mechanical properties of the extracellular matrix play a much more important role in cell differentiation (3) or responses of differentiated cells to various injurious insults (1, 6) than was previously thought. Cells can sense the stiffness of surrounding matrix and respond to mechanical cues in their microenvironment through a number of mechanisms, many of them mediated by integrins and signaling hubs localized at focal adhesions and leading to actomyosin remodeling, cell membrane rupture, etc. (9, 10). Stiffness-dependent expression of focal adhesion proteins and cytoskeletal remodeling (3) further illustrates this notion.
Pathological changes in biomechanical properties of the extracellular matrix may influence cell biological behaviors and be important factors in the pathogenesis of several diseases. Examples include matrix stiffness-dependent progression of malignant phenotype in cancer, exacerbation of LPS-induced lung inflammation, and pulmonary fibrosis (5–7).
In the respiratory system, contrasting alterations in lung matrix elasticity and structure are best represented in fibrosis or emphysema. The deregulated matrix deposition observed in lung parenchyma during idiopathic pulmonary fibrosis induces stiffening of the matrix, myofibroblast differentiation, and differential gene expression, thus leading to additional abnormal matrix deposition and progression of the disease (2, 5). On the other hand, pulmonary emphysema is characterized by enzymatic digestion of the major elastic components of the lung tissue, significantly decreasing the tissue stiffness and stability. A study by Takahashi et al. (8) proposed that reversal of this process by increasing alveolar proteoglycan deposition could reduce the progression of the disease by enhancing the stability of the extracellular matrix and decreasing the incidence of alveolar wall rupture. These studies highlight the importance of the lung tissue compliance as a key biomechanical factor contributing to lung pathologies.
In this issue of the Journal of Applied Physiology, Higuita-Castro and collaborators (4) used an in vitro model of lung epithelial cells grown on compliant substrates and exposed to mechanical stress produced by microbubble propagations that were used to stimulate airway reopening during respiratory cycles in inflamed lungs. This model allowed the authors to investigate how changes in airway wall stiffness alter the biomechanical mechanisms responsible for cellular injury during airway reopening. The presented results show that softer, more compliant substrates recapitulating airways in the emphysematous lungs led to increased cell detachment but lower cell necrosis, while stiffer airways relevant to lung fibrosis conditions resulted in increased cell necrosis and lower cell detachment. Such findings illustrate how matrix stiffness-dependent anchorage of lung epithelium may explain epithelial denudation and decreased cellular mass in emphysema, as well as the increased susceptibility of lung epithelium to shear-induced injury in fibrotic lungs with increased tissue stiffness.
This study further highlights a biomechanical dilemma: increased cell anchorage on stiffer substrates leading to enhancement of the actomyosin cytoskeleton, which, in turn, leads to more rigid monolayers and increased risk of injury, versus a more relaxed cytoskeleton, which is less susceptible to shear-induced mechanical injury, but at the expense of decreased epithelial anchorage and stability on softer substrates (Fig. 1).
Fig. 1.
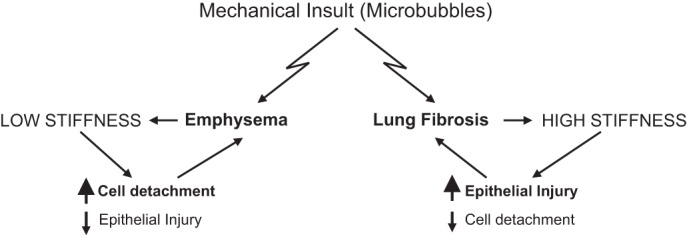
Contrasting changes in mechanical properties of lung parenchyma associated with emphysema and pulmonary fibrosis cause opposite effects on lung epithelial cell injury and detachment rates, leading to propagation of corresponding pathological condition.
Therefore, it is likely that normal lung mechanics represent a homeostatic equilibrium point, and that deviation from this point, as occurs during fibrosis or emphysema, may render the lung more susceptible to inflammatory insult or injurious mechanical ventilation. This type of biomechanical homeostasis has emerged as an important factor in maintaining and promoting healthy conditions in a number of physiological systems. A better understanding of the underlying mechanisms regulating lung biomechanical homeostasis might lead to new insights into the pathogenesis of pulmonary disorders and the development of specific therapies directed toward normalization of the mechanical microenvironment in the lung.
GRANTS
This paper was supported by National Heart, Lung, and Blood Institute Grants HL-87823 and HL-76259.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.G.B. drafted manuscript; K.G.B. edited and revised manuscript.
REFERENCES
- 1.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 42: 1114–1119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carver W, Goldsmith EC. Regulation of tissue fibrosis by the biomechanical environment. Biomed Res Int 2013: 101979, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Higuita-Castro N, Mihai C, Hansford DJ, Ghadiali SN. Influence of airway wall compliance on epithelial cell injury and adhesion during interfacial flows. J Appl Physiol; 10.1152/japplphysiol.00752.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mambetsariev I, Tian Y, Wu T, Lavoie T, Solway J, Birukov KG, Birukova AA. Stiffness-activated GEF-H1 expression exacerbates LPS-induced lung inflammation. PLos One 9: e92670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Majumdar A, Parameswaran H, Bartolak-Suki E, Suki B. Proteoglycans maintain lung stability in an elastase-treated mouse model of emphysema. Am J Respir Cell Mol Biol 51: 26–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi LU, Caldini EG, Velasco IT, Negri EM. Cytoskeleton and mechanotransduction in the pathophysiology of ventilator-induced lung injury. J Bras Pneumol 36: 363–371, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med 171: 1328–1342, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


