Abstract
Objectives
Delusional thoughts are common among patients with Alzheimer’s disease (AD) and may be conceptually linked to memory deficits (cannot recall accurate information which fosters inaccurate beliefs) and poor insight (unable to appreciate the illogic of beliefs). This study’s goals were to examine the clinical associations among delusions, memory deficits, and poor insight, explore neurobiological correlates for these symptoms, and identify shared mechanisms.
Design
Cross sectional analysis.
Setting
VA Greater Los Angeles Healthcare System.
Participants
Eighty-eight outpatients with AD (mean MMSE 19.3).
Measurements
Delusional thoughts were assessed with the Neuropsychiatric Inventory. Level of inaccurate insight was assessed with the Neurobehavioral Rating Scale (NRS). Memory was assessed with the Mattis Dementia Rating Scale (DRS) memory subscale. 18F-FDG PET imaging was used to measure regional cortical metabolism. Relationships between clinical ratings and regional cortical metabolic activity (voxel-based) were assessed using SPM2.
Results
Patients with delusions had lower DRS memory subscale scores. NRS inaccurate insight scores were no different in those with and without delusions. Cortical metabolic activity was lower in the right lateral frontal cortex, orbitofrontal cortex, and bilateral temporal cortex in patients with delusions. Low cortical metabolic activity in the right lateral, inferior, and medial temporal cortex was associated with poorer memory. This region partially overlapped the region of hypometabolism associated with delusions. In contrast, low cortical metabolic activity in bilateral medial frontal cortex was associated with poor insight.
Conclusions
Delusions in AD are associated with dysfunction in specific frontal and temporal cortical regions. Delusions are partially clinically and neurobiologically linked to memory deficits but not to poor insight.
Keywords: Alzheimer’s disease, delusions, insight, memory, FDG-PET, cerebral metabolism
Objective
Delusional thoughts are common among patients with Alzheimer’s disease (AD) (1). These non-cognitive symptoms have important clinical implications as they contribute prominently to behavioral disturbances, institutionalization, and caregiver burden (2, 3). However, the etiologic factors underlying delusions in AD remain unclear. Conceptually, delusional thoughts may be associated with memory deficits and poor insight. For example, the inability to recall accurate information may foster inaccurate beliefs. Poor insight, defined as reduced awareness of cognitive or functional deficits and impaired intuitive understanding, may undermine the patient’s appreciation of the illogical nature of these beliefs. While impaired memory and insight may be linked to delusional thoughts, the relationships are not necessarily causal and the extent of relationships and the neuropathological processes that mediate them are not known.
Studies of the clinical correlates of delusional thoughts have typically evaluated relationships with global cognition. Most studies find that delusions are more common among those AD patients with advanced cognitive deficits (1, 4, 5). Few studies have examined the relationship between delusional thoughts and memory deficits specifically (6) or the relationship between delusional thoughts and impaired insight. Two studies found a correlation between delusional thoughts and a lack of awareness of cognitive deficits in AD (7, 8). In addition, Kazui et al. (9) found that both memory impairment and delusions were significantly associated with lack of insight, suggesting that these three symptoms are clinically related in AD.
Although the exact pathophysiological mechanisms are unclear, neuroimaging studies indicate that delusional thoughts may be associated with dysfunction in frontal and temporal cortex in patients with AD (e.g., 10–15). Previous research has also shown an association between lack of insight in AD and right hemisphere and frontal lobe dysfunction that incompletely overlaps with regions of cortical dysfunction associated with delusions (16, 17). Thus, delusions and poor insight may have a shared relationship with right frontal cortex dysfunction. Further elucidation of the clinical and neuropathological associations among delusions, memory, and insight can help to identify critical mechanisms underlying cognitive and neuropsychiatric symptoms in AD and can help to inform clinical treatment.
The goals of this study were to examine the clinical associations among delusional thoughts, memory deficits, and poor insight in mild to moderate AD, and to explore the neurobiological basis and possible shared mechanisms for these symptoms using FDG-PET imaging. We hypothesized that both delusions and poor insight would be associated with right lateral/inferior frontal cortex hypometabolism and that memory deficits would be associated with inferior and medial temporal cortex hypometabolism.
Methods
Participants
This study included 88 participants with Alzheimer’s disease. Participants were recruited from outpatient clinics that included the VA Greater Los Angeles Healthcare System (VAGLAHS) Memory clinic and Geropsychiatry clinic. Participants had completed a clinical evaluation of neurological, psychiatric, and cognitive functioning and a structural neuroimaging scan. Final diagnosis was based on the criteria for probable Alzheimer’s disease established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association and is consistent with NIA/Alzheimer’s Association criteria for probable AD (18). Exclusion criteria included history of psychotic disorder unrelated to dementia, history of head trauma with loss of consciousness, history or neuroimaging evidence of stroke or cerebrovascular disease, seizure disorder, substance use disorder, severe aphasia, or systemic illness or other neurological illness that could account for cognitive deficits. Thirty-two of the participants were on a stable dose (3 months or more) of cholinesterase inhibitor medication and 21 were on a stable dose of selective serotonin reuptake inhibitor antidepressant. No participants were being treated with an antipsychotic, benzodiazepine, or other psychotropic medication. The study was reviewed and approved by the local institutional review board.
Clinical Assessment
Participants and their caregivers underwent a structured clinical assessment that included the Neuropsychiatric Inventory (NPI) (19) and the Neurobehavioral Rating Scale (NRS) (20). The NPI is a caregiver-based measure of 12 distinct psychiatric and behavioral disturbances in dementia. Presence or absence of delusions was based on question A of the NPI. For patients with delusions, frequency (1–4 scale) and severity (1–3 scale) of delusions were assessed. Delusions were classified as either paranoid-type (e.g., belief that others are stealing from him/her) or misidentification-type (e.g., belief that his/her house is not his/her home.)
The NRS consists of 28-items that measure the severity of a broad range of neuropsychiatric symptoms and behavioral disturbances based on information from the caregiver and a semistructured patient interview. Each NRS item is scored from 0 (not present) to 6 (extremely severe). Level of insight was measured with the inaccurate insight item of the NRS. The item score is based on a comparison of the patient’s assessment of his/her cognitive skills, functional abilities, and other circumstances along with future goals and plans. The participant’s ideas and plans are contrasted with objective evidence derived from clinical interview, cognitive testing, and caregiver report. The examiner’s overall assessment of the patient’s awareness and insight, using all information available, is captured on the item score. The NRS has been shown to have satisfactory interrater reliability (21) and to be a valid measure of psychopathology in individuals with dementia (20).
All participants completed a neuropsychological test battery as part of a comprehensive evaluation. The Mattis Dementia Rating Scale (DRS) (22) memory subscale was used to assess memory. The DRS has been shown to have satisfactory reliability and validity (23, 24). Memory subtest total scores range from 0 to 25, with a higher score indicating better performance. This subscale measures orientation, verbal recall, and verbal and visual recognition. Gross cognitive functioning was assessed using the MMSE.
FDG-PET Imaging
Patients completed PET imaging on the same day as the clinical assessment. Three different PET tomographs with similar imaging characteristics were used over the course of this study (Siemens 953/31, GE Advance PET-CT, and Phillips Gemini TF PET-CT scanners). Each scanner had in-plane and axial resolution of approximately 5 mm at full-width half maximum (FWHM). 18F-fluorodeoxyglucose (FDG) was synthesized at the VAGLAHS PET Imaging Facility according to the technique of Hamacher et al. (25). Patients received 5 to 10 mCi of FDG intravenously and rested with their eyes open during the 40-minute uptake phase. Participants were then placed in the scanner with the imaging plane parallel to the canthomeatal plane and data were acquired for 30 minutes.
Voxel-Based PET Image Analyses
PET data were analyzed using SPM2 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom) in Matlab 6.5 (MathWorks). Images were normalized to Montreal Neuroimaging Institute (MNI) space using trilinear interpolation and resampled to 2 × 2 × 2 mm voxels. Images were then smoothed using a 6-mm FWHM smoothing kernel. The t-test procedure in SPM2 was used to assess differences in metabolism between participants with and without delusions. To determine the contribution of insight and memory, an ANCOVA procedure was used, that included NRS inaccurate insight scores and DRS memory subscale scores as covariates in the model. In addition, the simple correlation procedure was used to assess the association between NRS inaccurate insight score and metabolic activity and the association between DRS memory subscale score and metabolic activity. Images were normalized using proportional scaling, and threshold masking was used to remove signal from structures outside of gray matter. The voxel-level statistical threshold was set at p<0.01 and results were considered significant for voxel clusters at the p<0.05 level, corrected for the search volume in each analysis.
Clinical Assessment Score Analyses
Univariate analyses of variance (ANOVAs) were used to assess differences between those with delusions and those without delusions for DRS total, DRS memory subscale, MMSE, and NRS inaccurate insight scores.
Results
Patient Characteristics
Eight-eight patients (71 men and 17 women) participated in the study. Mean (s.d.) age was 78 (8.0) years, mean education was 14 (3.6) years, and mean duration of dementia was 3.2 (2.4) years. Distribution of race/ethnicity was 56 white, 24 African American, 3 Hispanic, and 5 Asian/Pacific Islander.
Twenty-eight participants (32%) had recent delusional thoughts and 60 participants (68%) did not. Among participants with delusions, the mean NPI delusion frequency times severity score was 4.4 (3.5) out of 12, with a range of 1 to 12. Mean scores on the clinical measures are listed in Table 1. Participants with delusions were more impaired on measures of overall cognition (MMSE and DRS total) and had lower scores on the DRS memory subscale than participants without delusions. In contrast, inaccurate insight scores on the NRS did not differ in those with and without delusions. Inaccurate insight scores and DRS memory subscale scores showed a significant, albeit weak, negative correlation (r = −0.24, p=0.03, n=83). The duration of dementia was not significantly different in the group with no delusions (mean 3.1 (2.0) years) vs. those with delusions (mean 3.5 (2.0) years; t (83)=0.75, p=0.45).
TABLE 1.
Clinical assessment scores in the AD sample.
| No Delusions n= 60 |
Delusions n=28 |
F * | p-value | |
|---|---|---|---|---|
| MMSE | 20.9 (5.2) | 16.0 (5.0) | 17.6 | <0.001 |
| DRS Total | 110.5 (14.9) | 90.1 (22.9) | 23.9 | <0.001 |
| DRS Memory subscale | 13.3 (4.1) | 8.8 (3.5) | 24.7 | <0.001 |
| NRS Inaccurate Insight item | 2.3 (1.8) | 2.7 (1.7) | 0.8 | 0.36 |
Scores are displayed as mean (s.d.). Higher scores on the MMSE, DRS Total, and DRS memory subscale indicate better performance. Higher scores on NRS inaccurate insight item indicate poorer insight.
F statistic with d.f (1,86), (1,81), (1,82), and (1,85) for the MMSE, DRS Total, DRS Memory subscale, and NRS Inaccurate Insight item scores, respectively. d.f. varies due to occasional missing data.
With regard to the content of delusional thoughts, 26 participants presented with paranoid-type delusions and 11 presented with misidentification-type delusions. All but 2 of the participants with misidentification delusions also had paranoid delusions. Patients with both misidentification and paranoid delusions (n=9) had lower MMSE scores (mean 12.0 (5.4)) than those with only paranoid delusion (n=17; mean 17.9 (3.9); t (24)=3.22, p=0.004).
Cortical Metabolism in Patients with vs. without Delusions
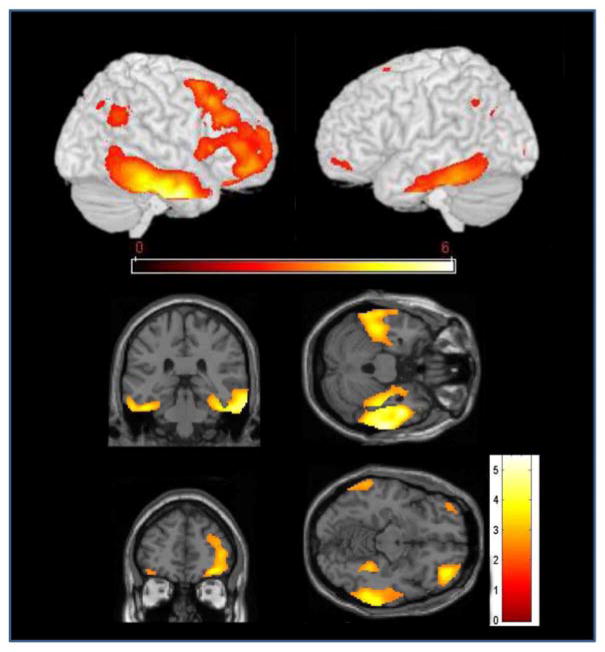
Cortical metabolic activity was lower in AD patients with delusions compared to those without delusions in three regions: right lateral frontal (middle and inferior frontal gyrus) and orbitofrontal cortex, and bilateral temporal cortex (Fig. 1, Table 2). Regions of lower cortical metabolic activity in the temporal cortex included the majority of the middle and inferior temporal gyri and the mid-portion of the parahippocampal gyrus, bilaterally. When DRS total score was included as a covariate in the analysis, the pattern of hypometabolism in those with delusions compared to those without delusions was unchanged, although only the difference in right temporal cortex remained statistically significant at the corrected cluster level. In a sensitivity analysis, participants with NPI delusion score =1 (infrequent and mild symptom) were switched from the delusion group to the no-delusion group and the between-group analysis of cortical metabolism was repeated. Results were unchanged. Finally, a post-hoc analysis compared cortical metabolism in patients with paranoid delusions only to those with both paranoid and misidentification delusions. Patients with combined delusions had lower metabolic activity than those with only paranoid delusions in the left inferior parietal lobule (results not shown).
Figure 1.
T-map of voxels where AD patients with delusions had lower metabolic activity than those without delusions. a) Rendering of the results of the SPM t-test analysis, projected onto the left and right cortical surface. b) SPM-2 maps showing coronal and axial view of bilateral temporal hypometabolism. c.) SPM-2 maps showing coronal and axial view of right frontal hypometabolism. Images are displayed at p<0.01.
TABLE 2.
Cortical regions where AD patients with delusions had lower metabolic activity than those without delusions.
| Region | BA | Side | x, y, z | k | T statistic (d.f.), peak voxel | p value, cluster level |
|---|---|---|---|---|---|---|
| Frontal | 11, 12, 47, 46, 8, 6 | R | 36, 46, −12 | 4589 | 3.81 (86) | <0.001 |
| Temporal | 20, 21 | R | 52, −4, −30 | 4847 | 5.49 (86) | <0.001 |
| Temporal | 20, 21 | L | −44, −32, −26 | 1958 | 3.75 (86) | 0.01 |
Abbreviations: BA= approximate Brodmann’s area. x, y, z = coordinates of peak voxel in MNI space. k = number of voxels within the cluster. Voxel size is 2 mm × 2 mm × 2 mm. Cluster-level p-values are corrected for the search volume.
Relationship between Cortical Metabolism and Insight and Memory
When NRS inaccurate insight scores and DRS memory subscale scores were included as covariates in the model assessing metabolic activity in those with versus those without delusions, the relationship between delusions and right temporal cortical hypometabolism was unchanged. Relationships with low metabolic activity in right lateral frontal and left temporal cortex persisted on SPM maps, although were not significant at the corrected cluster level.
Poor insight on the NRS inaccurate insight item was associated with lower metabolic activity in bilateral medial frontal cortex (Fig. 2, Table 3). There was no qualitative overlap between the regions of hypometabolism associated with delusions and those associated with poor insight.
Figure 2.
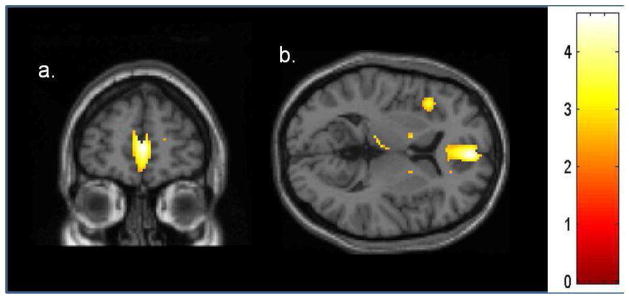
T-map of voxels showing direct correlation between NRS inaccurate insight item and cortical metabolism in AD patients. Inaccurate insight is correlated with low metabolic activity in bilateral medial frontal cortex in a.) coronal and b.) axial views. Image displayed at p<0.01.
TABLE 3.
Cortical region with significant correlation between NRS inaccurate insight item score and metabolic activity.
| Region | BA | Side | x, y, z | k | T statistic (d.f.), peak voxel | p value, cluster level |
|---|---|---|---|---|---|---|
| Frontal | 24, 32, 8, 9, 10 | R | 4, 54, 6 | 1926 | 4.57 (85) | 0.01 |
Abbreviations: BA= approximate Brodmann’s area. x, y, z, = coordinates of peak voxel in MNI space. k, number of voxels within the cluster. Voxel size is 2 mm × 2 mm × 2 mm. Cluster-level p-values are corrected for the search volume.
Lower scores (more impairment) on the DRS memory subscale were associated with lower metabolic activity in the right temporal cortex, including the middle, inferior, fusiform, and parahippocampal gyri. This region overlapped qualitatively with a region of hypometabolism associated with delusions (Fig. 3, Table 4). Low cortical metabolic activity in the left temporal, bilateral posterior cingulate, and right inferior parietal regions was also associated with lower scores on the DRS memory subscale, but was not significant at the cluster level.
Figure 3.
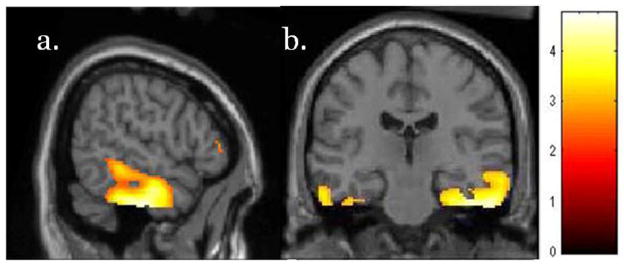
T-map of voxels showing direct correlation between DRS memory subscale score and lower metabolism in AD. Poor memory is associated with hypometabolism in right medial and inferior lateral temporal cortex in a.) sagittal and b.) coronal views. Image displayed at p<0.01.
TABLE 4.
Cortical region with significant correlation between DRS memory subscale score and metabolic activity.
| Region | BA | Side | x, y, z | k | T statistic (d.f.), peak voxel | p value, cluster level |
|---|---|---|---|---|---|---|
| Temporal | 20, 21, 28, 37 | R | 56, −16, −34 | 3408 | 4.74 (82) | 0.001 |
Abbreviations: BA= approximate Brodmann’s area. x, y, z, = coordinates of peak voxel in MNI space. k, number of voxels within the cluster. Voxel size is 2 mm × 2 mm × 2 mm. Cluster-level p-values are corrected for the search volume.
Discussion
The goals of this study were to examine the clinical associations among delusional thoughts, memory deficits, and poor insight in AD, and to assess the cortical metabolic activity associated with these symptoms. We observed that clinically, delusional thoughts were linked to poor memory but not to lower insight. Neurobiologically, delusions were associated with relative hypometabolism in right frontal and bilateral temporal cortex. Regions of hypometabolism associated with delusions did not qualitatively overlap with hypometabolic regions associated with poor insight. However, hypometabolism in right temporal cortex associated with memory impairment did overlap with a region of hypometabolism in AD patients with delusions. These results suggest that delusions in AD are at least partly related to memory deficits, both phenomenologically and neurobiologically, but not to poor insight.
Several neurobiological factors potentially contribute to psychosis in AD: genetic substrate (26), neurochemical alterations (27), neurofibrillary tangle density (28), and amyloid burden (29). The research findings vary considerably and study samples have generally been small. The in vivo neuroimaging literature has highlighted a relationship between frontal cortex dysfunction and psychosis (30, 31) or delusions specifically (10–12, 32, 33). Temporal dysfunction also has been found in AD patients with delusional thoughts (12–14, 34), although with less consistency in terms of laterality and subregions and with findings of both low and high metabolism or perfusion. Other regions found to be associated with delusions/psychosis in AD include anterior cingulate (12, 35), as well as parietal (11, 12, 35, 36) and occipital regions (34). Heterogeneity of previous results likely reflects small sample sizes, different assessment tools, and different neuroimaging techniques. Our results, based on a larger sample, showed lower cortical metabolic activity in AD patients with delusions in three regions: the right lateral frontal and orbitofrontal cortex, and bilateral lateral and inferior temporal cortex, suggesting a more prominent role of the temporal cortex in the neurobiology of delusions in AD than had been highlighted in previous research. Global cognition scores were lower in the delusions group than in the non-delusions group, and thus delusions and cortical hypometabolism could both reflect more advanced dementia. However, when global cognition was included as a covariate in the model, the pattern of results was unchanged, indicating that the relationship between delusions and regional hypometabolism is at least partly independent of global cognitive decline.
The content of delusional thoughts may be related to metabolic dysfunction in different cortical regions, which may also explain different findings across studies. We were not able to distinguish relationships between specific delusional thoughts and cortical metabolic activity, because there were insufficient patients with misidentification delusions only. However, when metabolic activity in those with paranoid delusions only was contrasted with that of AD patients with both paranoid and misidentification delusions, there were no metabolic differences in either frontal or temporal cortex, indicating that the frontal and temporal relationships observed in the overall group with delusions are not driven exclusively by the misidentification delusions. There was lower metabolic activity in the left inferior parietal lobule in patients with combined delusions vs. those with only paranoid delusions. However, this difference may reflect the greater cognitive impairment in the combined delusions group rather than a link to the content of delusional thoughts specifically, as prior studies have demonstrated greater parietal hypometabolism associated with more advanced cognitive deficits in AD.
The overlap of relationships between temporal hypometabolism and both poor memory and delusions suggests that an inability to recall information and delusional thoughts have shared functional underpinnings. Hypometabolism in the right lateral frontal and orbitofrontal cortex was also seen in AD patients with delusions, and was not associated with poor memory. Although speculative, these frontal areas have been shown to be involved in working memory and executive functioning (37, 38), suggesting that the inability to hold onto and successfully manipulate information may play a role in the failure to maintain and consider alternative possibilities necessary to successfully reject incorrect theories.
Many studies have found reduced medial temporal lobe (MTL) activity on memory tasks in patients with AD (e.g., 39–41). This is consistent with the course of AD pathology in the MTL, including the entorhinal cortex and hippocampus (42). Results from this study showed an association between low cortical metabolic activity and poor memory in the right lateral, inferior, and medial temporal cortex, including the parahippocampal and entorhinal regions. Memory in this study was assessed with the DRS memory subscale, which includes orientation and verbal recall items, as well as tasks of verbal and visual recognition. A recent quantitative meta-analysis of functional neuroimaging studies during encoding and retrieval phases of episodic memory tasks found that patients with AD failed to show significant engagement of MTL areas during retrieval (43). Recognition is associated with increased activity in hippocampal and parahippocampal areas, including the entorhinal cortex (44), in healthy individuals, and reduced activation in those regions in individuals with AD (43). Thus, our findings are consistent with pathological and neuroimaging studies of memory in AD. Moreover, qualitative overlapping of right temporal hypometabolism related to both delusions and impaired memory suggests a neurobiological association that can help the individual to contrast beliefs and mental imagery with memory for specific events, which can aid reality checking.
Our findings indicate that level of insight does not differ between AD patients with and without delusional thoughts. These results are in accordance with Lopez et al. (45) and Harwood et al. (46) who did not find a relationship between poor insight and psychotic symptoms in AD. However, other researchers have found a relationship between lack of awareness and delusions (7, 9, 47) and our finding may have reflected insufficient power to detect a small difference between the groups. We observed an association between low cortical metabolic activity and reduced insight in bilateral medial frontal cortex. This finding is consistent with one previous imaging study (48). However, these medial prefrontal relationships are not entirely consistent with other studies that have observed an association between poor awareness in AD and right hemisphere dysfunction, particularly involving frontal and temporal regions (16, 17, 49–52). There is no “gold standard” for assessment of insight in AD and instruments may include clinician ratings, questionnaire-based methods, performance-based methods, phenomenological methods, or a combination of methods (53). Also, the domain of awareness that is measured varies substantially from study to study, and has included insight related to memory functioning, overall cognitive functioning, behavioral problems, or activities of daily functioning (8). These differences may underlie inconsistencies in the research literature. Finally, insight encompasses complex cognitive processes such as awareness, understanding, and intuition, which may be less localizable to a discrete set of cortical regions compared to more elemental cognitions such as memory or language.
This study has several methodological limitations. Three different PET tomographs were used, although multiple scanners have been used successfully previously (54) and the SPM normalization procedure results in ratio scaling for each participant which limits the impact of different scanners (55). Another potential limitation was inclusion of participants on cholinesterase inhibitor and antidepressant medications. However, this is not atypical for an outpatient AD population and no participants had been treated with an antipsychotic or other psychotropic medication. The majority of participants were men, which limits the generalizability of the findings. Finally, all but two of our participants had paranoid delusions, which prohibited thorough comparisons by delusion type. The negative valence and suspicious nature seen in paranoid delusions may be related to temporal dysfunction seen in other disorders with persecutory delusions such as schizophrenia and temporal lobe epilepsy (56, 57). It is possible that delusions of misidentification are more closely linked to insight.
AD is a neurodegenerative disorder without specific focal lesions. Yet, our findings show that delusions and poor memory do overlap both clinically and neurobiologically. While delusions are not likely an exclusive product of memory deficits, the findings suggest that mechanisms may be partly shared and the inability to recall accurate information could contribute to the development or maintenance of inaccurate beliefs. Right lateral and inferior frontal cortical dysfunction was also associated with delusional thoughts, but not linked to either memory or insight, either clinically or neurobiologically, suggesting an independent role of right frontal dysfunction in the clinical expression of delusional thoughts in AD. The findings here also suggest that FDG-PET provides a biologically-based marker that may aid the development of treatments for psychosis in AD, by defining a more homogeneous subgroup of patients with delusions who may respond more consistently to a particular intervention. Moreover, regional cortical metabolism can change over the course of treatment (58, 59) and FDG-PET imaging may shed light on critical metabolic changes that occur with successful treatment. Successful psychosis treatment in AD may also benefit executive skills and memory via effects on frontal/temporal cortical dysfunction and thereby improve day-to-day living skills. Future studies can explore such metabolic changes with treatment, identify causal brain-behavior relationships using a longitudinal design, examine relationships with individual delusional thoughts in larger samples, and explore co-occurring neuropsychiatric symptoms that may influence the link between delusional thoughts and cortical function in AD.
Acknowledgments
This work was supported in part by the Department of Veterans Affairs (Merit Review Award, Dr. Sultzer; Career Development Award, Dr. Melrose) and the National Institute of Mental Health (R01MH56031).
Footnotes
This work was presented in part at the 2012 Annual Meeting of the American Association for Geriatric Psychiatry, Washington D.C.
Dr. Sultzer has received research support from Eli Lilly, and has served as a consultant to Otsuka Pharmaceutical and Eli Lilly. None of the other authors report potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990–2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson C, Tarrier N, Burns A. The impact of the symptoms of dementia on caregivers. Br J Psychiatry. 1997;170:62–68. doi: 10.1192/bjp.170.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Magni E, Binetti G, Bianchetti A, Trabucchi M. Risk of mortality and institutionalization in demented patients with delusions. J Geriatr Psychiatry Neurol. 1996;9:123–126. doi: 10.1177/089198879600900303. [DOI] [PubMed] [Google Scholar]
- 4.Mizrahi R, Starkstein SE, Jorge R, Robinson RG. Phenomenology and clinical correlates of delusions in Alzheimer’s disease. Am J Geriatr Psychiatry. 2006;14:573–581. doi: 10.1097/01.JGP.0000214559.61700.1c. [DOI] [PubMed] [Google Scholar]
- 5.Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, Fadda L, Canonico V, Trequattrini A, Pettenati C, Caltagirone C, Palmer K. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:1026–1035. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- 6.Koppel J, Goldberg TE, Gordon ML, Huey E, Davies P, Keehlisen L, Huet S, Christen E, Greenwald BS. Relationships between behavioral syndromes and cognitive domains in Alzheimer disease: the impact of mood and psychosis. Am J Geriatr Psychiatry. 2012;20:994–1000. doi: 10.1097/JGP.0b013e3182358921. [DOI] [PubMed] [Google Scholar]
- 7.Starkstein SE, Sabe L, Chemerinski E, Jason L, Leiguarda R. Two domains of anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;61:485–490. doi: 10.1136/jnnp.61.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aalten P, Van Valen E, Clare L, Kenny G, Verhey F. Awareness in dementia: a review of clinical correlates. Aging Ment Health. 2005;9:414–422. doi: 10.1080/13607860500143075. [DOI] [PubMed] [Google Scholar]
- 9.Kazui H, Hirono N, Hashimoto M, Nakano Y, Matsumoto K, Takatsuki Y, Mori E, Ikejiri Y, Takeda M. Symptoms underlying unawareness of memory impairment in patients with mild Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2006;19:3–12. doi: 10.1177/0891988705277543. [DOI] [PubMed] [Google Scholar]
- 10.Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, Cummings JL. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry. 2003;160:341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- 11.Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer’s disease: SPET evidence of right hemispheric dysfunction. Cortex. 1999;35:549–560. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- 12.Nakano S, Yamashita F, Matsuda H, Kodama C, Yamada T. Relationship between delusions and regional cerebral blood flow in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:16–21. doi: 10.1159/000089215. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, Vazquez S, Petracca G, Sabe L, Migliorelli R, Teson A, Leiguarda R. A SPECT study of delusions in Alzheimer’s disease. Neurology. 1994;44:2055–2059. doi: 10.1212/wnl.44.11.2055. [DOI] [PubMed] [Google Scholar]
- 14.Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, Furey M, Murphy DGM. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38:438–449. doi: 10.1016/0006-3223(94)00326-x. [DOI] [PubMed] [Google Scholar]
- 15.Ismail Z, Nguyen M-Q, Fischer CE, Schweizer TA, Mulsant BH. Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Research: Neuroimaging. 2012;202:89–95. doi: 10.1016/j.pscychresns.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Harwood DG, Sultzer DL, Feil D, Monserratt L, Freedman E, Mandelkern MA. Frontal lobe hypometabolism and impaired insight in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:934–941. doi: 10.1176/appi.ajgp.13.11.934. [DOI] [PubMed] [Google Scholar]
- 17.Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer’s disease: relationships to depression, cognitive function, and cerebral perfusion. J Clin Exp Neuropsychol. 1993;15:231–244. doi: 10.1080/01688639308402560. [DOI] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL. Assessment of cognitive, psychiatric, and behavioral disturbances in patients with dementia: the Neurobehavioral Rating Scale. J Am Geriatr Soc. 1992;40:549–555. doi: 10.1111/j.1532-5415.1992.tb02101.x. [DOI] [PubMed] [Google Scholar]
- 21.Sultzer DL, Berisford MA, Gunay I. The Neurobehavioral Rating Scale: reliability in patients with dementia. J Psychiatr Res. 1995;29:185–191. doi: 10.1016/0022-3956(95)00018-z. [DOI] [PubMed] [Google Scholar]
- 22.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu TB, editors. Geriatric Psychiatry. New York: Grune and Stratton; 1976. pp. 77–107. [Google Scholar]
- 23.Gardner R, Jr, Oliver-Munoz S, Fisher L, Empting L. Mattis Dementia Rating Scale: internal reliability study using a diffusely impaired population. J Clin Neuropsychol. 1981;3:271–275. doi: 10.1080/01688638108403130. [DOI] [PubMed] [Google Scholar]
- 24.Vitaliano PP, Breen AR, Russo J, Albert M, Vitiello MV, Prinz PN. The clinical utility of the dementia rating scale for assessing Alzheimer patients. J Chronic Dis. 1984;37:743–753. doi: 10.1016/0021-9681(84)90043-2. [DOI] [PubMed] [Google Scholar]
- 25.Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-18F-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27:235–238. [PubMed] [Google Scholar]
- 26.Sweet RA, Bennett DA, Graff-Radford NR, Mayeaux R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the NIA Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133:1155–1162. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai MKP, Lai O-F, Keene J, Esiri MM, Francis PT, Hope T, Chen CPL-H. Psychosis of Alzheimer’s disease is associated with elevated muscarinic M2 binding in the cortex. Neurology. 2001;57:805–811. doi: 10.1212/wnl.57.5.805. [DOI] [PubMed] [Google Scholar]
- 28.Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, Olney JW, McKeel DW., Jr Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000;57:1165–1173. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- 29.Murray PS, Kirkwood CM, Gray MC, Ikonomovic MD, Paljug WR, Abrahamson EE, Henteleff RA, Hamilton RL, Kofler JK, Klunk WE, Lopez OL, Penzes P, Sweet RA. Beta-amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging. 2012;33:2807–2816. doi: 10.1016/j.neurobiolaging.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotrla KJ, Chacko RC, Harper RG, Jhingran S, Doody R. SPECT findings on psychosis in Alzheimer’s disease. Am J Psychiatry. 1995;152:1470–1475. doi: 10.1176/ajp.152.10.1470. [DOI] [PubMed] [Google Scholar]
- 31.Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, Berisford MA. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995;7:476–484. doi: 10.1176/jnp.7.4.476. [DOI] [PubMed] [Google Scholar]
- 32.Staff RT, Venneri A, Gemmell HG, Shanks MF, Pestell SJ, Murray AD. HMPAO SPECT imaging of Alzheimer’s disease patients with similar content-specific autobiographic delusion: comparison using statistical parametric mapping. J Nucl Med. 2000;41:1451–1455. [PubMed] [Google Scholar]
- 33.Moran EK, Becker JA, Satlin A, Lyoo IK, Fischman AJ, Johnson KA. Psychosis of Alzheimer’s disease: Gender differences in regional perfusion. Neurobiol Aging. 2008;29:1218–1225. doi: 10.1016/j.neurobiolaging.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Hirono N, Mori E, Yasuda M, Ikejiri Y, Imamura T, Shimomura T, Ikeda M, Hashimoto M, Yamashita H. Factors associated with psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:648–652. doi: 10.1136/jnnp.64.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:167–171. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuhara R, Ikeda M, Nebu A, Kikuchi T, Maki N, Hokoishi K, Shigenobu K, Komori K, Tanabe H. Alteration of rCBF in Alzheimer’s disease patients with delusions of theft. Neuroreport. 2001;12:2473–2476. doi: 10.1097/00001756-200108080-00037. [DOI] [PubMed] [Google Scholar]
- 37.Barbey AK, Koenigs M, Grafman J. Orbitofrontal contributions to human working memory. Cereb Cortex. 2011;21:789–795. doi: 10.1093/cercor/bhq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 39.Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, Stebbins G, Gabrieli J. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- 40.Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipoloti L, Puel M, Demonet JF, Chollet F, Frackowiak RS. Alzheimer’s patients engage an alternative network during a memory task. Ann Neurol. 2005;58:870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- 41.Remy F, Mirrashed F, Campbell B, Richter W. Verbal episodic memory impairment in Alzheimer’s disease: a combined structural and functional MRI study. Neuroimage. 2005;25:253–266. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249 (Suppl 3):14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 43.Schwindt GC, Black SE. Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. Neuroimage. 2009;45:181–190. doi: 10.1016/j.neuroimage.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 45.Lopez OL, Becker JT, Somsak D, Dew MA, DeKosky ST. Awareness of cognitive deficits and anosognosia in probable Alzheimer’s disease. Eur Neurol. 1994;34:277–282. doi: 10.1159/000117056. [DOI] [PubMed] [Google Scholar]
- 46.Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:83–88. [PubMed] [Google Scholar]
- 47.Mangone CA, Hier DB, Gorelick PB, Ganellen RJ, Langenberg P, Boarman R, Dollear WC. Impaired insight in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 1991;4:189–193. doi: 10.1177/089198879100400402. [DOI] [PubMed] [Google Scholar]
- 48.Hanyu H, Sato T, Akai T, Shimizu S, Hirao K, Kanetaka H, Iwamoto T, Koizumi K. Neuroanatomical correlates of unawareness of memory deficits in early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:347–353. doi: 10.1159/000119594. [DOI] [PubMed] [Google Scholar]
- 49.Ott BR, Noto RB, Fogel BS. Apathy and loss of insight in Alzheimer’s disease: a SPECT imaging study. J Neuropsychiatry Clin Neurosci. 1996;8:41–46. doi: 10.1176/jnp.8.1.41. [DOI] [PubMed] [Google Scholar]
- 50.Starkstein SE, Vazquez S, Migliorelli R, Teson A, Sabe L, Leiguarda R. A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Arch Neurol. 1995;52:415–420. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- 51.Shibata K, Narumoto J, Kitabayashi Y, Ushijima Y, Fukui K. Correlation between anosognosia and regional cerebral blood flow in Alzheimer’s disease. Neurosci Lett. 2008;435:7–10. doi: 10.1016/j.neulet.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 52.Sedaghat F, Dedousi E, Baloyannis I, Tegos T, Costa V, Dimitriadis AS, Baloyannis SJ. Brain SPECT findings of anosognosia in Alzheimer’s disease. J Alzheimers Dis. 2010;21:641–647. doi: 10.3233/JAD-2010-090631. [DOI] [PubMed] [Google Scholar]
- 53.Clare L, Markova I, Verhey F, Kenny G. Awareness in dementia: A review of assessment methods and measures. Aging Ment Health. 2005;9:394–413. doi: 10.1080/13607860500142903. [DOI] [PubMed] [Google Scholar]
- 54.Melrose RJ, Ettenhofer ML, Harwood D, Achamallah N, Campa O, Mandelkern M, Sultzer DL. Cerebral metabolism, cognition, and functional abilities in Alzheimer disease. J Geriatr Psychiatry Neurol. 2011;24:127–134. doi: 10.1177/0891988711405333. [DOI] [PubMed] [Google Scholar]
- 55.Grady CL. Quantitative comparison of measurements of cerebral glucose metabolic rate made with two positron cameras. J Cereb Blood Flow Metab. 1991;11:A57–A63. doi: 10.1038/jcbfm.1991.38. [DOI] [PubMed] [Google Scholar]
- 56.Blackwood NJ, Howard RJ, Bentall RP, Murray RM. Cognitive neuropsychiatric models of persecutory delusions. Am J Psychiatry. 2001;158:527–539. doi: 10.1176/appi.ajp.158.4.527. [DOI] [PubMed] [Google Scholar]
- 57.Elliott B, Joyce E, Shorvon S. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res. 2009;85:172–186. doi: 10.1016/j.eplepsyres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Sultzer DL, Melrose RJ, Harwood DG, Campa O, Mandelkern MA. Effect of memantine treatment on regional cortical metabolism in Alzheimer’s disease. Am J Geriatr Psychiatry. 2010;18:606–614. doi: 10.1097/JGP.0b013e3181ca3a4e. [DOI] [PubMed] [Google Scholar]
- 59.Sultzer DL, Melrose RJ, Harwood DG, Campa O, Walston A, Narvaez TA, Ando T, Leskin LP, Mandelkern MA. Donepezil treatment and regional cortical metabolism in Alzheimer’s disease: FDG-PET findings. Am J Geriatr Psychiatry. 2011;19:S124–S125. [Google Scholar]