Abstract
Mechanical loading can increase tendon cross-sectional area (CSA), but the mechanisms by which this occurs are largely unknown. To gain a greater understanding of the cellular mechanisms of adult tendon growth in response to mechanical loading, we used a synergist ablation model whereby a tenectomy of the Achilles tendon was performed to induce growth of the synergist plantaris tendon. We hypothesized that after synergist ablation progenitor cells in the epitenon would proliferate and increase the size of the existing tendon matrix. Adult male mice were subjected to a bilateral Achilles tenectomy, and plantaris tendons were isolated from mice at 0, 2, 7, 14, and 28 days after surgery. Tendons were sectioned stained with either fast green and hematoxylin, prepared for fluorescent microscopy, or prepared for gene expression of scleraxis and type I collagen. After overload, there was a dramatic increase in total CSA of tendons, whereas the size of the original tendon matrix was not changed. Growth primarily occurred through the formation of a neotendon matrix between the original tendon and the epitenon, and contained cells that were proliferative and scleraxis positive. Additionally, an initial expansion of fibroblast cells occurred before the synthesis of new extracellular matrix. Fibroblasts in the original tendon did not re-enter the cell cycle. The results from this study provide new insight into the mechanisms of tendon growth, indicate tendon consists mostly of postmitotic cells, and that growth of tendon primarily occurs from the most superficial layers outward.
Keywords: neotendon, scleraxis, tendon
tendons are organized into functional cable-like units of primarily type I collagen and a surrounding matrix of network collagens, elastin, and various proteoglycans (2). Collagen fibers are arranged into fascicles in hierarchical order, which make up the tendon proper. Surrounding this is a basement membrane called the epitenon and loose peritendinous connective tissue, which contains the principal blood and nerve supply to the tendon. In sedentary mice, tendons continue to grow in size and length until ∼10 wk of age (15), and for humans the majority of tendon growth occurs in the first two decades of life (5). Mechanical loading can be a stimulus for tendon growth after adolescence, with vigorous exercise programs resulting in increases in cross-sectional area (CSA) of ∼30% in both mice (13) and humans (19).
While the phenomenon of gross tendon growth in adult animals has been reported, the cellular mechanisms that are responsible for this growth remain largely unknown. There is, however, much more known about the initial formation and growth of tendons during embryonic development and early postnatal stages of life. Scleraxis is a basic helix-loop-helix transcription factor required for the formation of limb tendons in embryonic development, with genetic inactivation of scleraxis resulting in a severe disruption in tendon formation (16). Scleraxis is highly regulated by both mechanical loading, and also the TGF-β/BMP superfamily (10, 14). Mice with a genetic inactivation of TGF-β signaling (Tgfbr2−/−) fail to form limb tendons and have severely disrupted scleraxis expression (18), and administration of TGF-β to cultured tendon fibroblasts increases scleraxis expression (10, 14). In Achilles tendons, scleraxis is expressed in the majority of fibroblasts throughout the tendon up through 2 mo of age, but past this point, scleraxis expression is confined to the epitenon region (13). Although chronic mechanical loading can increase tendon mass, CSA, and fibroblast density (13), it is not known whether the increase in CSA and fibroblast density occurs because of proliferation and expansion of existing tendon fibroblast cells throughout the tendon or whether growth occurs in distinct regions within the tendon.
To gain a greater understanding into the mechanism by which adult tendon adapts to mechanical loading and the role of scleraxis in adult tendon adaptation, we subjected adult mice to bilateral synergist ablation surgery. By removing the Achilles tendon, the synergist plantaris tendon receives a robust, supraphysiological increase in load and is solely responsible for all plantarflexion in the hindlimb (1, 11). We hypothesized that after synergist ablation, progenitor cells in the epitenon would proliferate and increase the size of the existing tendon matrix. Additionally, we hypothesized that synergist ablation would increase scleraxis expression throughout the period of rapid cell expansion and precede the synthesis of type I collagen.
METHODS
Animals.
All experiments were approved by the University of Michigan Committee on the Use and Care of Animals, and all procedures followed the ethical guidelines of the US Public Health Service and American Physiological Society. Approximately 6-mo-old male mice that express green fluorescent protein (GFP) under the control of the scleraxis promoter (ScxGFP mice, n = 6 per group), kindly provided by Dr. Ronen Schweitzer (17), were used in this study. To induce a supraphysiological load on plantaris tendons, mice were anesthetized with 1–2% isoflurane, and bilateral Achilles tenectomies were performed as previously described (1). A representative image of this surgical procedure is displayed in Fig. 1. After tenectomy, mice were allowed to recover and were permitted to freely ambulate about their cages. Buprenorphine (0.05 mg/kg) was administered subcutaneously for postoperative analgesia. At 2, 7, 14, or 28 days after the surgery, plantaris tendons were harvested from both limbs. These time points were selected based upon previous observations of ECM changes in the aponeurosis of muscles using a synergist ablation model (1). Age-matched mice that did not undergo surgery were used as controls. At 24 and 48 h before harvest, mice received an intraperitoneal injection of 100 μg of the thymidine analog 5-ethynyl-2′-deoxyuridine (EdU, Invitrogen) to mark proliferative cells. At the time of harvest, animals were anesthetized with pentobarbital sodium, and plantaris tendons were removed. The right tendon was then prepared for histology while RNA was isolated from the left tendon. Animals were then humanely euthanized with overdose of pentobarbital sodium.
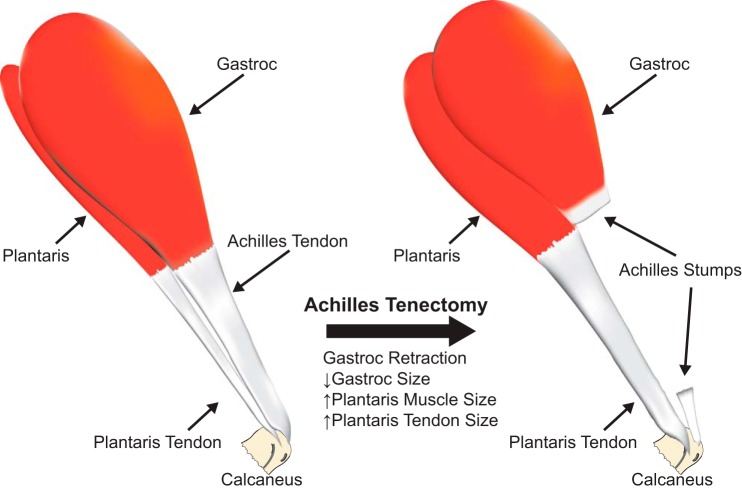
Fig. 1.
Representative image demonstrating the synergist ablation model. The Achilles tendon is removed, leaving the plantaris as the sole plantarflexor group. Over a short period of time, both the plantaris muscle and tendon increase in size.
Histology.
Plantaris tendons were placed in TissueTek (Sakura), snap frozen in isopentane cooled in liquid nitrogen, and stored at −80°C until use. Tendons were sectioned at a thickness of 10 μm on a cryostat, and either stained with fast green and hematoxylin or treated with DAPI (Sigma Aldrich) and a Click-iT kit with AlexaFluor 546 Azide (Invitrogen) to label EdU. Digital images of slides were taken using an Axiovert 200M microscope (Zeiss) outfitted with the ApoTome system and an 8-MP digital camera. CSA and cell density measurements were performed as previously described (12, 13) using ImageJ software (NIH) and calibrated high-resolution digital images. The CSA of tendons was calculated from fast green and hematoxylin sections by outlining the borders of the tendon and measuring the area of the resultant polygon. Cell density was measured by counting the number of nuclei present in each section and dividing by the CSA.
Gene expression.
Tendons were finely minced and homogenized in QIAzol (Qiagen), and RNA was extracted using a miRNeasy Mini kit (Qiagen). Total RNA was quantified using a NanoDrop (ThermoFisher), and RNA was reversed transcribed with an iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed using SsoFast EvaGreen reagents (Bio-Rad) in a CFX96 real-time thermal cycler (Bio-Rad) and primers to detect scleraxis (SCX) and type 1α2 collagen (COL1A2) and normalized to the stable housekeeping gene, β2-microglobulin (B2M). Primer sequences and cycling parameters have been previously described (3). Differences in gene expression between time points were quantified using the 2−ΔCt method (20).
Statistical analyses.
One-way ANOVAs (α = 0.05), followed by Holm-Sidak post hoc sorting, were used to assess differences between time points. Analysis was performed using Prism 6.0 (GraphPad). Values are presented as mean ± SE.
RESULTS
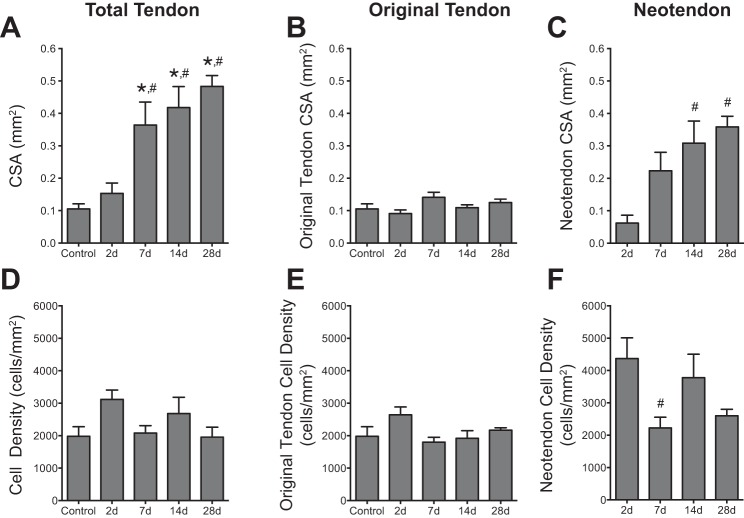
Supraphysiological overload of the plantaris tendon resulted in the generation of an immature tendon matrix, or neotendon matrix, between the epitenon and the original tendon matrix (Fig. 2). The initial formation of the neotendon matrix could be seen as early as 2 days postoverload, and this growth continued through 28 days (Fig. 2). Compared with controls, no significant differences in total tendon CSA were observed at 2 days, although there was a dramatic 2.5-fold increase in tendon CSA at 7 days, which increased to 3-fold at 14 days and 3.5-fold at 28 days (Fig. 3A). Throughout the overload period, the CSA of the original tendon matrix did not change compared with controls (Fig. 3B). The increase in the CSA of the neotendon was responsible for the overall increase in the total tendon CSA. Although no significant changes in neotendon CSA were observed at 2 and 7 days after overload, compared with the value at 2 days, the neotendon increased by fourfold at 14 days and by nearly fivefold at 28 days (Fig. 3C). Although widespread changes in CSA were observed throughout the overload period, the cell density in the tendon overall (Fig. 3D), as well as in the original tendon (Fig. 3E) and neotendon (Fig. 3F) regions, remained largely unchanged, with a nearly twofold decrease in cell density in the neotendon at 7 days compared with 2 days.
Fig. 2.
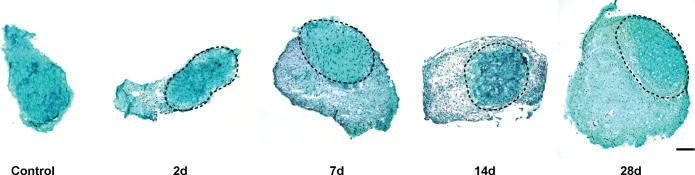
Representative cross sections of plantaris tendons from control and synergist ablation groups. Sections stained with fast green and hematoxylin. The original tendon in all groups subjected to synergist ablation is circled in a dashed line, and the neotendon matrix is located superficial to this line. Scale bar for all panels = 100 μm.
Fig. 3.
Quantitative histomorphometry of plantaris tendons from control and synergist ablation groups. Cross-sectional area (CSA) of the total tendon (A), original tendon (B), and neotendon (C). Cell density of the total tendon (D), original tendon (E), and neotendon (F). Values are means ± SE. Differences were tested with a one-way ANOVA and Holm-Sidak post hoc sorting (P < 0.05). *Significantly different from control; #from 2 days.
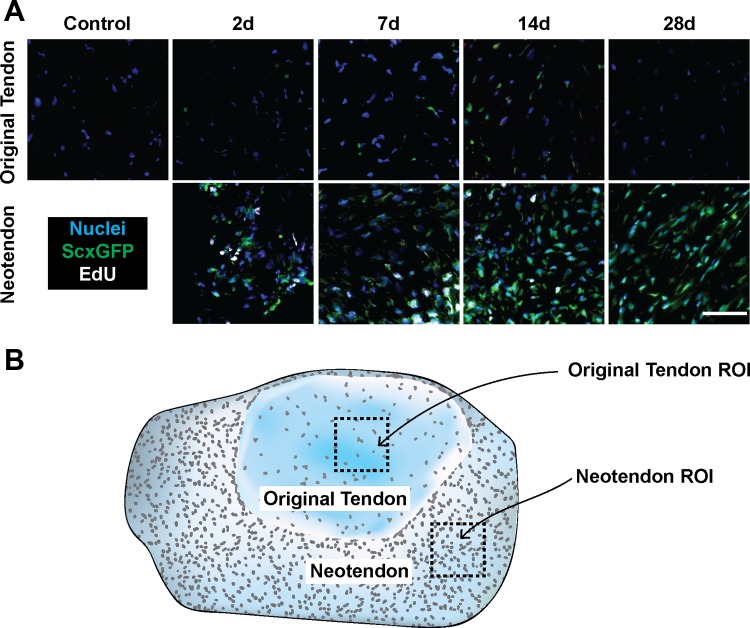
We next sought to determine which cells in the tendons were actively undergoing mitosis by labeling proliferating cells with EdU. As shown in Fig. 4A, the only EdU+ cells were observed in the neotendon region, and at no time did we detect EdU+ cells in the original tendon. Additionally, EdU was detected only in scleraxis-expressing fibroblasts in the neotendon, although not every scleraxis-expressing fibroblast was EdU+. In the original tendon, although scleraxis was not detected in fibroblasts in unloaded tendons, mechanical overload caused fibroblasts to induce scleraxis expression.
Fig. 4.
Representative immunohistochemistry of plantaris tendons from control and synergist ablation groups. A: high-magnification views of cells in the original tendon and neotendon. Blue, nuclei (DAPI); green, scleraxis-GFP; white, EdU. Scale bar for all panels = 50 μm. An illustration showing the regions of interest is shown in B.
Finally, we performed quantitative measures of scleraxis and type I collagen gene expression with qPCR. Compared with control tendons, scleraxis expression increased nearly 3-fold at 2 days, 5.5-fold at 7 days, 4-fold at 14 days, and 2.5-fold at 28 days (Fig. 5A). For type I collagen expression, compared with control tendons, no change was observed at 2 days, but expression increased fourfold at 7 days, sevenfold at 14 days, and back down to fivefold through 28 days (Fig. 5B).
Fig. 5.
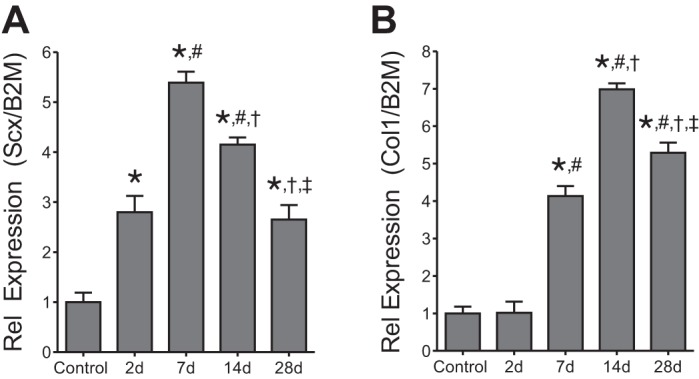
Gene expression of plantaris tendons from control and synergist ablation groups. Scleraxis expression (A) and collagen type Iα2 expression (B). Target gene expression was normalized to the stable housekeeping gene β2-microglobulin. Values are means ± SE. Differences were tested with a one-way ANOVA and Holm-Sidak post hoc sorting (P < 0.05). *Significantly different from control; #from 2 days; †from 7 days; ‡from 14 days.
DISCUSSION
The results from this study provide novel insight into the cellular mechanisms of tendon growth. The synergist ablation procedure is a commonly used technique used to study skeletal muscle growth (1, 11), and we postulated this approach could also be used to study rapid tendon growth. Although many previous studies used chronic exercise training as a model to study tendon growth (4), the current study was the first, to our knowledge, that reported synergist ablation as a model to study the rapid growth of mechanically overloaded tendon tissue. Additionally, we report novel findings regarding the proliferation capacities of cells in different regions of the tendon. The combined findings from the current study suggest that, in addition to classically used tendon growth schemes like chronic treadmill running, synergist ablation is a useful model to study tendon growth and that the fibroblasts within the core of the tendon do not re-enter the cell cycle to contribute to tendon growth.
Based on morphological appearance, Ippolito (7) postulated that the fibroblasts within tendon could be divided into two distinct categories—tenoblasts, which are proliferative fibroblasts that decline as organisms progress into adulthood, and tenocytes, which are terminally differentiated fibroblasts that are responsible for synthesizing and remodeling the tendon ECM. Although the terms tenoblast and tenocyte are often used in the adult tendon literature, there is little molecular evidence to support these cells as having the role ascribed to them by their suffix. However, during early and adolescent development, using the thymidine analog IdU, Tan and colleagues (21) demonstrated that tendon contains ∼10% IdU+ cells at 4 wk of age and this number decreases to ∼2% by 8 wk of age. Additionally, there is a gradient to this distribution, with the outer most layers of the tendon showing more IdU+ cells than the midsubstance. The work of Tan and colleagues (21) is in agreement with Michna (15) who reported a cessation of growth in rodent tendons at ∼10 wk of age. In adult humans, Heinemeier and colleagues (5) used the global increase in atmospheric 14C that came about due to above ground nuclear bomb testing to measure tissue growth rates in Achilles tendons and found that the core of tendon tissue appears to form and stop growing by 17 years of age, with no appreciable changes in 14C content observed after this time point. In this study, we demonstrate that in response to a mechanical growth stimulus, the existing fibroblasts within the midsubstance of tendons do not proliferate to contribute to tendon growth. Rather, there appears to be an expansion of fibroblasts in the outermost layers of tendon that likely arise from a progenitor cell population in or around the epitenon/peritenon region. Combined, the data from the current study, along with the work of Tan (21) and Heinemeier (5) suggest that the majority of fibroblasts within adult tendons are likely terminally differentiated and support the use of the term tenocyte to describe this cell population.
In addition to identifying populations of proliferative cells within tendon, this study also provided insight into the mechanisms of tendon ECM synthesis in the context of whole tendon growth. In Achilles tendons, scleraxis is expressed in nearly every fibroblast at 2 mo of age (13). By 3 to 4 mo of age, scleraxis expression declines in most cells throughout the tendon but persists in a population of cells in the epitenon (13). After supraphysiological overload, there was an increase in scleraxis-positive cells in the neotendon, some of which also were positive for EdU. Interestingly, although scleraxis was detected in the cells in the original tendon in response to overload, these cells did not appear to be proliferative. Furthermore, cell density in the neotendon was higher at 2 days compared with 7 days, and scleraxis expression was increased at 2 days, which preceded the increase in type I collagen expression at 7 days. This early expansion of cells, coupled with increased scleraxis expression, also occurred before the increase in CSA at 7, 14, and 28 days. Previous studies in cultured tendon fibroblasts have shown that scleraxis expression is correlated with increased cell proliferation and a reduction in scleraxis is correlated with hypocellular tendons (12) and that scleraxis directly regulates the expression of type I collagen (9). These results suggest that tendon growth likely occurs initially through an expansion of fibroblasts and is subsequently followed by an increase in ECM production. Based on in vitro studies of tendon fibroblasts and findings of the current study, scleraxis likely plays an important role in both of these processes.
Although we provided important insight into the changes in scleraxis expression after supraphysiological overload, there are several limitations to this study. First, although we show proliferating cells in the neotendon, we did not track cells over time using lineage tracing or dye. We also did not measure TGF-β superfamily signaling after plantaris overload. We focused on the plantaris tendon because of the fact that this is a highly established and simple synergist ablation model, but other tendons may behave very differently. We visualized an abundance of scleraxis-positive cells in the neotendon regions after overload but did not stain for other cell types inside the neotendon. Finally, a specific marker that identifies undifferentiated tendon progenitor cells in vivo is not yet known, and we did not identify one in this study. However, these progenitor cells are likely to be a type of mesenchymal stem cell, because injection of these cells into injured mouse Achilles tendon resulted in pockets of immature tendon matrix formation (6).
Tendons play an important role in transmitting and storing force in the musculoskeletal system and are among the most frequently injured and difficult to treat soft tissue injuries (8). Despite the importance of tendons in animal locomotion, relatively little is known about the fundamental cellular and molecular pathways that regulate tendon growth. The results from this study indicate that there appear to be at least two populations of cells that exist within tendon, and these different populations have altered responses after tendon mechanical loading. Although inducing a substantial growth stimulus caused an overall increase in tendon CSA, the existing fibroblasts within the tendon remained in a terminally differentiated state and the growth occurred entirely by the addition of new cells and matrix in the outermost layers of the tendon. Along with the work of Tan (21) and Heinemeier (5), the results from the current study suggest that tendons in adult animals grow from the most superficial layers out, in a fashion similar to the growth patterns of tree trunks. Additionally, gaining further understanding of the fundamental biology of tendon cell specification, proliferation, and migration will likely enhance our understanding of adult tendon mechanobiology and potentially inform future treatments for tendon injuries and diseases.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01-AR063649 and F31-AR065931.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P.G. and C.L.M. conception and design of research; J.P.G., A.C.P., D.G.R., A.C.N., and C.L.M. performed experiments; J.P.G., A.C.P., D.G.R., and C.L.M. analyzed data; J.P.G. and C.L.M. interpreted results of experiments; J.P.G. and C.L.M. prepared figures; J.P.G. and C.L.M. drafted manuscript; J.P.G. and C.L.M. edited and revised manuscript; J.P.G., A.C.P., D.G.R., A.C.N., and C.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The ScxGFP mice were a kind gift of Dr. Ronen Schewitzer.
REFERENCES
- 1.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol 303: C577–C588, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol 115: 884–891, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumucio JP, Flood MD, Phan AC, Brooks SV, Mendias CL. Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol 115: 539–545, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact 11: 115–123, 2011. [PubMed] [Google Scholar]
- 5.Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J 27: 2074–2079, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest 116: 940–952, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am 62: 583–598, 1980. [PubMed] [Google Scholar]
- 8.Kjaer M, Bayer ML, Eliasson P, Heinemeier KM. What is the impact of inflammation on the critical interplay between mechanical signaling and biochemical changes in tendon matrix? J Appl Physiol 115: 879–883, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Léjard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MHA, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-α1(I) collagen gene in tendon fibroblasts. J Biol Chem 282: 17665–17675, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol 21: 933–941, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci USA 105: 388–393, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30: 606–612, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendias CL, Gumucio JP, Lynch EB. Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fibroblasts. J Appl Physiol 113: 56–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res 236: 465–470, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134: 2697–2708, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236: 1677–1682, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136: 1351–1361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protocols 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Tan Q, Lui PPY, Lee YW. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev 22: 3128–3140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]