Abstract
BACKGROUND
Treatment regimens for omalizumab are guided by a dosing table that is based on total serum IgE and body weight. Limited data exist about onset and offset of omalizumab efficacy in children and adolescents or subgroups that most benefit from treatment.
OBJECTIVES
Post hoc analyses were conducted to (1) examine patient characteristics of those eligible and ineligible for omalizumab, (2) describe onset of effect after initiation of omalizumab and offset of treatment effect after stopping therapy, and (3) determine whether the efficacy differs by age, asthma severity, dosing regimen, and prespecified biomarkers.
METHODS
Inner-city children and adolescents with persistent allergic asthma were enrolled in the Inner-City Anti-IgE Therapy for Asthma trial that compared omalizumab with placebo added to guidelines-based therapy for 60 weeks.
RESULTS
Two hundred ninety-three of 889 participants (33%) clinically suitable for omalizumab were ineligible for dosing according to a modified dosing table specifying IgE level and body weight criteria. Baseline symptoms were comparable among those eligible and ineligible to receive omalizumab, but other characteristics (rate of health care utilization and skin test results) differed. The time of onset of omalizumab effect was <30 days and time of offset was between 30 and 120 days. No difference in efficacy was noted by age or asthma severity, but high exhaled nitric oxide, blood eosinophils, and body mass index predicted efficacy.
CONCLUSIONS
A significant portion of children and adolescents particularly suited for omalizumab because of asthma severity status may be ineligible due to IgE >1300 IU/mL. Omalizumab reduced asthma symptoms and exacerbations rapidly; features associated with efficacy can be identified to guide patient selection.
Keywords: Asthma exacerbations, Biomarkers, Dosing regimens, Inhaled corticosteroids, Omalizumab, Pharmacodynamics, Response predictors
For patients with persistent allergic asthma who fail to achieve control on the higher treatment steps of the National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3) guidelines, omalizumab, a humanized monoclonal anti-IgE antibody, is recommended.1 On the basis of previous studies, omalizumab reduces exacerbations, symptoms, and, in some patients, the dose of inhaled corticosteroids (ICS) needed to maintain asthma control.2–7
Because of the increased morbidity associated with a high prevalence of allergic sensitization and the heavy burden of allergen exposure among children, adolescents, and young adults living in inner-city environments,8–11 this population may particularly benefit from an IgE-targeted treatment. We therefore conducted a study and demonstrated the efficacy and safety of omalizumab when added to guidelines-based therapy among such inner-city residents with asthma in the National Institute of Allergy and Infectious Diseases Inner City Asthma Consortium, called the Inner-City Anti-IgE Therapy for Asthma (ICATA) trial.12 Among the 419 participants randomly assigned, omalizumab compared with placebo significantly reduced the number of days with asthma symptoms (24.5% decrease; P < .001) and reduced the proportion of participants who had one or more exacerbations from 48.8% to 30.3% (P < .001). These improvements occurred with omalizumab despite reductions in the use of ICS and long-acting β-agonists. Participants who were both sensitized and exposed to cockroach allergen were observed to have the greatest clinical benefits.
We have now conducted a post hoc analysis to learn more about the efficacy of omalizumab and its pharmacodynamics in children and adolescents. Our specific aims were to (1) examine patient characteristics of those eligible and ineligible for omalizumab, (2) further describe the apparent onset of effect after initiation of omalizumab and offset of treatment effect after stopping therapy, and (3) determine whether the efficacy of omalizumab differs by age, asthma severity, dosing regimen, and prespecified biomarkers.
METHODS
The design of this study is summarized in the primary outcome manuscript.12 Briefly, ICATA was a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial that compared omalizumab with placebo added to guidelines-based therapy in 419 inner-city children, adolescents, and young adults (ages of 6–20 years) with persistent allergic asthma. Participants had a physician’s diagnosis of asthma or symptoms for >1 year. Persons receiving long-term control therapy were also required to have symptoms of persistent asthma or evidence of uncontrolled disease as indicated by hospitalization or unscheduled urgent care in the 6 to 12 months preceding study entry. Those not receiving long-term control therapy were eligible for ICATA only if they met both of the above criteria. Finally, all were required to have at least one positive perennial allergen skin test and a weight and IgE suitable for dosing by an expanded dosing table described below (Table I). Allergen skin testing consisted of a panel of 14 extracts of indoor and outdoor allergens most relevant to inner-city environments. Written informed consent was obtained from each participant or their parent or legal guardian. Participants younger than 18 years of age provided assent. This trial is registered with www.clinicaltrials.gov, number NCT00377572.
TABLE I.
Omalizumab Dosing Table in ICATA
| Baseline IgE (IU/mL) | Weight (kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–25 | >25–30 | >30–40 | >40–50 | >50–60 | >60–70 | >70–80 | >80–90 | >90–125 | >125–150 | >150 | |
| 0–30 | Do Not Dose | ||||||||||
| ≥ 30–100 | 75*† | 75*† | 75*‡ | 150* | 150* | 150* | 150* | 150* | 300* | 300* | |
| >100–200 | 150*† | 150*† | 150*‡ | 300* | 300* | 300* | 300* | 300* | 225§ | 300§ | |
| >200–300 | 150*† | 150*† | 225*‡ | 300* | 300* | 225§ | 225§ | 225§ | 300§ | 375§ | |
| >300–400 | 225*† | 225*† | 300*‡ | 225§ | 225§ | 225§ | 300§ | 300§ | |||
| >400–500 | 225*† | 300*† | 225§‡ | 225§‡ | 300§ | 300§ | 375§ | 375§ | |||
| >500–600 | 300*† | 300*† | 225§‡ | 300§ | 300§ | 375§ | |||||
| >600–700 | 300*† | 225§† | 225§‡ | 300§‡ | 375 | ||||||
| >700–800 | 225§† | 225§† | 300§† | 375§† | Do Not Dose | ||||||
| >800–900 | 225§† | 225§† | 300§† | 375§† | |||||||
| >900–1000 | 225§† | 300§† | 375§† | ||||||||
| >1000–1100 | 225§† | 300§† | 375§† | ||||||||
| >1100–1200 | 300§† | 300§† | |||||||||
| >1200–1300 | 300§† | 375§† | |||||||||
| >1300 | Do Not Dose | ||||||||||
Values are milligrams per dose; empty cells are not eligible for dosing. This expanded omalizumab dosing table was consistent with the omalizumab FDA-approved safety and tolerability studies in ages 6 to 12 years, and approved for use in ICATA.
From N Engl J Med. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. Volume 364, pp. 1005–15. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission.12
Dosing once every 4 weeks.
Added from FDA dosing table.
Dose changed from FDA dosing table.
Dosing once every 2 weeks.
Study design
At screening visits, each participant was assessed for asthma symptoms, previous treatment, pulmonary function, allergen skin prick test sensitivity, total serum IgE, and allergen-specific IgE. From the ICATA treatment algorithm, study physicians determined participant eligibility along with the appropriate asthma regimen, based on symptoms, percentage of predicted FEV1, and current level of therapy, with the goal to achieve disease control. This regimen was given for a 4-week run-in period. Asthma medications covered by the participants’ insurance were prescribed but not directly supplied with the exception of omalizumab or placebo study injections and oral prednisone for exacerbations. Caregivers and participants received education about relevant environmental allergen remediation as well as bedding covers, traps, and a vacuum cleaner.
After the 4-week run-in period, each participant was randomly assigned to receive subcutaneous omalizumab or placebo injections every 2 or 4 weeks for a total of 60 weeks (15 or 30 injections). The omalizumab injection dose (75–375 mg) was based on weight and total serum IgE to ensure a minimum dose of 0.016 mg/kg/IgE (IU/mL) every month. The dosing table had an expanded range for weight (20–150 kg) and total IgE (30–1300 IU/mL) compared with the label dosing approved by the Food and Drug Administration (FDA) which is limited to weights of 30 to 150 kg and total IgE from 30 to 700 IU/mL (highlighted in Table I). This expanded dosage table was consistent with the omalizumab FDA-approved safety and tolerability studies in ages 6 to 12 years and approved for use in ICATA.6,13 Placebo was administered in the same volume and frequency as omalizumab by unblinded study nurses; all other study procedures were performed by study staff members masked to treatment assignment.
During the 60-week double-blind treatment, in addition to the 2- or 4-week injection visits, evaluation and management visits occurred every 3 months, at which time treatment adjustments were made on the basis of symptoms in the previous 2 weeks, estimated controller regimen adherence, and FEV1. A standardized 6-item Adherence Review Questionnaire was administered to query how many controller medications were taken in the previous 2 weeks. Asthma control was assessed and assigned a level that paralleled EPR-3 definitions: level 1 (well-controlled), levels 2 and 3 (not well-controlled), and level 4 (poorly controlled).1 Ongoing treatment adjustments were made to achieve well-controlled asthma. Six ICATA treatment steps were established12 to standardize prescribing patterns and corresponded to EPR-3—defined levels of asthma severity: mild (steps 1 and 2), moderate (step 3), and severe (steps 4–6).1
Study outcome measures
The primary ICATA outcome evaluated at each 4-week injection visit was the number of days with symptoms during the previous 2 weeks, as used in previous inner-city asthma studies.14,15 Symptom days is the largest of the following variables reported via standardized questionnaire over the previous 2 weeks: (1) number of days with wheezing, chest tightness, or cough; (2) number of nights of sleep disturbance; (3) number of days when activities were affected; (4) number of days of rescue albuterol use. For this post hoc analysis, the primary outcome measure was used along with 2 secondary outcomes, exacerbation rate and ICS usage, to evaluate the onset and offset of effect of omalizumab after starting and discontinuing this treatment, respectively. In addition, these outcome measures were used to determine whether the effect of omalizumab was equivalent in participants with moderate and severe asthma, and in participants <12 years and 12 to 20 years of age.
Statistical analysis
All reported analyses are post hoc comparisons. Recruitment eligibility group comparisons were made with analysis of variance and chi-square tests. On the basis of previous research,16 ICATA was designed with a 12-week wash-in period at the beginning of the double-blind period that was not included in primary intent-to-treat analysis that was previously reported12; the same convention was used here unless otherwise noted. Like previous analyses, longitudinal analyses were performed with linear mixed-effects models with random intercept and slope (to account for the within-subject correlation) and with visit and group as fixed effects; the models were adjusted for baseline symptom level, site, dosing schedule, and season. Subgroup analyses were performed with a baseline-adjusted statistical test for interaction and were considered significant at a P value < .10.17 Statistical analyses were performed with SAS software, version 9.2 (SAS Institute), and R, version 2.14.
RESULTS
Eligibility
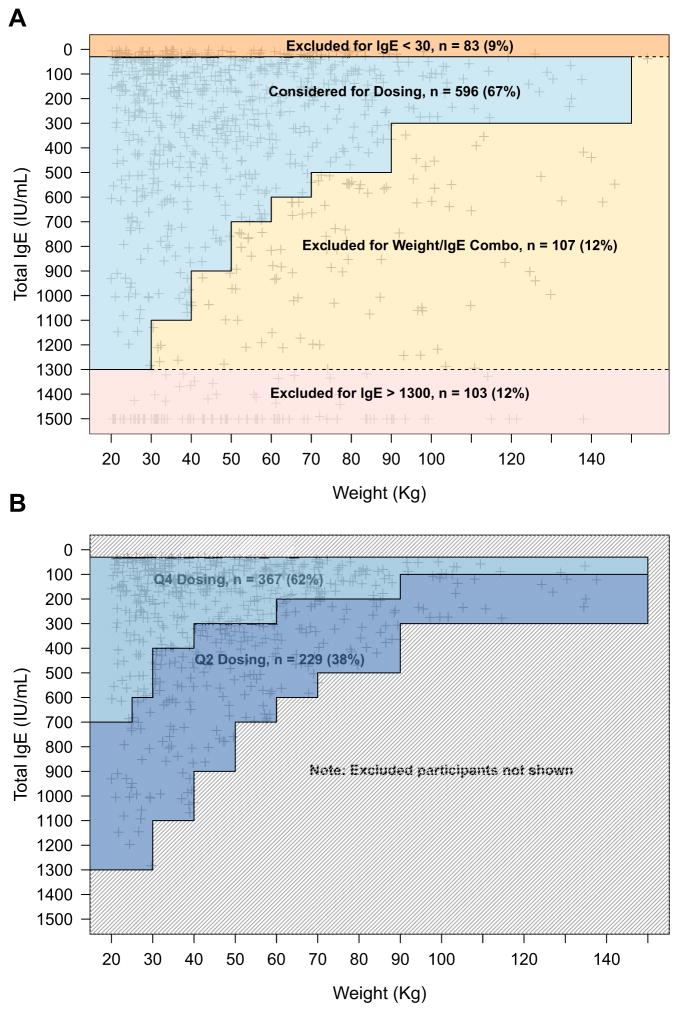
Dosing eligibility status and reasons for ineligibility (IgE too high, IgE too low, IgE/weight combination ineligible) are reported (Figure 1, A) and baseline characteristics (symptoms, health care utilization, and allergen skin test sensitivity percentages) for each of these classifications are provided (Table II).
FIGURE 1.
Dosing status for patients in the ICATA study. A, Shown is where weight and IgE measurements fall in the dosing chart for each of the 889 participants screened in ICATA. Ineligible participants are classified into 3 groups according to where they fall in the dosing chart (IgE <30 IU/mL; weight/IgE combo, and IgE >1300 IU/mL). B, Shown is how participants who are eligible for dosing break down into monthly and biweekly dosing groups. Q4, monthly; Q2, biweekly.
TABLE II.
Baseline participant characteristics by dosing eligibility status
| Eligible
|
Ineligible
|
P values*
|
|||||
|---|---|---|---|---|---|---|---|
| (1) | (2) IgE <30 IU/mL | (3) IgE/weight | (4) IgE >1300 IU/mL | (1) vs (2), (3) & (4) | (1) vs (2) | (1) vs (3) & (4) | |
| No. of participants | 596 | 83 | 107 | 103 | |||
|
| |||||||
| Age (y), mean ± SD | 10.7 ± 3.6 | 10.1 ± 3.9 | 11.0 ± 3.4 | 14.0 ± 3.0 | <.001 | .10 | <.001 |
|
| |||||||
| Symptoms, mean ± SD | |||||||
|
| |||||||
| Nights awoken (past 4 wk) | 11.5 ± 6.7 | 11.2 ± 6.6 | 11.3 ± 7.3 | 11.4 ± 6.9 | .80 | .58 | .99 |
|
| |||||||
| Days of wheeze (past 2 wk) | 4.3 ± 2.2 | 4.2 ± 2.3 | 4.7 ± 2.2 | 4.5 ± 2.2 | .39 | .53 | .15 |
|
| |||||||
| No. of albuterol uses (past 2 wk) | 14 ± 11 | 14 ± 12 | 14 ± 12 | 16 ± 12 | .88 | .68 | .66 |
|
| |||||||
| Health care utilization in past year (%) | |||||||
|
| |||||||
| Any hospitalizations | 28 | 12 | 25 | 25 | .03 | <.01 | .32 |
|
| |||||||
| Any unscheduled visits | 81 | 91 | 84 | 84 | .13 | .02 | .60 |
|
| |||||||
| On daily controller medications | 89 | 78 | 85 | 85 | .06 | <.01 | .42 |
|
| |||||||
| Allergen skin tests | |||||||
|
| |||||||
| No. positive (of 14), mean ± SD | 4.4 ± 2.7 | 2.6 ± 2.1 | 5.6 ± 2.8 | 5.6 ± 2.8 | .20 | <.001 | <.001 |
|
| |||||||
| Dog (%) | 30 | 19 | 29 | 35 | .49 | .03 | .67 |
|
| |||||||
| Cat (%) | 52 | 21 | 65 | 66 | .75 | <.001 | <.001 |
|
| |||||||
| Mouse (%) | 31 | 15 | 49 | 41 | .15 | <.01 | <.001 |
|
| |||||||
| Der f (%) | 38 | 27 | 58 | 57 | <.01 | .07 | <.001 |
|
| |||||||
| Der p (%) | 45 | 28 | 63 | 56 | .13 | <.01 | <.001 |
|
| |||||||
| German roach (%) | 50 | 17 | 61 | 62 | .70 | <.001 | <.01 |
|
| |||||||
| Roach mix (%) | 52 | 30 | 65 | 68 | .25 | <.001 | <.001 |
The 3 P values test for differences between: eligible and all ineligible participants, eligible participants and participants who were excluded because of low IgE, and eligible participants and participants who were excluded because of either high IgE or a combination of weight and IgE.
Of those potential participants who were screened as clinically suitable for ICATA, 33% (293 of 889 participants) were ineligible for protocol dosing because of their IgE and/or weight (Figure 1; Table II). Those excluded were categorized as follow: IgE <30 IU/mL (83 participants; 9.3%), IgE/weight combined (107; 12.0%), and IgE >1300 IU/mL (103; 11.6%). Eligible and ineligible participants had similar symptom levels, but other characteristics differed. Eligible participants had fewer positive allergen prick skin tests and sensitivity to specific allergens than groups ineligible because of IgE >1300 IU/mL or combined IgE/weight (P < .001 for number of positive skin tests), but eligible participants had more allergic activity than the group with IgE <30 IU/mL (P < .001). Participants who were excluded because of low IgE levels also had fewer hospitalizations in the previous year and lower rates of daily controller medication usage than eligible participants (both P < .01) and other ineligible participants (both P < .01; comparison not shown in Table II). Conversely, the participants excluded because of low IgE were more likely than others to have had an asthma-related unscheduled health care visit in the previous year (P = .03). Participants younger than the age of 12 years were less likely to be eligible for dosing than participants between 12 and 20 years old (61% vs 72% respectively; P < .001). Furthermore, participants excluded because of their IgE/weight combination or low IgE alone were younger (11.0 and 10.1 years, respectively) than participants excluded because of high IgE (14.0 years; P < .001) alone.
Onset and offset of omalizumab effect
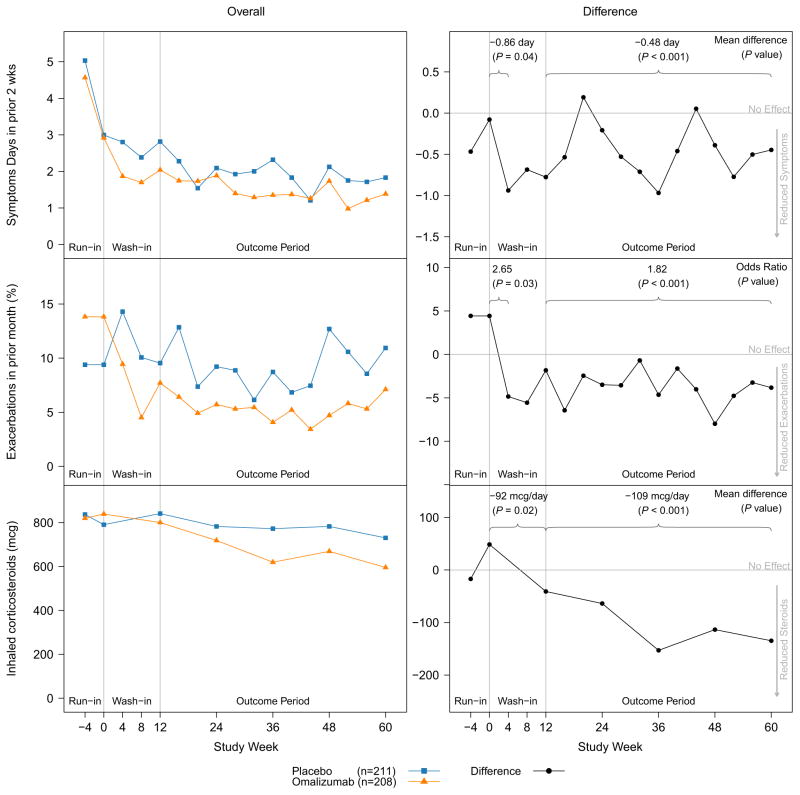
Figure 2 summarizes the time to effect for reduction in (1) asthma symptom days per 2 weeks, (2) exacerbations in the past month, and (3) ICS usage for participants receiving omalizumab compared with participants who received placebo. As previously reported,12 compared with placebo, omalizumab significantly decreased symptoms, exacerbations, and ICS usage (Figure 2) during the 12-month double-blind period. Although the protocol specified a 3-month wash-in period to guarantee omalizumab had time to achieve its maximum effect, similar results are seen with or without the wash-in data included.
FIGURE 2.
Omalizumab time to effect for exacerbations and symptom days. Shown are changes in overall symptoms (top row), exacerbations (middle row), and ICS use (bottom row) between enrollment (week −4) and the end of the double-blind (week 60) in ICATA. Changes in group means (left column) are compared with effect size (right column) to better emphasize the timing of efficacy. The left column shows average effect sizes at the first symptom assessment (4 weeks for symptom days and exacerbations, 12 weeks for ICS) and over the course of the outcome period starting at week 12. During the outcome period, the relative improvement in symptoms was 24.5%, reduction in exacerbations was 37.9%, and reduction in ICS dose was 14.1%. From N Engl J Med. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. Volume 364, pp. 1005–15. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission.12
Figure 2 shows that omalizumab treatment reduced both symptoms and exacerbations within the first 30 days of treatment and that these benefits were sustained over the remainder of the study. At the first visit after random assignment, 4 weeks after the initial injection, participants taking omalizumab had symptom days and exacerbation rates that were significantly lower than those observed in the placebo group (4-week treatment effect of 0.86 days per 2 weeks for symptom days; P = .04) and odds ratio of 2.65 for exacerbations (P = .03). Four-week effects cannot be measured for ICS because treatment was adjusted in 3-month intervals, but the 12-week effect for ICS was also significant (92 μg/day of budesonide equivalent; P = .02).
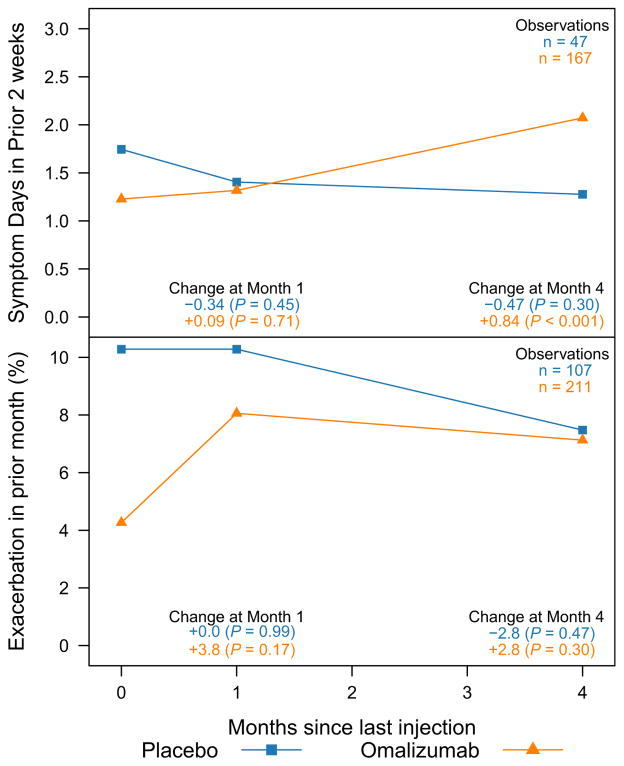
There were 318 ICATA participants, 211 receiving omalizumab and 107 receiving placebo, who had at least 4 months of open-label follow up after their last study injection. Of those, 167 active and 47 placebo participants completed symptom assessments at their last injection, and again 1 and 4 months later. Participants stopping omalizumab injections saw a larger increase in symptom days than participants who stopped taking placebo (P for interaction = .01; Figure 3). On average, participants saw a 0.84 day increase per 2 weeks in symptoms 4 months after stopping omalizumab (P < .001), whereas participants stopping placebo showed no significant change in symptoms (P = .30). By 4 months, the treatment effect for exacerbations also appeared to be waning, but the interaction between the groups stopping placebo and omalizumab was not significant (P = .23; Figure 3).
FIGURE 3.
Time for omalizumab to lose effect. Shown are changes in symptoms and exacerbations after cessation of injections (omalizumab or placebo) during the ICATA open-label follow-up period. Within 4 months of the final injection, participants taking omalizumab saw increases in symptoms and exacerbations (symptoms: 0.84-day increase, P <.001; exacerbations: 2.8% increase, P =.30) whereas the placebo group remained stable (both P > .30).
Efficacy based on dosing regimen, participant age, level of asthma severity, and prespecified biomarkers
Table III summarizes the efficacy of omalizumab as differentiated by dosing interval (2 week versus 4 week), age (<12 versus 12–20 years), and by asthma severity level (as indicated by treatment at randomization). Participants receiving biweekly injections saw greater reductions in both exacerbations (odds ratio = 2.54) and ICS usage (−204.8 μg/day) than participants receiving monthly injections (1.42 and −50.2 μg/day; interaction P values of .08 and 0.02, respectively). Omalizumab efficacy for symptom days per 2 weeks did not differ by dosing regimen (P = .62). Similar reductions were seen for all outcomes regardless of whether the treatment regimen was changed from the FDA-approved omalizumab dosing chart. Omalizumab was more efficacious in reducing exacerbations for children age 12 years and older (P = .09), but no corresponding differences were observed in ICS dose or symptom effects according to age. ICATA participants at all treatment step levels benefitted similarly from omalizumab on the basis of all 3 outcomes. Participants with total IgE ≥700 IU/mL had the greatest reduction in ICS usage (−504.6 μg/day) because of omalizumab, a population that exceeds the limits in the FDA-approved product information.
TABLE III.
Level of omalizumab efficacy and baseline participant characteristics
| Subgroup | No. | Symptom days per 2 weeks
|
ICS taken
|
Exacerbations/month
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | P* | P for interaction† | Effect | P* | P for interaction† | OR (95% CI) | P* | P for interaction† | ||
| All participants | ||||||||||
|
| ||||||||||
| ITT with wash-in period | 419 | −0.48 | <0.01 | N/A | −108.6 | <0.01 | N/A | 1.82 (1.33–2.50) | <0.01 | N/A |
|
| ||||||||||
| ITT without wash-in period | 419 | −0.52 | <0.01 | N/A | −108.6 | <0.01 | N/A | 1.85 (1.38–2.47) | <0.01 | N/A |
|
| ||||||||||
| Dosing group | ||||||||||
|
| ||||||||||
| 2-Wk dosing group | 166 | −0.39 | 0.09 | 0.62 | −204.8 | <0.01 | 0.02 | 2.54 (1.56–4.15) | <0.01 | 0.08 |
|
| ||||||||||
| 4-Wk dosing group | 253 | −0.54 | <0.01 | −50.2 | 0.22 | 1.42 (0.94–2.15) | 0.09 | |||
|
| ||||||||||
| FDA dosing chart | ||||||||||
|
| ||||||||||
| Standard | 227 | −0.46 | 0.02 | 0.71 | −71.32 | 0.10 | 0.35 | 2.43 (1.53–3.84) | <0.01 | 0.21 |
|
| ||||||||||
| Dose changed | 87 | −0.70 | 0.03 | −118.5 | 0.09 | 1.27 (0.65–2.46) | 0.48 | |||
|
| ||||||||||
| Addition to chart | 105 | −0.35 | 0.24 | 0.71 | −184.7 | <0.01 | 0.35 | 1.50 (0.82–2.75) | 0.19 | |
|
| ||||||||||
| Age | ||||||||||
|
| ||||||||||
| Age 11 y or younger | 250 | −0.56 | <0.01 | 0.50 | −128.0 | <0.01 | 0.44 | 1.52 (1.04–2.21) | 0.03 | 0.09 |
|
| ||||||||||
| Age 12 y or older | 169 | −0.36 | 0.13 | −75.9 | 0.14 | 2.76 (1.54–4.96) | <0.01 | |||
|
| ||||||||||
| Treatment at randomization‡ | ||||||||||
|
| ||||||||||
| Steps 1–2 | 113 | −0.30 | 0.28 | 0.68 | −128.6 | 0.03 | 0.58 | 2.16 (1.03–4.50) | 0.04 | 0.44 |
|
| ||||||||||
| Steps 3–4 | 152 | −0.48 | 0.05 | −54.0 | 0.28 | 1.44 (0.87–2.39) | 0.16 | |||
|
| ||||||||||
| Steps 5–6 | 154 | −0.64 | 0.01 | −105.5 | 0.04 | 2.21 (1.36–3.59) | <0.01 | |||
|
| ||||||||||
| Exhaled nitric oxide | ||||||||||
|
| ||||||||||
| <20 ppb | 158 | −0.38 | 0.08 | 0.55 | −40.5 | 0.45 | 0.11 | 1.15 (0.66–1.98) | 0.63 | 0.05 |
|
| ||||||||||
| ≥20 ppb | 155 | −0.45 | 0.05 | −164.2 | 0.03 | 2.57 (1.46–4.54) | <0.01 | |||
|
| ||||||||||
| Blood eosinophils | ||||||||||
|
| ||||||||||
| <2% | 69 | −0.55 | 0.13 | 0.81 | −94.1 | 0.25 | 0.76 | 0.56 (0.24–1.30) | 0.18 | <0.01 |
|
| ||||||||||
| ≥2% | 337 | −0.46 | <0.01 | −120.5 | <0.01 | 2.13 (1.50–3.02) | <0.01 | |||
|
| ||||||||||
| BMI | ||||||||||
|
| ||||||||||
| <25 | 302 | −0.55 | <0.01 | 0.44 | −126.7 | <0.01 | 0.36 | 1.46 (1.00–2.12) | 0.05 | 0.03 |
|
| ||||||||||
| ≥25 | 117 | −0.30 | 0.27 | −61.0 | 0.32 | 3.17 (1.73–5.80) | <0.01 | |||
|
| ||||||||||
| Dust mite sIgE | ||||||||||
|
| ||||||||||
| Negative | 213 | −0.14 | 0.50 | 0.01 | −48.4 | 0.29 | 0.03 | 2.15 (1.34–3.44) | <0.01 | 0.43 |
|
| ||||||||||
| Positive (>0.35 KUA/mL) | 195 | −0.87 | <0.01 | −191.7 | <0.01 | 1.66 (1.06–2.60) | 0.03 | |||
|
| ||||||||||
| Total IgE | ||||||||||
|
| ||||||||||
| <700 IU/mL | 384 | −0.51 | <0.01 | 0.52 | −75.5 | 0.02 | <0.01 | 1.66 (1.19–2.31) | 0.23 | 0.75 |
|
| ||||||||||
| ≥700 IU/mL | 35 | −0.14 | 0.79 | −504.6 | <0.01 | 4.16 (1.41–12.3) | <0.01 | |||
|
| ||||||||||
| Prestudy unscheduled visit? | ||||||||||
|
| ||||||||||
| No | 91 | −0.20 | 0.53 | 0.30 | 46.4 | 0.50 | 0.01 | 1.11 (0.54–2.29) | 0.77 | 0.13 |
|
| ||||||||||
| Yes | 328 | −0.57 | <0.01 | −153.0 | <0.01 | 2.06 (1.45–2.93) | <0.01 | |||
OR, Odds ratio.
Effects are differences between intervention and control participants during the double blind (not including the 3-month wash-in period) for symptom days/2 weeks and ICS taken (budesonide μg/day equivalents) and ORs for exacerbations. The omalizumab group serves as the OR reference population, so a value greater than 1 indicates a greater reduction in exacerbations in the treatment group.
Indicates with-in subgroup effect.
Indicates between-group interactions and were adjusted for baseline levels.
ICATA medication treatment steps are as follows: step 1, budesonide DPI 180 μg daily; step 2, budesonide DPI 80 μg twice daily; step 3, budesonide DPI 360 μg twice daily; step 4, Advair 250 μg/50 μg twice daily; step 5, Advair 250 μg/50 μg twice daily plus montelukast daily; step 6, Advair 500 μg/50 μg twice daily plus montelukast daily.
Table III summarizes the association of prespecified biomarkers with the efficacy of omalizumab; cut points for these subgroup analyses were set a priori. Participants with exhaled nitric oxide ≥20 ppb, blood eosinophils ≥2%, and body mass index (BMI; calculated as weight divided by height; kg/m2) ≥25 were more likely to benefit from omalizumab, based on exacerbation measures.
DISCUSSION
A significant finding in the ICATA study12 was the marked reduction in seasonal asthma exacerbations experienced by the omalizumab-treated group. In a post hoc analysis, the average monthly rate of exacerbations nearly doubled in the placebo group during the fall and spring compared with summer (9.0% and 8.1%, respectively, vs 4.6%; P < .001). This seasonal spike in exacerbations was not observed in the omalizumab group (4.3% in fall and 4.2% in spring vs 3.3% in summer), and the difference between the treatment groups was significant (P for interaction < .001). Although this effectiveness of omalizumab was noted across the study population as a whole, subgroup analysis found that participants both sensitized and exposed to cockroach allergen had the greatest benefit. This article presents further post hoc analyses to describe the pharmacodynamics and other biomarkers of response for omalizumab in the enrolled inner-city children and adolescents.
First, one-third of participants suitable for omalizumab therapy according to the clinical entry criteria of the ICATA trial, aeroallergen sensitivity, and asthma symptoms and history were ineligible for dosing on the basis of the body weight/serum IgE dosing table restrictions. This observation was in the face of utilization of an expanded dosage table. This ineligibility was primarily driven by high serum IgE (>1300 IU/mL), with and without higher body weight, a highly atopic and clinically relevant population.
In the ICATA study,12 the first 12 weeks of the double-blind phase served as a wash-in period, and data were not included in the analysis to make sure that enough time was provided for omalizumab to achieve maximum effect, based on the observations of Bousquet et al.16 Omalizumab is absorbed slowly after subcutaneous administration, reaching peak serum concentrations after a mean of 7 to 8 days. Clearance is slow (mean, 2.4 ± 1.1 mL/kg/day) with a terminal half-life estimated to be 26 days. No clinically important changes in the pharmacokinetics of omalizumab have been observed as a result of differences of age, sex, or race.18
Unexpectedly, this secondary analysis of ICATA suggests onset of effect sooner than previously reported. Omalizumab treatment reduced both asthma symptoms and exacerbations within the first 30 days, with improvement maintained throughout the 48 weeks of the study. The offset of effect of 1 to 4 months was consistent with previous observations.
Previous published studies of omalizumab in the treatment of allergic asthma in children, adolescents, and young adults have been somewhat limited,6,13 thus the importance of the ICATA study.12 The post hoc analyses presented here suggest that efficacy for exacerbations and ICS treatment is comparable in children 6 to 12 years of age compared with older children (>12 years). Our data suggest that omalizumab may be efficacious in both severe disease (steps 5–6 treatments) and more moderate disease (steps 1–4). Certain subgroups of persons, for example, those with higher exhaled nitric oxide, blood eosinophils, and BMI were more likely to benefit from omalizumab according to the secondary analysis. Confirmation of these findings could be useful in additional trials, particularly with pharmacoeconomic outcomes and in populations beyond the inner-city cohort we enrolled in ICATA, to validate their use in clinical studies to individualize therapy.
In summary, this secondary analysis of the primary National Institute of Allergy and Infectious Diseases ICATA study provides some new insights for inner-city children and adolescents with persistent allergic asthma. Omalizumab reduces both asthma symptoms and exacerbations rapidly. Predictors of clinical efficacy can be identified to guide patient selection.
What is already known about this topic?
Omalizumab is recommended for patients with persistent allergic asthma inadequately controlled on higher treatment steps. Omalizumab reduces exacerbations, symptoms, and, in some patients, the dose of inhaled and oral corticosteroids needed to maintain asthma control.
What does this article add to our knowledge?
This study provides new insights on dosing information and interpretation of onset and offset of omalizumab efficacy for children/adolescents who qualify for treatment on the basis of our defined entry criteria. Predictors of efficacy are presented.
How does this study impact current management guidelines?
This study suggests that the onset of efficacy of omalizumab is sooner than previously reported. Omalizumab treatment reduced both asthma symptoms and exacerbations within the first 30 days of treatment.
Acknowledgments
This project was funded in whole or in part by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health (contracts NO1-AI-25496 and NO1-AI-25482); the National Center for Research Resources, National Institutes of Health (grants M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, 5M01RR020359-04, UL1RR031988, 1UL1RR025780, and UL1 RR024982); Novartis Pharmaceuticals, under a clinical trial agreement with the University of Wisconsin-Madison, Dey Pharma (which provided EpiPens), and SC Johnson (which provided household pest control).
Abbreviations used
- BMI
Body mass index
- EPR-3
Expert Panel Report 3
- FDA
Food and Drug Administration
- ICATA
Inner-City Anti-IgE Therapy for Asthma
- ICS
Inhaled corticosteroids
Footnotes
Cite this article as: Sorkness CA, Wildfire JJ, Calatroni A, Mitchell HE, Busse WW, O’Connor GT, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol: In Practice 2013;163–71. http://dx.doi.org/10.1016/j.jaip.2013.01.011.
Conflicts of interest: C. A. Sorkness has received research support from Novartis. W. W. Busse is on the Merck advisory board; has received consultancy fees from Amgen, AstraZeneca, Novartis, GlaxoSmithKline, MedImmune, and Genentech; and has received research support from the National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases (NIAID), and the National Heart, Lung, and Blood Institute (NHLBI). J. A. Pongracic has received research support from the NIAID. M. Kattan has received research support from the NIH. W. J. Morgan has received consultancy fees from Genentech and the Cystic Fibrosis Foundation and has received research support from the NIH-NHLBI Asthma Net and the Cystic Fibrosis Foundation. S. J. Teach has received research support from the NIH-NIAID, NIH-NHLBI, and NIH—National Center for Research Resources (NCRR) and has received consulting fees from the Merck Childhood Asthma Network. A. H. Liu has received speaker’s honoraria from Merck and is on the GlaxoSmithKline safety monitoring board. S. J. Szefler has received consulting fees from GlaxoSmithKline, Genentech, Merck, Schering, Boehringer Ingelheim, and Novartis and has received research support from the NIH-NHLBI Asthma Clin Res Network, NIH-NIAID Inner City Asthma Consortium, NIH-NHLBI Asthma Net, National Institute of Environmental Health Sciences/Environmental Protection Agency Childhood Environmental Health Center, NIH-NHLBI Childhood Management Program, and NHLBI Childhood Asthma Research and Education. The other authors declare that they have no relevant conflicts.
References
- 1.National Heart, Lung, and Blood Institute. [Accessed December 1, 2011];Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma Full Report 2007. 2007 Aug 28; Available from: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 2.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 3.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–34. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–16. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 8.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan WJ, Rangsithienchai PA, Wood RA, Rivard D, Chinratanapisit S, Perzanowski MS, et al. Pest and allergen exposure and abatement in inner-city asthma: a work group report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee. J Allergy Clin Immunol. 2010;125:575–81. doi: 10.1016/j.jaci.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 14.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 15.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–86. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 17.Gabler NB, Duan N, Liao D, Elmore JG, Ganiats TG, Kravitz RL. Dealing with heterogeneity of treatment effects: is the literature up to the challenge? Trials. 2009;10:43. doi: 10.1186/1745-6215-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holgate S, Buhl R, Bousquet J, Smith N, Panahloo Z, Jimenez P. The use of omalizumab in the treatment of severe allergic asthma: a clinical experience update. Respir Med. 2009;103:1098–113. doi: 10.1016/j.rmed.2009.03.008. [DOI] [PubMed] [Google Scholar]