Trousseau's syndrome): Review of the literature and experience of a single center of internal medicine. Clin Exp Med. 2013;13:85–97. doi: 10.1007/s10238-013-0230-0. [DOI] [PubMed] [Google Scholar]
- 3.Sack GH, Jr, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: Clinical, pathophysiologic, and therapeutic features. Medicine. 1977;56:1–37. [PubMed] [Google Scholar]
- 4.Cestari DM, Weine DM, Panageas KS, Segal AZ, DeAngelis LM. Stroke in patients with cancer: Incidence and etiology. Neurology. 2004;62:2025–2030. doi: 10.1212/01.wnl.0000129912.56486.2b. [DOI] [PubMed] [Google Scholar]
- 5.Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: A multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 6.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine. 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, et al. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034. doi: 10.1161/STROKEAHA.112.658625. [DOI] [PubMed] [Google Scholar]
- 8.Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med. 1987;83:746–756. doi: 10.1016/0002-9343(87)90908-9. [DOI] [PubMed] [Google Scholar]
- 9.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: Pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 10.Dutta T, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97:894–898. doi: 10.1016/j.amjcard.2005.09.140. [DOI] [PubMed] [Google Scholar]
- 11.Bal S, Menon B, Demchuk A. Relationship of endocarditis, disseminated intravascular coagulation, and embolic signals in cancer with stroke. Ann Neurol. 2012;71:146. doi: 10.1002/ana.22306. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 13.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. [Accessed June 12, 2014];Neurology. 2014 doi: 10.1212/WNL.0000000000000539. published online ahead of print May 21, 2014. 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494–501. doi: 10.1212/WNL.0b013e3181cef837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein SR. Medical record reviews in emergency medicine: The blessing and the curse. Ann Emerg Med. 2005;45:452–455. doi: 10.1016/j.annemergmed.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 17.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Feldman DR, Patil S, Trinos MJ, Carousso M, Ginsberg MS, Sheinfeld J, et al. Progression-free and overall survival in patients with relapsed/refractory germ cell tumors treated with single-agent chemotherapy: Endpoints for clinical trial design. Cancer. 2012;118:981–986. doi: 10.1002/cncr.26375. [DOI] [PubMed] [Google Scholar]
- 20.Rogers LR. Cerebrovascular complications in patients with cancer. Sem Neurol. 2010;30:311–319. doi: 10.1055/s-0030-1255224. [DOI] [PubMed] [Google Scholar]
- 21.Redfors P, Jood K, Holmegaard L, Rosengren A, Blomstrand C, Jern C. Stroke subtype predicts outcome in young and middle-aged stroke sufferers. Acta Neurol Scand. 2012;126:329–335. doi: 10.1111/j.1600-0404.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- 22.Vallejos J, Jaramillo A, Reyes A, Illanes S, Orellana P, Manterola J, et al. Prognosis of cryptogenic ischemic stroke: A prospective single-center study in chile. J Stroke Cerebrovasc Dis. 2012;21:621–628. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219. doi: 10.1002/ana.22050. [DOI] [PubMed] [Google Scholar]
- 24.Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: Predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34:122–126. doi: 10.1161/01.str.0000047852.05842.3c. [DOI] [PubMed] [Google Scholar]


