Abstract
Data regarding the ventilatory response to exercise in adolescents with mild-to-moderate cystic fibrosis (CF) are equivocal. This study aimed to describe the ventilatory response during a progressive cardiopulmonary exercise test (CPET) up to maximal exertion, as well as to assess the adequacy of the ventilatory response for carbon dioxide (CO2) exhalation. Twenty-two adolescents with CF (12 boys and 10 girls; mean ± SD age: 14.3 ± 1.3 years; FEV1: 78.6 ± 17.3% of predicted) performed a maximal CPET. For each patient, data of a sex- and age matched healthy control was included (12 boys and 10 girls; mean ± SD age: 14.3 ± 1.4 years). At different relative exercise intensities of 25%, 50%, 75%, and 100% of peak oxygen uptake (VO2peak), breathing pattern, estimated ventilatory dead space ventilation (VD/VT ratio), minute ventilation (VE) to CO2 production relationship (VE/VCO2-slope), partial end-tidal CO2 tension (PETCO2), and the VE to the work rate (VE/WR) ratio were examined. VO2peak was significantly reduced in CF patients (P = 0.01). We found no differences in breathing pattern between both groups, except for a significantly higher VE at rest and a trend towards a lower VE at peak exercise in patients with CF. Significantly higher values were found for the estimated VD/VT ratio throughout the CPET in CF patients (P < 0.01). VE/VCO2-slope and PETCO2 values differed not between the two groups throughout the CPET. VE/WR ratio values were significantly higher in CF during the entire range of the CPET (P < 0.01). This study found an exaggerated ventilatory response (high VE/WR ratio values), which was adequate for CO2 exhalation (normal VE/VCO2-slope and PETCO2 values) during progressive exercise up to maximal exhaustion in CF patients with mild-to-moderate airway obstruction.
Keywords: Pulmonary physiology, Ventilation, Breathing pattern, Children
Background
Peak oxygen uptake (VO2peak) is reported to be limited in patients with cystic fibrosis (CF) (Bongers et al. 2012; Hjeltnes et al. 1984; Keochkerian et al. 2008; Shah et al. 1998; Wideman et al. 2009). This reduction seems to have a multifactorial cause (Selvadurai et al. 2002). Respiratory, cardiovascular, as well as peripheral muscle function are reported as potential exercise limiting mechanisms (Almajed and Lands 2012). In patients with mild to moderate pulmonary disease, non-pulmonary factors, such as low muscle mass, impaired skeletal muscle function and centrally mediated oxygen delivery, seem to predominate in limiting exercise capacity (Regnis et al. 1996; Moorcroft et al. 2005; Saynor et al. 2014). In more severe patients with CF (forced expiratory volume in one second (FEV1) <60% of predicted), ventilatory constraints and impaired gas exchange become more important determinants.
Due to continuous airflow obstruction, as reflected by a decreased FEV1 and dynamic hyperinflation, adolescents with CF have been described to develop a rapid shallow breathing pattern at rest (Hart et al. 2002) and during exercise (Keochkerian et al. 2005). This can be accompanied with a decreased ventilatory capacity and concomitant reduced VO2peak (Keochkerian et al. 2008). Children and adolescents with CF with static hyperinflation at rest (residual volume to total lung capacity ratio (RV/TLC) >30%) seem to be more prone to a ventilatory limitation during exercise, which appears to be associated with decreased exercise performance (Sovtic et al. 2013; Werkman et al. 2010).
A recent study found that exercise limitation in adult patients with CF is multifactorial and that it was dominantly correlated with FEV1 and nutritional and inflammatory status, but also with the magnitude of the overall ventilatory response during exercise (Pastré et al. 2014). In patients with severe airway obstruction (FEV1 < 50% of predicted), multivariate analysis revealed the FEV1 to be a significant independent predictor of exercise capacity, whereas the ratio between minute ventilation and carbon dioxide exhalation (VE/VCO2 ratio) at peak exercise was the major determinant of exercise limitation in patients with mild-to-moderate disease (FEV1 > 50% of predicted) (Pastré et al. 2014). However, knowledge about the ventilatory response to exercise in adolescent patients with mild to moderate CF is ambiguous and inconclusive. Several studies in mild-to-moderate adolescents with CF describe an exaggerated ventilatory response with a rapid shallow breathing pattern at rest (Hart et al. 2002) and during exercise (Keochkerian et al. 2008). On the contrary, a recent study in adolescents with mild CF did not find any evidence for a different ventilatory response and/or rapid shallow breathing pattern during exercise (Borel et al. 2014). As the two studies demonstrating exaggerated ventilatory responses were performed in adolescents with CF with lower FEV1 values, this suggests that the ventilatory response to exercise is at least partially affected by the degree of airway obstruction.
Moreover, questions can be raised whether the adopted rapid shallow breathing pattern is beneficial as higher breathing frequencies seem to increase ventilatory dead space ventilation (VD/VT ratio), as has been reported in patients with CF waiting for lung transplantation (Thin et al. 2004). However, adding additional dead space volume during exercise in patients with mild CF lung disease had no influence on VO2peak and the duration of the exercise test, and it even increased ventilation which was attributed to an increased tidal volume with no change in respiratory rate (Dodd et al. 2006). The increased dead space volume was accompanied by higher VE/VCO2 ratios during exercise (Dodd et al. 2006). This finding in patients with mild CF suggests that the ventilatory response during exercise in adolescents with CF might differ with healthy controls and that this might alter during the course of the disease. Unfortunately the recent study of Borel et al. (2014), which focussed on ventilation during the entire range of exercise, only mentioned mechanical constraints influencing ventilation and did not discuss metabolic issues related to ventilation. Moreover, they included small and unequal groups of only prepubertal children.
As lung function decreases over time in most patients with CF, exercise capacity eventually becomes limited by the lungs reaching their mechanical limits to expand (approximately at FEV1 values ≤60%pred) (Almajed and Lands 2012). Insight in the breathing pattern during progressive exercise in adolescents with CF in a broad spectrum of lung function deterioration, as well as the adequacy of this ventilatory response for carbon dioxide (CO2) exhalation, is clinically relevant for future therapeutic interventions. Therefore, the aim of the current study was 1) to describe the ventilatory response during a progressive cardiopulmonary exercise test (CPET) up to maximal exertion in adolescents with mild-to-moderate CF and 2) to assess the adequacy of the ventilatory response for CO2 exhalation (determined from partial end-tidal carbon dioxide tension (PETCO2)) throughout the CPET in mild-to-moderate patients with CF. Our hypothesis is that adolescents with CF develop an obstructive breathing pattern, combined with a relatively large VD/VT ratio, which limit CO2 wash out.
Methods
Participants
Exercise data of twenty-two adolescents (12 boys and 10 girls from 12 to 17 years of age, mean ± SD age: 14.3 ± 1.3 years) with mild-to-moderate CF were randomly selected from the exercise database from the CF Center at the University Medical Center Utrecht. The database contained anonymous patient data of anthropometry, lung function and exercise capacity which was measured as part of usual care at the routine annual check-up. Therefore, all patients were free from acute pulmonary or gastrointestinal exacerbation at the time of testing. Testing procedures used in this study met the assumptions for standard of practice for the routine care of patients with CF. For each patient with CF, an age, sex and anthropometrically matched healthy control who performed a maximal CPET in our hospital retrospectively was selected (untrained and normal physical activity level). All participants and their guardians provided approval for inclusion of the data in research studies. After evaluation, the medical ethical committee of the University Medical Center Utrecht determined that inclusion of the data conformed to the regulations of the Dutch CF Registration and that inclusion of the data in this study met the ethical polices of the University Medical Center Utrecht, as well as the regulations of the Dutch government.
Anthropometric measures
Body mass and body height were determined using an electronic scale (Seca, Hamburg, Germany) and a stadiometer (Ulmer Stadiometer, Ulm, Germany) respectively. Body mass index (BMI) was calculated as the body mass in kilograms divided by the square of the body height in meters. Standard deviation (SD) scores were calculated for body height for age, body mass for age, and BMI for age using Dutch normative values (Fredriks et al. 2000). The equation of Haycock et al. (1978), validated in infants, children, and adults, was used to obtain the participants’ body surface area (BSA).
Spirometry and plethysmography
In the patients with CF, spirometry and plethysmography were performed by qualified lung function analysts of the CF Center at the University Medical Center Utrecht. Since the healthy controls were not known with any disease, spirometry and plethysmography was not performed in this group. In order to prevent the potential influence of bronchial reactivity during exercise, spirometry and plethysmography were performed after bronchodilation with salbutamol (800 μg). FEV1 was obtained from flow volume curves (Masterscreen, Jaeger, Würzburg, Germany). Residual volume (RV) and total lung volume (TLC) were determined in a body plethysmograph (Master Lab system, Jaeger, Würzburg, Germany). The RV was expressed as a percentage of TLC (RV/TLC ratio) as well. In order to improve comparative possibilities with the reports of other studies in CF, we used the commonly used reference values of Zapletal et al. (1987) to express lung function values as percentage of predicted values.
Cardiopulmonary exercise test
All participants completed a maximal CPET using an electronically braked cycle ergometer (Jaeger Physis, Carefusion, Houten, The Netherlands) after bronchodilation with salbutamol. After three minutes of rest measurements, cycling started at a workload of 0 W. Then, the work rate (WR) was linearly incremented with a 15 W∙min−1 ramp protocol (Godfrey 1974) until the patient stopped due to volitional exhaustion, despite strong verbal encouragement. The test effort was considered maximal when the participant showed objective (heart rate (HR) at peak exercise (HRpeak) >180 beats · min−1 and/or a respiratory exchange ratio (RER) at peak exercise (RERpeak) >1.0) (Armstrong and Welsman 2008) and subjective (unsteady biking, sweating, facial flushing, and clear unwillingness to continue despite encouragement) signs of maximal effort.
Throughout the CPET, participants breathed through a facemask (dead space volume 63 or 72 mL, dependent on size) (Hans Rudolph Inc, Kansas City, MO) that was connected to a calibrated metabolic cart (Oxycon Pro, Carefusion, Houten, the Netherlands). Gas analyzers were calibrated using gases of known concentration, whereas the flow meter was calibrated using a three-liter syringe (Hans Rudolph Inc, Kansas City, MO). Expired gas passed through a flow meter and a gas analyzer connected to a computer, which calculated breath-by-breath minute ventilation (VE), oxygen uptake (VO2), VCO2 and RER from conventional equations. Output from the flow meter and gas analyzers were averaged over ten-second intervals and stored for further use. Relative VO2peak (VO2peak/kg) was calculated by dividing VO2peak by body mass. HR was monitored continuously by a three-lead electrocardiogram (Hewlett-Packard, Amstelveen, Netherlands) and transcutaneous O2 saturation at the index finger was measured by pulse oximetry (Nellcor 200 E, Nellcor, Breda, the Netherlands). Peak exercise parameters were defined as the highest values achieved within the last 30 seconds prior to exhaustion.
Data analysis
The ventilatory threshold was defined as the point at which the ventilatory equivalent for oxygen and the partial end-tidal oxygen tension reached a minimum and thereafter began to rise in a consistent manner, coinciding with an unchanged ventilatory equivalent for carbon dioxide and a peak in the PETCO2 course (American Thoracic Society, American College of Chest Physicians 2003; Ohuchi et al. 1996). When this ventilatory equivalents method appeared to provide uncertain results for a participant’s ventilatory threshold (n = 4, 18%, in the CF patients, n = 0 in the healthy controls), the point at which the linear slope of the relation between the VCO2 and VO2 changed was taken as the ventilatory threshold, according to the V-slope method (Beaver et al. 1986). The ventilatory threshold (VO2) was expressed as an absolute value, relative value (VO2 normalized for body mass) and as a percentage of the attained VO2peak. The estimated VD/VT ratio was calculated by using the PETCO2. The graphical presentation of VE as a function of VCO2 during the progressive CPET was used to determine the point at which VE increased out of proportion to VCO2, the respiratory compensation point.
Next to resting values, tidal volume, breathing frequency, VE, VD/VT ratio, and PETCO2 were determined as an average of 30 seconds at different exercise intensities of 25%, 50%, 75% and 100% of VO2peak. Both VE and tidal volume were adjusted for body mass as well. To allow for fair comparison, resting VO2 was subtracted from VO2peak. Subsequently, 25%, 50%, 75% and 100% of this delta VO2 was calculated and summed with the resting VO2 for each participant (CF patients and healthy controls). Furthermore, we calculated the VE to VO2 relationship (VE/VO2-slope) and the VE to VCO2 relationship (VE/VCO2-slope) at the same different exercise intensities by linear regression of the exercise data up to 25%, 50%, 75%, and 100% of VO2peak using the least squares approach. We examined the participants’ response of the VE to the WR (VE/WR ratio) at similar intensities. Data from the first minute of exercise were excluded since the breathing pattern during the first minute of exercise frequently appears to be unstable.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, version 15.0; SPSS Inc., Chicago, IL) was used for the data-analysis. Shapiro-Wilk tests for normality were performed in order to evaluate the data distribution of each variable. Differences between adolescents with CF and their healthy counterparts in anthropometry and in exercise data at different exercise intensities were examined with Mann–Whitney U tests. Data are presented as mean values ± SD. A P-value <0.05 was considered as statistically significant.
Results
Anthropometric data for the patients with CF and the healthy controls are presented in Table 1, with no significant anthropometric between-group differences. Lung function characteristics of the adolescents with CF are also shown in Table 1. Patients had mild-to-moderate airway obstruction (mean FEV1 expressed as a percentage of predicted of 79 ± 17%) and a mild-to-moderate degree of static hyperinflation (mean RV/TLC ratio of 34 ± 9%). More specifically, 14 patients (5 boys and 9 girls) had an absolute RV/TLC ratio greater than 30%, which suggests static hyperinflation (Eid et al. 2000).
Table 1.
Participant characteristics
| Healthy (n = 22) | CF (n = 22) | P-value | |
|---|---|---|---|
| Sex (boy/girl) | 12/10 | 12/10 | NA |
| Age (years) | 14.3 ± 1.4 | 14.3 ± 1.3 | 0.99 |
| CF mutation class (I/II/IV/unknown)a | NA | 9/27/1/6 | NA |
| PA colonization (never/free of infection/intermittent/chronic)b | NA | 6/1/6/9 | NA |
| Body height (m) | 1.67 ± 0.10 | 1.65 ± 0.09 | 0.61 |
| Body height for age SDSc | 0.17 ± 0.86 | −0.18 ± 0.99 | 0.24 |
| Body mass (kg) | 53.9 ± 12.2 | 50.2 ± 7.2 | 0.31 |
| Body mass for age SDSc | 0.02 ± 0.83 | −0.37 ± 0.64 | 0.13 |
| BMI (kg · m−2) | 19.1 ± 2.6 | 18.5 ± 2.0 | 0.51 |
| BMI for age SDSc | −0.09 ± 0.80 | −0.34 ± 0.85 | 0.37 |
| BSA (m2) | 1.57 ± 0.22 | 1.54 ± 0.14 | 0.72 |
| FEV1 (L) | NA | 2.52 ± 0.67 | NA |
| FEV1 (%pred)d | NA | 78.6 ± 17.3 | NA |
| RV/TLC (%) | NA | 33.5 ± 9.3 | NA |
| VO2peak/kg (mL · kg−1 · min−1) | 49.1 ± 7.2 | 42.4 ± 8.7 | <0.01** |
Values are presented as mean ± SD.
Abbreviations: BMI body mass index, BSA body surface area, CF cystic fibrosis, CFTR cystic fibrosis transmembrane conductance regulator, FEV 1 forced expiratory volume in one second, NA not applicable, NS not statistically significant, RV/TLC residual volume to total lung capacity ratio, SDS standard deviation score.
**P < 0.01.
aBased on the classification of CFTR alleles used by Green et al. (2010).
bBased on the criteria of Lee et al. (2003).
cReference values of Fredriks et al. (2000).
dReference values of Zapletal et al. (1987).
All participants performed a maximal effort during the CPET (mean test duration of 623 ± 139 and 659 ± 194 seconds for the CF patients and healthy controls respectively), and the results are presented in Table 2. Compared with the healthy controls, adolescents with CF attained significantly lower values for HRpeak, peak WR (WRpeak), WRpeak/kg, VO2peak, VO2peak/kg, VO2peak/m2 (normalized for BSA), peak VCO2 (VCO2peak), VCO2peak/kg, and peak VE (VEpeak) normalized for body mass (VEpeak/kg), whereas they attained significantly higher values for the estimated VD/VT ratio at peak exercise, the VE/VO2-slope up to the ventilatory threshold, PETCO2 at rest, and the VE/WR ratio at peak exercise.
Table 2.
Exercise performance in the healthy adolescents and adolescents with CF
| Healthy (n = 22) | CF (n = 22) | P-value | |
|---|---|---|---|
| Maximal values | |||
| HRpeak (beats · min−1) | 192 ± 7 | 186 ± 8 | 0.02* |
| RERpeak | 1.15 ± 0.08 | 1.19 ± 0.09 | 0.16 |
| WRpeak (W) | 222 ± 62 | 164 ± 32 | <0.01** |
| WRpeak/kg (W · kg−1) | 4.2 ± 0.6 | 3.3 ± 0.5 | <0.001*** |
| VO2peak (mL · min−1) | 2638 ± 685 | 2126 ± 516 | 0.01* |
| VO2peak/kg (mL · kg−1 · min−1) | 49.1 ± 7.2 | 42.4 ± 8.7 | <0.01** |
| VO2peak/BSA (mL · m2 · min−1) | 1667 ± 259 | 1378 ± 274 | <0.01** |
| VCO2peak (mL · min−1) | 2963 ± 871 | 2409 ± 540 | 0.03* |
| VCO2peak/kg (mL · kg−1 · min−1) | 54.9 ± 8.9 | 48.1 ± 9.7 | 0.02* |
| VE @ VO2peak (L · min−1) | 89.5 ± 27.2 | 75.8 ± 18.8 | 0.08 |
| VE/kg @ VO2peak (L · kg−1 · min−1) | 1.7 ± 0.3 | 1.5 ± 0.3 | 0.04* |
| Absolute tidal volume @ VO2peak (mL) | 1736 ± 339 | 1616 ± 373 | 0.32 |
| Relative tidal volume @ VO2peak (mL · kg−1) | 32.7 ± 4.8 | 32.2 ± 6.4 | 0.98 |
| Breathing frequency @ VO2peak (breaths · min−1) | 51 ± 9 | 48 ± 9 | 0.25 |
| Estimated VD/VT ratio @ VO2peak (%) | 17 ± 2 | 21 ± 4 | <0.001*** |
| PETCO2 @ VO2peak (mmHg) | 36.3 ± 3.2 | 38.2 ± 3.7 | 0.10 |
| VE/WR ratio @ VO2peak (mL · min−1 · W−1) | 399 ± 57 | 476 ± 71 | <0.001*** |
| Submaximal values | |||
| Absolute ventilatory threshold (mL · min−1) | 1439 ± 381 | 1216 ± 253 | 0.07 |
| Relative ventilatory threshold (mL · kg−1 · min−1) | 27.1 ± 6.1 | 24.4 ± 5.1 | 0.13 |
| Ventilatory threshold (%VO2peak) | 55 ± 9 | 58 ± 9 | 0.32 |
| PETCO2 @ rest (mmHg) | 31.8 ± 2.4 | 33.5 ± 3.2 | 0.03* |
| PETCO2 @ the ventilatory threshold (mmHg) | 39.3 ± 3.6 | 40.0 ± 2.6 | 0.50 |
| VE/VO2-slope up to the ventilatory threshold | 20.5 ± 3.2 | 23.7 ± 5.1 | 0.02* |
| VE/VCO2-slope up to the respiratory compensation point | 26.6 ± 2.8 | 27.1 ± 2.9 | 0.60 |
Values are presented as mean ± SD.
Abbreviations: BSA body surface area, CF cystic fibrosis, HR peak peak heart rate, NS not statistically significant, P ET CO 2 partial end-tidal carbon dioxide tension, RER peak peak respiratory exchange ratio, VCO 2peak peak carbon dioxide output, VCO 2peak /kg peak carbon dioxide output normalized for body mass, VD/VT ratio physiological dead space ventilation, VE minute ventilation, VE/kg minute ventilation normalized for body mass, VE/VCO 2 -slope slope of the relationship between minute ventilation and carbon dioxide output, VE/VO 2 -slope slope of the relationship between minute ventilation and oxygen uptake, VE/WR minute ventilation to work rate ratio, VO 2peak peak oxygen uptake, VO 2peak /kg peak oxygen uptake normalized for body mass, WR peak peak work rate, WR peak /kg peak work rate normalized for body mass.
*P < 0.05; **P < 0.01; ***P < 0.001.
Breathing pattern components, tidal volume and breathing frequency, adopted during exercise did not differ significantly between patients with CF and healthy adolescents, except for a significantly higher breathing frequency at rest (19 ± 3 versus 22 ± 5 breaths · min−1; P = 0.02) and a trend for lower absolute tidal volume values at or near maximal exercise in patients with CF (1.74 ± 0.34 versus 1.62 ± 0.37 L; P = 0.32). Consequently, VE at rest was significantly higher (11.5 ± 2.1 versus 13.8 ± 3.4 L · min−1; P = 0.02), whereas VEpeak (75.8 ± 18.8 versus 89.5 ± 27.2 L.min−1; P = 0.08) tended to be lower in patients with CF. VE normalized for body mass (VE/kg) was significantly higher at rest (0.2 ± 0.05 versus 0.3 ± 0.06 L · kg−1 · min−1; P < 0.01) and at 25% of VO2peak (0.4 ± 0.07 versus 0.5 ± 0.08 L · kg−1 · min−1; P = 0.03) in the patients with CF. Values for breathing frequency divided by the tidal volume (rapid shallow breathing index) tended to be higher in patients with CF at rest (32 ± 13 versus 38 ± 13; P = 0.14) and at 25% of VO2peak (27 ± 10 versus 32 ± 15; P = 0.31). Furthermore, estimated VD/VT ratio values were significantly higher during all exercise intensities in patients with CF (see Figure 1, P < 0.01, P < 0.01, P < 0.01, and P < 0.001 at 25%, 50%, 75%, and 100% of VO2peak respectively).
Figure 1.

Changes in the estimated VD/VT ratio during exercise at similar percentages of VO 2peak in the healthy adolescents and the adolescents with CF. Dashed lines correspond to the ventilatory threshold in both groups. Abbreviations: CF = cystic fibrosis; VD/VT ratio = physiological dead space ventilation; VO2peak = peak oxygen uptake. **P < 0.01; ***P < 0.001.
Figure 2 shows significantly higher VE/VO2-slope values at 25% (P = 0.01) and 50% (P < 0.01) of VO2peak in patients with CF, whereas VE/VCO2-slope values differed not significantly between the two groups throughout the entire range of the CPET. Furthermore, Figure 2 demonstrates that, except for resting values (P = 0.03), PETCO2 values differed not significantly between the two groups during the CPET.
Figure 2.
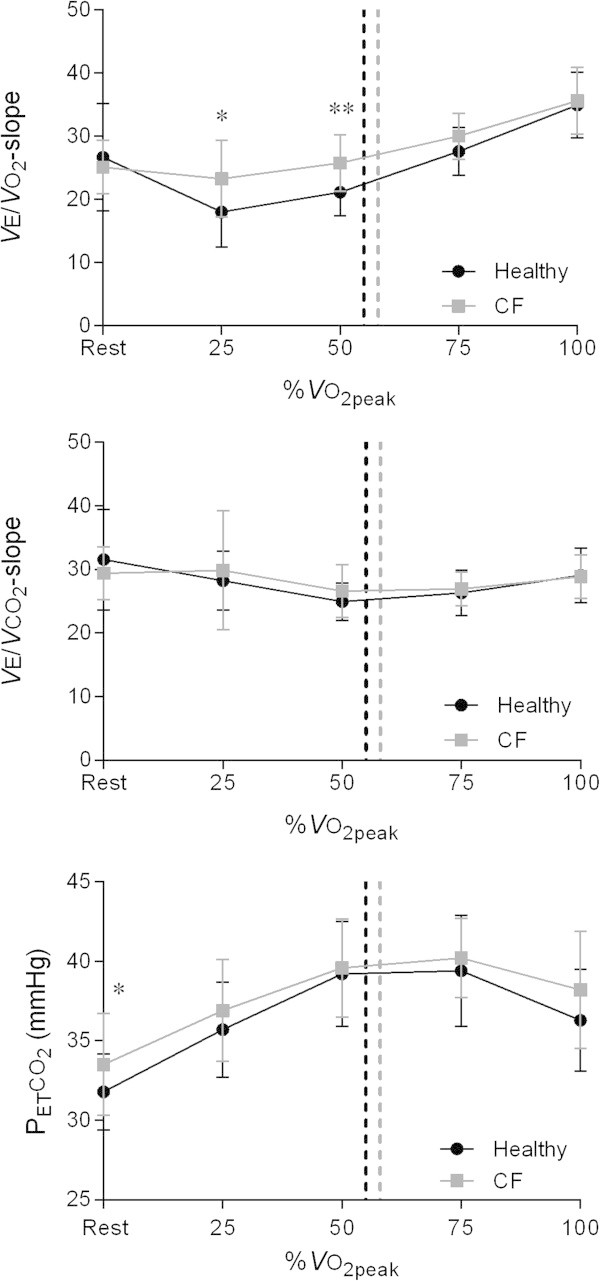
Changes in VE/ VO 2-slope, VE/ VCO 2 -slope, and P ET CO 2 during exercise at similar percentages of VO 2peak in the healthy adolescents and the adolescents with CF. Dashed lines represent the ventilatory threshold in both groups. Abbreviations: CF = cystic fibrosis; PETCO2 = partial end-tidal carbon dioxide tension; VE/VCO2-slope = slope of the relationship between minute ventilation and carbon dioxide output; VE/VO2-slope = slope of the relationship between minute ventilation and oxygen uptake; VO2peak = peak oxygen uptake. *P < 0.05; **P < 0.01.
Figure 3 represents the RER at rest and throughout progressive exercise in order to elucidate the higher VE/VO2 ratios during sub-maximal exercise. At rest and during sub-maximal exercise, patients with CF attained significantly higher RER values (P < 0.001, P < 0.001, P < 0.001, P < 0.01, and P = 0.26 at rest, 25%, 50%, 75%, and 100% of VO2peak respectively). The VE/WR ratio depicted in Figure 4 was significantly higher in CF during the entire range of the CPET (P < 0.01, P < 0.001, P < 0.001, and P < 0.001 at 25%, 50%, 75%, and 100% of VO2peak respectively).
Figure 3.

RER during exercise at similar percentages of VO 2peak in healthy adolescents and patients with CF. Vertical dashed lines correspond to the ventilatory threshold in both groups. Abbreviations: CF = cystic fibrosis; RER = respiratory exchange ratio; VO2peak = peak oxygen uptake. **P < 0.01; ***P < 0.001.
Figure 4.
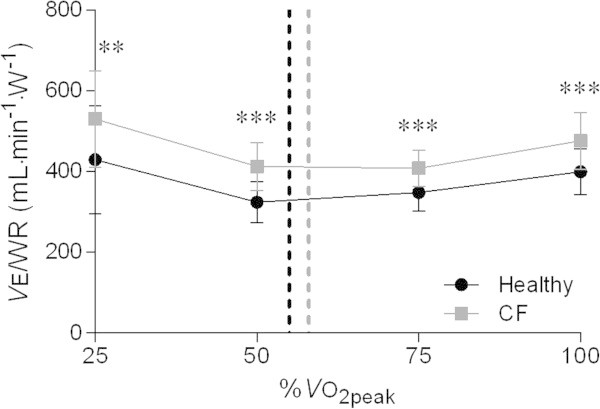
Changes in the VE / WR ratio during exercise at similar percentages of VO 2peak in healthy adolescents and patients with CF. Dashed lines correspond to the ventilatory threshold in both groups. Abbreviations: CF = cystic fibrosis; VE/WR = minute ventilation to work rate ratio; VO2peak = peak oxygen uptake. **P < 0.01; ***P < 0.001.
Discussion
The present study aimed to 1) describe the ventilatory response during a progressive CPET and 2) assess the adequacy of the ventilatory response for CO2 exhalation during exercise in mild-moderate adolescents with CF. First, we found an exaggerated ventilatory response during exercise. Second, this ventilatory response to exercise seems to be adequate for CO2 exhalation in patients with mild-to-moderate CF. The latter is illustrated by a similar course of the VE/VCO2-slope throughout exercise, higher RER values during sub-maximal exercise, and the ability to maintain PETCO2 values within normal limits throughout the entire range of the CPET.
The basic physiological factors that determine and modify the ventilatory response to exercise are: 1) CO2 output of the exercising muscles, 2) the arterial CO2 set-point, 3) the VD/VT ratio, and 4) the change in the arterial pressure of CO2 (PaCO2) during exercise (Wasserman et al. 1996). Several of these concepts are addressed to in this study. First, muscular CO2 output during (sub-)maximal exercise seems to be increased in patients with CF (Bongers et al. 2012; Hebestreit et al. 2005; Nguyen et al. 2014), which is illustrated in the current study by a higher RER. The higher RER in patients with CF is suggested to reflect a higher reliance on glucose oxidation to meet energy demands during exercise (Hebestreit et al. 2005; Nguyen et al. 2014). Altered substrate utilization in CF (de Meer et al. 1995; Moser et al. 2000; Selvadurai et al. 2003) might explain the increased RER at rest and for the lower exercise intensities. In addition, slowed VO2 kinetics during exercise and recovery in patients with CF might also be a possible explanation for an increased RER during sub-maximal exercise (Kusenbach et al. 1999; Massin et al. 2000; Pouliou et al. 2001; Hebestreit et al. 2005; Stevens et al. 2009; Saynor et al. 2014). This results in a greater dependency on glycolytic energy systems at lower exercise intensities resulting in an increased VCO2. Higher aerobic or anaerobic glycolytic energy expenditure during exercise increases ventilation as supported by a study in patients with familial mitochondrial myopathy (Heinicke et al. 2011). The current study showed an exaggerated VE relative to metabolic rate, indicated by high VE/VO2-slope values and VE/WR ratios, as well as an elevated RER, with no apparent signs of pulmonary insufficiency.
Second, the VD/VT was significantly higher at all exercise intensities in patients with CF, which seems to be exaggerated with disease progression (post-hoc analysis, data not shown). High VD/VT values during exercise have previously been reported in patients with CF (Cerny et al. 1982; Coates et al. 1988; Thin et al. 2004; Wilkens et al. 2010). This, in combination with the trend of higher PETCO2 in patients with CF, indicates an abnormal alveolar dead space ventilation (Wilkens et al. 2010), which might explain the relatively high VE values at rest and during sub-maximal exercise in patients with CF. Unfortunately, the exaggerated ventilatory response we found and the shift from oxidative to glycolytic energy metabolism, as shown by the attenuation of the RER slope at exercise intensities above 50% of VO2peak when compared to healthy controls, seems not to be able to totally compensate the limited exercise capacity in patients with CF (significantly reduced VO2peak/kg and WRpeak/kg). Our results are in agreement with the study of Borel et al. (2014) who found no effect of mild CF on breathing pattern and breathing strategy during an incremental CPET. Although breathing pattern and breathing strategy of adolescents with CF were comparable with healthy controls, most studies still report a reduced exercise capacity in adolescents with CF (Almajed and Lands 2012; Rand and Prasad 2012; Saynor et al. 2014). Saynor et al. (2014) recently suggested that centrally mediated oxygen delivery might be the principally limiting the aerobic function of pediatric CF patients with mild-to-moderate airway obstruction during ramp incremental cycling exercise. However, based on the results of the study of Keochkerian et al. (2008) on the ventilatory response in children with CF, one would still expect that CF could alter the ventilatory response during a progressive CPET. The current study demonstrated no significant effect of CF on the ventilatory response for CO2 exhalation (VE/VCO2-slope and PETCO2) to exercise in patients with CF with mild-to-moderate airway obstruction.
As described above, we found higher values for RER, VE/WR, and VE/VO2-slope values during sub-maximal exercise. These results suggest a higher ventilatory demand rather than a higher ventilatory response during sub-maximal exercise in patients with mild CF. An explanation for the absence of an impact of CF on the ventilatory response to exercise is the mild-to-moderate severity of the lung disease in our population. Keochkerian et al. (2008) reported that the more severe the airway obstruction, the more rapid and shallow the breathing pattern.
The current study has some limitations. Firstly, by categorizing exercise intensity by %VO2peak, there is no standardization of exercise intensity relative to the ventilatory response for each participant. However, standardizing for exercise intensity relative to VO2 (%VO2peak) was also done by other studies (Borel et al. 2014; Keochkerian et al. 2008). Since the ventilatory threshold occurred at a similar percentage of VO2peak in the pediatric CF patients and the healthy controls (58 ± 9% versus 55 ± 9%; P = 0.32), we believe that we did compare groups within the same physiological exercise intensity domain. Secondly, the sample size was relatively small and included mainly patients with CF with mild to moderate airflow obstruction. For this reason, these findings cannot be generalized to patients with severe airflow obstruction. Nevertheless, the current study sample is representative for the CF population in a tertiary CF Center. Thirdly, the estimated VD/VT ratio cannot be accurately predicted from the PETCO2 in patients with an increased VD/VT ratio due to lung disease (Wasserman et al. 2005), so caution must be taken with the interpretation of these results. Fourth, unfortunately we were not able to correct VO2peak for fat free mass as this variable was not routineously measured in patients with CF in the CF Center at the University Medical Center Utrecht. Finally, the used criteria for a maximal effort are subject to debate, especially in patients with CF. It has previously been demonstrated that traditional testing protocols and verification criteria significantly underestimate VO2peak in both healthy (Barker et al. 2011) and children with CF (Saynor et al. 2013). We did not verify the attainment of a true VO2peak with a supramaximal exercise testing procedure.
Implications and future research
As a higher ventilatory demand seems to be present during submaximal exercise in mild-moderate patients with CF, a small decline in ventilatory capacity might hamper the precarious balance between ventilation and homeostasis with further disease progression in patients with CF. The main findings presented in this study highlight the importance for the clinician to aim for attenuation of lung function decline even in patients with CF with a relatively preserved lung function (“normal” FEV1). For future research it would be interesting to evaluate the latter hypothesis in patients with CF with more severe airway obstruction. Moreover, the differences we found during the course of sub-maximal exercise highlight the importance to evaluate the submaximal exercise response as well when interpreting a CPET, and not just focus on peak exercise parameters.
Conclusions
The current study found an exaggerated, but adequate ventilatory response to exercise for CO2 exhalation in patients with CF with mild-to-moderate airway obstruction. The higher RER, VE/WR ratios, and VE/VO2-slope values during sub-maximal exercise point towards a higher ventilatory demand during sub-maximal exercise in patients with CF and mild-to-moderate lung disease.
Acknowledgements
Dr. Bongers and Dr. Werkman were supported by unconditional research grants from the Educational Foundation of the University Medical Center Utrecht (BB) and the Scientific Committee Physiotherapy of the Royal Dutch Society for Physiotherapy (MW).
Abbreviations
- BMI
Body mass index
- BSA
Body surface area
- CF
Cystic fibrosis
- CO2
Carbon dioxide
- CPET
Cardiopulmonary exercise test
- FEV1
Forced expiratory volume in one second
- HR
Heart rate
- HRpeak
peak heart rate
- PETCO2
Partial end-tidal carbon dioxide tension
- RER
Respiratory exchange ratio
- RERpeak
Respiratory exchange ratio at peak exercise
- RV
Residual volume
- RV/TLC ratio
Residual volume to total lung capacity ratio
- SD
Standard deviation
- TLC
Total lung capacity
- VCO2
Carbon dioxide production
- VCO2peak
Peak carbon dioxide production
- VD/VT ratio
Ventilatory dead space ventilation ratio
- VE
minute ventilation
- VEpeak
peak minute ventilation
- VE/VO2-slope
minute ventilation to oxygen uptake slope
- VE/VCO2 ratio
minute ventilation to carbon dioxide production ratio
- VE/VCO2-slope
minute ventilation to carbon dioxide production slope
- VE/WR
minute ventilation to work rate ratio
- VO2
Oxygen uptake
- VO2peak
peak oxygen uptake
- WR
Work rate
- WR
peak work rate.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BB participated in the design of the study, carried out data analysis and drafted the manuscript. MW carried out data analysis and drafted the manuscript. TT and EH participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Bart C Bongers, Email: b.c.bongers-3@umcutrecht.nl.
Maarten S Werkman, Email: mwerkman@dekinderkliniek.nl.
Tim Takken, Email: t.takken@umcutrecht.nl.
Erik H J Hulzebos, Email: h.hulzebos@umcutrecht.nl.
References
- Almajed A, Lands LC. The evolution of exercise capacity and its limiting factors in cystic fibrosis. Paediatr Respir Rev. 2012;13:195–199. doi: 10.1016/j.prrv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society, American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Welsman JR. Aerobic fitness. In: Armstrong N, van Mechelen W, editors. Paediatric Exercise Science and Medicine. Oxford: Oxford University Press; 2008. p. 101. [Google Scholar]
- Barker AR, Williams CA, Jones AM, Armstrong N. Establishing maximal oxygen uptake in young people during a ramp cycle test to exhaustion. Br J Sports Med. 2011;45:498–503. doi: 10.1136/bjsm.2009.063180. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Bongers BC, Hulzebos HJ, Arets HGM, Takken T. Validity of the oxygen uptake efficiency slope in children with cystic fibrosis and mild-to-moderate airflow obstruction. Pediatr Exerc Sci. 2012;24:129–141. doi: 10.1123/pes.24.1.129. [DOI] [PubMed] [Google Scholar]
- Borel B, Leclair E, Thevenet D, Beghin L, Gottrand F, Fabre C. Mechanical ventilatory constraints during incremental exercise in healthy and cystic fibrosis children. Pediatr Pulmonol. 2014;49:221–229. doi: 10.1002/ppul.22804. [DOI] [PubMed] [Google Scholar]
- Cerny FJ, Pullano TP, Cropp GJ. Cardiorespiratory adaptations to exercise in cystic fibrosis. Am Rev Respir Dis. 1982;126:217–220. doi: 10.1164/arrd.1982.126.2.217. [DOI] [PubMed] [Google Scholar]
- Coates AL, Canny G, Zinman R, Grisdale R, Desmond K, Roumeliotis D, Levison H. The effects of chronic airflow limitation, increased dead space, and the pattern of ventilation on gas exchange during maximal exercise in advanced cystic fibrosis. Am Rev Respir Dis. 1988;138:1524–1531. doi: 10.1164/ajrccm/138.6.1524. [DOI] [PubMed] [Google Scholar]
- de Meer K, Jeneson JA, Gulmans VA, van der Laag J, Berger R. Efficiency of oxidative work performance of skeletal-muscle in patients with cystic-fibrosis. Thorax. 1995;50:980–983. doi: 10.1136/thx.50.9.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JD, Barry SC, Gallagher CG. Respiratory factors do not limit maximal symptom-limited exercise in patients with mild cystic fibrosis lung disease. Respir Physiol Neurobiol. 2006;152:176–185. doi: 10.1016/j.resp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–358. doi: 10.1542/peds.105.2.354. [DOI] [PubMed] [Google Scholar]
- Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP. Body index measurements in 1996–7 compared with 1980. Arch Dis Child. 2000;82:107–112. doi: 10.1136/adc.82.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey S. Exercise testing in children: applications in health and disease. London: W.B. Saunders Company Ltd; 1974. [Google Scholar]
- Green DM, McDougal KE, Blackman SM, Sosnay PR, Henderson LB, Naughton KM, Collaco JM, Cutting GR. Mutations that permit residual CFTR function delay acquisition of multiple respiratory pathogens in CF patients. Respir Res. 2010;11:140. doi: 10.1186/1465-9921-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N, Polkey MI, Clément A, Boulé M, Moxham J, Lofaso F, Fauroux B. Changes in pulmonary mechanics with increasing disease severity in children and young adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:61–66. doi: 10.1164/rccm.2112059. [DOI] [PubMed] [Google Scholar]
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/S0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- Hebestreit H, Hebestreit A, Trusen A, Hughson RL. Oxygen uptake kinetics are slowed in cystic fibrosis. Med Sci Sports Exerc. 2005;37:10–17. doi: 10.1249/01.MSS.0000150065.97657.7B. [DOI] [PubMed] [Google Scholar]
- Heinicke K, Taivassalo T, Wyrick P, Wood H, Babb TG, Haller RG. Exertional dyspnea in mitochondrial myopathy: clinical features and physiological mechanisms. Am J Physiol Regul Integr Comp Physiol. 2011;301:R873–884. doi: 10.1152/ajpregu.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjeltnes N, Stanghelle JK, Skyberg D. Pulmonary function and oxygen uptake during exercise in 16 year old boys with cystic fibrosis. Acta Paediatr Scand. 1984;73:548–553. doi: 10.1111/j.1651-2227.1984.tb09969.x. [DOI] [PubMed] [Google Scholar]
- Keochkerian D, Chlif M, Delanaud S, Gauthier R, Maingourd Y, Ahmaidi S. Timing and driving components of the breathing strategy in children with cystic fibrosis during exercise. Pediatr Pulmonol. 2005;40:449–56. doi: 10.1002/ppul.20266. [DOI] [PubMed] [Google Scholar]
- Keochkerian D, Chlif M, Delanaud S, Gauthier R, Maingourd Y, Ahmaidi S. Breathing pattern adopted by children with cystic fibrosis with mild to moderate pulmonary impairment during exercise. Respiration. 2008;75:170–177. doi: 10.1159/000097772. [DOI] [PubMed] [Google Scholar]
- Kusenbach G, Wieching R, Barker M, Hoffmann U, Essfeld D. Effects of hyperoxia on oxygen uptake kinetics in cystic fibrosis patients as determined by pseudo-random binary sequence exercise. Eur J Appl Physiol Occup Physiol. 1999;79:192–196. doi: 10.1007/s004210050494. [DOI] [PubMed] [Google Scholar]
- Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Massin MM, Leclercq-Foucart J, Sacre JP. Gas exchange and heart rate kinetics during binary sequence exercise in cystic fibrosis. Med Sci Monit. 2000;6:55–62. [PubMed] [Google Scholar]
- Moorcroft AJ, Dodd ME, Morris J, Webb AK. Symptoms, lactate and exercise limitation at peak cycle ergometry in adults with cystic fibrosis. Eur Respir J. 2005;25:1050–1056. doi: 10.1183/09031936.05.00011404. [DOI] [PubMed] [Google Scholar]
- Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med. 2000;162:1823–1827. doi: 10.1164/ajrccm.162.5.2003057. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Obeid J, Baker JM, Takken T, Pedder L, Parise G, Timmons BW. Reduced fat oxidation rates during submaximal exercise in boys with cystic fibrosis. J Cyst Fibros. 2014;13:92–98. doi: 10.1016/j.jcf.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakajima T, Kawade M, Matsuda M, Kamiya T. Measurement and validity of the ventilatory threshold in patients with congenital heart disease. Pediatr Cardiol. 1996;17:7–14. doi: 10.1007/BF02505805. [DOI] [PubMed] [Google Scholar]
- Pastré J, Prévotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med. 2014;14:74. doi: 10.1186/1471-2466-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliou E, Nanas S, Papamichalopoulos A, Kyprianou T, Perpati G, Mavrou I, Roussos C. Prolonged oxygen kinetics during early recovery from maximal exercise in adult patients with cystic fibrosis. Chest. 2001;119:1073–1078. doi: 10.1378/chest.119.4.1073. [DOI] [PubMed] [Google Scholar]
- Rand S, Prasad SA. Exercise as part of a cystic fibrosis therapeutic routine. Expert Rev Respir Med. 2012;6:341–351. doi: 10.1586/ers.12.19. [DOI] [PubMed] [Google Scholar]
- Regnis JA, Donnelly PM, Robinson M, Alison JA, Bye PT. Ventilatory mechanics at rest and during exercise in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1418–1425. doi: 10.1164/ajrccm.154.5.8912758. [DOI] [PubMed] [Google Scholar]
- Saynor ZL, Barker AR, Oades PJ, Williams CA. A protocol to determine valid VO2max in young cystic fibrosis patients. J Sci Med Sport. 2013;16:539–544. doi: 10.1016/j.jsams.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Saynor ZL, Barker AR, Oades PJ, Williams CA. Impaired aerobic function in young cystic fibrosis patients during ramp exercise. Med Sci Sports Exerc. 2014;ᅟ:ᅟ. doi: 10.1249/MSS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- Selvadurai HC, McKay KO, Blimkie CJ, Cooper PJ, Mellis CM, Van Asperen PP. The relationship between genotype and exercise tolerance in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:762–765. doi: 10.1164/ajrccm.165.6.2104036. [DOI] [PubMed] [Google Scholar]
- Selvadurai HC, Allen J, Sachinwalla T, Macauley J, Blimkie CJ, Van Asperen PP. Muscle function and resting energy expenditure in female athletes with cystic fibrosis. Am J Respir Crit Care Med. 2003;168:1476–1480. doi: 10.1164/rccm.200303-363OC. [DOI] [PubMed] [Google Scholar]
- Shah AR, Gozal D, Keens TG. Determinants of aerobic and anaerobic exercise performance in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1145–1150. doi: 10.1164/ajrccm.157.4.9705023. [DOI] [PubMed] [Google Scholar]
- Sovtic AD, Minic PB, Kosutic J, Markovic-Sovtic GP, Gajic MB. Static hyperinflation is associated with decreased peak exercise performance in children with cystic fibrosis. Respir Care. 2013;58:291–297. doi: 10.4187/respcare.01946. [DOI] [PubMed] [Google Scholar]
- Stevens D, Oades PJ, Armstrong N, Williams CA. Early oxygen uptake recovery following exercise testing in children with chronic chest diseases. Pediatr Pulmonol. 2009;44:480–488. doi: 10.1002/ppul.21024. [DOI] [PubMed] [Google Scholar]
- Thin AG, Dodd JD, Gallagher CG, Fitzgerald MX, Mcloughlin P. Effect of respiratory rate on airway deadspace ventilation during exercise in cystic fibrosis. Respir Med. 2004;98:1063–1070. doi: 10.1016/j.rmed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Zhang YY, Riley MS. Ventilation during exercise in chronic heart failure. Basic Res Cardiol. 1996;91(Suppl. 1):1–11. doi: 10.1007/BF00810518. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Werkman MS, Hulzebos HJ, Arets HG, van der Net J, Helders PJ, Takken T. Is static hyperinflation a limiting factor during exercise in adolescents with cystic fibrosis? Pediatr Pulmonol. 2010;46:119–124. doi: 10.1002/ppul.21329. [DOI] [PubMed] [Google Scholar]
- Wideman L, Baker CF, Brown PK, Consitt LA, Ambrosius WT, Schechter MS. Substrate utilization during and after exercise in mild cystic fibrosis. Med Sci Sports Exerc. 2009;41:270–278. doi: 10.1249/MSS.0b013e318188449b. [DOI] [PubMed] [Google Scholar]
- Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, Aliverti A. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010;65:808–814. doi: 10.1136/thx.2009.131409. [DOI] [PubMed] [Google Scholar]
- Zapletal A, Samenek M, Paul T. Lung function in children and adolescents: methods, reference values. In: Herzog H, editor. Progress in respiration research. Basel: Karger; 1987. pp. 114–218. [Google Scholar]


