Abstract
Inputs from the two sides of the brain interact to create maps of interaural time difference (ITD) in the nucleus laminaris of birds. How inputs from each side are matched with high temporal precision in ITD-sensitive circuits is unknown, given the differences in input path lengths from each side. To understand this problem in birds, we modeled the geometry of the input axons and their corresponding conduction velocities and latencies. Consistent with existing physiological data, we assumed a common latency up to the border of nucleus laminaris. We analyzed two biological implementations of the model, the single ITD map in chickens and the multiple maps of ITD in barn owls. For binaural inputs, since ipsi- and contralateral initial common latencies were very similar, we could restrict adaptive regulation of conduction velocity to within the nucleus. Other model applications include the simultaneous derivation of multiple conduction velocities from one set of measurements and the demonstration that contours with the same ITD cannot be parallel to the border of nucleus laminaris in the owl. Physiological tests of the predictions of the model demonstrate its validity and robustness. This model may have relevance not only for auditory processing but also for other computational tasks that require adaptive regulation of conduction velocity.
Keywords: auditory brain stem, axons, conduction velocity, models, sound localization
barn owls are nocturnal hunters (Payne 1971; Takahashi 2010), with highly developed auditory systems (Wagner et al. 2003; Kubke et al. 2004), mediating precise sound localization (Bala et al. 2003; Hausmann et al. 2009; Fischer and Peña 2011). Their sound localization in the azimuthal plane depends on detection of interaural time differences (ITD) (Moiseff and Konishi 1981; Poganiatz et al. 2001; Hausmann et al. 2009). Owls are very sensitive to ITDs, with minimal audible angles of ∼3° (Bala et al. 2003), corresponding to ITDs of ∼7.5 μs (Campenhausen and Wagner 2006). Neural precision is in the same range, as determined from field potentials in the nucleus laminaris (NL; Wagner et al. 2005) and from ITD tuning (Bala et al. 2003; Peña et al. 1996).
In birds, ITDs are extracted in a network made up of axons from the nucleus magnocellularis (NM) and neurons of NL (Rubel and Parks 1975; Carr and Konishi 1990; Carr and Boudreau 1993b) (Fig. 1A). The network resembles a model proposed by Jeffress (1948) (for review, see Ashida and Carr 2011; Joris and Yin 2007). In this model, detection of ITD is based on coincidence detection and delay lines. The neurons in NL act as coincidence detectors, while the magnocellular axons function as delay lines. Good evidence for magnocellular axons acting as delay lines comes from slice experiments in the chicken (Overholt et al. 1992) and emu (MacLeod et al. 2006). Overholt et al. (1992) showed that the response latency of the recorded field potential evoked by midline stimulation of the contralateral fibers increased linearly as the recording electrode is moved from medial to lateral. For ipsilateral fibers, no systematic change in response latency was observed. In barn owls, evidence for delay lines is indirect, since parallel recordings from many points along the mediolateral axis are difficult in vivo (Carr and Konishi 1988). In owls, delay line axons extend along two axes, mediolateral and dorsoventral, angled at ∼90° to each other (Fig. 1A), unlike in the chicken where the majority of the extent of the axonal arbor is along a single axis.
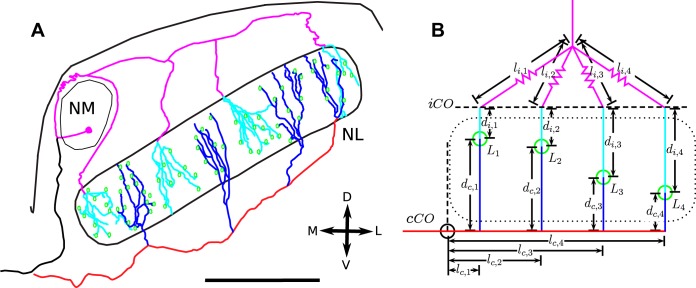
Fig. 1.
A: tracings of nucleus magnocellularis (NM) axons entering nucleus laminaris (NL) in the barn owl. The axons take stereotypical paths and run along approximately straight lines inside and outside NL and exhibit sharp angles at the point of entry into NL. The sections that are assumed to be straight share the same color. The contralateral axon is colored red outside and blue inside NL. The ipsilateral axon is colored magenta outside and cyan inside. NL somata are shown in green. Scale bar = 1 mm. B: schematic diagram of the linear model. NL border indicated as a dotted slab. The colored lines correspond to the axon sections in A. The green circles mark the points at which the measurements are taken and to which the contra- and ipsilateral latencies are Lc,n and Li,n, respectively. The black lines show the lengths of the relevant axon segments. The left border of the schematic, at which the measurements of the lc,n start, correspond to the contralateral circuit onset point, termed cCO, which the signal reaches at time LcCO. The line consisting of the points where the ipsilateral axons enter NL was termed iCO. Note the dashed line characterizing point iCO is drawn here for reasons of clarity slightly above the dorsal border of NL. The zig-zag of the lines for the Li,n highlights their inconsistent length. For definition of the further symbols used in the diagram, see also text. D, dorsal; M, medial; L, lateral; V, ventral.
Even in the comparatively simple chicken and emu cases, how inputs from the two brain sides are synchronized is still unclear (Seidl et al. 2010; Seidl 2013). Recent work by Seidl et al. (2014) suggests that velocity is regulated differentially in ipsi- and contralateral portions of the efferent axons, and speculates on neuron-glia interactions as a possible mechanism for synchronization, although the precise method of modulation remains unclear (Cheng and Carr 2007). Mechanisms of input synchronization are similarly obscure in the mammalian equivalent of the NL, the medial superior olive (Karino et al. 2011). In mammalian brain slices, the internal latency difference for the two excitatory pathways to the medial superior olive remains poorly understood (Jercog et al. 2010), while anatomical reconstructions of the length differences, and thereby internal delays, cannot account for the distribution of best delays in the cat (Karino et al. 2011).
There are therefore major questions about the structure and function of auditory delay lines that require investigation. Here we provide a framework for how delays between the ipsi- and contralateral signals might be adaptively regulated. This functional model of the magnocellular-laminaris network shows that the complex problem of delays can be linearized and that adaptive regulation of ipsilateral and contralateral delays can be achieved locally within the nuclei.
MATERIALS AND METHODS
This section will be divided into four parts: model outline, model analysis, methods used for data analysis, and acquisition of experimental data.
Outline of the Model
In the barn owl, the delay line circuit (Fig. 1A) is composed of axons originating in NM and projecting to an isofrequency lamina in NL. This organization has been clearly shown for the high-frequency regions of NL above ∼3 kHz (Carr and Konishi 1990; Carr and Boudreau 1993b, 1996; Kubke et al. 2002; Cheng and Carr 2007). Each isofrequency lamina contains the axons of many NM neurons forming a two-dimensional slab of brain tissue of ∼700 × 2,500 μm in size. The slab is oriented at ∼25–45° to the upper brainstem surface and inclined by ∼45 towards the rostro-caudal axis. The shorter axis of the slab is oriented from dorsomedial to ventrolateral (Carr and Boudreau 1993a). For reasons of simplicity we shall refer to the longer axis as being oriented from medial to lateral and the shorter axis as being oriented from dorsal to ventral, although we are aware that ventromedial to dorsolateral and ventrolateral to dorsomedial are, respectively, more precise.
NL receives input from both the contra- and ipsilateral NM. Contralateral axons cross the midline and then run parallel to the ventral border of the long axis of the slab, giving off collaterals that invade NL to travel from the ventral to the dorsal border of the slab (Carr and Konishi 1990; Carr and Boudreau 1996). We term the points where the collaterals originate contralateral bifurcation points. Ipsilateral axons enter the slab from the dorsal surface. Briefly, within NL the ipsilateral fibers run from dorsal to ventral, while the contralateral fibers run from ventral to dorsal. Magnocellular fibers from each side of the brain interdigitate and synapse on stubby somatic spines of the sparsely distributed laminaris neurons (Fig. 1A).
The situation in the chicken and emu differs from that in the owl (Fig. 2A). In the higher frequency regions of chickens and emus (above ∼500–1,000 Hz), laminaris neurons form a single row running from ventromedial to dorsolateral (Rubel and Parks 1975). These neurons have dendrites extending dorsally and ventrally. Contralateral magnocellular axons run parallel to the row of laminaris neurons on their ventral side and synapse on the ventral dendrites, while ipsilateral magnocellular axons fan out from a point dorsal to NL and contact the dorsal dendrites of the same neurons (Young and Rubel 1983; Jhaveri and Morest 1982; Burger and Rubel 2008).
Fig. 2.
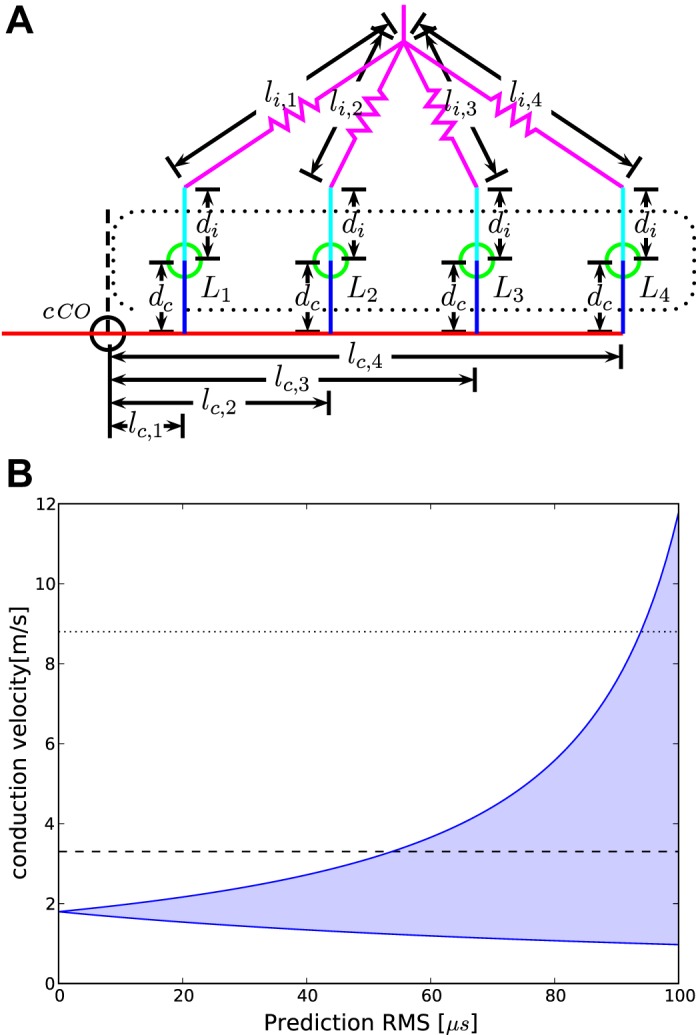
A: schematic of the linear model in the chicken case. Colors are the same as in Fig. 1B. Note that since NL consists of a single layer of cells in the chicken, the distances within NL are now equal on each side. B: application of the model to the chicken case. Shown are the possible ranges of the conduction velocity as a function of the maximal fitting error, starting from the analytical solution [root mean square (RMS) = 0] to a fitting error of RMS <100 μs. At each maximum RMS, the shaded range indicates the velocities which produce an RMS lower than the maximum RMS. Dashed horizontal lines show the range of velocities reported by Seidl (2013).
In the construction of the model for one isofrequency lamina, we took into account the most important characteristics of the circuit: its morphology and its function as a network that detects ITD. Earlier work (e.g., Gerstner et al. 1996) mainly concentrated on modeling ITD detection by considering the time it takes from the eardrum to the coincidence-detector neuron, the arrival time, or latency L, as a whole. Here we extend this approach and divide the latency L into three components:
| (1) |
where Ld is the time spent inside NL, Ll the time spent in the NL circuit before entering NL itself, while LCO is the time from the eardrum to the onset of the NL circuit (Fig. 1). The indexes indicate the predominant direction of the axons: d for dorsoventral and l for mediolateral. The point of contralateral circuit onset is defined here as the medial edge of NL. The exact point was determined by drawing a line that extended the medial edge of NL to the middle of the fiber tract below NL (dashed line in Fig. 1B). It will turn out later that the exact dorsoventral location of the point in the fiber tract is not important, because a shift of this line only adds a constant latency to every collateral (see Eq. 2). In the model we denote this point as contralateral circuit onset (cCO) (Fig. 1B, 2), analogous to point BP2c in Seidl et al. (2010). Similarly, the dorsal edge of NL, where the ipsilateral axons enter NL, is denoted as ipsilateral circuit onset (iCO) (Fig. 1B). The third temporal component of latency, LCO, is the time it takes the signal to reach the points cCO and iCO. In other words, we lump all the processes before the points cCO and iCO. We assume common latencies that we call LcCO and LiCO, respectively. This implies the signal is transmitted along an initial common pathway until the beginning of the circuit, here defined as the medial edge of NL for contralateral processing and the dorsal edge of NL for ipsilateral processing. Note that the contra- and ipsilateral common latencies may be different.
The latencies Ld and Ll are readily specified in relation to the geometry of the circuit by distances along axons and conduction velocities inside and around the nucleus. For latency LCO this is not as easy, if not impossible, because it is comprised of several components including cochlear and synaptic as well as axonal and other biophysical propagation delays. In the specification of these latencies L, we discriminate between different locations in NL that we denote by subscript n and between the ipsi- and contralateral sides of the circuit that we separate by subscripts i and c. As an example, Li,n refers to the latency at the n-th measurement location for ipsilateral stimulation. Statements made omitting the subscripts are generally applicable to several locations or to both sides, unless stated otherwise.
We first describe the conversion of the circuit geometry in the barn owl NL (Fig. 1A) into the model (Fig. 1B). The tracings in Fig. 1A show that the contralateral axons generally follow a straight line and take 90° turns only when they bifurcate. This is also in accordance with general findings from axons in other animals, such as the chicken, the frog (Katz 1985) and mammals such as the cat (Beckius et al. 1999). In the contralateral case, we therefore approximate the axon tract as a single straight line and each of the collaterals as a further line. These lines slightly underestimate the actual lengths of the axons, because a straight line is always the shortest possible distance between two points, while the actual axons are not perfectly straight. In the model, the lengths along the axon tract from point cCO up to a bifurcation point n are considered as lc,n and the corresponding collaterals as dc,n (Fig. 1B, 2). On the ipsilateral side, we only have to consider the distance inside the nucleus, dn,i, because point iCO lies at the dorsal border of the nucleus. The point iCO was chosen to lie at the dorsal border of NL, because the trajectories of the ipsilateral axons to the border of the nucleus are not well defined (indicated by zig-zag lines in Fig. 1A), while they are clear within the nucleus. In the chicken, this choice is further justified by the absence of systematic variation of ipsilateral delays in the mediolateral direction found by Overholt et al. (1992).
We further assume constant conduction velocities in the mediolateral and dorsoventral direction along the axon paths. The main factors influencing the conduction velocity in a typical axon are the diameter and, if the axon is myelinated, the distance between the nodes of Ranvier. In the NL of the barn owl, the internodal distance decreases after the axon enters the nucleus, indicating a reduction in the conduction velocity compared with outside the nucleus (Carr and Konishi 1990). Therefore, the velocities inside and outside the nucleus are assigned different variables. We discriminate between the contralateral velocity in the mediolateral direction, vc,l and contralateral velocity in the dorsoventral direction vc,d, and the ipsilateral velocity in the dorsoventral direction vi,d, respectively. Thus Ll may be replaced by , while Ld is equal . This leads to the following extension of Eq. 1:
| (2) |
Note that the ipsilateral equation only contains two latencies, because the point iCO is at the border of NL, rendering li,n zero.
Up to now we have considered the ipsi- and contralateral processing separately. The ipsi- and contralateral fibers interdigitate in NL and synapse on the coincidence-detector neurons. These neurons are sensitive to the difference of the arrival times from the left and right brain sides, the ITD (Goldberg and Brown 1968; Carr and Konishi 1990; Joseph and Hyson 1993; Nishino et al. 2008; Yin and Chan 1990). We therefore extend our model to binaural processing and introduce the variable ITDn = Li,n − Lc,n for each observation n. By doing so, we assume that no additional temporal asymmetries are introduced by the synapses and intracellular pathways (see also Kuokkanen et al. 2010, 2013). If only this information is retained, the equation takes the following form:
| (3) |
Since both LiCO and LcCO appear as constant terms in this system of equations, it is not possible to solve for their individual values. Therefore, we can simplify Eq. 3 by introducing ΔLCO = LiCO − LcCO. This results in:
| (4) |
Analyzing the Model
How should the model best be analyzed? We first note that the model equations have six variables for the contralateral side and four variables for the ipsilateral side (Eq. 2). On the contralateral side, three of the variables differ by location (denoted by the subscript n), while three are shared across locations. In the ipsilateral case there are two variables that differ between locations and two that are shared. We could conduct simulations to explore the entire parameter space to find possible solutions, consisting of combinations of locations, latencies, and velocities. On the other extreme, we could measure all parameters, including velocities and circuit onset latencies, and test the model by calculating whether they are consistent with the model equations. However, examination of the anatomical and physiological circumstances shows that the parameters Ln, ln, and dn can more easily be measured than the velocities vl, vd, and the initial delay LCO. Thus we take an approach where we measure parameters that vary between locations and leave remaining parameters free, exploring their biologically realistic value space.
For the remainder of this article, we will use this combination of measured and free parameters. It should be noted that this approach is one of many possible, because we could have measured one or more of the global parameters, such as LCO or vd in addition. This would have left us with only one or two free parameters for the contralateral case. We chose this set of measured parameters because our experimental setup was most suited to measure latencies within NL. The measurement of LCO or vd would be more feasible in an in vitro study.
Methods Used for Data Analysis
Since biological data are noisy, it is desirable to obtain as many measurements for a given set of unknown parameters as possible to improve the accuracy of the results. In systems of equations, this leads to overdetermination and typically not an exact solution. For such a situation, we consider the least-squares method to find solutions that minimize the root mean squared (RMS) differences between predicted and observed values of L and best ITD.
The calculated latencies derived from these least squares solution parameters, or other parameter combinations, by reinserting them into Eq. 2, are denoted Lncalc. The corresponding measured values are denoted Lnmeas.
The RMS error of a model based on a given set of parameters, denoted as RMS (vc,l, vc,d, LcCO) in the contralateral case and analogously in the ipsilateral and bilateral cases, compared with a set of N measurements, is then defined as
| (5) |
The root mean square error between the model and the measurement will be called fitting error in the following sections. The same measure is defined for the ITD case using ITD instead of L and model predictions based on Eq. 4. The corresponding fitting error can then be calculated for different parameter ranges. Examples of the resulting error distributions are shown by the colors in the heatmaps (see Figs. 3–7). All heatmaps were produced using the Python programming language (Python Programing Language, RRID:nif-0000–30053) and the Matplotlib package (Python Plotting, RRID:nif-0000–31991).
Fig. 3.
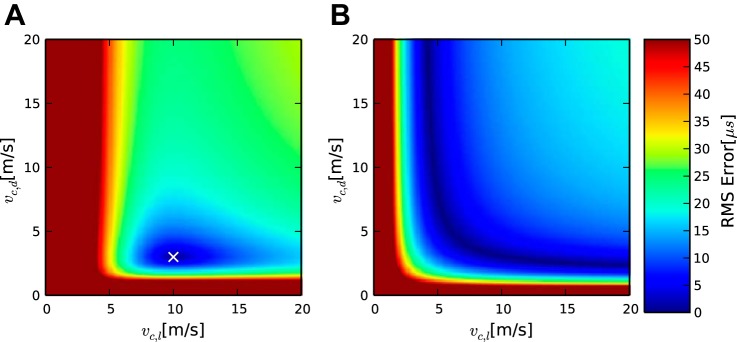
Fitting error distributions as defined in Eq. 5 for 2 simulated cases. In both cases the true value was vc,d = 3 m/s and vc,l = 10 m/s. The measurements were square (A) and linear (B) as shown in Table 1. Despite the underlying velocities being identical, the first case has a clear local minimum in the fitting error (A), while the second has the same fitting error for many parameter combinations (B).
Fig. 7.
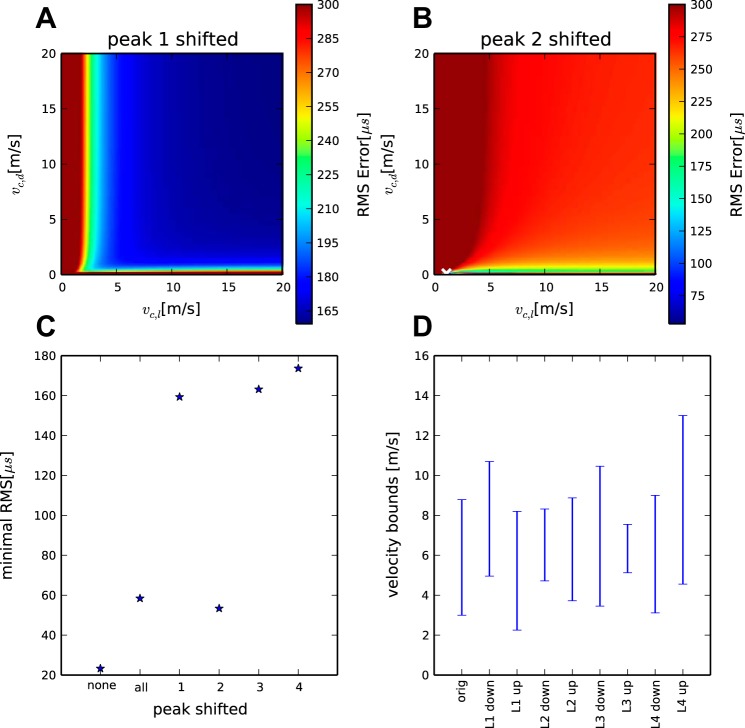
A–C: influence of peak shifting and peak matching. D: robustness of the results. Shown are predicted vl velocity ranges in the different conditions specified at the bottom of the diagram: orig: reference condition as shown in Fig 4A. Ln down/up refer to shifting the respective tip position of the electrode by 100 μm up or down.
To asses the influence of measurement uncertainties, we calculated a confidence interval for each calculated variable. This was done numerically by randomly drawing measurement perturbations from a normal distribution with zero mean and a standard deviation corresponding to our assumed measurement errors. These were then added to the measurement values, and the results were tabulated. This was done 104 times, and confidence intervals were calculated from the distribution of the resulting values. The standard deviation of the assumed measurement error was 20 μm in the spatial dimension, 20 μs for unilateral time measurements, and 10 μs for ITD measurements. These values were based on the sampling interval and the shape of the lesions in the reconstructions (see following subsection).
Acquisition of Experimental Data
Two data sets were used in the analysis, one from the chicken and one from the barn owl (Tyto furcata pratincola, formerly called Tyto alba). The chicken data were collected in the laboratory of C. Köppl and are part of the data set published in Köppl and Carr (2008). The owl data were collected at the University of Maryland in the laboratory of C. Carr. The experimental procedures have been described in detail in the respective publications (chicken: Köppl and Carr 2008; owl: Wagner et al. 2009). The protocols were approved by the University of Maryland, the government of Upper Bavaria, and the University of Sydney, respectively. Briefly, working with anesthetized birds, extracellular data were obtained using high-impedance tungsten (owls) or glass (chickens) electrodes. Acoustic stimuli were digitally generated. After passing a digital-to-analog converter [DD1 (TDT)] and an anti-aliasing filter [FT6–2, corner frequency of 20 kHz (TDT)], the signals were variably attenuated [PA4 (TDT)], impedance matched [HB6 (TDT)], and attenuated by an additional fixed amount before being fed to commercial miniature earphones. Two separate signals could be generated, passing through separate channels of associated hardware and driving two separate earphones. The earphones were housed in custom-built, calibrated, and closed sound systems, inserted into the bird's ear canals. Sound pressure levels were calibrated individually at the start of each experiment, using built-in miniature microphones (Knowles EM3068, Ithaca, IL).
Analog waveforms of recordings were saved in most experiments. Auditory stimuli were clicks, noise, and tones. All stimuli were digitally created with a sampling rate of 48,077 Hz (sampling interval: 20.8 μs). The latencies Ln were obtained from stimulation with clicks, while the tuning to interaural time difference was obtained from noise or tone stimulation. Calculating latencies from clicks is highly nontrivial due to the ringing nature of the response (Wagner et al. 2005). We here used the latency value of the phase delay, which was previously found to be the most precise measure. It is measured by calculating the group delay of the response and then picking the peak closest to the group delay. For a more detailed description, see Wagner et al. (2005).
Recording sites were histologically verified by either electrolytic lesions (owls) or iontophoretic deposition of the marker substance neurobiotin (chickens). The distances l and d were taken from cresyl-violet stained sections (Köppl and Carr 2008; Carr et al. 2013). Since the owl NL contains multiple maps of ITDs in each tonotopic band (Sullivan and Konishi 1986; Carr et al. 2013), we used as reference points for the different penetrations, the position where the ITD tuning curve had its maximum at 0 μs. This position was marked by an electrolytic lesion after the data were recorded. The lesions were recovered. A line running along the electrode path was drawn through the center of the lesion. The distances from the center of the lesion to the ventral and dorsal borders, respectively, were measured on this line, rendering dc and di. To obtain lc, the distance parallel to the mediolateral border was measured from the point where this line through the lesion crosses the ventral border of NL to the mediolateral border of NL (point cCO).
RESULTS
The results presented are based on both experimental data and simulations. We provide a general analysis of different special cases of the model: assuming that all d values are the same, all latencies L are the same, or all ITD values are the same. As a last general point we then examine the implications of the model for the role of the absolute value of LCO and the consequences of different sampling scenarios on the solutions. Finally, we perform proof of principle analyses of experimental data and conclude with comments on robustness of the model.
Constant Dorsoventral Distance d
Generally, if we consider locations within NL for which all d values, or dorsoventral axonal lengths, are the same, does not vary. This makes it possible to reduce the model equations, since can then be viewed as an additional constant temporal offset in addition to the constant
| (6) |
may be lumped with LcCO and treated as one constant.
A special case would be if all ds were zero, i.e., no collaterals exist. The network would then consist of a one-dimensional row of cells along a straight axon tract.
The case of constant d values has a biological realization in the chicken and the emu NL (Rubel and Parks 1975; MacLeod et al. 2006; Jhaveri and Morest 1982), where a row of NL cells extends from medial to lateral and the contralateral magnocellular axons run from medial to lateral in a fiber tract along the ventral border of NL (Fig. 2). The ventral terminal arborizations from this mediolaterally oriented fiber tract, from the first bifurcation point to the most lateral terminal in the fiber tract, have been measured in the chicken. In one study, the distance was 885 μm (Seidl et al. 2010) and between 950 and 1,180 μm in another study (Köppl and Carr 2008). The axons forming this tract give off short dorsoventral (mean length 135 ± 35 μm) collaterals (Seidl et al. 2010). The lengths of these collaterals do not appear to change significantly from medial to lateral (Seidl et al. 2010), and the collaterals contact NL cells on the ventrally extending dendrites (Young and Rubel 1983). Because of this spatial arrangement we chose this as an example of the reduced case described in Eq. 6 and set all dns to be constant. In a mathematical sense, the result is a one-dimensional version of the model. Delays measured in the NL cells depend linearly on the distance from the end of the initial common pathway or point cCO, while collaterals in the d direction should contribute a constant delay. The linearity is due to the constant vl along the fiber tract from medial to lateral.
Available in vitro measures of delays (Overholt et al. 1992; Görlich et al. 2010; MacLeod et al. 2006) were used as a first test for the simplifications of the model. In a slice preparation of the chicken brainstem, Overholt et al. (1992) measured delays by recording field potentials at progressively more lateral locations in NL, after stimulating in the contralateral NM or in NM axons in the crossed dorsal cochlear tract. MacLeod et al. (2006) made similar measurements in the emu. In all cases, a linearly increasing delay was observed, with data fit well by a straight line. We interpret these data such that the slope of linearly increasing delay corresponds to a constant vl, while the y-offset reflects LcCO + . Note that for the in vitro data, the constant LcCO is somewhat arbitrary, since it depends on the position of the stimulation electrode and on other factors such as slice temperature. Nevertheless, it represents a constant delay for a given experimental configuration, which keeps these factors unchanged. Similarly, slice temperature would also affect conduction velocity but would not affect the overall structure of the circuit. Overholt et al. (1992) and MacLeod et al. (2006) recorded the response delays using successive recordings along the mediolateral dimension of NL. By contrast, Görlich et al. (2010) recorded simultaneously from many positions in NL, using a multielectrode array. Both sets of authors observed a similar linear increase in conduction delay from medial to lateral, following stimulation of the contralateral fibers.
These in vitro studies relied on electrical stimulation to evoke responses, while here we use data from signals evoked by auditory stimulation. The substrate is, however, the same, and we assume that these observations of constant delay hold for both types of stimulation. The main conclusion of this section is that the concept of a constant LcCO and a constant mediolateral velocity is justified in the chicken and in the emu. This assumption is not trivial, because velocity could vary along the extent of the delay line or there could be different pathway lengths in different fibers, meaning the absence of a common latency LcCO along the initial common pathway to point cCO.
Wavefront Condition
After analyzing the case of constant d, we considered constant values of the latency L. All points that share a common latency form a wavefront, sometimes called an isodelay line. The neurophonic potential (Snyder and Schreiner 1984; Weinberger et al. 1970) is a field potential that propagates through owl NL and behaves like a wave, thus forming wavefronts (Sullivan and Konishi 1986; Wagner et al. 2005, 2009; Laughlin et al. 2010). Due to the linear nature of the model described here, all such wavefronts are straight lines, meaning that dn may be described as a straight-line function of ln:
| (7) |
where a is the slope and b an offset. This allows the following simplification of the system of equations shown in Eq. 2:
| (8) |
Since all terms except ln are constant across measurements n, it follows that for Ln to be constant across all n, the derivative of Ln with respect to ln must be zero. This is the wavefront condition:
| (9) |
In the following we consider different values of the slope, a, which reflects the rate of change of ITDs. First, if the wavefront runs parallel to the ventral border of NL, i.e., the slope a of the line is zero, the wavefront condition reduces to = 0. This condition is only fulfilled if vl becomes infinitely large and as such is not physically or biologically plausible. Thus points parallel to the ventral border of NL cannot form a wavefront or isodelay line, because this would require an infinitely large mediolateral velocity. Second, if a is not zero, the wavefront condition in Eq. 9 can be stated in terms of the ratio of velocities:
| (10) |
Thus the ratio of the velocities inside and outside of NL determines the slope of the wavefront with respect to the ventral border of NL. Since both velocities must be positive, the slope a can only be negative. The possible ratios of the velocities correspond to the value of a. Lastly, if a is small, e.g., a < 0.5, we see from Eq. 10 that vd must be considerably lower than vl. The data from Carr and Konishi (1990) support this, since internodal distances within NL were shorter than internodal distances outside NL. The reduction of internodal distances implies lower conduction velocities inside NL compared with outside NL (Brill et al. 1977).
Iso-ITD lines in the barn owl.
The two-dimensional map of ITDs in the owl NL has been characterized by iso-ITD lines (Sullivan and Konishi 1986). Iso-ITD lines represent those lines in the body of NL along which the best ITD does not change. A special iso-ITD line is the one where the best ITD is 0 μs, the 0-ITD-line, corresponding to the location of sound directly in front of or directly behind the animal. Sullivan and Konishi (1986) concluded from their data that the 0-ITD-line runs parallel to the ventral and dorsal borders of NL. We asked whether the model could reproduce this finding and generated iso-ITD lines. This analysis is similar to the analysis of the straight line wavefront in Eq. 9, in that ITD is constant along an iso-ITD line and the iso-ITD lines are straight. Unlike for the wavefront, however, we here analyze a binaural phenomenon, meaning that we include both ipsi- and contralateral conduction times. Using Eq. 4 and proceeding analogously to Eqs. 9 and 10, we can formulate for an iso-ITD condition:
| (11) |
Note that an iso-ITD line parallel to the border of NL corresponds to a = 0. Again, in this case, the mediolateral velocity vc,l would have to be infinitely high, which is biologically implausible. Thus the main conclusion from this section is that, in contrast to Sullivan and Konishi (1986), iso-ITD lines cannot be parallel to the ventral border of NL.
Absolute and Difference Delays
The last general point addresses the role of the initial common latency LCO. Distances and conduction velocities were only formulated in respect to Ld = and Ll = , but not for LCO, which appears as a constant in Eq. 2. Since we assumed that the three latency components are summed linearly to form the total latency L (Eq. 1), they are mathematically independent of each other. This means that the relationship between conduction velocities and distance may be fully described without taking into account the latency along the common initial pathway LCO. Latency LCO can be eliminated by considering the difference in response latency between two recording locations. Such response latency differences are denoted with a double index, one for each lesion: Lab = La − Lb, and distance differences dab and lab analogously. This requires at least three measurements as may be shown starting from Eq. 2:
| (12) |
| (13) |
We substitute distances and velocities for the latencies for the contralateral case:
| (14) |
or, in the matrix formulation of the system of equations, using the difference variables defined earlier:
| (15) |
The solution in terms of vd and vl, obtained by inverting the matrix above and then taking the reciprocal of the entries of the resulting vector, is:
| (16) |
| (17) |
In conclusion, location and response latency differences are sufficient to determine the velocities vd and vl.
In an analogous fashion, we can proceed for ITD, starting from Eq. 4 and subtracting ITD1 from ITD2:
| (18) |
For four measurement points we can obtain an analytical solution for the three velocities in consideration. Thus model equations can be solved without knowing the initial common delays, the LCOs. Although the values of the LCOs are not necessary to calculate the velocities, we note that knowing the velocities makes it possible to calculate the LCOs.
A further point merits mentioning. The axon tract below NL runs parallel to the ventral border of NL. For our model we regard the tract as being faithfully represented by a line (red in Fig. 1B). It is somewhat arbitrary where this line is placed. If we place the line close to the border, the ds are small, if we place the line further away, a constant length is added to all ds. Since this corresponds to a constant additional latency, it cancels out in Eq. 15 and thus does not influence the values of vl and vd. Similarly, the points iCO and cCO can be chosen independently, because any differences are subsumed into ΔLCO. Thus the exact positioning of these lines is not critical for model solutions.
Collinear Sampling
A principle of experimental design is that correlations between predictor variables, sometimes referred to as multicollinearities, should be avoided to maximize their informativeness (Chatterjee and Hadi 2006). This principle affects how measurement points are chosen. For the system of equations first introduced in Eq. 2 to have a solution, the matrix in Eq. 15 must be nonsingular and thus the determinant must be nonzero: d32l21 − l32d21 ≠ 0. This is equivalent to the sample points selected from a two-dimensional slab not lying on a straight line (otherwise dij = alij ⇒ d32l21 − l32d21 = al21l32 − l21al32 = 0). This holds true whenever there is more than one velocity to be determined. In the case of the chicken and emu, there is effectively only one dimension to choose sample points from, but also only one velocity, and the system is always solvable.
Apart from the extreme case of no solution existing when the measurement points form a perfectly straight line, the sensitivity of the model to small changes depends on the collinearity of the sample points, i.e., how close they are to lying on a straight line. An appropriate measure is the condition number κ of the sample matrix squared, κ(AAT), where the sample matrix A has columns corresponding to measurement points and rows corresponding to measurement dimensions. The condition number is a measure for the increase in the error of the solution induced by a certain error in the measurement (Kincaid and Cheney 1992). The lowest, optimal value for the condition number is one. The higher the value of the condition number is, the worse is the influence of the propagated error on the solution. In special cases the condition number is related to the correlation coefficient. In the simplest case of two distances being measured and the samples measured such that they have their mean at zero and their variance is one, the condition number can be expressed in terms of the correlation coefficient between the measurements: κ(AAT) = . This number then reaches the value one for no correlation (best possible condition number) and tends toward infinity as the correlation coefficient approaches 1. It may be seen from Table 1 that high correlations yield high condition numbers. Specifically, if all points lie exactly on a straight line and the correlation coefficient is 1, their condition number is infinitely large, and there is no solution. For a correlation coefficient of 0.95, a very high condition number results (Table 1, rightmost column). On the other hand, if the measurement points lie at the edges of a square or form a zigzag line, the correlation is zero and the condition numbers take values <10 (Table 1, two left columns).
Table 1.
Different layouts and their correlation coefficients and condition numbers
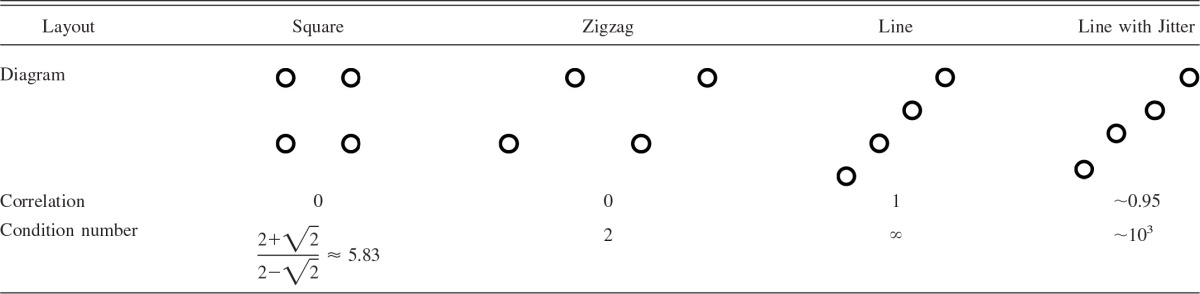
In each case 4 measurement points as indicated are used.
To assess the effects of different measurement layouts, we calculated the fitting error values for the square and linear layout (Fig. 3). It can be seen that in the case of the linear layout (Fig. 3B), which has a high correlation and condition number, there is no global minimum of the fitting error and many solutions are equally plausible, while the square layout (Fig. 3A) has a clearly localized minimum at the true solution. This means that in the square layout the underlying conduction velocities can be inferred, while this is impossible in the linear case.
Experimenters should take this restriction into consideration and choose measurement layouts with low condition numbers (e.g., square or zigzag as in Table 1). It should also be noted that the layout of the sampling points is important and not the shape of the structure in which the sampling points are placed. We shall use the measure of the condition number in the analysis of the biological cases from the owl used here.
Analysis of Biological Samples Cases
After these general analyses, we consider two real biological data sets, one from the chicken and one from the barn owl. These data were collected for other purposes without having the limitations of the model equations in mind. They may, therefore, be regarded as good test cases for the power and limitations of the theory presented here.
Chicken sample data.
The data set used for the chicken is presented in Table 2. The case analyzed contained two penetrations. Latencies and distances were measured from stained sections. The data are part of the data set published in Köppl and Carr (2008).
Table 2.
Chicken data
| Penetration No. | Lc, μs | lc, μm |
|---|---|---|
| 8-1 | 3,485 | 118 |
| 8-2 | 3,603 | 330 |
Penetration No. specifies the penetration. The other parameters have been explained in materials and methods.
In the contralateral case, we note that the contralateral chicken version of the model (Eq. 6) has only two unknown variables, vc,l and LcCO, thus two observations are sufficient to derive an analytical solution according to
| (19) |
Solving this system of two equations using the data set from Table 2 yields a velocity vc,l = = 1.80 m/s. Using this value for the velocity we can calculate LcCO = − Lc,1 − Lc,2 = 3.42 ms. Thus 3.42 ms, or 98%, of the conduction time to the first measurement at 3.485 ms is consumed along the initial common pathway. An analytical solution facilitates estimation of the possible ranges of the conduction velocities as a function of the fitting error (Fig. 2B). Starting from the analytical solution, the possible ranges widen in an asymmetrical fashion. For a fitting error of 41.8 μs that corresponds to ±1-sample interval in our recordings, the possible range is 1.33–2.78 m/s. For a fitting error of 60 μs, this range increases and allows values of 1.19–3.66 m/s. The corresponding ranges for LcCO are 3.38–3.46 and 3.36–3.48 ms, respectively. This again demonstrates that most of the conduction time passes before the signal reaches NL. The main conclusion from the analysis of this sample case is that the model can produce reasonable conduction velocities.
Barn owl sample data.
The owl data set (Table 3) is more complex than that of the chicken, since in the owl d distances are added that were not needed in the chicken. The data are part of the data set published in Carr et al. (2013). This case contained four penetrations. Latencies were determined from click responses and distances were measured from stained sections. We begin by analyzing the contralateral portion of the dataset based on the difference delays as described in Eq. 15 and calculate the fitting error from Eq. 5.
Table 3.
Owl data
| Penetration No. | Lc*, μs | Lc**, μs | dc, μm | lc, μm | Li*, μs | di, μm | ITD, μs |
|---|---|---|---|---|---|---|---|
| 1 | 2,494 | 2,806 | 145 | 580 | 2,494 | 241 | 8 |
| 2 | 2,536 | 2,806 | 175 | 683 | 2,557 | 365 | −5 |
| 3 | 2,557 | 2,827 | 74 | 1,345 | 2,557 | 456 | 7 |
| 4 | 2,640 | 2,931 | 30 | 1,773 | 2,661 | 445 | −19 |
Parameters are as in Table 2 or as explained in materials and methods. ITD, interaural time difference. Asterisks refer to 2 different delay conditions:
phase delay (see also Wagner et al. 2005);
phase delay plus 1 period of the oscillating response. Best frequencies were measured as 3.4 kHz in penetrations 1 and 2 and 3.6 kHz in penetrations 3 and 4.
This case is overdetermined, since data from four penetrations were available (Table 3), while three parameters (vc,d, vc,l, LcCO) had to be determined. To search the parameter space for two velocities, we constructed a heatmap showing the relationship between the two velocities for different fitting errors (Fig. 4). Figure 4 shows that the minimal fitting error occurs when vc,d = 1.1 m/s and vc,l = 4.9 m/s and has a value of 24.6 μs. Using these values, we can derive an implied value of LcCO of 2.23 ms. The 95% confidence interval on vc,l lies between 2.5 and 9.1 m/s and for vc,d between 0.4 and 6.0 m/s. The uncertainties in the velocities are not independent, and the resulting confidence interval for the ratio is 0.14 to 0.59, meaning that the velocities were significantly different despite their individual confidence intervals overlapping.
Fig. 4.
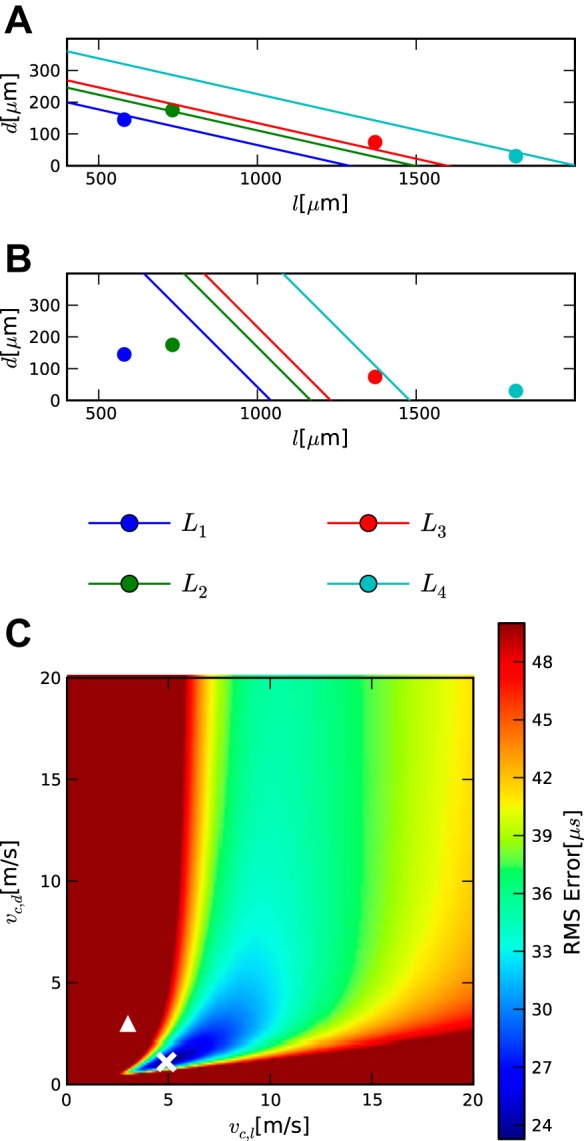
Application of the model to the barn owl case. Analysis of the contralateral case for measurements points in the 3,600-Hz region. To compare different possible velocity combinations, we calculated latencies in NL. A and B: 2 latency maps for such combinations. The lines show isolatency lines, and the colored dots show the locations at which the corresponding latencies were measured. A: good match, with vc,d = 1.1 m/s and vc,l = 4.9 m/s, the latencies match their observation locations well. B: bad match, in which both velocities take the same value, the average of the 2 velocities in the previous case: vc,d = vc,l = 3 m/s. C: heatmap of the fitting error for combinations of vc,d and vc,l. The white cross marks the combination of velocities with the lowest fitting error shown in A. The triangle show a worse combination, shown in B. Fitting errors >50 μs are all shown in the same color. There are several solutions with a similarly low fitting error when the ratio of the velocities is kept constant, but the velocities are still relatively well constrained (blue oval in heatmap). Condition number: 150.9.
Again, as in the chicken case, most of the response latency is consumed in the initial common pathway. This is consistent with the literature (Köppl 1997b). This author presented a formula to calculate conduction time up to NM based on click response latencies. Since NM is a processing stage before NL, it can be used as a lower bound on LcCO. With the use of this formula for the best frequency determined at the recording locations, e.g., 3,500 Hz, yields a value of 1.71 ms. An even more restricted lower bound would be the latency at the midline. Since midline recordings were not previously available, in a separate experiment latencies were measured from single unit recordings in vivo in one owl and were ∼0.1 ms longer than in NM, arriving at 1.835 ms (SD 0.075 ms, n = 16). These values are slightly shorter than our estimated LcCO, which is to be expected since there is a small conduction time between the midline and the medial border of NL.
The relationship between the two velocities remains well defined for fitting errors up to ∼30 μs (Fig. 4). The dorsoventral velocity vc,d is always smaller than the mediolateral velocity vc,l. For even higher fitting errors, many possible combinations of the two velocities can reproduce the data. For example, for a fitting error of 42 μs, either vc,d could be larger than vc,l or smaller. Thus, for such high-fitting errors, the model does not produce a clear relationship. One reason for this may be that the four measurement points in this case were too close to a straight line with a condition number of 150.9.
In the analysis of the ipsilateral data, only one velocity needed to be derived. The velocity that produced the lowest fitting error was vi,d = 1.9 m/s, and an optimal LiCO of 2.37 ms, in agreement with the values from (Köppl 1997b).
The velocity of contralateral axons outside of NL vc,d is higher than that in the chicken (4.9 vs. 1.9 m/s). This is consistent with anatomical measurements of the axon diameters. Chicken axon diameters are thinner, with average axon diameters above NL (1.104 ± 0.417 μm) and below NL (1.470 ± 0.646 μm), while both were smaller than the mean axon diameter along the midline (∼2 μm) (Seidl et al. 2010). In barn owls, mean axon diameters of ipsilateral axons above the NL are 3.18 ± 0.74 μm, within the nucleus for both ipsilateral and contralateral axons are 2.89 ± 0.66 μm (n = 412), and below the nucleus for the contralateral axons are 3.5 ± 0.98 μm (n = 193) (Carr and Boudreau 1993a).
The main conclusion from this analysis is that for small fitting error values, vc,l is larger than vc,d, consistent with earlier analyses (Carr and Konishi 1990). A second conclusion is that LcCO is larger compared with both Lc,d and Lc,l.
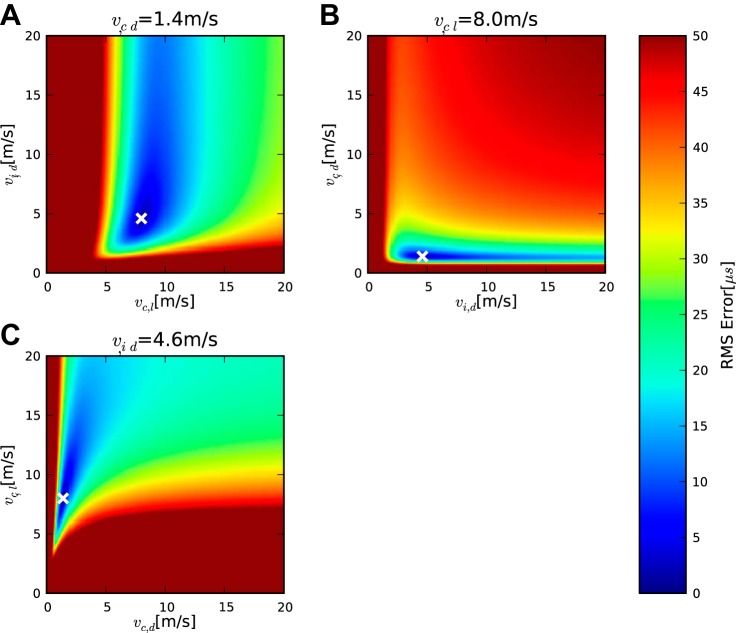
The binaural case was analyzed further for the barn owl sample data (Fig. 5). Since there are now three velocities to find, data from four measurement sets are needed for a solution according to Eq. 18. This analysis yielded values of vc,l = 8.0 m/s, vc,d = 1.4 m/s, and vi,d = 4.6 m/s. The largest discrepancy between the velocities derived from monaural and binaural cases is found for vi,d, which can be understood by observing that the regions of low fitting error (blue) extend furthest in the direction of vi,d in Fig. 5, A and B, which means that the value is not strongly constrained by the data in this case. If the dependence of the relationship between the pairs of velocities on the fitting error is considered, it becomes obvious that vi,d is less correlated with either vc,d or vc,l than the two other velocities with each other, because the relationship between vc,d and vc,l, which produces the lowest error (blue area in Fig. 5C), is oriented diagonally, while the region of lowest error in Fig. 5, A and B, runs roughly parallel to the axis representing vi,d. Thus, for the error to remain low, the ratio between the contralateral velocities vc,d and vc,l should be maintained, while the ipsilateral velocity vi,d can be altered independently. Analogous to the implied values of LiCO and LcCO, we calculated the value of ΔLCO that produced the lowest fitting error, obtaining 0.13 ms.
Fig. 5.
Application of the model to bilateral barn owl case. A–C, symbols, and colors are the same as in Fig. 4. Heatmaps represent the variation of 2 velocities with the third velocity fixed at the local error minimum, the value of which is indicated in the title.
The discrepancies between the results from the contralateral and bilateral data were within the confidence intervals. The confidence bounds derived from the bilateral data were larger, as can be seen from the larger regions of low fitting error in Fig. 5. The confidence interval on vc,l lies between 1.5 and 26.5 m/s, for vc,d between 0.7 and 22.2 m/s, and for vi,d between 0.2 and 6.6. Again the resulting confidence interval for the ratio was below one, at 0.1 to 0.35.
The main conclusion of the analysis of the binaural case is that the model can be applied in the case where more than two independent variables need to be estimated. In this section we used the model to calculate three conduction velocities simultaneously, and our results were in accordance with the previous contralateral velocity calculations. Reasons for the variation between ipsi- and contralateral velocities may lie in axonal structure (see discussion).
Uniform Peak Shifting
A key theoretical result of the linear model employed in this study is the invariance of the velocities when the dimensions of the systems are shifted by a fixed amount. This is true in the temporal as well as the spatial sense. Specifically, an addition of a constant to the latencies will only affect LCO and not the velocities. The oscillatory nature of the click-evoked neurophonic (Wagner et al. 2005) allowed for a further test of this theoretical result with the experimental data. We used recordings of contralateral latencies in the owl as a test case. In the previous section, the latencies were taken from the peaks that represented the phase delay in Table 2). Here we select the latencies of the subsequent peaks (Lc** in Table 2). This shift of peaks retains the spatial relationships of the measurement points as specified by the ds and ls constant but changes L almost uniformly.
In these simulations, the estimated values of vc,l and vc,d do not change much for the lowest fitting error (Fig. 6) and demonstrate a similar curve progression as in Fig. 4. Importantly, vc,l is again larger than vc,d. Note, however, that the fitting error is higher than in the nonshifted cases. The lowest fitting error was 59 μs, an increase by more than a factor of 2 (Fig. 7). The relationship between vc,l and vc,d becomes unclear for larger values of the fitting error (Fig. 4). The value of LcCO was increased, as expected from the peak shifting.
Fig. 6.
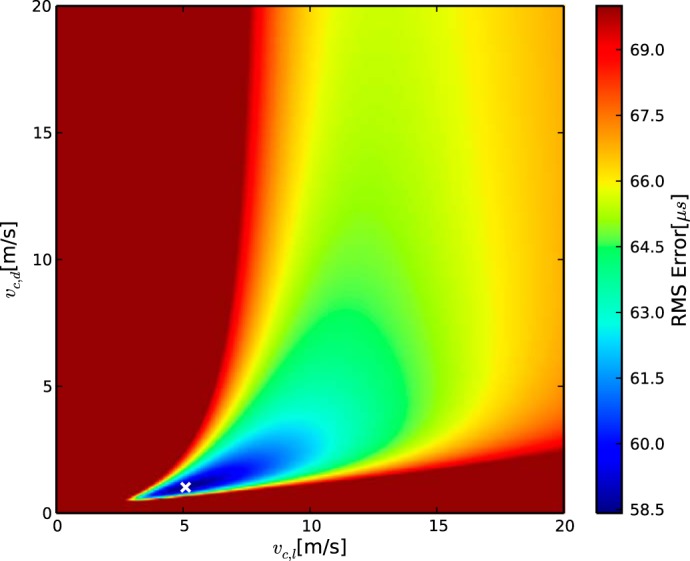
Fitting error heatmap for latencies uniformly shifted by 1 period at all measurement points. A–C and symbols are the same as in Fig. 4. Note that the ratio between the velocities remains well defined after the uniform latency shift.
The main conclusion from this analysis is that uniform peak shifting does not alter the values of vc,l and vc,d that produce the lowest fitting error, consistent with previously described sensitivity to interaural phase differences (Wagner et al. 2005; Carr and Konishi 1990; Moiseff and Konishi 1981; Fischer and Peña 2009). The relationship between vc,l and vc,d outside the optimum also remains similar if a (constant) delay corresponding to the distance between the peaks is added to the common delay LcCO. The temporal shifting performed here is mathematically equivalent to shifting the point of the circuit onset cCO by a fixed amount. Thus the exact choice of cCO is not crucial.
Mismatched Peak Shifting
Responses to ITD are oscillatory and a peak in one trace can correspond to several others from different recording locations (Kuokkanen et al. 2013). Uniform peak shifting does not greatly change the relationship between the velocities but increases minimal values of the fitting errors. A practical problem that arises in the analysis of the electrophysiological data is that it is not always obvious which peaks from different recording locations correspond to each other. This makes the consideration of the performance of the model in the case of incorrectly matched peaks interesting.
The fitting errors should change much more if noncorresponding peaks are selected for the analysis. We again used the contralateral data from the owl as a test case. In these tests we left the latency of three peaks as in the analysis shown in Fig. 4 and shifted the latency of the remaining peak. For example, in the analysis shown in Fig. 7B, the following latencies were used: penetration 1: 2,494 μs; penetration 3: 2,557 μs; penetration 4: 2,640 μs; and penetration 2: 2,806 μs, i.e., the peak from the second penetration was shifted compared with the other peaks. The results show a reasonably low minimal fitting error. The relationships between the velocities changed, however, with estimated values for vc,d <1 m/s for fitting errors <150 μs. In the reasonable range of >1 m/s for both velocities, the fitting error is >200 μs. The changes seen for mismatched shifts of the other penetrations (Fig. 7, A and C) produced a minimal fitting error >160 μs for the entire space of possible velocities.
In conclusion, mismatched peak-shifting changed the relation between the velocities and generally increased fitting errors, allowing model performance to be used as a selection criterion for the matching of peaks.
Robustness Regarding Possible Reconstruction Errors
The spatial measurements depend on the ability to determine the position of the electrode tip during recording. The position of the electrode tip was identified from the center of the electrolytic lesions (see materials and methods). Since these lesions have a diameter of ∼200 μm, an uncertainty of up to 100 μm exists about the exact position of the tip of the electrode. Therefore, we tested the effect of shifting the supposed position of the electrode tip 100 μm up or down. The value of vd was constrained to values between 0.5 and 5.0 m/s, and the values of vl were allowed to vary freely. The resulting velocity ranges (Fig. 7D), based on the same fitting error limit as used earlier (41.8 μs), show that the possible ranges for vl change slowly. In the reference condition (Fig. 7D, orig), the possible range was from 3 to 9 m/s. In the shifted conditions the lower bounds varied from 2 to 5 m/s while the upper bound varied more (7–13 m/s). None of the estimated upper bound velocities were unreasonably high, however. In summary, the model predicts consistent velocity ranges for location shifts up to 100 μm.
DISCUSSION
Our linear model describes the role of delays in the representation of ITD. Provided an initial common latency exists up to NL, differential adjustment of velocities within NL is sufficient to obtain latency matching for ITD detection. Proof of principle tests from both chicken and owl data justify the assumptions of the model and demonstrate its robustness.
Relationship Between the Model and the Biological Circuits
While the mathematical formulation of the model does not contain restrictions, it seemed reasonable to restrict the parameter space to biologically plausible values such as positive values for velocities and distances. Furthermore, because the model was mathematically simple, we were also able to incorporate measurement errors to reveal a potential range of solutions in addition to the analytical solution. Measurement error also took into account the finite sampling interval of 20.8 μs (Ashida and Carr 2011; Ashida et al. 2010) and the temporal jitter or dispersion, which amounts to several tens of microseconds (Köppl 1997a).
Key model assumptions of initial common latency, constant conduction velocity, and axonal path lengths were derived from experimental measurements. We first consider the initial common latency. In the barn owl, Carr and Konishi (1990) found a common latency from the ear to the borders of NL in the 5-kHz region. They reported latencies of 2.82 ms (±0.24) for ipsilateral axons at the dorsal border and 2.87 ms (±0.21) for contralateral axons at the ventral border of the 5-kHz region. Fewer data are available for the chicken in vivo, but similar latency matches between ipsilateral and contralateral responses were observed in the data from Köppl and Carr (2008).
Assumptions of constant conduction velocities were also derived from experimental measurements. The constant mediolateral velocity observed in in vitro studies in chicken and emu (Overholt et al. 1992; Görlich et al. 2010; MacLeod et al. 2006) was the basis for the assumption of a constant mediolateral velocity in the model. The values of 1.8 m/s and the range up to 3.6 m/s observed with the model were at the lower range of what Overholt et al. (1992) calculated from their chicken slice recordings and were lower than the adjusted conduction velocities proposed by Seidl et al. (2010). In the barn owl Carr and Konishi (1988) provided experimental evidence for a constant dorsoventral conduction velocity. Conduction delays and distances were measured from systematic dorsoventral changes in phase (Carr and Konishi 1990). These authors calculated velocities between 3.8 and 4.7 m/s. Our data supported these dorsoventral measurements, since they lay between the inferred values for vc,d and vi,d (1.1–5.5 m/s). Mediolateral velocity has not yet been measured in the barn owl, although our analyses support the hypothesis that mediolateral velocities were higher than dorsoventral velocities (Carr and Konishi 1990).
Our final assumption of linear axonal path length segments cannot be strictly true, since real axon paths are not straight and show variation between neighboring axons (Carr and Konishi 1988, 1990; Seidl et al. 2010; Karino et al. 2011; Beckius et al. 1999; Smith et al. 1993). Nevertheless, our model produced robust results within at least ±100 μm, indicating that deviations from strictly linear paths should not influence the predictive power of the model. A strong model prediction concerns the representation of ITD in the barn owl. In the barn owl NL, ITDs are mapped along the dorsoventral extent of NL, while lines of constant ITDs extend mediolaterally (Carr et al. 2013). Sullivan and Konishi (1986) reported that these iso-ITD lines were parallel to the lower border of NL. Our simulations show this cannot be the case, because it would require an infinitely large mediolateral conduction velocity, and our recent data indicate that the iso-ITD lines are not parallel to ventral border of NL (Carr et al. 2013).
Adaptive Regulation of Latency
Adaptive, or developmental, regulation of latency should occur in all ITD circuits, since circuits are formed before auditory cues stabilize (for reviews, see Kubke and Carr 2005; Rubel and Fritzsch 2002; Cheng and Carr 2007; Parks and Rubel 2004; Seidl et al. 2014). Latency regulation may take place at all levels of the ITD detection circuit. Seidl et al. (2010) has suggested that regulation of conduction time across the midline would be necessary for the ipsi- and contralateral fibers to arrive at the same time in NL, i.e., that coincidence detection required inputs to be both synchronous in time as well as matched in phase. His recent review (Seidl 2013) suggests that activity-dependent processes may shape the organization of myelination along the NM axons in the chicken. Similar predictions have been made for the barn owl, where myelination of the delay line axons occurs in at least two steps/periods of development. Myelination of the auditory nerve and NM axons up to the borders of NL occurs early in development, while myelination of the vc,d and vi,d segments coincides with the period of head growth and attainment of stable ITD cues (Cheng and Carr 2007).
Adaptive regulation of delay could provide the adjustments in conduction velocity required for simultaneous activation of NL coincidence detector neurons (Leibold et al. 2002). It is an open question to what degree coincidence detection requires inputs to be synchronous in time as well as matched in phase. Our mathematical analysis shows that where a common initial pathway exists, binaural tuning may be obtained by local regulation of conduction velocities within NL. The uniform peak shifting simulations described above suggest that difference delays and not the absolute value of the latencies are important, just as observed experimentally (Wagner et al. 2005). Recordings from NL neurons further show that NL neurons respond over a wide range of integer multiples of their preferred interaural phase difference (Christianson and Peña 2006). Thus the auditory system seems to match ipsilateral and contralateral latencies, so that inputs arrive at NL at about the same time, but responses from the postsynaptic coincidence detectors and our simulations suggest these latencies could vary by multiples of 2π without ill effect. Precise measurements of individual fiber latencies are required to show if this occurs in nature. Data support our assumption of an initial common latency and support the hypothesis of Seidl (2013) that conduction velocity of the delay line inputs is regulated to match ipsi- and contralateral delays (Carr et al. 2013; Seidl 2013).
The barn owl has a two-dimensional layout of NL isofrequency slabs, while many other birds and mammals have a one-dimensional or monolayer organization. This two-dimensional layout allows for a redundant representation of ITD and thus an improvement of the representation of ITD cues (Carr and Boudreau 1993b; Kubke et al. 2002). We add another advantage; vi,d and vc,d may be individually adjustable, creating additional degrees of freedom that may allow for more precise matching of ipsilateral and contralateral delays. We note also that delay may also be adjusted on a faster time scale, through mechanisms other than regulation of latency (Stange et al. 2013). Further discussion of this and related topics may be found in recent reviews (Grothe et al. 2010).
Regulation of latency in the mammalian medial superior olive may be multifaceted. Like in the avian NL, excitatory inputs to the medial superior olive are thought to be matched in latency, and current models of ITD processing assume that propagation times between the ipsi- and contralateral ears to the MSO neurons are equal. Few data are available, although measures of delay to the lateral superior olive show some similar conduction times from ipsi- and contralateral inputs (Joris and Yin 1998). In vitro, Jercog et al. (2010) found an internal latency difference between ispi- and contralateral inputs consistent with the position of the medial superior olive to one side of the brainstem and the shorter ipsilateral pathway. Careful reexamination of axonal branching patterns of physiologically characterized axons of spherical bushy cells of the cat anteroventral cochlear nucleus, which project to binaural coincidence detectors in the medial superior olive, led Karino et al. (2011) to conclude that the anatomical patterns could not account for the frequency-dependent distribution of best delays in the cat (Hancock and Delgutte 2004; Joris et al. 2005), and they suggest other mechanism(s) must contribute to the internal delays. Bremen and Joris (2013) further point out the importance of the relationship between delay and characteristic frequency, which constrains potential mechanism(s) that could generate best delays in the medial superior olive.
Latency changes with frequency as a function of cochlear delay in birds and mammals (Köppl 1997a; Pfeiffer and Kim 1975; Ruggero et al. 1986), so our assumption of matched ipsilateral and contralateral latencies is contingent upon matched best frequencies. Notably, delays arising from mismatched best frequencies have been used to generate an alternate model of ITD sensitivity termed stereausis (Shamma et al. 1989; Bonham and Lewis 1999). Systematic delays from frequency mismatches have not, however, been observed in ITD sensitive circuits in barn owl (Singheiser et al. 2010; Fischer and Peña 2009) and chicken (Köppl and Carr 2008). In mammals, the stereausis model has not been ruled out, with the suggestion that small differences in frequency tuning between ears in neurons in the superior olivary complex could contribute to adaptive regulation of delays (Fitzpatrick et al. 2002; Joris et al. 2005; Hancock and Delgutte 2004; Day and Semple 2011). The model presented here therefore conforms with the experimental findings in avians and provides a framework to reason about the consequences of different aspects of latency regulation.
Extensions of the Model
Our approach demonstrates that introduction of complex anatomical data can yield additional insight and produce consistent results. However, there is also one caution; the robustness of the predicted velocities depends on the positioning of the measurement points in relation to each other. Measurement points should not lie on a straight line, because this prevents precise estimation of velocities, even when they are well defined.
In the binaural barn-owl test case analyzed here, we observed a large difference between the ipsilateral and contralateral dorsoventral velocities, which is different from the observations of Carr and Konishi (1990). This might mean that our model is too simple. Specifically, we positioned the point iCO at the dorsal edge of NL, which may be an oversimplification. It would be more consistent with the anatomical data to move this point from the border of NL to the dorsal bifurcation point above NL, from which the axons start to fan out, thereby taking slightly different lengths in the routes to NL.
It is possible to relax some of the restrictions of the model. If, for example, a linearly changing velocity is included in both the mediolateral and dorsoventral direction, two new parameters are added but the structure of the model is not changed. This addition of two new parameters would require two additional independent measurements. The model would fail as such if different individual axons in the same path had different velocities, and if no common path existed. An extension of the model would be possible, if more structural data were available, like the exact path from NM to NL and associated conduction times.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants DCD-00436 (to C. Carr) and DCD T32-DC00046 (to the University of Maryland Center for the Evolutionary Biology of Hearing), Deutsche Forschungsgemeinschaft Grants WA 606/21 (to H. Wagner) and KO 1143/12-2 (to C. Köppl), and University of Sydney R&D Grant (to C. Köppl).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M., S.S., C.K., C.C., and H.W. conception and design of research; T.M., S.S., C.K., C.C., and H.W. analyzed data; T.M., C.K., C.C., and H.W. interpreted results of experiments; T.M. prepared figures; T.M., C.C., and H.W. drafted manuscript; T.M., C.K., C.C., and H.W. edited and revised manuscript; T.M., S.S., C.K., C.C., and H.W. approved final version of manuscript; S.S., C.K., C.C., and H.W. performed experiments.
ACKNOWLEDGMENTS
We thank Sandra Brill for help with data analysis. This work profited from helpful comments by Richard Kempter and Paula Kuokkanen.
REFERENCES
- Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr Opin Neurobiol 21: 745–751, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida G, Wagner H, Carr C. Processing of phase-locked spikes, and periodic signals. In: Analysis of Parallel Spike Trains, edited by Grün S, Rotter S. New York: Springer, vol. 7 of Springer Series in Computational Neuroscience, 2010, p. 59–74. [Google Scholar]
- Bala ADS, Spitzer M, Takahashi TT. Prediction of auditory spatial acuity from neural images on the owl's auditory space map. Nature 424: 771–774, 2003. [DOI] [PubMed] [Google Scholar]
- Beckius GE, Batra R, Oliver DL. Axons from anteroventral cochlear nucleus that terminate in medial superior olive of cat: observations related to delay lines. J Neurosci 19: 3146–3161, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham BH, Lewis ER. Localization by interaural time difference (ITD): effects of interaural frequency mismatch. J Acoust Soc Am 106: 281–290, 1999. [DOI] [PubMed] [Google Scholar]
- Bremen P, Joris PX. Axonal recordings from medial superior olive neurons obtained from the lateral lemniscus of the chinchilla (Chinchilla laniger). J Neurosci 33: 17506–17518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MH, Waxman SG, Moore JW, Joyner RW. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry 40: 769–774, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R, Rubel E. Encoding of interaural timing for binaural hearing. In: The Senses: a Comprehensive Reference. San Diego, CA: Academic, 2008, p. 613–630, 2008. [Google Scholar]
- Campenhausen M, Wagner H. Influence of the facial ruff on the sound-receiving characteristics of the barn owl's ears. J Comp Physiol A 192: 1073–1082, 2006. [DOI] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. An axon with a myelinated initial segment in the bird auditory system. Brain Res 628: 330–334, 1993a. [DOI] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Organization of the nucleus magnocellularis and the nucleus laminaris in the barn owl: Encoding and measuring interaural time differences. J Comp Neurol 334: 337–355, 1993b. [DOI] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Development of the time coding pathways in the auditory brainstem of the barn owl. J Comp Neurol 373: 467–483, 1996. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. Axonal delay lines for time measurement in the owl's brainstem. Proc Natl Acad Sci USA 85: 8311–8315, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C, Shah S, Ashida G, McColgan T, Wagner H, Kuokkanen PT, Kempter R, Köppl C. Maps of ITD in the nucleus laminaris of the barn owl. In: Basic Aspects of Hearing, edited by Moore BJ, Patterson RD, Winter IM, Carlyon RP, Gockel HE. Springer, NY, vol. 787 of Advances in Experimental Medicine and Biology, 2013, p. 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Hadi AS. Analysis of Collinear Data. Hoboken, NJ: John Wiley & Sons, 2006. [Google Scholar]
- Cheng SM, Carr CE. Functional delay of myelination of auditory delay lines in the nucleus laminaris of the barn owl. Dev Neurobiol 67: 1957–1974, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson GB, Peña JL. Noise reduction of coincidence detector output by the inferior colliculus of the barn owl. J Neurosci 26: 5948–5954, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Semple MN. Frequency-dependent interaural delays in the medial superior olive: implications for interaural cochlear delays. J Neurophysiol 106: 1985–1999, 2011. [DOI] [PubMed] [Google Scholar]
- Fischer BJ, Peña JL. Owl's behavior and neural representation predicted by Bayesian inference. Nat Neurosci 14: 1061–1066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Peña J. Bilateral matching of frequency tuning in neural cross-correlators of the owl. Biol Cybern 100: 521–531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Batra R. Transformations in processing interaural time differences between the superior olivary complex and inferior colliculus: beyond the Jeffress model. Hear Res 168: 79–89, 2002. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature 383: 76–78, 1996. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Functional organization of the dog superior olivary complex: an anatomical and electrophysiological study. J Neurophysiol 31: 639–656, 1968. [DOI] [PubMed] [Google Scholar]
- Görlich A, Illy M, Friauf E, Wagner H, Luksch H, Löhrke S. Development of the delay lines in the nucleus laminaris of the chicken embryo revealed by optical imaging. Neuroscience 168: 564–572, 2010. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Delgutte B. A physiologically based model of interaural time difference discrimination. J Neurosci 24: 7110–7117, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann L, von Campenhausen M, Endler F, Singheiser M, Wagner H. Improvements of sound localization abilities by the facial ruff of the barn owl (Tyto alba) as demonstrated by virtual ruff removal. PLoS One 4: e7721, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffress LA. A place theory of sound localization. J Comp Physiol Psychol 41: 35–39, 1948. [DOI] [PubMed] [Google Scholar]
- Jercog PE, Svirskis G, Kotak VC, Sanes DH, Rinzel J. Asymmetric excitatory synaptic dynamics underlie interaural time difference processing in the auditory system. PLoS Biol 8: e1000406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Morest DK. Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: a Golgi study. Neuroscience 7: 837–853, 1982. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. III. Comparison with afferent pathways. J Neurophysiol 79: 253–269, 1998. [DOI] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci 30: 70–78, 2007. [DOI] [PubMed] [Google Scholar]
- Joris P, Heijden M, Louage D, Sande B, Kerckhoven C. Dependence of binaural, and cochlear “ best delays” on characteristic frequency In: Auditory Signal Processing, edited by Pressnitzer D, Cheveigné A, McAdams S, Collet L. New York: Springer, 2005 p. 477–483. [Google Scholar]
- Joseph AW, Hyson RL. Coincidence detection by binaural neurons in the chick brain stem. J Neurophysiol 69: 1197–1211, 1993. [DOI] [PubMed] [Google Scholar]
- Karino S, Smith PH, Yin TC, Joris PX. Axonal branching patterns as sources of delay in the mammalian auditory brainstem: a re-examination. J Neurosci 31: 3016–3031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ. How straight do axons grow? J Neurosci 5: 589–595, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid D, Cheney W. Numerical analysis-mathematics of scientific computing. Math Comput 59: 297, 1992. [Google Scholar]
- Köppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci 17: 3312–3321, 1997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C. Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. J Neurophysiol 77: 364–377, 1997b. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Maps of interaural time difference in the chicken's brainstem nucleus laminaris. Biol Cybern 98: 541–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Carr C. Development of the auditory centers responsible for sound localization. In: Sound Source Localization, edited by Popper A, Fay R. New York: Springer, vol. 25 of Springer Handbook of Auditory Research, 2005. p. 179–237. [Google Scholar]
- Kubke MF, Massoglia DP, Carr CE. Developmental changes underlying the formation of the specialized time coding circuits in barn owls (Tyto alba). J Neurosci 22: 7671–7679, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Massoglia DP, Carr CE. Bigger brains or bigger nuclei? Regulating the size of auditory structures in birds. Brain Behav Evol 63: 169–180, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen PT, Ashida G, Carr CE, Wagner H, Kempter R. Linear summation in the barn owl's brainstem underlies responses to interaural time differences. J Neurophysiol 110: 177–130, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen PT, Wagner H, Ashida G, Carr CE, Kempter R. On the origin of the extracellular field potential in the nucleus laminaris of the barn owl (Tyto alba). J Neurophysiol 104: 2274–2290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MM, Verschooten E, Joris PX. Oscillatory dipoles as a source of phase shifts in field potentials in the mammalian auditory brainstem. J Neurosci 30: 13472–13487, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold C, Kempter R, van Hemmen JL. How spiking neurons give rise to a temporal-feature map: from synaptic plasticity to axonal selection. Phys Rev E Stat Nonlin Soft Matter Phys 65: 051915, 2002. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Soares D, Carr CE. Interaural timing difference circuits in the auditory brainstem of the emu (Dromaius novaehollandiae). J Comp Neurol 495: 185–201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci 1: 40–48, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci 28: 7153–7164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt EM, Rubel EW, Hyson RL. A circuit for coding interaural time differences in the chick brainstem. J Neurosci 12: 1698–1708, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Overview: development and plasticity of the central auditory system In: Plasticity of the Auditory System. New York: Springer, 2004. p. 1–7. [Google Scholar]
- Payne RS. Acoustic location of prey by barn owls (Tyto Alba). J Exp Biol 54: 535–573, 1971. [DOI] [PubMed] [Google Scholar]
- Peña JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16: 7046–7054, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer RR, Kim DO. Cochlear nerve fiber responses: distribution along the cochlear partition. J Acoust Soc Am 58: 867–869, 1975. [DOI] [PubMed] [Google Scholar]
- Poganiatz I, Nelken I, Wagner H. Sound-localization experiments with barn owls in virtual space: influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol 2: 1–21, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Parks TN. Organization and development of brain stem auditory nuclei of the chicken: tonotopic organization of n. magnocellularis and n laminaris. J Comp Neurol 164: 411–433, 1975. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25: 51–101, 2002. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Robles L, Rich NC. Basilar membrane mechanics at the base of the chinchilla cochlea. II. Responses to low-frequency tones and relationship to microphonics and spike initiation in the VIII nerve. J Acoust Soc Am 80: 1375–1383, 1986. [DOI] [PubMed] [Google Scholar]
- Seidl AH. Regulation of conduction time along axons. Neuroscience 276C: 126–134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Barría A. Differential conduction velocity regulation in ipsilateral and contralateral collaterals innervating brainstem coincidence detector neurons. J Neurosci 34: 4914–4919, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Harris DM. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J Neurosci 30: 70–80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma SA, Shen N, Gopalaswamy P. Stereausis: binaural processing without neural delays. J Acoust Soc Am 86: 989–1006, 1989. [DOI] [PubMed] [Google Scholar]
- Singheiser M, Fischer BJ, Wagner H. Estimated cochlear delays in low best-frequency neurons in the barn owl cannot explain coding of interaural time difference. J Neurophysiol 104: 1946–1954, 2010. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol 331: 245–260, 1993. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Schreiner CE. The auditory neurophonic: basic properties. Hear Res 15: 261–280, 1984. [DOI] [PubMed] [Google Scholar]
- Stange A, Myoga MH, Lingner A, Ford MC, Alexandrova O, Felmy F, Pecka M, Siveke I, Grothe B. Adaptation in sound localization: from GABAB receptor-mediated synaptic modulation to perception. Nat Neurosci 16: 1840–1847, 2013. [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Neural map of interaural phase difference in the owl's brainstem. Proc Natl Acad Sci USA 83: 8400–8404, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT. How the owl tracks its prey–II. J Exp Biol 213: 3399–3408, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Brill S, Kempter R, Carr CE. Microsecond precision of phase delay in the auditory system of the barn owl. J Neurophysiol 94: 1655–1658, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Brill S, Kempter R, Carr CE. Auditory responses in the barn owl's nucleus laminaris to clicks: impulse response and signal analysis of neurophonic potential. J Neurophysiol 102: 1227–1240, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Güntürkün O, Nieder B. Anatomical markers for the subdivisions of the barn owl's inferior collicular complex and adjacent peri- and subventricular structures. J Comp Neurol 465: 145–159, 2003. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Kitzes LM, Goodman DA. Some characteristics of the “auditory neurophonic”. Experientia 26: 46–48, 1970. [DOI] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990. [DOI] [PubMed] [Google Scholar]
- Young SR, Rubel EW. Frequency-specific projections of individual neurons in chick brainstem auditory nuclei. J Neurosci 3: 1373–1378, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]