Abstract
Background and purpose
In the discussion on the cost-effectiveness of screening precise estimates of severe asymptomatic carotid stenosis (ACAS) are vital. Accordingly, we assessed the prevalence of moderate and severe ACAS by age and sex using pooled cohort data.
Methods
We performed an individual participant data meta-analysis (23,706 participants) of four population-based studies (MDCS, Tromsø, CAPS and CHS). Outcomes of interest were asymptomatic moderate (≥50%) and severe carotid stenosis (≥70%).
Results
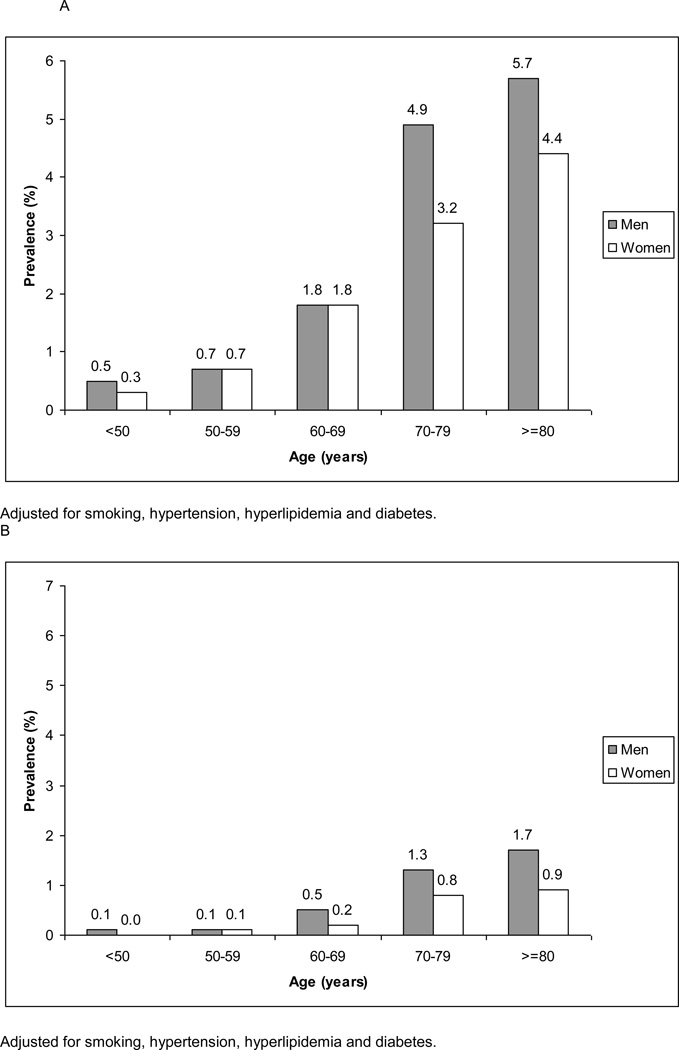
Prevalence of moderate ACAS ranged from 0.5% (95% CI, 0.3% to 1.1%) in men aged below 50 years to 5.7% (4.5% to 7.1%) in men aged 80 years and above. For women this prevalence increased from 0.3 (0.1% to 0.6%) to 4.5% (2.8% to 6.8%). Prevalence of severe ACAS ranged from 0.1% (0.0% to 0.4%) in men aged below 50 years to 1.7% (0.8% to 3.4%) in men aged 80 and above. For women this prevalence increased from zero (0.0% to 0.2%) to 0.9% (0.4% to 2.4%).
Conclusions
Prevalence of severe ACAS in the general population ranges from zero to 1.6% which is useful information in the discussion on the cost-effectiveness of screening.
Keywords: Carotid Stenosis, Epidemiology, Stroke
Introduction
Studies have reported an annual stroke risk of approximately 2–5% for patients with severe asymptomatic carotid stenosis (ACAS)1,2. Two randomized controlled trials in subjects with ACAS showed a benefit from carotid endarterectomy in men3,4, whereas uncertainty persisted in women5. These prompted the discussion on non-invasive screening for ACAS in the general population5,6. Since precise and valid prevalence estimates are important for recommendations regarding population-based screening, we initially sought to determine age- and sex-specific prevalence estimates for ACAS through systematic literature review and meta-analysis7. However, good stratified estimates appeared difficult to extract due to the variety in definition used for ACAS. Therefore, we set out to determine the prevalence of moderate and severe ACAS in the general population using individual participant data from four population-based cohort studies.
Methods
Data from four population-based studies of clinically asymptomatic patients were used; these cohorts have been previously detailed elsewhere8–12. In brief, the Tromsø Study is a population-based prospective study in Tromsø, Norway. All inhibitants aged 55 to 74 years and 5–10% samples of other 5-year-age groups aged ≥25 years were invited. In total 6,727 participants (attendance rate 77%) were screened and informed consent was obtained from 6,659 participants8. In the population-based Malmö Diet and Cancer Study (MDCS) a total of 28,449 participants attended between 1991 and 1996 (attendance rate 41%). A random sample of 6,103 (20%) participants had an ultrasound examination9,10. In the Carotid Atherosclerosis Progression Study (CAPS), members of a German primary healthcare scheme were invited of whom 6,962 participants (attendance rate 21%) agreed to take part11. The Cardiovascular Health Study is a community-based, prospective study of people aged ≥65 years including 5,888 subjects (attendance rate 57%)12.
The following baseline characteristics were recorded: age, sex, history of vascular disease, body mass index (BMI), waist-hip ratio (WHR), blood pressure, hypertension, diabetes mellitus, smoking status, blood lipids and methods of measuring stenosis. Hypertension was defined as ≥140/90 mmHg or treatment with antihypertensive drugs. Diabetes mellitus was defined as fasting blood glucose level ≥7 mmol/l or treatment with insulin or oral glucose-lowering drugs. Hyperlipidemia was defined as total cholesterol ≥4.5 mmol/L, LDL-cholesterol ≥2.5 mmol/L or use of lipid-lowering medication13. Moderate ACAS was defined as ≥50% stenosis and severe ACAS as ≥70% stenosis, measured by Doppler ultrasonography supported by B-mode sound imaging in three of the four studies (Table 1). When both carotid arteries were measured, we used the largest stenosis observed14.
Table 1.
General characteristics of the study population, by cohort.
| Tromsø | MDCS | CAPS | CHS | Total | |
|---|---|---|---|---|---|
| Nr. of participants | 6659 | 6103 | 5056 | 5888 | 23706 |
| Mean age, y (sd) | 60.2 (10.1) | 57.5 (5.9) | 50 (13.1) | 72.8 (5.6) | 60.5 (12.1) |
| Male sex, n (%) | 3298 (49.5) | 2572 (42.1) | 2471 (48.9) | 2495 (42.4) | 10836 (45.7) |
| History of disease, n (%) | |||||
| Coronary heart disease, n (%) | 822 (12.3) | 102 (1.7) | 108 (2.1) | 1154 (19.6) | 2186 (9.2) |
| Cerebrovascular disease, n (%) | 182 (2.7) | 69 (1.2) | 52 (1.0) | 349 (5.9) | 652 (2.8) |
| Body Mass Index mean kg/m2 (sd) | 26.1 (3.9) | 25.9 (4.0) | 26.6 (4.1) | 26.7 (4.7) | 26.3 (4.2) |
| Waist-Hip Ratio, mean (sd) | 0.87 (0.08) | 0.85 (0.09) | 0.95 (0.11) | 0.93 (0.09) | 0.9 (0.1) |
| Hypertension, n (%) | 2257 (33.9) | 2659 (43.6) | 707 (14.0) | 2511 (42.6) | 7760 (32.7) |
| Mean systolic BP (sd) | 145 (22.5) | 141 (19) | 128 (17) | 136.5 (21.8) | 141.2 (21.5) |
| Mean Diastolic BP, (sd) | 83 (12) | 87 (9.5) | 77.3 (10.1) | 70.7 (11.4) | 79.9 (12.8) |
| Diabetes, n (%) | 217 (3.3) | 157 (2.6) | 134 (2.7) | 722 (12.3) | 1230 (5.2) |
| Smoker, n (%) | 2116 (31.8) | 1618 (28.1) | 2717 (53.8) | 700 (11.9) | 4161 (17.6) |
| Lipids, mean (sd) | |||||
| Total Cholesterol | 6.75 (1.29) | 6.2 (1.1) | NR | 5.4 (1.1) | 6.1 (1.3) |
| HDL Cholesterol | 1.5 (0.43) | 1.4 (0.4) | 1.54 (0.44) | 1.37 (0.37) | 1.46 (0.42) |
| LDL Cholesterol | NR | 4.2 (1.0) | 3.35 (0.93) | 4.16 (0.98) | 3.6 (1.02) |
| Triglycerides | 1.7 (1.1) | 1.4 (0.8) | 1.5 (0.99) | 1.37 (0.8) | 1.55 (0.95) |
| Methods of measure stenosis | |||||
| Duplex Ultrasonography | yes | yes | yes | no | |
| -Lumen diameter method | yes | yes | no | . | |
| -Cross sectional lumen method | yes | no | yes | . |
NR=Not Reported, MDCS = Malmö Diet and Cancer Study, CAPS = Carotid Atherosclerosis Progression Study, CHS = Cardiovascular Health Study, HDL = high density lipoprotein, LDL = low density lipoprotein
We determined the prevalence of moderate and severe ACAS, by age and sex in the complete dataset and among those without a history of coronary heart disease or cerebrovascular disease. Analysis of variance was used to estimate age- and sex-specific prevalence estimates adjusted for hypertension, hyperlipidemia, diabetes mellitus and smoking. We assessed whether the overall prevalence estimates differed among current smokers, hyperlipidemic, hypertensive and diabetic subjects or the combination of one or more of these vascular risk factors compared to those without risk factors.
Results
General characteristics are shown in Table 1. In men the prevalence of moderate ACAS increased with age from 0.5% (95% Confidence Interval, 0.3% to 0.9%) to 5.7% (4.5% to 7.1%) for severe ACAS the prevalence increased from 0.1% (0.0% to 0.4%) to 1.7% (0.8% to 3.4%) (Figure 1 and Webtable 1). For women, the prevalence of moderate ACAS increased from 0.3% (0.1% to 0.6%) to 4.4% (2.8% to 7.1%); for severe ACAS this prevalence increased from zero (−0.2% to 0.3%) to 0.9% (0.4% to 6.8%). The prevalence estimates were almost similar in participants without a history of vascular disease (Webtable 2). The prevalence of severe ACAS was higher in participants with vascular risk factors (Figure 2).
Figure 1.
Age- and sex-specific prevalence estimates of moderate (A) and severe ACAS (B) in men and women
Figure 2.
Prevalence of severe stenosis in subgroups
Discussion
The prevalence of moderate ACAS varied from 0.3% to 5.7% and the prevalence of severe ACAS from zero to 1.7%. Prevalence estimates increased with age and were slightly higher in men. Age- and sex-specific estimates in the present study are smaller than the prevalence estimates reported in our previous literature-based meta-analysis7. This differences in prevalence may have been introduced by the selection process of individual papers in the literature-based meta-analysis. Only a few studies reported age- and sex-specific data7. Also, it was not possible to correct for heterogeneity in baseline characteristics between studies. These aspects were overcome in the present analyses in which a large number of persons was involved, giving us the ability to present precise estimate of the ACAS prevalence by age and sex. This study has some limitations. Our meta-analysis suffers from non-participation in the individual cohorts. When non-response is related to the more sick or high-risk patients, which is supported by the non-participant analyses in the MDCS cohort15, our estimates reflect an underestimation of the actual ACAS prevalence. The volunteer approach in CAPS, however, did not select participants with a particularly low vascular risk16. Although, differences exist in the methods for determination of stenosis-degree between studies9–13, the regression analyses using the Tromso data indicated that different approaches were unrelated to the prevalence estimate of moderate ACAS. Therefore, it is unlikely that the different methods used to measure stenosis degree have affected our results.
For the discussion about the feasibility and cost-effectiveness of screening the general population for ACAS, our findings are important. Some reported that screening for severe ACAS was cost-effective when the prevalence of severe ACAS was at least 20%17. Using that cut-off point and given our estimates, population screening is unlikely to become worthwhile. Yet, we recommend the development of a prediction rule estimating the risk of having severe carotid stenosis to evaluate whether we can select a high risk group of participants that might benefit from screening.
In conclusion, overall the prevalence of severe ACAS in the general population ranges from zero to 1.6%. Its prevalence increases with age and with risk factor levels. These results are of relevance for the discussion on screening for severe asymptomatic carotid artery stenosis.
Supplementary Material
Acknowledgement
Source of Funding
This study is supported by an unconditional grant from the Netherlands Organization for Health Research and Development (ZonMW, project No. 6230.0046).
Footnotes
Disclosures
None.
Reference List
- 1.Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485–1490. doi: 10.1161/01.str.22.12.1485. [DOI] [PubMed] [Google Scholar]
- 2.Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000;342:1693–1700. doi: 10.1056/NEJM200006083422302. [DOI] [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM. ACST: which subgroups will benefit most from carotid endarterectomy? Lancet. 2004;364:1122–1123. doi: 10.1016/S0140-6736(04)17090-6. [DOI] [PubMed] [Google Scholar]
- 6.Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev. 2005:CD001923. doi: 10.1002/14651858.CD001923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke. 2009;40:1105–1113. doi: 10.1161/STROKEAHA.108.532218. [DOI] [PubMed] [Google Scholar]
- 8.Mathiesen EB, Joakimsen O, Bonaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromso Study. Cerebrovasc Dis. 2001;12:44–51. doi: 10.1159/000047680. [DOI] [PubMed] [Google Scholar]
- 9.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Life-course perspective on socioeconomic differences in carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1704–1711. doi: 10.1161/01.atv.0000032006.75577.24. [DOI] [PubMed] [Google Scholar]
- 10.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17:299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 13.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 14.Chappell FM, Wardlaw JM, Young GR, et al. Carotid artery stenosis: accuracy of noninvasive tests--individual patient data meta-analysis. Radiology. 2009;251:493–502. doi: 10.1148/radiol.2512080284. [DOI] [PubMed] [Google Scholar]
- 15.Manjer J, Carlsson S, Elmstahl S, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10:489–499. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Sitzer M, Skutta M, Siebler M, Sitzer G, Siegrist J, Steinmetz H. Modifiable stroke risk factors in volunteers willing to participate in a prevention program. Neuroepidemiology. 1998;17:179–187. doi: 10.1159/000026171. [DOI] [PubMed] [Google Scholar]
- 17.Derdeyn CP, Powers WJ. Cost-effectiveness of screening for asymptomatic carotid atherosclerotic disease. Stroke. 1996;27:1944–1950. doi: 10.1161/01.str.27.11.1944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.