Abstract
Abnormal heart rate recovery (HRR) after maximal exercise may indicate autonomic dysfunction and is a predictor for cardiovascular mortality. HRR is attenuated with aging and in middle-age hypertensive patients, but it is unknown whether HRR is attenuated in older-age adults with hypertension. This study compared HRR among 16 unmedicated stage 1 hypertensive (HTN) participants [nine men/seven women; 68 ± 5 (SD) yr; awake ambulatory blood pressure (BP) 149 ± 10/87 ± 7 mmHg] and 16 normotensive [control (CON)] participants (nine men/seven women; 67 ± 5 yr; 122 ± 4/72 ± 5 mmHg). HR, BP, oxygen uptake (V̇o2), cardiac output (Qc), and stroke volume (SV) were measured at rest, at two steady-state work rates, and graded exercise to peak during maximal treadmill exercise. During 6 min of seated recovery, the change in HR (ΔHR) was obtained every minute and BP every 2 min. In addition, HRR and R-R interval (RRI) recovery kinetics were analyzed using a monoexponential function, and the indexes (HRRI and RRII) were calculated. Maximum V̇o2, HR, Qc, and SV responses during exercise were not different between groups. ΔHR was significantly different (P < 0.001) between the HTN group (26 ± 8) and the CON group (36 ± 12 beats/min) after 1 min of recovery but less convincing at 2 min (P = 0.055). BP recovery was similar between groups. HRRI was significantly lower (P = 0.016), and there was a trend of lower RRII (P = 0.066) in the HTN group compared with the CON group. These results show that in older-age adults, HRR is attenuated further with the presence of hypertension, which may be attributable to an impairment of autonomic function.
Keywords: hypertension, heart rate recovery, autonomic function, exercise
a delayed heart rate recovery (HRR) following maximal exercise indicates an abnormal reactivation of the parasympathetic nervous system and/or withdrawal of sympathetic nervous activity (13, 17, 26, 30). Although the relative roles of parasympathetic reactivation and sympathetic withdrawal remain in question, attenuated HRR may be a sign of autonomic dysfunction (20). Attenuated HRR has been linked to increased mortality in healthy adults (9), as well as those with cardiovascular disease (8, 24) and diabetes (6).
HRR in young to middle-aged hypertensive adults is attenuated compared with age-matched normotensive adults (11). This difference is thought to reflect the decreased parasympathetic activity observed in hypertensive adults through HR variability (HRV) analysis (27). Likewise, sympathetic hyperactivity has been observed in hypertensive adults (1, 12, 32). However, we are unaware of any studies that have investigated the effect of hypertension on HRR in a cohort of older-age subjects. Aging is independently associated with increased sympathetic activity (16, 22, 28), reduced influence of parasympathetic activity as measured by HRV (2), and delayed HRR (5). Whether hypertension attenuates HRR further in older-age adults is unknown.
Like HRR, chronotropic incompetence (CI) or an attenuated rise in HR during exercise has also been linked to mortality (19, 21). When CI is combined with HRR, the combination may be more predictive of mortality than either measurement on its own (23). CI may also be a function of altered autonomic regulation, specifically, delayed parasympathetic withdrawal and sympathetic activation (15). However, the diminished parasympathetic activity and increased sympathetic activity in hypertensive adults may preserve the chronotropic response to exercise but attenuate HRR.
Accordingly, the purpose of the current study was to compare HRR and CI from a maximal treadmill exercise test between normotensive and hypertensive older-age adults. We hypothesized that HRR would be attenuated in the hypertensive (HTN) group, but CI would be similar between the HTN and normotensive [control (CON)] groups.
METHODS
Ethical approval.
The protocol was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. Each subject was informed of the purpose and risks associated with the study and gave written, informed consent in accordance with the standards set by the Declaration of Helsinki.
Participants.
Thirty-two older participants (60-78 yr) were recruited for the study. To be eligible for the study, participants could be normotensive or have treated or untreated stage 1 hypertension. Participants were screened with a careful medical history, physical examination, and a 12-lead ECG assessment. Participants were excluded from the study if they smoked, had a history of cardiovascular disease other than hypertension, had any history of neuromuscular or renal disease, or exercised more than three times/week, >30 min/session. The women were postmenopausal and not receiving estrogen-replacement therapy.
Following the screening, participants who were using antihypertensive medication were weaned off their medication and then remained medication free until they completed the study. All participants then had a 3-wk run-in, during which participants received counseling and were encouraged to maintain healthy lifestyle interventions, as recommended by The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines (7). At any stage during the medication washout or the 3-wk run-in, participants were excluded from the study and asked to resume their previous antihypertensive medication immediately if systolic blood pressure (SBP) ≥160 mmHg or diastolic blood pressure (DBP) ≥100 mmHg, as measured in the office, appeared. During the run-in, participants were required to come to the laboratory for a once/wk BP check, as well as to measure and record their BP daily at home.
At the conclusion of the 3-wk run-in, the awake average of a 24-h ambulatory BP measurement (SunTech Oscar 2; SunTech Medical, Morrisville, NC) was used to assign participants as normotensive (SBP < 135 mmHg; DBP < 85 mmHg), isolated systolic hypertensive (SBP 135–159 mmHg; DBP < 85 mmHg), or combined systolic-diastolic hypertensive (SBP 135–159 mmHg; DBP ≥ 85 mmHg) (7). Sixteen participants were classified as hypertensive (HTN group; nine men and seven women; 60–76 yr of age), and 16 participants were classified as normotensive (CON group; nine men and seven women; 61–78 yr of age]. In the HTN group, six participants (two men and four women) were classified as isolated systolic hypertensive, and 10 participants (seven men and three women) were classified as combined systolic-diastolic hypertensive.
Protocol.
Upon arriving at the laboratory, participants were instrumented and rested in a standing position for no less than 20 min. The participant then walked and/or ran on a treadmill for 5 min at two individually chosen, steady-state speeds: the first speed corresponding with 30% maximum oxygen uptake (V̇o2max) and the second speed at 60% V̇o2max. After 15 min of seated rest, participants then completed an incremental test to exhaustion on the treadmill. The speed of steady-state 2 was chosen, and the gradient increased 2% every 2 min to maximal exertion. Immediately upon cessation of the exercise, participants rested in a seated position for 6 min.
Beat-by-beat HR and R-R interval (RRI) were measured continuously throughout the protocol (12-lead ECG). Cardiac output (Qc; C2H2 rebreathing method) (18, 29), V̇o2 (Douglas bag method), and BP (electrosphygmomanometer) were measured at rest, during each steady-state stage, and at maximal exercise. Stroke volume (SV) was calculated as SV (ml) = Qc/HR. Expired gas fractions were analyzed using mass spectrometry and ventilator volumes by a Tissot spirometer. V̇o2 was defined as the maximal V̇o2 measurement from at least a 30-s Douglas bag measurement. Mean arterial pressure (MAP) was calculated as MAP (mmHg) = [(SBP − DBP)/3] + DBP. Total peripheral resistance (TPR) was calculated as TPR (dyn/s/cm5) = (MAP/Qc) × 80 (14).
Data analysis.
CI was assessed as the percentage of calculated HR reserve reached at peak exercise using the following equation (19).
CI (%) = (measured HR reserve/calculated HR reserve) × 100.
where measured HR reserve represents the difference between the measured peak and resting HR; calculated HR reserve represents the difference between calculated peak HR (i.e., 220 − age) and measured resting HR.
HR and RRI recovery were calculated as the absolute difference in HR (ΔHR) and RRI (ΔRRI) compared with peak exercise values; recovery values were recorded at the end of each minute (10 s average) for 6 min postexercise. In addition to conventional analysis strategies that are designed to provide simple yet meaningful clinical applications, the dynamic HR and RRI recovery profiles were characterized using a monoexponential function (4) to describe both the amplitude and time-course domains of the response simultaneously. Thus off-transient responses for HR and RRI were modeled using the following equation
Y(t) = YBSLN + A (1 − e−t/τ).
where Y(t) represents the HR or RRI at any given time (t); YBSLN is the prerecovery value of Y before exercise cessation [e.g., maximum HR (HRmax)]; A is the amplitude of the change in Y from YBSLN; and τ is the recovery time constant (mean response time) and represents the time required to attain 63% of the steady-state amplitude (4). Data were modeled from exercise cessation to the end of the recovery period. The model parameters were estimated by least-squares nonlinear regression (Origin; OriginLab, Northampton, MA), in which the best fit was defined by minimization of the residual sum of squares and minimal variation of residuals around the y-axis (Y = 0). In addition, an overall index of the HRR profile [incorporating both amplitude and τ, which we have termed the “HRR index” (HRRI)] is given as the quotient of A/τ and expressed as beats/min2, where a greater HRRI is indicative of a more efficient postexercise recovery.
Statistical analysis.
Individual means between groups were analyzed using an unpaired t-test. All other measurements were analyzed by two-way repeated-measures ANOVA with Bonferroni t-test post hoc analysis. In the event that normality tests and/or equal variance tests failed, the data were log transformed and analyzed again using the same two-way repeated-measures ANOVA. Statistical significance was set at P < 0.05. Results are shown as mean ± SD.
RESULTS
Subject characteristics.
There were no significant differences in age, height, weight, or body mass index between the HTN and CON groups (Table 1). Twenty-four-hour ambulatory SBP, DBP, and MAP were all significantly higher in the HTN group.
Table 1.
Subject characteristics
| Variable | CON Group, n = 16 | HTN Group, n = 16 | P |
|---|---|---|---|
| Age, yr | 67 ± 5 | 68 ± 5 | 0.652 |
| Sex, % men | 56 | 56 | |
| Height, cm | 171 ± 10 | 171 ± 9 | 0.986 |
| Weight, kg | 75 ± 10 | 78 ± 16 | 0.541 |
| BMI, kg/m2 | 26 ± 2 | 27 ± 5 | 0.637 |
| Avg. awake HR, beats/min | 72 ± 7 | 72 ± 8 | 0.981 |
| Avg. awake SBP, mmHg | 122 ± 4 | 149 ± 10 | <0.001 |
| Avg. awake DBP, mmHg | 72 ± 5 | 87 ± 7 | <0.001 |
| Avg. awake MAP, mmHg | 83 ± 5 | 107 ± 9 | <0.001 |
Data are shown as mean ± SD. CON, normotensive (control); HTN, hypertensive; BMI, body mass index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic BP; MAP, mean arterial pressure. HR and BP measurements were calculated from 24-h ambulatory BP monitoring.
Resting and exercise data.
V̇o2, Qc, HR, and SV were similar between the HTN and CON groups at rest and during both steady states (Table 2). V̇o2max as well as HR, Qc, and SV at peak exercise were also similar between groups. SBP, DBP, and MAP were all significantly higher during exercise in the HTN group than the CON group, but there was no group difference for TPR (Table 2).
Table 2.
Resting and exercise testing data
| Variable | CON Group |
HTN Group |
Group × Protocol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | SS1 | SS2 | Max | Rest | SS1 | SS2 | Max | ||
| V̇O2, ml/kg/min | 3.2 ± 0.4 | 11.9 ± 1.6 | 15.5 ± 2.6 | 25.5 ± 5.0 | 3.2 ± 0.5 | 11.8 ± 1.2 | 15.0 ± 2.4 | 24.0 ± 5.3 | P = 0.709 |
| HR, beats/min | 83 ± 11 | 99 ± 10 | 116 ± 10 | 165 ± 11 | 83 ± 15 | 98 ± 14 | 114 ± 13 | 161 ± 12 | P = 0.974 |
| Qc, l/min | 3.5 ± 0.9 | 7.9 ± 1.7 | 9.3 ± 2.0 | 12.5 ± 3.2 | 3.5 ± 0.9 | 8.0 ± 1.9 | 9.6 ± 2.4 | 12.5 ± 3.0 | P = 0.867 |
| SV, ml | 43 ± 10 | 81 ± 20 | 81 ± 19 | 76 ± 18 | 44 ± 17 | 83 ± 23 | 86 ± 23 | 78 ± 17 | P = 0.724 |
| SBP, mmHg | 118 ± 14 | 149 ± 22 | 157 ± 20 | 196 ± 23 | 134* ± 21 | 162* ± 16 | 176* ± 23 | 208* ± 28 | P = 0.558 |
| DBP, mmHg | 80 ± 9 | 79 ± 11 | 75 ± 12 | 87 ± 16 | 87* ± 11 | 85* ± 12 | 90* ± 12 | 95* ± 21 | P = 0.180 |
| MAP, mmHg | 93 ± 9 | 93 ± 9 | 102 ± 12 | 103 ± 11 | 102* ± 14 | 111* ± 10 | 119* ± 12 | 133* ± 20 | P = 0.178 |
| TPR, dyn/s/cm5 | 2,200 ± 538 | 1,078 ± 263 | 909 ± 216 | 845 ± 259 | 2,481 ± 629 | 1,180 ± 310 | 1,046 ± 256 | 893 ± 218 | P = 0.800 |
Data are shown as mean ± SD. SS1 and -2, steady-state 1 and 2 work rates, respectively; Max, peak exercise; V̇O2, oxygen uptake; Qc, cardiac output; SV, stroke volume; TPR, total peripheral resistance.
Significant group effect (P < 0.05).
Chronotropic incompetence.
The rise in HR (79 ± 17 vs. 82 ± 11 beats/min; P = 0.527) and SBP (74 ± 25 vs. 78 ± 21 mmHg; P = 0.571) from rest to peak exercise was similar in the HTN and CON groups, respectively. CI in the HTN group (114 ± 14%) was not significantly different than the CON group (118 ± 18%; P = 0.387).
Exercise recovery data.
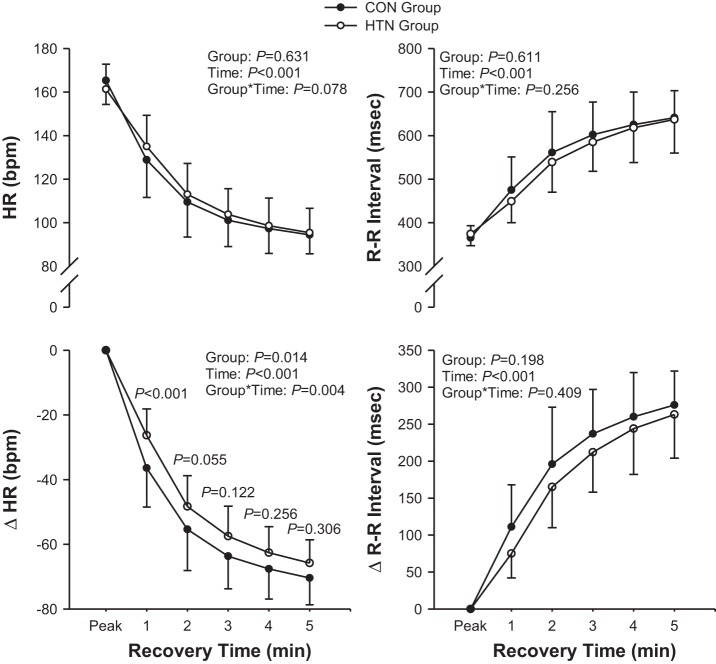
Peak exercise HR and RRI were not significantly different between the HTN and CON groups. ΔHR was significantly lower in the HTN group than the CON group at 1 min, although the difference between groups was less convincing at 2 min (P = 0.055; Fig. 1). There were no group differences in the postexercise ΔRRI response (Fig. 1).
Fig. 1.
Heart rate (HR) and R-R interval (RRI) recovery for the normotensive [control (CON)] and hypertensive (HTN) groups. ΔHR and ΔRRI represent the change in HR and RRI, respectively, from peak values. P values at individual data points represent the P value between groups at that specific time point. Data are shown as mean ± SD. Group*Time, Group × Time.
According to the categorical criteria of an abnormal HRR response at 1 min (≤18 beats/min considered a greater cardiovascular risk), as set by Watanabe et al. (31), one in the CON group (zero men and one woman) and four in the HTN group (three men and one woman) had an attenuated HRR response (χ2, P = 0.333). With the use of the criteria (≤42 beats after 2 min considered a greater risk of mortality), outlined by Cole et al. (9), two in the CON group (one man and one woman) and four in the HTN group (two men and two women) had an attenuated HRR response (χ2, P = 0.651).
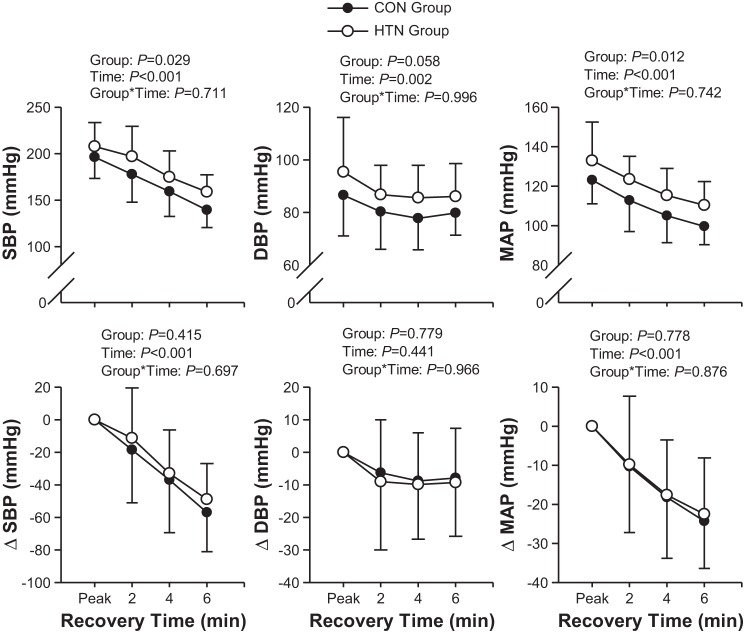
Postexercise SBP and MAP were higher in the HTN group than the CON group, but there was only a trend of higher DBP in the HTN group (Fig. 2). There were no group differences in SBP, DBP, and MAP changes in peak values during recovery (Fig. 2).
Fig. 2.
Systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressure for the CON and HTN groups. ΔSBP, ΔDBP, and ΔMAP represent the change in SBP, DBP, and MAP, respectively, from peak values. Data are shown as mean ± SD.
Monoexponential modeling.
In general, the individual coefficient of determination for the monoexponential models used was excellent for HR (mean r2 > 0.950) and RRI (mean r2 > 0.890).
The monoexponential modeling of the HR kinetics response revealed similar A (HTN = 74 ± 8; CON = 76 ± 12 beats/min; P = 0.409) values during postexercise recovery. However, τ was higher (HTN = 126 ± 35; CON = 102 ± 68 s; P = 0.047), and HRRI was lower in the HTN group (38 ± 12 beats/min2) compared with the CON group (58 ± 30 beats/min2; P = 0.016). As a result, the percentage of overall HRR that occurred in the 1st min was lower in the HTN group (39 ± 9%) than the CON group (52 ± 16%; P = 0.012). Furthermore, the time to attain a clinically meaningful ΔHR of 18 beats/min (ΔHR18) was slower in the HTN group (ΔHR18 = 27 ± 7 s) than the CON group (ΔHR18 = 21 ± 11 s; P = 0.017), and there was a trend for the HTN group to attain ΔHR42 (ΔHR42 = 57 ± 15 s) slower than the CON group (ΔHR42 = 43 ± 23 s; P = 0.019).
Interestingly, there was a single outlier in the CON group whose HRR profile was not well characterized by a monoexponential model. This participant had a greater reduction in HR between 2 and 3 min postexercise (20 beats/min) than either HRmax − 1 min postexercise (13 beats/min) or between 1 and 2 min postexercise (18 beats/min). When this subject was removed from the statistical analyses, differences in τ (CON = 88 ± 39 s; P = 0.008), HRRI (60 ± 30 beats/min2; P = 0.009), ΔHR18 (19 ± 7 s; P = 0.003), and ΔHR42 (39 ± 15 s; P = 0.003) all achieved a higher degree of probability.
The dynamic adjustment of RRI during postexercise recovery was similar between the HTN (A = 377 ± 124 ms; τ = 237 ± 124 s) and CON (A = 370 ± 154 ms, P = 0.748; τ = 213 ± 245 s, P = 0.115) groups, yet there was a trend (P = 0.066) for a lower RRI index (RRII) in the HTN (107 ± 44 ms/min) compared with the CON (168 ± 115 ms/min) group. Again, when comparisons were made after removing data from a single outlier in the CON group, differences in the RRII (176 ± 114 ms/min; P = 0.022) and τ (157 ± 104 s; P = 0.015) were significant.
DISCUSSION
The key finding of this study was evidence of delayed HRR in older-age adults with stage 1 hypertension compared with age-matched normotensive adults. The HTN group had a smaller reduction in HR from peak HR during exercise to 1 min postexercise when compared with the CON group. Monoexponential modeling showed a reduced HRRI, as well as a lower percentage of overall HRR in the 1st min in the HTN group compared with the CON group. These results suggest that postexercise autonomic function is altered in the HTN group compared with the CON group, potentially due to attenuated parasympathetic reactivation and/or sympathetic withdrawal.
This study is the first to compare HRR between older-age normotensive and hypertensive adults. HRR has been shown to be attenuated in middle-aged adults with hypertension compared with age-matched normotensive adults (11). However, increased age has also been shown to attenuate HRR following maximal exercise (5).
The attenuated HRR in the HTN group was shown through both traditional analysis, i.e., the decrease in HR from peak exercise HR to HR at specific time points (e.g., 1, 2, 3 min and so on), as well as the kinetics analyses of HRR. The faster adjustment (i.e., smaller τ) and the greater HRRI both point to a faster postexercise recovery in the CON group. These data were in spite of a similar chronotropic response to exercise and HRmax in each group. However, the differences between the HTN and CON groups for τ were only significant when the data for one outlier from the CON group were removed from the analysis. Together, the ΔHR and kinetic analysis data of the present study show that even in older-age adults, HRR is attenuated further with hypertension.
HR is derived from the RRI, and therefore, we also compared the RRI response during recovery. From what we are aware of, this is the first study to compare the RRI recovery following exercise in hypertensive adults. Because the relationship between RRI and calculated HR is an exponential decline, for each regular decrement in RRI, there is an exponential increase in HR. This explains why we observed a significant difference between the HTN and CON groups for ΔHR but not ΔRRI. However, the strong trend of an increased RRII recovery in the CON group was similar to the significantly increased HRRI in the CON group compared with the HTN group.
The attenuated HRR in the HTN group is not due to between-group differences in cardiorespiratory fitness. HRR is improved with increased V̇o2max (10, 17); however, in the present study, V̇o2max was not significantly different between groups. Qc, SV, and TPR were also similar between groups at rest and during exercise. Therefore, the attenuated HRR and RRI recovery in the HTN group suggests that autonomic regulation during postexercise recovery is altered in the HTN group compared with the CON group.
The attenuated HRR in the HTN group is probably due to altered sympathovagal “balance” following exercise. However, the relative contribution of parasympathetic reactivation and sympathetic withdrawal to postexercise HRR and how this changes at different time points remain unclear (13, 17, 26, 30). Hypertension is associated with a reduction in parasympathetic activity (27) and sympathetic hyperactivity (1, 12, 32) at rest; thus the attenuated HRR in the HTN group is probably due to delays in both parasympathetic reactivation and sympathetic withdrawal.
Previous studies in large subject cohorts have established HRR “thresholds,” in which the failure to attain a given decrease in HR within a specific time following exercise is associated with a significantly increased risk of mortality. Watanabe et al. (31) suggested a decrease in HR of ≤18 beats/min after 1 min as a cutoff for decreased survival in a wide variety of patients, including hypertensive patients. Alternatively, in a cohort of healthy participants without cardiovascular disease, Cole et al. (9) found that a decrease of ≤42 beats/min, 2 min after ceasing exercise, was a strong predictor of mortality. In the present study, the HTN group had a greater but insignificant proportion of the patients who did not meet these criteria compared with the CON group. In contrast, the kinetic analysis of the data found that the calculated time to achieve a reduction in both ΔHR18 and ΔHR42 was slower in the HTN group compared with the CON group.
However, specific thresholds should be used cautiously, as consideration must be given to the exercise and recovery protocols. In particular, the position of recovery is critical, as supine recovery accelerates HRR via greater central blood volume and earlier restoration of central command. The parameters of this study (HRR measured in a seated position after an immediate ceasing of maximal exercise) do not match with the parameters of the Watanabe et al. (31) (recovery in a supine position) or the Cole et al. (9) (submaximal exercise) study.
An important consideration in the present study is that all subjects had been free of medication for at least 6 wk, and patients were classified as hypertensive or normotensive following the medication-free period. Therefore, these results are only representative of older-age adults who are not receiving pharmaceutical treatment for hypertension. β-Blockers have been shown to attenuate (3) or have no effect on (25) HRR; however, this disconnect is likely due to the β-blocker-induced reduction in HRmax during exercise. Six weeks was considered sufficient time for the effects of the medications to be washed out; however, the concentration of the medications in the blood was not measured at the end of the 6 wk.
Another consideration in the present study is the practical implications for the kinetic analysis strategy used. Although the kinetics analyses used in this study offer distinct advantages in terms of identifying and describing physiological differences between the HTN and CON participants, the practical implications for a clinician and patient are less certain. We are unaware of any study that has compared the results of a kinetic analysis with future participant morbidity and mortality.
In summary, the present study shows that in older-age adults, HRR is attenuated with hypertension. These results indicate altered autonomic function following maximal exercise in the HTN group, which may include reduced parasympathetic activation and sustained sympathetic hyperactivity.
GRANTS
Support for this study was provided by the National Heart, Lung, and Blood Institute (grant RO1 HL091078 to Q.F.) and by the National Institute on Aging (grant RO1 AG017479 to B.D.L.).
DISCLOSURES
There are no competing interests for the authors to declare.
AUTHOR CONTRIBUTIONS
Author contributions: S.A.B., T.B.B., B.D.L., and Q.F. conception and design of research; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., B.D.L., and Q.F. performed experiments; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., T.T., B.D.L., and Q.F. analyzed data; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., T.T., B.D.L., and Q.F. interpreted results of experiments; S.A.B., T.B.B., and Q.F. prepared figures; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., B.D.L., and Q.F. drafted manuscript; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., T.T., B.D.L., and Q.F. edited and revised manuscript; S.A.B., T.B.B., M.D.P., K.N.B., M.M.G., Y.O., G.C-R., N.F., S.S., J.L.H., M.D.S., T.T., B.D.L., and Q.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for their participation. The authors also thank Rhonda L. Meier and Sheryl A. Livingston for their valuable laboratory assistance, as well as Drs. Rong Zhang and Jason Ng for their expertise and assistance.
REFERENCES
- 1.Anderson E, Sinkey C, Lawton W, Mark A. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Antelmi I, De Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol 93: 381–385, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. The prognostic value of the heart rate response during exercise and recovery in patients with heart failure: influence of beta-blockade. Int J Cardiol 138: 166–173, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bosquet L, Gamelin F, Berthoin S. Reliability of postexercise heart rate recovery. Int J Sports Med 29: 238–243, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Chen JY, Yungling LL, Wei-Chuan T, Cheng-Han L, Po-Sheng C, Yi-Heng L, Liang-Miin T, Jyh-Hong C, Li-Jen L. Cardiac autonomic functions derived from short-term heart rate variability recordings associated with heart rate recovery after treamill exercise test in young individuals. Heart Vessels 26: 282–288, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YJ, Lauer MS, Earnest CP, Church TS, Kampert JB, Gibbons LW, Blair SN. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care 26: 2052–2057, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jr., Jones D, Materson B, Oparil S, Wright J, Jr., Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 132: 552–555, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Darr KC, Bassett DR, Morgan BJ, Thomas DP. Effects of age and training status on heart rate recovery after peak exercise. Am J Physiol 23: H340–H343, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Erdogan D, Gonul E, Icli A, Yucel H, Arslan A, Akcay S, Ozaydin M. Effects of normal blood pressure, prehypertension, and hypertension on autonomic nervous system function. Int J Cardiol 151: 50–53, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy. Hypertension 45: 513–521, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger JJ, Kiet Le F, Lahiri MK, Kannankeril PJ, Ng J, Kadish AH. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290: H2446–H2452, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Guyton AC, Hall JE. Overview of the circulation: medical physics of pressure, flow and resistance. In: Textbook of Medical Physiology 10th Edition. Philadelphia: W. B. Saunders, 2000, p. 144–151. [Google Scholar]
- 15.Hammond HK, Froelicher VF. Normal and abnormal heart rate responses to exercise. Prog Cardiovasc Dis 27: 271–296, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension 54: 127–133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24: 1529–1535, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis SS, Levine BD, Prisk G, Shykoff B, Elliott A, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (Metoprolol or Atenolol). Am J Cardiol 96: 1328–1333, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease. J Am Coll Cardiol 51: 1725–1733, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 281: 524–529, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol 275: R1600–R1604, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil 14: 215–221, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 284: 1392–1398, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Racine N, Blanchet M, Ducharme A, Marquis J, Boucher JM, Juneau M, White M. Decreased heart rate recovery after exercise in patients with congestive heart failure: effect of β-blocker therapy. J Card Fail 9: 296–302, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol Respir Environ Exerc Physiol 53: 1572–1575, 1982. [DOI] [PubMed] [Google Scholar]
- 27.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 32: 293–297, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Triebwasser J, Johnson R, Burpo R, Campbell J, Reardon W, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med 48: 203–209, 1977. [PubMed] [Google Scholar]
- 30.Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppanen T, Tiinanen S, Makikallio TH, Huikuri H. Sympatho-vagal interaction in the recovery phase of exercise. Clin Physiol Funct Imaging 31: 272–281, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 104: 1911–1916, 2001. [PubMed] [Google Scholar]
- 32.Yamada Y, Miyajima E, Tochikubo O, Matsukawa T, Ishii M. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension 13: 870–877, 1989. [DOI] [PubMed] [Google Scholar]