Abstract
The ability to accurately measure skeletal muscle functional performance at the single-cell level would be advantageous for exercise physiology studies and disease modeling applications. To that end, this study characterizes the functional response of individual skeletal muscle myotubes derived from adult rodent tissue to creatine treatment and chronic exercise. The observed improvements to functional performance in response to these treatments appear to correlate with alterations in hypertrophic and mitochondrial biogenesis pathways, supporting previously published in vivo and in vitro data, which highlights the role of these pathways in augmenting skeletal muscle output. The developed system represents a multiplexed functional in vitro assay capable of long-term assessment of contractile cellular outputs in real-time that is compatible with concomitant molecular biology analysis. Adoption of this system in drug toxicity and efficacy studies would improve understanding of compound activity on physical cellular outputs and provide more streamlined and predictive data for future preclinical analyses.
Keywords: in vitro, adult skeletal muscle, cantilevers, serum free, exercise
the development of advanced cell culture systems, capable of accurately measuring the functional output of a variety of cell and tissue types, is one of the targets of recent efforts to engineer complex, physiologically relevant in vitro assay systems for drug development and disease modeling applications (36). Skeletal muscle is one tissue for which in vitro analogs have been developed that are capable of effectively recapitulating the functional maturation and performance of the native tissue through controlled stimulation and measurement of the contractile output of embryonic (8, 39), neonatal/adult (2, 3, 16), and human (38) cultured cells. Such systems represent exciting possibilities for drug toxicity/efficacy studies, as well as for the modeling of human disease states in vitro. New in vitro systems that extend these studies to allow functional assessment of muscle maturation and recovery from injury would also be beneficial for advanced exercise physiology applications.
Importantly, the integration of these functional skeletal muscle models with established long-term stimulation parameters and/or hypertrophic drug treatments, such as creatine, will allow investigators to effectively simulate and assess muscle building and exercise in vitro (16, 29–31). Application of such technology could have important implications for the study of the training regimes used by elite athletes, as well as for developing appropriate therapies for patients with muscle wasting conditions, such as muscular dystrophy and sarcopenia. Previous work in this field has focused on assessment of the molecular responses and metabolic output of skeletal muscle cells subjected to bouts of in vitro exercise and/or drug treatment (5, 18, 20, 34). In creatine studies, increased transcript expression for contractile machinery proteins, such as myosin heavy chain (MHC), have been observed in treated cultures (18, 19). Similarly, an increase in gene expression for markers of mitochondrial biogenesis and measurements suggesting increased glucose metabolism in cells subjected to in vitro exercise protocols has been reported (29–31). However, to date, very little work has been done on directly measuring the cell's functional response to such in vitro stimulation regimes. Hence, there remains some ambiguity as to whether the molecular and metabolic changes observed in response to in vitro exercise correspond to a measureable change in the contractile behavior of cultured cells. Moreover, no published work has yet studied the effect of in vitro exercise on muscle fiber (myotube) fatigue, meaning little is known about the fatigability of cultured muscle, or how it compares to the in vivo tissue. Verifying an improvement in cultured skeletal muscle myotube contractility in response to exercise, as well as establishing a physiologically accurate model of fatigue, would be an important step in the development of a functional systems to model exercise physiology in vitro.
In this study, a previously reported (33, 35) serum-free, multiplexed assay system utilizing silicon cantilevers was used to record the contractile output of adult rat skeletal muscle cells subjected to long-term stimulation protocols and creatine treatment. Both long-term stimulation and creatine treatment produced improved peak force (PF) and time-to-fatigue (TTF) data, as well as increased transcript expression for MHC and mitochondrial biogenesis markers, in line with previously published reports. These data highlight not only the functional improvement of muscle cells subjected to bouts of in vitro exercise, but also their biologically correct hypertrophic response to treatment with a known muscle building compound (15, 18, 19, 27). The use of multiplexed microscale cantilevers facilitates the interrogation of individual myotubes, allowing analysis of the effects of exercise and/or drug treatment at the single-cell level, but, since each cantilever is a separate experiment, with a high statistical power. Mathematical scaling of measured outputs is possible using this model (33, 39), enabling data obtained at the single-cell level to be used in predictions of whole tissue responses to physical and chemical challenges.
MATERIALS AND METHODS
Cantilever fabrication and surface preparation.
Cantilever chips were fabricated from silicon wafers, using previously published methods (7, 39). Briefly, 100-mm silicon-on-insulator wafers had a 4-μm-thick device layer and buried oxide layer of 1 μm. In the device layer, the cantilevers were patterned using S1818 photoresist and etched using deep reactive ion etching. To protect the cantilevers during processing, a 1-μm-layer of silicon dioxide was deposited on the top surface. The bottom of the wafer was similarly patterned and etched so that the silicon beneath the cantilevers was removed, producing a large window underneath. The oxide layers were removed using a buffered oxide etch solution. The resulting structures were silicon cantilevers that could be imaged from above and interrogated with a laser from below.
These cantilevers, as well as the coverslip controls used in this study, were coated with an amine-terminated alkylsilane, (3-trimethoxysilyl propyl) diethylenetriamine (DETA) (United Chemical Technologies, Bristol, PA) using methods previously described (6, 13, 14, 39). DETA is an analog of spermidine, which is known to promote long-term survival of cells in vitro, and has been previously validated in the culture of various cell types (9, 23). Thus it was used in this study as a surface coating, to promote cell adhesion and survival.
The silanization of the cantilever surfaces with DETA was performed using a solution of 0.1% (vol/vol) DETA-silane in toluene, heated to 70°C for 30 min, followed by a series of toluene rinses, and reheating to 70°C for 30 min in fresh toluene to remove any unreacted silane. The surfaces were oven cured at 110°C for 2 h and stored in a desiccator until use. The surface coating composition was verified using X-ray photoelectron spectroscopy and contact angle goniometry.
Dissection and cell culture.
Adult rats were euthanized using excess CO2 in accordance with Institutional Animal Care and Use Committee standards. All care and experimental procedures were performed under the approval of the University of Central Florida Institutional Animal Care and Use Committee project no. 14–35, approved annually, and expiring on 9/4/2015. The tibialis anterior muscles were isolated from the left and right hindlimbs. The muscles were placed in a dish containing phosphate-buffered saline (PBS), and scalpels were used to remove excess skin and hair. The muscles were then transferred to a Petri dish containing an enzyme solution consisting of 20 ml Dulbecco's modified Eagle's medium (DMEM), 25 mg collagenase type I (Worthington, Lakewood, NJ), and 1 mg neutral protease (Worthington). The tissue was minced in this dish, and the resulting fragments were transferred to a 50-ml conical tube. This tube was placed horizontally in a 37°C water bath and left for 1 h at 100 rpm. Following this enzymatic digestion, the tube was removed from the water bath, and its contents were mechanically triturated and filtered through a 100-μm mesh to remove undigested tissue fragments. The tube was then centrifuged at 400 g for 4 min. The cell pellet was resuspended in 20 ml of DMEM and then separated into two 100-mm Petri dishes. These dishes were placed in an incubator for 45 min to allow adhesion of fibroblasts to the surfaces and so enrich the nonadherent population for myogenic cells. The dishes' contents were then collected in a 50-ml tube and centrifuged again at 400 g for 4 min. The cell pellet was resuspended in 4 ml of proliferation medium (Table 1) and plated onto four DETA coverslips in a 12-well plate and was left to incubate overnight. The following day, the medium was collected into a 50-ml tube and centrifuged at 400 g for 4 min. The supernatant was removed, and the cell pellet was again resuspended in proliferation medium. The cells were plated onto seven coverslips and six cantilever chips and left overnight. The following day, the medium on these plates was aspirated, and 1 ml fresh proliferation medium was added to each well. Once the plated cells achieved confluency and myotubes began to form (at about 6 days after the first plating), 500 μl of differentiation medium (50:50 neurobasal-L15 medium plus 10 ng/ml insulin-like growth factor) were added to each well. Every 4 days subsequently, one-half of the culture medium on each well was replaced with fresh differentiation medium. Cultures were maintained for 7–9 days after addition of differentiation medium before analysis.
Table 1.
Composition of serum-free medium components in 50:50 neurobasal-L15 medium
| Component | Company | Catalogue No. | Quantity |
|---|---|---|---|
| Antibiotic-antimycotic | Invitrogen | 15240062 | 5 ml |
| aFGF | Invitrogen | 13241-013 | 20 ng/ml |
| Calcium chloride | Fisher | 10035-04-8 | 500 ng/ml |
| VEGF | RND systems | 293-ve-010 | 20 ng/ml |
| bFGF | RND Systems | 3339-FB-025 | 40 ng/ml |
| CNTF | Cell Sciences | CRC 401B | 40 ng/ml |
| NT-3 | Cell Sciences | CRN 500B | 20 ng/ml |
| NT-4 | Cell Sciences | CRN 501B | 20 ng/ml |
| GDNF | Cell Sciences | CRG 400B | 20 ng/ml |
| BDNF | Cell Sciences | CRB 600B | 20 ng/ml |
| CT-1 | Cell Sciences | CRC 700B | 20 ng/ml |
| LIF | Sigma | L5158 | 20 ng/ml |
| Vitronectin | Sigma | V0132 | 100 ng/ml |
aFGF and bFGF, acidic and basic fibroblast growth factor, respectively; CNTF, ciliary neurotrophic factor; NT-3 and NT-4, neurotrophin-3 and -4, respectively; GDNF, glial-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; CT-1, cardiotrophin-1; LIF, leukemia inhibitory factor.
Chronic low-frequency stimulation protocol and creatine treatment.
After 7–9 days in differentiation medium, two cantilevers and one coverslip were placed into a chronic low-frequency stimulation (CLFS) apparatus. The remaining cantilevers and coverslips were used as unstimulated controls. The apparatus consisted of carbon electrodes housed in a six-well plate and connected to a pulse stimulator. Electrical pulses had a magnitude of 3 V, with a pulse width of 1.5 ms; five pulses were delivered to the cultures at 20 Hz every 4 s. This stimulation protocol was devised based on previously published work (17, 32). The cultures were incubated at 37°C within this apparatus for 4–7 days, and the medium was replaced every other day. Similarly, cultures were fed with medium supplemented with 40 mM creatine (Sigma) following 7–9 days in differentiation medium. Cultures were maintained in creatine-containing medium for a further 4–7 days before analysis, and the medium was replaced every other day.
PF and TTF calculation.
A laser and photo-detector system, adapted from Atomic Force Microscopy technology, was utilized to calculate PF of individual myotube contractions, as well as the myotubes' TTF (Fig. 1). Application of this system for similar studies has been described in detail elsewhere (33, 35). Each cantilever chip was transferred to a heated stage housed within a modified electrophysiology rig. The culture dish on this heated stage was filled with differentiation medium (+10 mM HEPES) to maintain the cells during analysis.
Fig. 1.
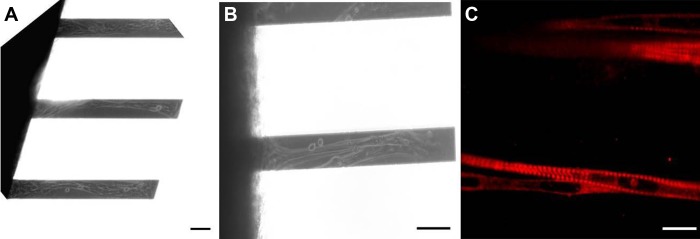
Images of adult-derived skeletal muscle myotubes. Phase-contrast images are shown of myotubes on silicon cantilevers at ×10 (A) and ×20 (B) magnification. C: immunostaining of cultures using an antibody against myosin heavy chain (MHC) with all classes (A4.1025, DSHB) indicating highly striated and mature muscle fibers. Scale bar = 100 μm.
Briefly, the automated system consisted of a class 2 photodiode laser positioned below the stage so that the beam was focused on the cantilever tip and reflected into the center of a four-quadrant photo-detector (8, 40). A pulse stimulator was used to generate pulses capable of eliciting contraction in the cultured myotubes. The biphasic pulses were 3 V in magnitude and had a pulse width of 40 ms, with a frequency of 1 Hz. The resulting cantilever deflection was measured in terms of laser displacement (in V). Software was written in LabVIEW (National Instruments) to control laser and photo-detector positioning to allow scanning across all cantilevers on each chip for contractile activity. The acquired raw data were converted into a direct measurement of force using a modified Stoney's equation. Previous work (33) had demonstrated that measurements analyzed using the Stoney equation with a similar cantilever system exhibited strong correlation with data generated using finite-element analysis, which takes into account a more detailed set of cell dimensions when calculating force production. The assumptions made for analysis using the Stoney equation were, therefore, deemed acceptable for determining changes in force production per cell in response to creatine treatment and long-term stimulation.
To determine which cantilevers had contracting myotubes, the chip in the culture dish was first subjected to broad field electrical stimulation using the stimulation parameters listed above. The cantilevers were scanned for 5 s each to elicit and record contractile responses. The active cantilevers were then scanned, with continuous electrical stimulation, until their PF was reduced by one-half. Thus the TTF was calculated by measuring the time elapsed in which a 50% reduction in PF was observed. Subsequent bright-field images were collected of each contracting cantilever, and those supporting multiple myotubes were discounted from further analysis to ensure the calculation of force generated per myotube was accurate. Preliminary studies were performed to ensure that this 50% reduction in PF was indicative of myotube fatigue and not physical damage to the myotubes, such as tearing of the sarcolemma induced by stimulation. After a 7-min rest and recovery period, ∼88% of original PF was observed, suggesting force reduction was indeed due to cellular exhaustion (data not shown).
Immunocytochemistry.
Cells were fixed for 10 min in 4% paraformaldehyde in PBS. These cultures were then permeabilized with 0.1% Triton 100 in PBS for 20 min, followed by a 20-min incubation in 5% donkey serum with 0.1% bovine serum albumin in PBS (blocking solution). The blocking solution was then removed, and the cells were further incubated overnight at 4°C in a primary antibody solution consisting of a mouse monoclonal antibody against MHC (DSHB A4.1025) at a 1:10 dilution in blocking solution. The cells were washed three times with PBS and then incubated in the dark for 2 h at room temperature with a secondary antibody solution consisting of a donkey anti-mouse Alexafluor-594 conjugate diluted 1:200 in blocking solution. The cells were washed three times in PBS before being mounted on microscope slides using a hard-set DAPI-containing mounting medium (Vectashield) for visualization.
Quantitative polymerase chain reaction.
Samples designated for PCR analysis were homogenized in TRIzol reagent, and the RNA isolated according to the manufacturer's protocol. Purified RNA was converted to cDNA, and the quantitative polymerase chain reactions (qPCR) were run using the BIO-RAD iScript One-Step RT-PCR kit with SYBR Green (BIO-RAD Laboratories, Hercules, CA; catalog no. 170–8892), according to the manufacturer's protocol. Briefly, the PCR reaction mixture (12.5 μl 2 × SYBR Green RT-PCR reaction mix, 0.5 μl iScript reverse transcriptase, 0.75 μl forward primer, 0.75 μl reverse primer, 10 ng RNA template, and nuclease-free water to a total reaction volume of 25 μl) was prepared for each gene to be analyzed from each sample in triplicate wells of a 48-well plate. PCR primers used in this study were taken from those previously tested and verified (Table 2) (12). The reaction plate was then transferred to an MJ Mini thermal cycler (Bio-Rad) and incubated at 50°C for 10 min and 95°C for 5 min before being cycled 40 times at 95°C for 10 s, followed by 60°C for 30 s. The qPCR protocol and fluorescent output from the reactions were implemented and recorded using Opticon Monitor software (Bio-Rad).
Table 2.
PCR primer sequences
| Gene | Sequence (5′ to 3′) |
|---|---|
| MHC 1 FWD | GCCAACTATGCTGGAGCTGATGCCC |
| MHC 1 REV | GGTGCGTGGAGCGCAAGTTTGTCATAAG |
| MHC 2A FWD | GGCACAAAACTGCTGAAGCAGAGGC |
| MHC 2A REV | GGTGCTCCTGAGGTTGGTCATCAGC |
| MHC 2B FWD | GCAGCTACTGGATGCCAGTGAGCGC |
| MHC 2B REV | CTGGACGATGTCTTCCATCTCTCC |
| MHC 2X FWD | GGCAGCAGCAGCTGCGGAAGCAGAGTCTGG |
| MHC 2X REV | GAGTGCTCCTCAGATTGGTCATTAGC |
| PGC-1α FWD | GTGCAGCCAAGACTCTGTATGG |
| PGC-1α REV | GTCCAGGTCATTCACATCAAGTTC |
| ERR-γ FWD | TGACTTGGCTGATCGAG |
| ERR-γ REV | CCCAGGATCAAGATTTCC |
MHC, myosin heavy chain; FWD, forward; REV, reverse; PGC-1α, peroxisome proliferator-activated receptor-α coactivator-1; ERR-γ, estrogen-related receptor-γ.
The threshold cycle (CT) was defined as the fractional cycle at which the fluorescence generated by the binding of SYBR Green molecules to double-stranded DNA exceeded a fixed threshold above the baseline. The amount of each target gene present in the sample was quantitated using the comparative CT method (21). Mean CT values from triplicate reactions for each sample were determined and normalized to that of an endogenous housekeeping gene (β-actin) run in parallel. The amount of target amplification relative to the endogenous control was calculated using the formula 2−ΔΔCT.
Image analysis.
The width of myotubes cultured on cantilevers was measured as a means to estimate cross-sectional area (CSA). Phase-contrast images were collected using a Zeiss Axiovert 200 inverted microscope and analyzed using ImageJ software. Lines were drawn vertically across each image at points one-third from each edge of the field of view. The width of each myotube that crossed either of these lines was measured at that point to eliminate selection bias.
Statistical analysis.
All experiments were repeated at least three times using cultures prepared on different days, with tissue from different animals. Differences in force and TTF measurements among the three conditions (“untreated”, “CLFS”, “creatine treated”) were each evaluated statistically using one-way repeated-measures ANOVA (α = 0.05). Following the repeated-measures ANOVA with a statistically significant F-statistic, means were compared using Tukey's honestly significant difference test for multiple comparisons (α = 0.05). qPCR expression data for CLFS and creatine-treated samples were expressed relative to untreated controls; as such, control values were always 1. For these experiments, a one-sample T-test was used to calculate whether or not mean expression levels were statistically different from 1. All values noted in the text are expressed as means ± SE.
RESULTS
Primary adult rat skeletal muscle myotubes were maintained on silicon cantilevers for 14 days in differentiation medium before being analyzed for contractile function. Immunocytochemical analysis at this time point demonstrated the development of a distinct striated morphology, indicating the formation of mature and functionally capable sarcomeres within these cells (Fig. 1). This end point enabled maximal levels of myotube differentiation and maturation (∼70% of cultured myotubes responded to broad-field stimulation), while minimizing myotube loss due to detachment and thereby maximizing data collection.
Functional assessment of cultured myotubes.
Using the described protocol, contractile activity was recorded from the cultured myotubes, verifying the ability for this system to measure the functional contraction (Fig. 2A). Continuous recording of stimulated cantilever cultures indicated substantial reductions in PF measurements over extended periods that enabled the assessment of skeletal muscle fatigue in real-time (Fig. 2B). Analysis of functional data from the experimental conditions demonstrated that both creatine treatment and long-term stimulation produced significant increases in PF compared with untreated controls (Fig. 3A, P < 0.02). No significant differences were observed between creatine treated and CLFS cultures over 4- or 7-day applications (P > 0.5). Similarly, creatine and CLFS regimens were found to statistically increase the TTF capacities of cultured myotubes roughly fivefold, compared with untreated controls (Fig. 3B, P < 0.001). No significant differences in TTF were observed between any of the treatment regimens examined (P > 0.6). These combined data suggest that each treatment regime examined was capable of promoting the same magnitude of functional improvement in myotube performance over the time course investigated in this study.
Fig. 2.
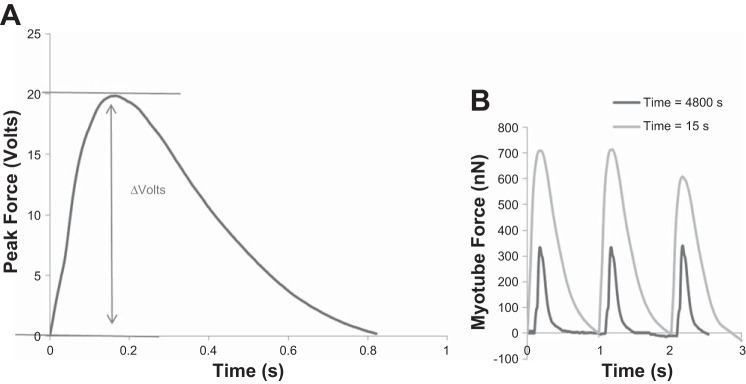
Measurement of force generation and fatigue. A: representative peak force trace indicating how peak force is measured as a change in voltage by the photo-detector. This change in voltage can be converted using a modified version of Stoney's equation to force in nN. B: representative traces of peak force at initial time (15 s) and when peak force has reached a 50% reduction in force (4,800 s). Recordings were measured in volts, then converted to force using Stoney's equations, and replotted.
Fig. 3.
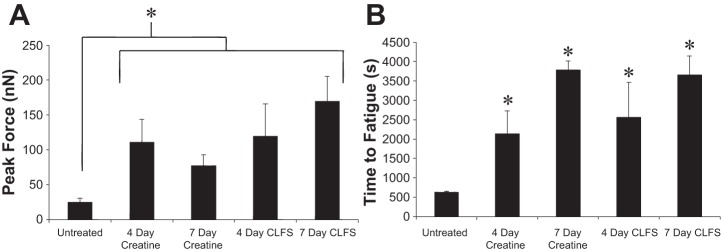
Comparison of peak force and fatigue. A: graphical representation of peak force changes of 4- and 7-day creatine and chronic low-frequency stimulation (CLFS) treatment regimens, *P < 0.02. B: graphical representation of time-to-fatigue changes of 4- and 7-day creatine and CLFS treatment regimens, *P < 0.001.
Assessment of hypertrophy in cultured myotubes.
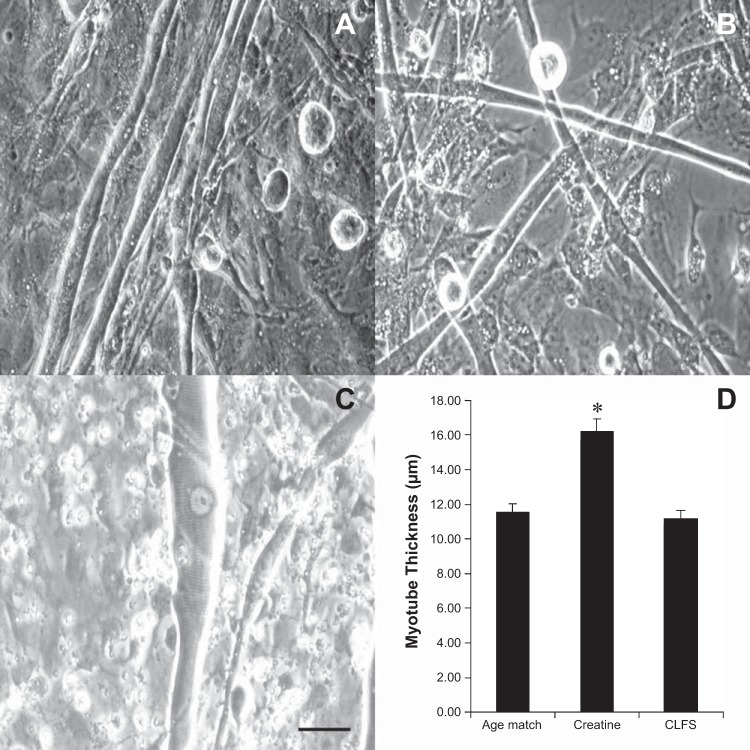
During the culture of the primary adult skeletal muscle cells, it was observed that creatine-treated cultures developed substantially thicker myotubes than all other conditions examined (Fig. 4). Image analysis confirmed that 4- and 7-day creatine-treated cultures promoted a significant increase in myotube CSA compared with both CLFS and untreated controls (n = 3, P < 0.0001). Both creatine treatment and CLFS were found to improve functional performance, but only creatine treatment produced a significant hypertrophic response in the cultured myotubes. The collected data, therefore, implied that the observed differences in functional performance between treated and untreated cultures were due to the adaptation of different molecular mechanisms activated through the two methods of myotube stimulation employed.
Fig. 4.
Assessment of hypertrophy in cultured myotubes. Phase images of multinucleated myotubes in control (A), CLFS (B), and creatine-treated (C) cultures are shown. Scale bar = 50 μm. D: graphical comparison of myotube thickness for comparison of hypertrophy, *P < 0.001.
Gene expression analysis of cultured myotubes.
Data collected from qPCR experiments highlight that both creatine treatment and CLFS have a significant effect on transcription profiles within primary adult myotubes in vitro. Application of creatine to cultured cells for 4 days produced a two- to fourfold increase in all MHC transcripts examined (n = 4, P < 0.002, Fig. 5), whereas 4-day CLFS protocols were found to promote an upregulation in estrogen-related receptor-γ transcripts (n = 3, P < 0.03). The differences observed in upregulated genes within these cultures highlight the alternative pathways activated by the treatment regimens examined. The collected data confirmed the physiological differences in myotube CSA discussed earlier and are in line with previously published work assessing the hypertrophic effect of creatine on skeletal muscle and the cellular response to chronic exercise regimes in vivo and in vitro (15, 22, 27).
Fig. 5.
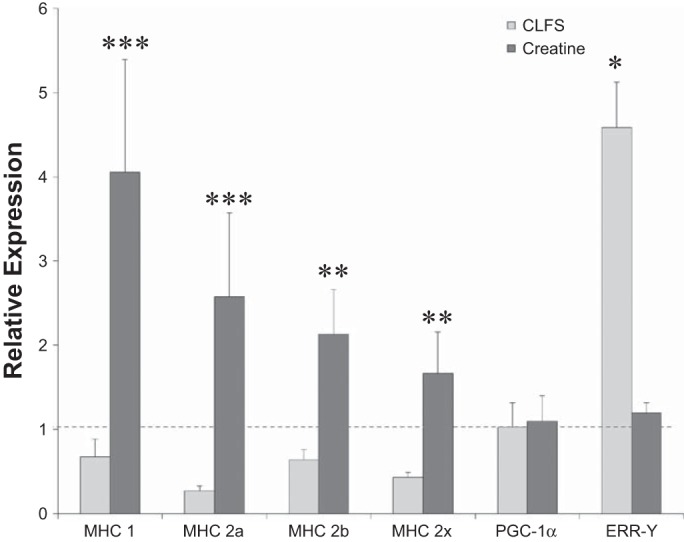
Gene expression analysis of cultured myotubes. The gene expression profiles for myotubes undergoing a 4-day treatment of either CLFS or creatine were assessed using real-time quantitative PCR analysis. All MHC genes were significantly upregulated under creatine treatment, **P < 0.002, ***P < 0.0001. CLFS treatment resulted in a greater than fourfold upregulation in estrogen-related receptor-γ (ERR-γ) transcripts, *P < 0.03. This PCR analysis suggests different mechanisms of action for equivalent changes in functional outputs. PGC-1α, peroxisome proliferator-activated receptor-α coactivator-1.
DISCUSSION
The development and widespread application of an in vitro model of exercise and fatigue capable of modeling skeletal muscle functional response would be of substantial benefit to exercise physiology studies and investigations of muscle building and wasting. While three-dimensional cultures have obvious advantages for modeling whole tissue behavior, multiplexed, two-dimensional systems are more easily adapted for application in high-throughput drug efficacy/toxicity studies. Furthermore, the ability to record contractile data from individual myotubes is useful for examining alterations in individual muscle fiber phenotype in healthy and diseased states. Multiplexed cantilever assays provide information on maturation and performance variability between myotubes and provide multiple data points for the generation of more statistically relevant observations. Analysis of three-dimensional myotube bundles either relies on integration of invasive force transducers or lower resolution optical mapping. The noninvasive nature of the cantilever analysis system means cultures can be reanalyzed at multiple time points for continued assessment of individual cells. The high-resolution data-acquisition system, utilizing laser and photo-detector hardware, enables higher sampling rates (1,000 Hz) than optical techniques (5–500 Hz), facilitating more in depth analysis of changes in functional contractile waveforms. Finally, two-dimensional systems, such as the one described here, are likely to be more readily integrated with complex, multicell-type platforms for body-on-a-chip applications (10, 36, 41) and for developing phenotypic assays for drug evaluation.
Previous work in this laboratory has developed and optimized this cantilever system for assessing embryonic myotube development (35, 40). While such a culture model is beneficial as a proof of principle, application of this technology to investigate age-related myopathies and exercise regimens in aged tissue would benefit from integration of the cantilever platform with cells derived from adult tissue. This study builds on the published data to utilize a cell population derived from adult tissue within a functional in vitro assay system designed to quantify contractile responses in real-time and over extended periods in response to both drug and exercise treatments.
The collected data demonstrate that this multiplexed cantilever array can be used to effectively monitor rates of muscle fatigue in vitro. While previous work from a number of groups (17, 24, 30, 32) has used long-term stimulation and creatine treatment to evaluate molecular changes during cellular development in vitro, this study is the first to assess the effect of such treatment applications on long-term functional performance. Such data are important for confirming that the molecular changes observed in vitro correlate to alterations in functional output, thereby validating such studies for producing predictions of whole tissue responses in vivo.
Analysis of the cultured cells highlights that both PF generation and rates of fatigue improve significantly in response to either creatine or long-term stimulation protocols. The observed improvements to myotube output and endurance correlate to established data evaluating the effects of similar treatments in vivo (1, 11, 25, 28, 37). Assessment of gene transcription profiles in treated cells suggests that the improvements to functional output induced by these treatments are facilitated by activation of different molecular mechanisms. Creatine application promoted a hypertrophic response in the cultured cells, leading to the upregulation of contractile protein apparatus and the maturation of sarcomeric structures, resulting in myotubes with significantly larger CSAs compared with untreated controls (Figs. 4 and 5, P < 0.0001). Long-term stimulation is known to initiate alterations in transcription of genes relating to mitochondrial biogenesis, such as estrogen-related receptor-γ (5, 22, 26). Upregulation of these genes likely induces increased mitochondrial presence within these cells, facilitating a greater endurance capacity (5, 34). This understanding of the effects of long-term stimulation on skeletal muscle is supported by the data presented in this study (Fig. 5).
Improvements to PF observed in CLFS cultures suggest the repeated stimulation likely also had an effect on sarcomeric development in these cultures. No significant difference was observed in PF production between creatine-treated cultures and those subjected to CLFS. However, significant differences in myotube CSA were observed between these culture conditions. Together, these data imply that creatine treatment promoted a hypertrophic response, leading to greater myotube volumes and, therefore, more cytoplasmic space for sarcomere formation, resulting in greater PF production. Since no hypertrophic response or upregulation of MHC transcripts was observed in CLFS cultures, the observed improvements in force likely result from improved development of the sarcomeres already present within these cells. Previously reported results indicate evidence for the upregulation of myosin synthesis in electrically stimulated in vitro cultures utilizing chick (4) and C2C12 cell lines (21). On the surface, these data seem contradictory to those presented in this paper. However, there are significant differences between this work and those published previously that inhibit direct comparison. Our protocol provides a defined, serum-free medium formulation that eliminates many unknown factors contributed to the culture system by fetal bovine serum addition. The use of serum in the cited work may have led to the generation of significantly altered responses to electrical stimulation, due to the activation of different pathways by unknown factors in the medium. The use of our defined culture system obviates this issue, thereby providing a more controlled environment for investigating the direct effects of electrical stimulation on culture maturation and development. Furthermore, our functional assay system utilizes cells derived from adult rat tissue, which may demonstrate significantly different physiological responses to long-term electrical stimulation than alternate cell sources.
This study provides baseline parameters for measuring endurance of single fibers in vitro. While previous studies have characterized fatigue parameters in vivo, this study is the first to do so for in vitro muscle fibers. As such, it was necessary to determine an appropriate level of PF decline, which could be taken as evidence of muscle fatigue. Using the described functional assay, it is not possible to characterize a 100% force reduction, since the baseline fluctuations make such small recordings unreliable. Muscle failure, as is measured in vivo, is not possible to investigate in this system, since failure does not necessarily confirm that the muscle fibers are no longer contracting, but rather that they are no longer able to generate sufficient force to overcome the mass of the animal. It is not clear at what point this occurs and how much variability exists between different muscle types and between individuals. The parameters assessed in this study provide the means to compare between experimental repeats and facilitate normalization of measurements to a consistent evaluation of cellular exhaustion to potentially produce a more reliable assay of muscle function than some in vivo models. Measurement of a 50% reduction in PF is sufficient to reliably compare fatiguability between experimental samples while restraining experimental lengths to a time-frame that would facilitate investigation of multiple cellular phenotypes and/or treatments on a given day.
This system demonstrates, for the first time, the capacity to directly measure both absolute PF and rates of fatigue in individual cultured myotubes. The developed system thereby allows full functional assessment of skeletal muscle responses to both small-molecule treatment and exercise regimes controlled via broad-field electrical stimulation. The successful application of cells derived from adult tissue and maintained in serum-free defined culture conditions makes this model appropriate for investigations of adult myopathies and/or drug efficacy and toxicity studies by utilizing a phenotypic assay system. The model is also appropriate to combine with molecular biological techniques for advanced mechanistic studies, as well as target identification in drug evaluations. Further development of this model to improve its high-throughput nature will likely be beneficial to future preclinical screening technologies and exercise physiology protocols.
GRANTS
This study was supported by National Institutes of Health Grants R01-NS-050452 and R01-EB-009429.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.W.M. and J.J.H. conception and design of research; C.W.M., S.N., and K.P. performed experiments; C.W.M. and A.S.T.S. analyzed data; C.W.M., A.S.T.S., and C.J.L. interpreted results of experiments; C.W.M. prepared figures; C.W.M. and A.S.T.S. drafted manuscript; J.J.H. edited and revised manuscript; J.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Stephen Lambert for guidance and review of the manuscript.
REFERENCES
- 1.Balsom PD, Söderlund K, Sjödin B, Ekblom B. Skeletal muscle metabolism during short duration high-intensity exercise: influence of creatine supplementation. Acta Physiol Scand 154: 303–310, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Bian W, Juhas M, Pfeiler TW, Bursac N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng Part A 18: 957–967, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc 4: 1522–1534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brevet A, Pinto E, Peacock J, Stockdale FE. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science 193: 1152–1154, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y, Hazen BC, Russell AP, Kralli A. Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (PERM1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J Biol Chem 288: 25207–25218, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials 31: 4880–4888, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, Riedel L, Guo X, Hickman JJ. Embryonic motoneuron-skeletal muscle co-culture in a defined system. Neuroscience 146: 481–488, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc 2: 1795–1801, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11: 1305–1314, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 13: 55–72, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Francaux M, Poortmans JR. Effects of training and creatine supplement on muscle strength and body mass. Eur J Appl Physiol 80: 165–168, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, Olson EN, Kralli A, Kelly DP. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest 123: 2564–2575, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Das M, Rumsey J, Gonzalez M, Stancescu M, Hickman J. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods 16: 1347–1355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 32: 9602–9611, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hespel P, Op't Eijnde B, Leemputte MV, Ursø B, Greenhaff PL, Labarque V, Dymarkowski S, Hecke PV, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol 536: 625–633, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32: 3575–3583, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YC, Dennis RG, Baar K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol 291: C11–C17, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Ingwall JS. Creatine and the control of muscle-specific protein synthesis in cardiac and skeletal muscle. Circ Res 38: I115–I123, 1976. [PubMed] [Google Scholar]
- 19.Ingwall JS, Morales MF, Stockdale FE. Creatine and the control of myosin synthesis in differentiating skeletal muscle. Proc Natl Acad Sci U S A 69: 2250–2253, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48: 963–970, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Ito A, Yamamoto Y, Sato M, Ikeda K, Yamamoto M, Fujita H, Nagamori E, Kawabe Y, Kamihira M. Induction of functional tissue-engineered skeletal muscle constructs by defined electrical stimulation. Sci Rep 4: 4781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M, Lundby C. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115: 785–793, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Kaeberlein M. Spermidine surprise for a long life. Nat Cell Biol 11: 1277–1278, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Khodabukus A, Baar K. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle. Tissue Eng Part C Methods 18: 349–357, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Laforest S, St-Pierre DM, Cyr J, Gayton D. Effects of age and regular exercise on muscle strength and endurance. Eur J Appl Physiol 60: 104–111, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Lai L, Wang M, Martin OJ, Leone TC, Vega RB, Han X, Kelly DP. A Role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J Biol Chem 289: 2250–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis M, Van Beneden R, Dehoux M, Thissen JP, Francaux M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C2C12 cells. FEBS Lett 557: 243–247, 2004. [DOI] [PubMed] [Google Scholar]
- 28.McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci 50A: B97–B104, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295: E1191–E1204, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Nikolic N, Bakke SS, Kase ET, Rudberg I, Flo Halle I, Rustan AC, Thoresen GH, Aas V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLos One 7: e33203, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H, Bhalla R, Saigal R, Radisic M, Watson N, Langer R, Vunjak-Novakovic G. Effects of electrical stimulation in C2C12 muscle constructs. J Tissue Eng Regen Med 2: 279–287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pette D, Dusterhoft S. Altered gene expression in fast-twitch muscle induced by chronic low-frequency stimulation. Am J Physiol Regul Integr Comp Physiol 262: R333–R338, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Pirozzi KL, Long CJ, McAleer CW, Smith AST, Hickman JJ. Correlation of embryonic skeletal muscle myotube physical characteristics with contractile force generation on an atomic force microscope-based bio-microelectromechanical systems device. Appl Phys Lett 103: 83108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. Estrogen-related receptor γ is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem 285: 22619–22629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AST, Long CJ, Pirozzi K, Hickman JJ. A functional system for high-content screening of neuromuscular junctions in vitro. Technology (Singap World Sci) 1: 37–48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, Shuler ML. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 13: 1201–1212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc 47: 1208–1214, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Vandenburgh H. High-content drug screening with engineered musculoskeletal tissues. Tissue Eng Part B Rev 16: 55–64, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson K, Das M, Wahl KJ, Colton RJ, Hickman J. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLos One 5: e11042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson K, Molnar P, Hickman J. Integration of functional myotubes with a Bio-MEMS device for non-invasive interrogation. Lab Chip 7: 920–922, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Zhao Z, Abdul Rahim NA, van Noort D, Yu H. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9: 3185–3192, 2009. [DOI] [PubMed] [Google Scholar]