Abstract
The genotoxicity of a complex mixture [neutral fraction (NF)] from a wood preserving waste and reconstituted mixture (RM) mimicking the NF with seven major polycyclic aromatic hydrocarbons (PAHs) and benzo(a)pyrene (BaP) was investigated by determining DNA adducts and tumor incidence in male B6C3F1 mice exposed to 3 different doses of the chemical mixtures. The peak values of DNA adducts were observed after 24 h and the highest levels of PAH-DNA adducts were exhibited in mice administered NF+BaP, and the highest tumor incidence and mortality were also observed in this group. DNA adduct levels after 1, 7, or 21 d were significantly correlated with animal mortality and incidence of total tumors including liver, lung, and forestomach. However, only hepatic DNA adducts after 7 d significantly correlated with liver tumor incidence. Most proteins involved in DNA repair including ATM, pATR, Chk1, pChk1, DNA PKcs, XRCC1, FANCD2, Ku80, Mre11 and Brca2 were significantly lower in liver tumor tissue compared to non-tumor tissue. Expression of proteins involved in apoptosis and cell cycle regulation were also significantly different in tumor vs non-tumor tissues and it is possible that PAH-induced changes in these gene products are important for tumor development and growth.
Keywords: Polycyclic aromatic hydrocarbon mixtures, DNA adducts, Tumor incidence, Reverse Phase Protein Array, 32P-postlabeling assay, Protein expression
Introduction
Cancer is a major public health problem in United States and worldwide and it is estimated that 1,665,540 new cancer cases will be diagnosed and 585,720 deaths from cancer will occur in the United States in 2014 (Siegel et al. 2014). Epidemiological and experimental data show that some cancers are caused by environmental factors, including exposure to complex mixtures such as carcinogenic PAHs from cigarette smoke, air and water pollution. Several PAHs, including BaP, have been listed by the U.S. Environmental Protection Agency as probable human carcinogens (Agency 2003).
PAHs are precarcinogens that are converted into carcinogens by drug metabolizing enzymes and the resulting reactive metabolites alkylate DNA or induce DNA damage that play essential roles in the initiation phase of carcinogenesis (Hecht 2003; Wogan et al. 2004). For example, DNA adducts can cause miscoding events in critical genes (Hecht 2012). Many DNA adducts serve as biomarkers of carcinogenic PAH exposure and cancer risk (Phillips and Venitt 2012; Poirier 1997; Urban et al. 2012). Significant positive linear correlations between levels of PAH-DNA adducts and cancer incidence have been reported in both epidemiological studies (Agudo et al. 2012; Tang et al. 2001; Veglia et al. 2008) and animal experiments (Culp et al. 1998). However, negative correlations between PAH-DNA adducts and tumor incidence have also been observed (Saieva et al. 2011; Siddens et al. 2012) and the mechanic relationships between carcinogen-DNA adducts and tumor formation are still not fully understood.
Gene/protein expression involved in DNA repair, apoptosis, and cell cycle regulation play very important roles in chemical carcinogenesis (Henkler et al. 2012; Suzuki et al. 2012) and treatment with BaP alters expression of genes involved in xenobiotic metabolism, DNA repair, apoptosis, and cell cycle regulation (Hamouchene et al. 2011). Initial recognition of DNA adducts is a key event regulating DNA damage repair (Sugasawa 2011) and this includes nucleotide excision repair (NER). BaP-DNA adducts in liver and esophagus were significantly increased in DNA repair deficient mice compared to wild-type mice (John et al. 2012). It was reported that apoptosis may also be associated with decreased BaP adduct levels (Zhou et al. 2010) and failure to induce apoptosis in human bronchial epithelial cells exposed to cigarette smoke resulted in enhanced neoplastic transformation (Du et al. 2012).
PAH mixtures are known human carcinogens and the potency of several individual compounds has been defined in animal studies, however, minimal information is available on potential interactions of complex mixtures of PAHs. Mixture interactions may alter the absorption, distribution, metabolism or excretion of chemicals and some chemicals in PAH mixtures may either inhibit or promote chemical carcinogenesis. Therefore, these interactions may effectively increase or decrease the potential toxicity or genotoxicity of the mixture (Tarantini et al. 2011).
The current study investigated the genotoxicity and carcinogenicity of a single PAH---BaP, BaP plus a complex PAH mixture (NF) isolated from a wood preserving waste, and a reconstituted mixture (RM) containing BaP and six other major carcinogenic PAHs. The relationships between genotoxicity and carcinogenicity were studied and expression of proteins involved in DNA repair, apoptosis, and cell cycle regulation was also investigated in tumor and normal liver tissues.
Materials and Methods
Chemicals
Benz(a)anthracene (BaA), BaP, benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), chrysene, and dibenz(a,h)anthracene (DA)) were obtained from Sigma-Aldrich (St. Louis, MO). Indeno(1,2,3-c,d)pyrene (IP) was purchased from Absolute Standards (Hamden, CT). Materials for DNA extraction (Gupta 1984; Randerath et al. 1981; Reddy and Randerath 1986; Zhou et al. 1999), and 32postlabeling analysis (Phillips and Arlt 2007; Randerath et al. 1999; Reddy and Randerath 1986; Zhou et al. 2004) have been reported previously.
Extraction and PAH fractionation from complex chemical mixture
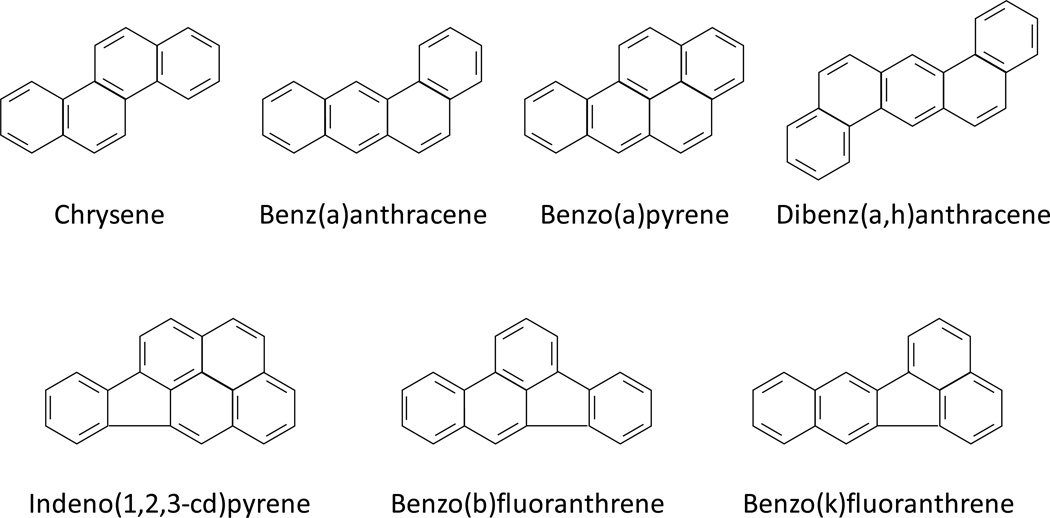
Wood preserving waste (WPW), a non-aqueous phase liquid was initially collected from a contaminated aquifer beneath a wood treatment plant. A liquid-liquid separation technique was used to separate this material into acid, base, and neutral fractions following the USEPA 3650B method ((EPA) 1996). A 30 ml volume of WPW oil, 60 ml of methylene chloride (CH2Cl2), and 60 ml sodium hydroxide (NaOH), pH 12, were added to a 2,000 ml separating funnel and shaken for 2 min. Once phases were separated, the upper aqueous layer (acid fraction) was removed, and the lower organic layer (base/NF) was extracted two more times. One volume of base/NF, 3 volumes of 1N H2SO4 and 1.5 volumes of CH2Cl2 were shaken in a new separating funnel for 2 min. After the phases were separated, the organic phase was removed and aqueous phase was extracted one more time with CH2Cl2 (same volume as first extraction). The two organic layers were combined to form the NF. The NF contains major carcinogenic PAHs (Figure 1), including BaA, BaP, BbF, chrysene, BkF, DA, and IP.
Fig 1.
Chemical structures of the seven PAHs categorized as class B2 carcinogens according to the USEPA. These were present in the neutral fraction (NF) extracted from a wood preserving waste and were included in the reconstituted mixture (RM).
Preparation of a reconstituted mixture
Based on the composition of PAHs in the NF, seven major carcinogenic PAHs, which are defined as class B2 carcinogens by USEPA, were selected for a RM. The concentrations of each PAH in the RM were simulated to those in the NF. The final concentrations for animal treatment are listed in Table 1.
Table 1.
Quantities of PAHs used in mouse treatments
| Group | Chemical composition (µg/mouse) | |||||||
|---|---|---|---|---|---|---|---|---|
| BaP | BaA | Chrysene | BbF | BkF | DA | IP | ||
| Control | - | - | - | - | - | - | - | |
| BaP | Low | 50 | - | - | - | - | - | - |
| Middle | 125 | - | - | - | - | - | - | |
| High | 375 | - | - | - | - | - | - | |
| NF+BaP | Low | 50 | 0.85 | 0.85 | 0.34 | 0.21 | 0.02 | 0.11 |
| Middle | 125 | 2.12 | 2.12 | 0.84 | 0.52 | 0.04 | 0.28 | |
| High | 375 | 5.3 | 5.3 | 2.1 | 1.3 | 0.1 | 0.7 | |
| RM | Low | 50 | 0.62 | 0.77 | 0.34 | 0.21 | 0.02 | 0.10 |
| Middle | 125 | 1.56 | 1.92 | 0.84 | 0.52 | 0.04 | 0.24 | |
| High | 375 | 3.9 | 4.8 | 2.1 | 1.3 | 0.1 | 0.6 | |
Benzo(a)pyrene (BaP), benz(a)anthracene (BaA), chrysene, benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), dibenz(a,h)anthracene (DA), and indeno(1,2,3-c,d)pyrene (IP).
Chemical analysis of the complex mixture
The analysis of the PAHs in the sample extracts was accomplished using a modified USEPA Method 8270C (GC/MS-SIM) as reported previously (Cizmas et al. 2003). The quantification of the analytes was based on authentic standards and internal standards. The identification of the analytes was based on retention times and mass spectra (primary and confirmation ions).
Animals
B6C3F1 male mice (21 days old, 7–11 g) were obtained from Harlan Sprague Dawley (Houston, TX). Animals were housed in the Program for Animal Resources of the Institute of Biosciences and Technology, Texas A&M University System Health Science Center. All procedures were conducted with the approval of the Institutional Animal Care and Use Committee of the Texas A&M University System Health Science Center (Protocol Number: 06001-R1).
Animal experiments
Mice were housed in cages in a temperature and humidity controlled facility with a daily photoperiod of 12 h light and 12 h dark. Animals were acclimatized for a week to the new environment, and then the mice were randomly allocated to ten experimental groups with 35 mice for each group. Mice had free access to water and fresh diet was provided in clean food bowls daily. The animals were subjected to a single intraperitioneal (i.p.) injection with BaP or a mixture of seven PAHs. Table 1 shows doses of PAHs applied in animal experiments. These chemicals, except for BaP, and their concentrations were similar to the residues extracted from the dense non-aqueous phase liquid collected at a wood preserving waste (WPW) Superfund Site (Phillips 2006). These chemicals were dissolved in DMSO:Corn oil (50:50). The vehicle volume for ip injection was about 7 µl per g body weight. Five animals were sacrificed by CO2 asphyxiation and cervical dislocation at 1, 7, and 21 d after the PAH treatment. Five mice served as a control and therefore, were injected with the vehicle. Twenty to thirty mice for each group were maintained in the same environment for 280 d to observe tumor formation. Liver tissues were collected and were frozen in liquid nitrogen immediately and then stored at –8°C until extractions of DNA and protein. Part of each tissue was fixed in paraformaldehyde (4%) for histological examination.
Analyses of DNA adducts
DNA extraction and 32P-postlabeling analyses were performed as in previous experiments (Phillips and Arlt 2007; Zhou et al. 2005). DNA was isolated by solvent extraction combined with enzymatic digestion of protein and RNA (Gupta 1984; Randerath et al. 1981) and stored at –80°C until analysis. The nuclease P1-enhanced bisphosphate version of the 32P-postlabeling method (Reddy and Randerath 1986) was used for analysis, with modifications of the chromatographic conditions (Mabon et al. 1996). Briefly, DNA (10 µg) was enzymatically degraded to normal (Np) and modified (Xp) deoxyribonucleoside 3’-monophosphates with micrococcal nuclease and spleen phosphodiesterase at pH 6.0 and 37°C for 3.5 h. After treatment of the mixture with nuclease P1 to convert normal nucleotides to nucleosides, modified nucleotides (Xp) were converted to 5’-32P-labeled deoxyribonucleoside 3’,5’-bisphosphates (pXp) by incubation with carrier-free [γ-32P]ATP and polynucleotide kinase.
Radioactively labeled digests were applied to modified polyethyleneimine (PEI)-cellulose thin layers and chromatographed overnight (15–16 h) with 2.3 M sodium phosphate, pH 5.75 (D1), to purify bulky adducts. Labeled PAH-adducts retained in the lower (L, 2.8×1.0 cm) part of the D1 chromatogram were, after brief autoradiography on Cronex 4 X-ray film, each contact-transferred to individual acceptor sheets and resolved by two-dimensional thin-layer chromatography (TLC). The bulky DNA adducts were separated with 3.82 M lithium formate, 6.75 M urea, pH 3.35 and 0.72 M sodium phosphate, 0.45 M Tris–HCl, 7.65 M urea, pH 8.2 in the first (D3) and second (D4) dimensions, respectively. 32P-labeled DNA adducts were visualized by screen-enhanced autoradiography at -80°C using Kodak XAR-5 film or with the aid of an InstantImager (Packard Instruments) (Zhou et al. 1999). Appropriate blank count rates were automatically subtracted by the instrument from sample values. The extent of covalent DNA modification was calculated from corrected sample count rates, the amount of DNA assayed (expressed as pmol DNA monomer units or DNA-P), and the specific activity of [γ-32P]ATP according to the following formula:
Quantitative data represented minimum estimates because 100% recovery presumably was not achieved.
Reverse phase protein array (RPPA) assay
The assay of RPPA (Byers et al. 2012; Malinowsky et al. 2013) was performed in the Functional Proteomics Reverse Phase Protein Array Core Facility at MD Anderson Cancer Center, Houston, TX. Briefly, tissue was lysed with RPPA lysis buffer and diluted in five 2-fold serial dilutions in dilution buffer (lysis buffer containing 1% SDS). Serial diluted lysates were arrayed on nitrocellulose-coated FAST slides (Whatman, Inc, Piscataway, NJ) by an Aushon 2470 Arrayer (Aushon BioSystems, Billerica, MA). Each slide was probed with a validated primary antibody plus a biotin-conjugated secondary antibody. The signal was amplified using a Dako Cytomation–catalyzed system (Dako North America, Inc. Varpinteria, CA) and visualized by DAB colorimetric reaction. The slides were scanned, analyzed, and quantified using the customized-software Microvigene (VigeneTech Inc. North Billerica, MA) to generate spot intensity (He et al. 2012; Tibes et al. 2006).
Histological examination
At 280 days of age surviving mice were euthanized by CO2 asphyxiation, followed by cervical dislocation, and a number of tissues (liver, lung, and forestomach) examined first by gross necropsy and then fixed in 10% formalin. Fixed tissues were routinely processed to paraffin blocks, and hematoxylin and eosin-stained sections were analyzed by a board-certified histopathologist as previously described (Shorey et al. 2012).
Statistical analysis
The relationships between values of PAH-DNA adducts at 1, 7, and 21 d and the mortality and tumor incidence were analyzed by linear regression analysis (Zar 2009). Statistical analysis of the data from individual mice was performed by using the unpaired and paired Student’s t-tests (Zar 2009).
Results
Profiles and levels of hepatic PAH-DNA adducts
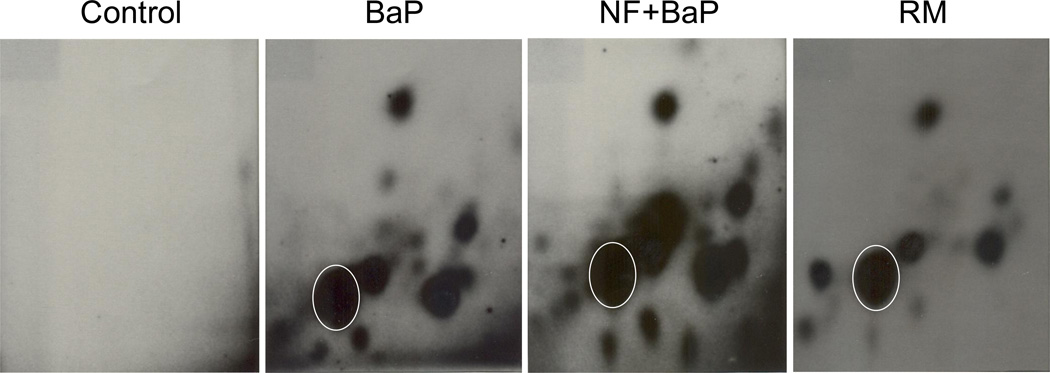
For acute experiments, animals were terminated at 1, 7, and 21 d after PAH treatment. Profiles and levels of hepatic DNA adduct formation were determined. Representative profiles of hepatic DNA adducts in mice treated with BaP, BaP+NF, or RM are displayed in Figure 2. Typical patterns of PAH-DNA adducts are exhibited in all three groups with only slight differences. Total levels of PAH-DNA adducts for all chemical groups at three time points, and three different doses are summarized in Table 2. PAH-DNA adducts formed quickly within one day after PAH treatment. High levels of adducts were observed after 24 h and they gradually decreased after 7 and 21 d. The NF+BaP groups showed higher levels of hepatic DNA adducts compared to the BaP or RM groups. Adduct levels at high doses were 81.6±23.4, 276.5±19.0, and 94.6±14.9 in 109 normal nucleotides, respectively, for the BaP, NF+BaP, and RM groups. Analysis of all nine treatment groups (three doses and three time points) and comparison of DNA adduct levels between the NF+BaP and RM treatment by paired-sample t-test (Zar 2009) showed that DNA adduct levels were significantly higher among the mice dosed with NF+BaP than those dosed with RM (P=0.05). DNA adducts were also detected in mice 280 d after treatment with PAHs, however, hepatic PAH-DNA adducts were not observed at this time point (data not shown).
Fig 2.
Typical representative profiles of 32P-postlabeled PAH-DNA adducts detected in the livers of B6C3F1 mice. Animals were terminated at 1 d after PAH treatments. The circled spot is the benzo(a)pyrene-7,8-dihyrodiol-9,10-epoxide deoxy-guanosine (BPDE-dG) adduct.
Table 2.
Total DNA adduct levels in liver of B6C3F1 male mice
| Chemical | Group | DNA adducts in 109 nucleotides* | ||
|---|---|---|---|---|
| 1 day | 7 days | 21 days | ||
| Control | CO:DMSO | ND** | ND** | ND** |
| BaP | Low | 64.6±6.5 | 25.0±2.2 | 19.3±1.9 |
| Middle | 74.9±10.8 | 74.1±34.8 | 26.7±3.4 | |
| High | 81.6±23.4 | 61.9±9.2 | 29.5±3.4 | |
| NF+BaP | Low | 45.4±2.3 | 27.9±1.8 | 30.4±2.8 |
| Middle | 85.1±14.5 | 76.9±6.5 | 40.3±5.9 | |
| High | 276.5±19.0 | 157.3±41.5 | 53.5±3.5 | |
| RM | Low | 31.3±3.7 | 27.1±3.4 | 25.5±3.6 |
| Middle | 42.1±11.2 | 36.5±4.5 | 33.8±5.2 | |
| High | 94.6±14.9 | 47.8±5.4 | 37.6±5.1 | |
Mean±SEM, n=4;
Not detectable.
Histological observations
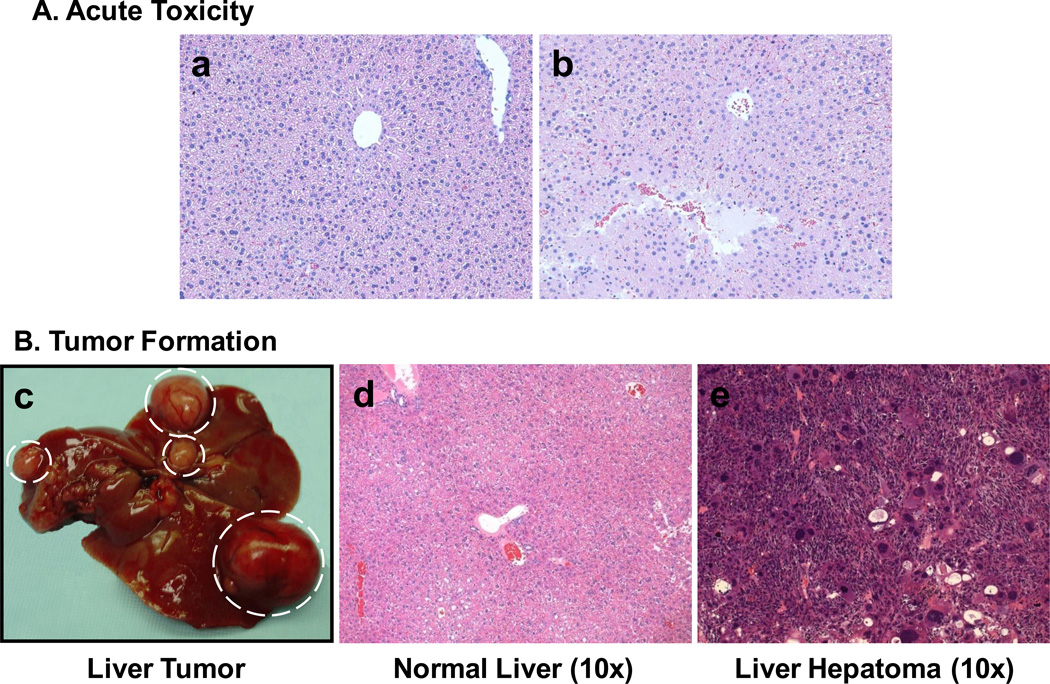
Acute histopathological changes were observed in the high dose RM group (Figure 3A, panel b). PAHs caused liver damage 7 d after treatment compared to the control group (Figure 3A, panel a). These results suggest that the high dose of carcinogenic PAHs not only induced genotoxicity, but also caused toxicity-based histological alterations.
Fig 3.
Acute histological changes (A) in the liver of mice treated with reconstituted mixture (RM). Panel a: control, mouse treated with corn oil/DMSO (50:50). Panel b: mouse treated with the high dose of the reconstituted mixture (RM). Seven days after treatment, focal hemorrhage and tissue necrosis were observed. (B) PAH-induced hepatic tumor formation. Panel c: gross tumor formation observed in the high dose NF+BaP group during dissection. Panel d: liver tissue from control group treated with corn oil/DMSO (50:50). Panel e: liver hepatoma observed in the high dose NF+BaP group. All samples were obtained at 280 d after PAH treatment. NF, neutral fraction.
Forty weeks after i.p treatment with PAHs, tumor formation was observed in the liver, lung, and forestomach of B6C3F1 mice. The tumor incidence was greater in liver than in lung or forestomach (Table 3). Upon dissection, gross liver tumor formation was observed (Figure 3B, panel c) and histological analysis indicated that most liver tumors were hepatomas (Figure 3B, panel e). High hepatic tumor incidence was observed in all three chemical groups.
Table 3.
Mortality and tumor incidence in B6C3F1 male mice
| Chemical | Group | n | Mortality (%) | Tumor incidence (%) | |
|---|---|---|---|---|---|
| Liver | Multiple organs* | ||||
| Control | CO:DMSO | 20 | 5 | 0 | 5 |
| BaP | Low | 20 | 0 | 25 | 30 |
| Middle | 22 | 3 | 58 | 63 | |
| High | 22 | 6 | 31 | 44 | |
| NF+BaP | Low | 20 | 15 | 28 | 33 |
| Middle | 20 | 25 | 35 | 47 | |
| High | 20 | 75 | 50 | 75 | |
| RM | Low** | 26 | 0 | - | - |
| Middle | 26 | 8 | 13 | 13 | |
| High | 28 | 14 | 31 | 42 | |
Multiple organs include liver, lung, and forestomach.
The results of tumor incidence including liver tumor incidence and multiplicity tumor incidence are outliers and need to be further investigated in the future.
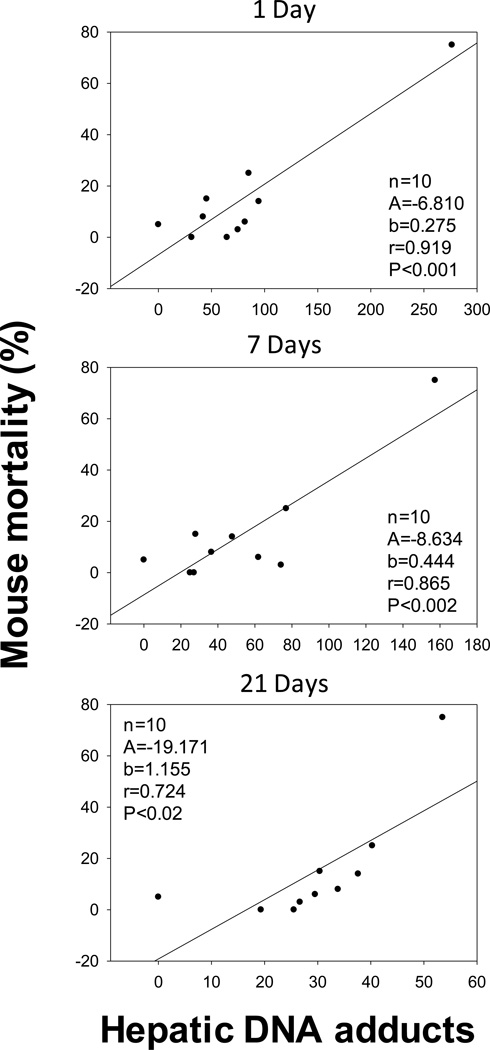
The relationships between DNA adducts and mortality
Mortality was measured by the number of dead animals observed prior to the end (280 d) of the study. The groups that were challenged with NF+BaP had higher mortality than the groups dosed with either BaP or the RM. Interestingly, levels of DNA adducts at 1, 7 and 21 d were significantly correlated with animal mortality (Figure 4). The higher the levels of hepatic DNA adducts displayed at early time points, the higher was the mortality. Hepatic DNA adduct values after 1 d exhibited the most significant correlation with mouse mortality (P<0.001) compared to 7 (P<0.002) and 21 d (P<0.02). These results suggested that PAHs induced acute toxicity including genotoxicity could be associated with increased cancer related mortality. PAH-DNA adducts could be also significant biomarkers for such mortality.
Fig 4.
Correlations between hepatic DNA adduct levels (adducts in 109 normal nucleotides) at 1, 7, and 21 d, and mouse mortality prior to 280 d. Linear correlations were statistically significant for 1 d (P<0.001), 7 d (P<0.002) and 21 d (P<0.02).
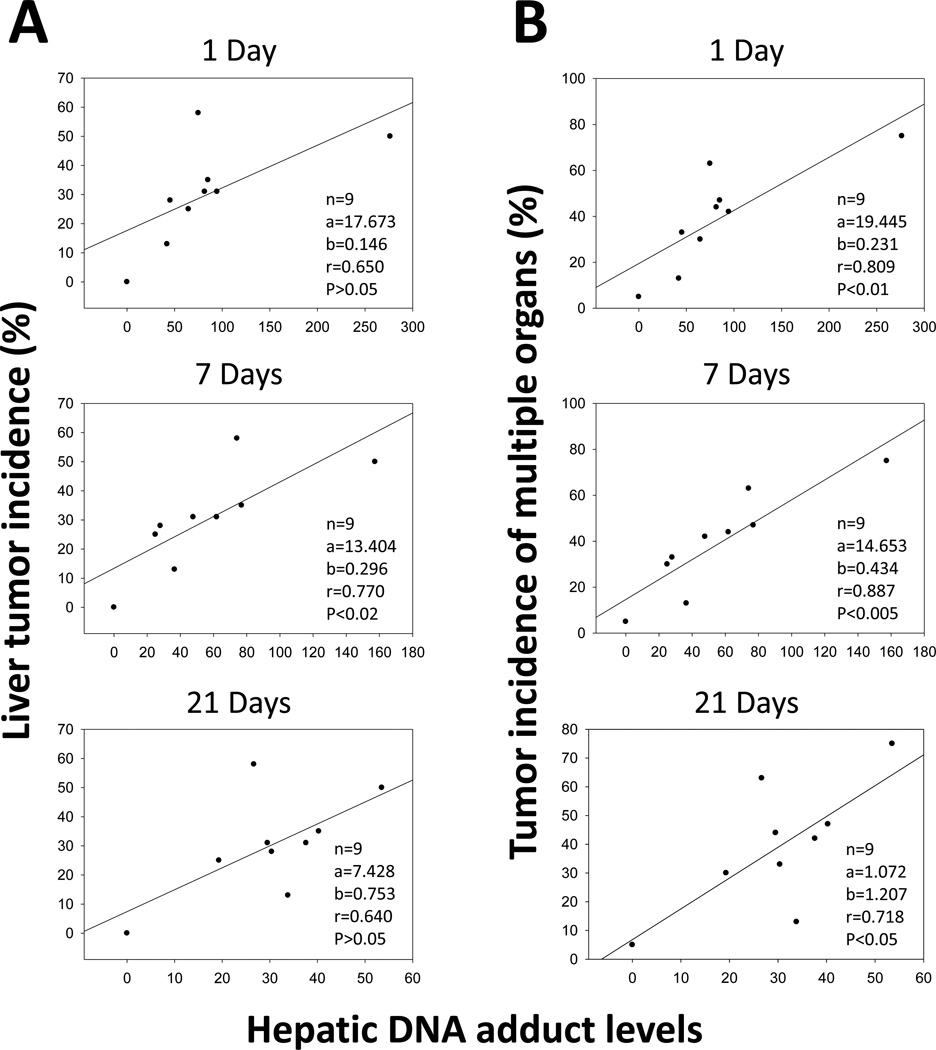
The relationships between DNA adducts and tumor incidence
Tumor incidence for all groups was determined based on histological observation. Linear regression between PAH-DNA adducts and tumor incidence was determined and hepatic DNA adduct levels after 7 d significantly correlated with liver tumor incidence with P-values being <0.02 (Figure 5A). Interestingly, hepatic DNA adducts may be not only biomarkers of liver tumors, but also biomarkers for tumors formed in other organs. Figure 5B shows that levels of hepatic DNA adducts after 1, 7, and 21 d were significantly correlated with tumor incidence in multiple organs (liver, lung and forestomach). The P-values are <0.01, <0.005, and <0.05 for 1 d, 7, d and 21 d, respectively.
Fig 5.
Correlation between hepatic DNA adduct levels (adducts in 109 normal nucleotides) at 1, 7, and 21 d, and liver tumor incidence (A). Adduct levels detected at 7 d, but not at 1 or 21 d, were significantly correlated with liver tumor incidence. (B) Correlation between hepatic DNA adduct levels (adducts in 109 normal nucleotides) at 1, 7, and 21 d, and tumor incidence in multiple organs (liver, lung, and forestomach). Levels of hepatic DNA adducts at 1, 7, and 21 d were all significantly associated with tumor incidence in multiple organs (liver, lung and forestomach).
Comparisons of protein expression involved in DNA repair, apoptosis, and cell cycle regulation between tumor and normal tissues
A panel of 4 normal and 4 tumor tissues was profiled by RPPA to identify differences in expression of protein involved in chemical carcinogenesis such as DNA repair, apoptosis, and cell cycle regulation. Protein lysate was collected from hepatic tumor samples and normal liver tissues and it was evident that most DNA repair proteins including ATM, pATR, Chk1, pChk1, DNA PKcs, XRCC1, FANCD2, Ku80, Mre11 and Brca2 were expressed at significantly lower levels in tumor vs normal tissues (Table 4). Expression of proteins involved in apoptosis and cell cycle regulation were also significantly different in tumor vs non-tumor tissues. In tumor tissues, proteins PARP1, caspase3, caspase7, XIAP, p53, p-Rb (Ser807/811), and Cyclin B1 were downregulated. In contrast, Bcl-xL, Bax, pBad (Ser112), and P16/INK4A were upregulated.
Table4.
Protein expression in liver tumor and normal liver of B6C3F1mice treated with carcinogenic PAHs
| Pathway | Protein | Tumor (T) | Normal (N) | T/N | P value |
|---|---|---|---|---|---|
| DNA repair |
ATM | 0.590±0.046 | 0.761±0.126 | 0.967 | 0.0432 |
| pATR | 0.569±0.037 | 0.748±0.019 | 0.761 | 0.0001 | |
| Chk1 | 0.788±0.028 | 0.961±0.042 | 0.820 | 0.0005 | |
| Chk2 | 1.640±0.128 | 1.493±0.115 | 1.098 | 0.1386 | |
| pChK1 | 0.743±0.033 | 0.894±0.017 | 0.831 | 0.0002 | |
| DNA PKcs | 0.564±0.035 | 0.772±0.015 | 0.731 | <0.0001 | |
| ERCC1 | 4.413±0.347 | 3.450±0.554 | 1.279 | 0.0257 | |
| XRCC1 | 0.508±0.032 | 0.640±0.011 | 0.794 | 0.0002 | |
| FANCD2 | 1.543±0.093 | 1.896±0.192 | 0.814 | 0.0162 | |
| Ku80 | 0.585±0.050 | 0.696±0.027 | 0.841 | 0.0081 | |
| MSH2 | 2.510±0.218 | 2.274±0.313 | 1.104 | 0.2629 | |
| Mre11 | 0.870±0.040 | 1.194±0.160 | 0.729 | 0.0077 | |
| RAD50 | 1.465±0.152 | 1.362±0.166 | 1.076 | 0.3985 | |
| Brca2 | 0.513±0.036 | 0.680±0.025 | 0.754 | 0.0003 | |
| Apoptosis | PARP1 (Cleaved) | 0.905±0.108 | 1.105±0.072 | 0.819 | 0.0217 |
| Caspase3 | 0.363±0.012 | 0.466±0.015 | 0.779 | <0.0001 | |
| Caspase7 (Cleaved) |
0.480±0.024 | 0.584±0.017 | 0.822 | 0.0004 | |
| Bcl-xL | 1.501±0.143 | 1.075±0.056 | 1.396 | 0.0014 | |
| Bcl-2 | 1.301±0.074 | 1.234±0.136 | 1.054 | 0.4207 | |
| Bax | 1.485±0.180 | 0.910±0.015 | 1.632 | 0.0007 | |
| pBad(Ser112) | 0.988±0.074 | 0.778±0.031 | 1.270 | 0.0019 | |
| XIAP | 1.224±0.141 | 2.495±0.059 | 0.491 | 0.0006 | |
| Cell cycle regulation |
P16/INK4A | 1.314±0.117 | 1.108±0.099 | 1.186 | 0.0363 |
| p53 | 0.693±0.036 | 0.884±0.071 | 0.784 | 0.0030 | |
| Phopho-Rb (Ser807/811) |
0.756±0.043 | 0.992±0.061 | 0.762 | 0.0007 | |
| Rb | 1.244±0.087 | 1.057±0.099 | 1.177 | 0.0293 | |
| Cyclin B1 | 0.726±0.033 | 1.050±0.030 | 0.691 | <0.0001 | |
| Cyclin D1 | 0.947±0.068 | 0.885±0.110 | 1.070 | 0.3744 | |
| Others | Nrf2 | 1.013±0.046 | 1.142±0.030 | 0.887 | 0.0034 |
| Egfr | 0.405±0.034 | 0.455±0.013 | 0.890 | 0.0329 | |
| Cox2 | 0.527±0.029 | 0.609±0.050 | 0.865 | 0.0291 |
Normal tissues were obtained from control group, n=4.
Tumor tissues were from the BaP group (3 samples) and RM group (1 sample).
Discussion
Environmental PAHs are formed by the incomplete burning of hydrocarbons such as coal, gas, oil, and wood. They are generated from smoking, automobile exhaust, industrial activities, forest fires, and even grilled meats and are always found as mixtures in the environment and in biological tissues (Guo et al. 2011). In our current studies, we found there were seven major carcinogenetic PAHs in a NF extracted from a WPW dense non-aqueous phase liquid. This complex PAH mixture was carcinogenic in vivo and similar results were previously reported for this mixture by Culp and coworkers (Culp et al. 1998). RM was prepared based on the chemical compositions and relative PAH concentrations in the NF (Table 1). However, the biological effects caused by these two mixtures were significantly different. Levels of DNA adducts, mortality, and tumor incidence in the NF groups were much higher compared to those in RM groups (Table 2). These results suggest that some PAHs or other unknown compounds that were present in the NF, but not in the RM, played an important role in the formation of DNA adducts and tumors induced by the NF. It was reported that PAHs in binary mixtures modulate the efficiency of BaP to form DNA adducts in human cells (Tarantini et al. 2011) and chromium (VI) exposure can greatly enhance the mutagenicity and cytotoxicity of PAHs by inhibiting the nucleotide excision repair (Hu et al. 2004).
Environmental factors including tobacco smoke, diet, and exposure to environmental and occupational carcinogens are believed to be responsible for many cancers (J.J. 2008), and for humans PAHs are among most abundant chemical carcinogens in terms of levels and exposure. Most cancers are still not curable and therefore, cancer prevention is an important anticancer strategy, particularly in high risk populations such as smokers and others exposed to environmental and occupational carcinogens. Therefore, biomarkers of tumor formation are critical for the early detection of disease-related changes and the prediction of cancer risk (Kyrtopoulos 2006).
The 32P-postlabeling assay was first developed in 1982 (Gupta et al. 1982) and was modified with nuclease P1-enhanced bisphosphate in 1986 (Reddy and Randerath 1986). Because of its high sensitivity and requirement of less than 10 µg DNA, the 32P-postlabeling assay (Phillips and Arlt 2007) has been applied in a number of human and animal studies of detection for DNA adducts. However, it is difficult for the 32P-postlabeling assay to identify the structures of most carcinogen-DNA adducts. BPDE-dG (Jeffrey et al. 1977; Randerath et al. 1998) is one of structure identified carcinogen-DNA adducts (Fig. 3). Our results showed that the BPDE-dG adduct has very similar trends with total DNA adducts. In future studies, high resolution mass spectrometry (Klaene et al. 2012) or a combination of 32P-postlabeling assay and mass spectrometry should be considered in detection and structure identification of DNA adducts (Farmer and Singh 2008).
It was reported that complex PAH mixtures have been associated with increased cancer mortality (Warshawsky et al. 1996). In the current study we observed that levels of PAH-DNA adducts detected at 1, 7 and 21 d after PAH treatment were significantly correlated with animal mortality. Peak values of DNA adducts detected after 1 d may be a suitable predictor for general toxicity that is associated with the chronic adverse effects, including mortality.
The correlation between levels of PAH-DNA adducts and tumor incidence was also observed in this investigation. For example, hepatic PAH-DNA adducts detected after 7 d significantly correlated with liver tumor incidence at 280 d. Adduct levels after 1 and 21 d showed the trends of linear regressions but the correlations were not significant (0.05<P<0.10). Interestingly, hepatic PAH-DNA adducts were significantly correlated with total tumor incidence in multiple organs (liver, lung, and forestomach) at 280 d, with P values being <0.01, <0.005, and <0.05 for 1, 7 and 21 d, respectively. Adduct levels after 7 d showed the best correlation with both liver tumor incidence and tumor incidence of multiple organs. One possible mechanism for these correlations could be that DNA repair systems that play important role after initial DNA damage from PAH exposure may be compromised. PAH-DNA adducts rapidly activate nucleotide excision repair (Henkler et al. 2012). Adducted nucleotides that were not repaired or removed within 7 days could lead to mutations. Accumulation of mutations is an important step for the tumor development (Vogelstein and Kinzler 2004).
Data obtained from RPPA indicated that some proteins involved in DNA repair such as ATM, pATR, Chk1, pChk1, Xrcc1, Ku80, Mre11 and apoptosis (PARP1, caspase3, caspase7, XIAP) were significantly decreased in liver tumors compared to normal liver tissues of B6C3F1 mice (Table 4). These results suggest that genetic alterations, especially genes related to DNA repair and apoptosis pathways, may play important roles in PAH-mediated carcinogenesis. It was reported (Shi et al. 2004) that women with reduced DNA repair capacity were at an increased risk for developing breast cancer. Attenuation of DNA repair and apoptosis could increase the formation of carcinogen-DNA adducts (John et al. 2012) followed by DNA mutations and tumor formation. Our results indicate that proteins involved in cell cycle regulation showed both upregulation and downregulation. Similar results were reported by Hamouchene and coworkers (Hamouchene et al. 2011) who showed that BaP exposure increased gene expression of GDF15, JUN, RGC32, and EGR1, but deceased HDAC4 and Scaper. Thus, PAH-induced alterations in DNA repair and apoptotic pathways may facilitate persistent DNA adduct formation and changes in expression of these proteins or their corresponding mRNAs and may serve as biomarkers of exposure and risk. Future development and validation of these biomarkers at early time point after exposure is warranted.
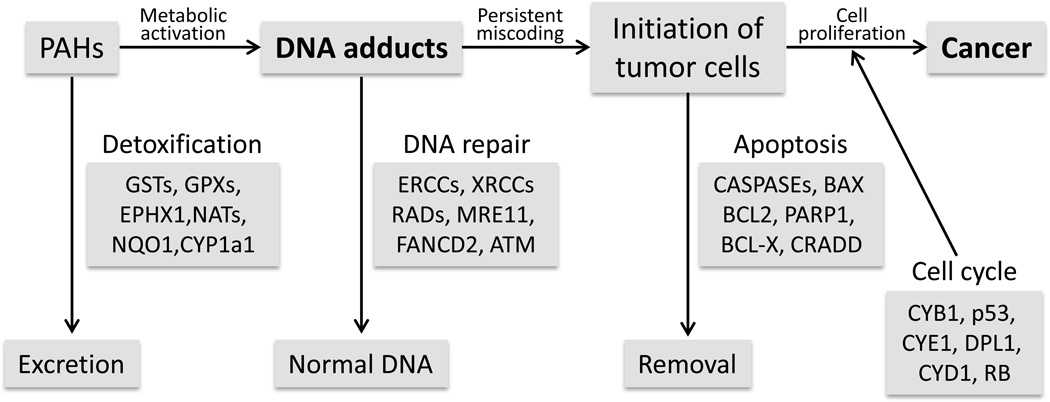
DNA adducts and their levels are important for tumor formation in rats after exposure to 2-nitrofluorene (Cui et al. 1995) and our results also confirmed that DNA adducts could be useful biomarkers for tumor risk in mice after exposure to complex PAH mixtures. However, there are many factors involved in chemical carcinogenesis between formation of DNA adducts and tumor formation. Figure 6 shows a number of proteins/genes involved in DNA repair, apoptosis, and cell cycle regulation that may play important roles in chemical carcinogenesis. Dynamic observations of these proteins/genes after exposure to chemical carcinogens may help predict future tumor risk, and facilitate understanding the mechanisms of chemical carcinogenesis and will be investigated in future studies.
Fig 6.
Possible mechanisms by which various proteins/genes involved in DNA repair, apoptosis, and cell cycle regulation play important roles in PAH-mediated carcinogenesis.
Acknowledgements
We thank Dr. Annika Gillespie and Rebecca Lingenfelter for their efforts during animal experiments. This research work was supported by National Institute of Health grants P30-ES09106, P42-ES04917, ES-09132, and ES-019689, HL-087174, HL-112516 (to BM), and the Center for Translational Environmental Health Research (CTEHR), P30ES023512.
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
Contributor Information
Tracie D. Phillips, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843
Molly Richardson, School of Rural Public Health, Texas A&M University Health Science Center, College Station, TX 77843.
Yi-Shing Lisa Cheng, Baylor College of Dentistry, Texas A&M University Health Science Center, Dallas, TX 75246.
Lingyu He, School of Rural Public Health, Texas A&M University Health Science Center, College Station, TX 77843.
Thomas J. McDonald, School of Rural Public Health, Texas A&M University Health Science Center, College Station, TX 77843
Stephen H. Safe, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843
Kirby C. Donnelly, School of Rural Public Health, Texas A&M University Health Science Center, College Station, TX 77843
Fen Wang, Institute of Biosciences and Technology, Texas A&M University Health Science Center, Houston, TX 77030.
Bhagavatula Moorthy, Department of Pediatrics, Baylor College of Medicine, Houston, TX 77030.
Guo-Dong Zhou, Institute of Biosciences and Technology, Texas A&M University Health Science Center, Houston, TX 77030; School of Rural Public Health, Texas A&M University Health Science Center, College Station, TX 77843.
References
- 1.(EPA) EPA. Method 3650B, Acid-base partition cleanup. 1996 http://wwwepaorg/osw/hazard/testmethods/sw846/pdfs/3650bpdf. [Google Scholar]
- 2.Agency EP. U.S. EPA integrated risk information system (IRIS) Washington, DC: Environmental Protection Agency; 2003. [Google Scholar]
- 3.Agudo A, Peluso M, Munnia A, et al. Aromatic DNA adducts and risk of gastrointestinal cancers: a case-cohort study within the EPIC-Spain. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research cosponsored by the American Society of Preventive Oncology. 2012;21(4):685–692. doi: 10.1158/1055-9965.EPI-11-1205. [DOI] [PubMed] [Google Scholar]
- 4.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2(9):798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cizmas L, Barhoumi R, Burghardt RC, et al. A comparison of two methods for fractionating complex mixtures in preparation for toxicity analysis. J Toxicol Environ Health A. 2003;66(14):1351–1370. doi: 10.1080/15287390306392. [DOI] [PubMed] [Google Scholar]
- 6.Cui XS, Torndal UB, Eriksson LC, Moller L. Early formation of DNA adducts compared with tumor formation in a long-term tumor study in rats after administration of 2-nitrofluorene. Carcinogenesis. 1995;16(9):2135–2141. doi: 10.1093/carcin/16.9.2135. [DOI] [PubMed] [Google Scholar]
- 7.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19(1):117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 8.Du H, Sun J, Chen Z, Nie J, Tong J, Li J. Cigarette smoke-induced failure of apoptosis resulting in enhanced neoplastic transformation in human bronchial epithelial cells. J Toxicol Environ Health A. 2012;75(12):707–720. doi: 10.1080/15287394.2012.690088. [DOI] [PubMed] [Google Scholar]
- 9.Farmer PB, Singh R. Use of DNA adducts to identify human health risk from exposure to hazardous environmental pollutants: the increasing role of mass spectrometry in assessing biologically effective doses of genotoxic carcinogens. Mutation research. 2008;659(1–2):68–76. doi: 10.1016/j.mrrev.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Wu K, Huo X, Xu X. Sources, distribution, and toxicity of polycyclic aromatic hydrocarbons. J Environ Health. 2011;73(9):22–25. [PubMed] [Google Scholar]
- 11.Gupta RC. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RC, Reddy MV, Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- 13.Hamouchene H, Arlt VM, Giddings I, Phillips DH. Influence of cell cycle on responses of MCF-7 cells to benzo[a]pyrene. BMC Genomics. 2011;12:333. doi: 10.1186/1471-2164-12-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Zhou Z, Hofstetter WL, et al. Aberrant expression of proteins involved in signal transduction and DNA repair pathways in lung cancer and their association with clinical parameters. PLoS One. 2012;7(2):e31087. doi: 10.1371/journal.pone.0031087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature reviews. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS. Lung carcinogenesis by tobacco smoke. International journal of cancer Journal international du cancer. 2012;131(12):2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkler F, Stolpmann K, Luch A. Exposure to polycyclic aromatic hydrocarbons: bulky DNA adducts and cellular responses. Exs. 2012;101:107–131. doi: 10.1007/978-3-7643-8340-4_5. [DOI] [PubMed] [Google Scholar]
- 18.Hu W, Feng Z, Tang MS. Chromium(VI) enhances ()-anti-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a ]pyrene-induced cytotoxicity and mutagenicity in mammalian cells through its inhibitory effect on nucleotide excision repair. Biochemistry. 2004;43(44):14282–14289. doi: 10.1021/bi048560o. [DOI] [PubMed] [Google Scholar]
- 19.JJ SSMaJ. The Effects of Environmental Factors on Cancer Prevalence Rates and Specific Cancer Mortality Rates in a Sample of OECD Developed Countries. International Journal of Applied Economics. 2008;5(2):24. [Google Scholar]
- 20.Jeffrey AM, Weinstein IB, Jennette KW, et al. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- 21.John K, Pratt MM, Beland FA, et al. Benzo[a]pyrene (BP) DNA adduct formation in DNA repair-deficient p53 haploinsufficient [Xpa(−/−)p53(+/−)] and wild-type mice fed BP and BP plus chlorophyllin for 28 days. Carcinogenesis. 2012;33(11):2236–2241. doi: 10.1093/carcin/bgs247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaene JJ, Sharma VK, Glick J, Vouros P. The analysis of DNA adducts: The transition from (32)P-postlabeling to mass spectrometry. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyrtopoulos SA. Biomarkers in environmental carcinogenesis research: striving for a new momentum. Toxicology letters. 2006;162(1):3–15. doi: 10.1016/j.toxlet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Mabon N, Moorthy B, Randerath E, Randerath K. Monophosphate 32P-postlabeling assay of DNA adducts from 1,2:3,4-diepoxybutane, the most genotoxic metabolite of 1,3-butadiene: in vitro methodological studies and in vivo dosimetry. Mutation research. 1996;371(1–2):87–104. doi: 10.1016/s0165-1218(96)90098-1. [DOI] [PubMed] [Google Scholar]
- 25.Malinowsky K, Wolff C, Schott C, Becker KF. Characterization of signalling pathways by reverse phase protein arrays. Methods Mol Biol. 2013;1049:285–299. doi: 10.1007/978-1-62703-547-7_21. [DOI] [PubMed] [Google Scholar]
- 26.Phillips DH, Arlt VM. The 32P-postlabeling assay for DNA adducts. Nat Protoc. 2007;2(11):2772–2781. doi: 10.1038/nprot.2007.394. [DOI] [PubMed] [Google Scholar]
- 27.Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. International journal of cancer Journal international du cancer. 2012 doi: 10.1002/ijc.27827. [DOI] [PubMed] [Google Scholar]
- 28.Phillips TD. PhD Thesis. Genotoxicity of Complex Chemical Mixtures, Texas A&M University; 2006. The relationships between levels of DNA adducts and tumor incidence in different tissues of B6C3F1 male mice treated with benzo(a)pyrene and a reconstituted PAH mixture. [Google Scholar]
- 29.Poirier MC. DNA adducts as exposure biomarkers and indicators of cancer risk. Environmental health perspectives. 1997;105(Suppl 4):907–912. doi: 10.1289/ehp.97105s4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randerath K, Randerath E, Zhou GD, et al. Genotoxicity of complex PAH mixtures recovered from contaminated lake sediments as assessed by three different methods. Environmental and molecular mutagenesis. 1999;33(4):303–312. doi: 10.1002/(sici)1098-2280(1999)33:4<303::aid-em7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Randerath K, Reddy MV, Gupta RC. 32P-labeling test for DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randerath K, Sriram P, Moorthy B, et al. Comparison of immunoaffinity chromatography enrichment and nuclease P1 procedures for 32P-postlabelling analysis of PAH-DNA adducts. Chemico-biological interactions. 1998;110(1–2):85–102. doi: 10.1016/s0009-2797(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 33.Reddy MV, Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- 34.Saieva C, Peluso M, Masala G, et al. Bulky DNA adducts and breast cancer risk in the prospective EPIC-Italy study. Breast Cancer Res Treat. 2011;129(2):477–484. doi: 10.1007/s10549-011-1472-8. [DOI] [PubMed] [Google Scholar]
- 35.Shi Q, Wang LE, Bondy ML, Brewster A, Singletary SE, Wei Q. Reduced DNA repair of benzo[a]pyrene diol epoxide-induced adducts and common XPD polymorphisms in breast cancer patients. Carcinogenesis. 2004;25(9):1695–1700. doi: 10.1093/carcin/bgh167. [DOI] [PubMed] [Google Scholar]
- 36.Shorey LE, Castro DJ, Baird WM, et al. Transplacental carcinogenesis with dibenzo[def,p]chrysene (DBC): timing of maternal exposures determines target tissue response in offspring. Cancer letters. 2012;317(1):49–55. doi: 10.1016/j.canlet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddens LK, Larkin A, Krueger SK, et al. Polycyclic aromatic hydrocarbons as skin carcinogens: comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse. Toxicology and applied pharmacology. 2012;264(3):377–386. doi: 10.1016/j.taap.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 39.Sugasawa K. Multiple DNA damage recognition factors involved in mammalian nucleotide excision repair. Biochemistry (Mosc) 2011;76(1):16–23. doi: 10.1134/s0006297911010044. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Umemura T, Ishii Y, et al. Possible involvement of sulfotransferase 1A1 in estragole-induced DNA modification and carcinogenesis in the livers of female mice. Mutation research. 2012;749(1–2):23–28. doi: 10.1016/j.mrgentox.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Tang D, Phillips DH, Stampfer M, et al. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Cancer research. 2001;61(18):6708–6712. [PubMed] [Google Scholar]
- 42.Tarantini A, Maitre A, Lefebvre E, Marques M, Rajhi A, Douki T. Polycyclic aromatic hydrocarbons in binary mixtures modulate the efficiency of benzo[a]pyrene to form DNA adducts in human cells. Toxicology. 2011;279(1–3):36–44. doi: 10.1016/j.tox.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5(10):2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 44.Urban AM, Upadhyaya P, Cao Q, Peterson LA. Formation and repair of pyridyloxobutyl DNA adducts and their relationship to tumor yield in A/J mice. Chemical research in toxicology. 2012;25(10):2167–2178. doi: 10.1021/tx300245w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veglia F, Loft S, Matullo G, et al. DNA adducts and cancer risk in prospective studies: a pooled analysis and a meta-analysis. Carcinogenesis. 2008;29(5):932–936. doi: 10.1093/carcin/bgm286. [DOI] [PubMed] [Google Scholar]
- 46.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature medicine. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 47.Warshawsky D, Talaska G, Xue W, Schneider J. Comparative carcinogenicity, metabolism, mutagenicity, and DNA binding of 7H-dibenzo[c,g]carbazole and dibenz[a,j]acridine. Crit Rev Toxicol. 1996;26(2):213–249. doi: 10.3109/10408449609017932. [DOI] [PubMed] [Google Scholar]
- 48.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Seminars in cancer biology. 2004;14(6):473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Zar JH. Biostatistical Analysis. Fifth edition. New Jersey: Prentice-Hall; 2009. [Google Scholar]
- 50.Zhou GD, Hernandez NS, Randerath E, Randerath K. Acute elevation by short-term dietary restriction or food deprivation of type I I-compound levels in rat liver DNA. Nutrition and cancer. 1999;35(1):87–95. doi: 10.1207/S1532791487-95. [DOI] [PubMed] [Google Scholar]
- 51.Zhou GD, Popovic N, Lupton JR, Turner ND, Chapkin RS, Donnelly KC. Tissue-specific attenuation of endogenous DNA I-compounds in rats by carcinogen azoxymethane: possible role of dietary fish oil in colon cancer prevention. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1230–1235. doi: 10.1158/1055-9965.EPI-04-0759. [DOI] [PubMed] [Google Scholar]
- 52.Zhou GD, Randerath K, Donnelly KC, Jaiswal AK. Effects of NQO1 deficiency on levels of cyclopurines and other oxidative DNA lesions in liver and kidney of young mice. International journal of cancer. 2004;112(5):877–883. doi: 10.1002/ijc.20375. [DOI] [PubMed] [Google Scholar]
- 53.Zhou GD, Richardson M, Fazili IS, et al. Role of retinoic acid in the modulation of benzo(a)pyrene-DNA adducts in human hepatoma cells: implications for cancer prevention. Toxicology and applied pharmacology. 2010;249(3):224–230. doi: 10.1016/j.taap.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]