Abstract
To replace damaged or diseased tissues, large tissue-engineered constructs can be prepared by assembling modular components in a bottom-up approach. However, a high speed method is needed to produce sufficient numbers of these modules for full-sized tissue substitutes. To this end, we have devised a novel production technique that combines air shearing and a plug flow reactor-style design to rapidly produce large quantities of hydrogel-based (here type I collagen) cylindrical modular components with tunable diameters and length. Using this technique, modules containing NIH 3T3 cells showed greater than 95% viability while endothelial cell surface attachment and confluent monolayer formation was demonstrated. Additionally, the rapidly-produced modules were used to assemble large tissue constructs (> 1 cm3) in vitro. Module building blocks containing luciferase-expressing L929 cells were packed in full size adult rat liver-shaped bioreactors and perfused with cell medium, to demonstrate the capacity to build organ-shaped constructs; bioluminescence demonstrated sustained viability over 3 days. Cardiomyocyte embedded modules were also used to assemble electrically stimulatable contractile tissue.
Keywords: Scale-up, endothelial cells, cardiomyocytes, bioreactor, vasculature
1. Introduction
In tissue engineering, scaffolds are often used to spatially organize cells for the construction of replacement tissue. Scaffolds are useful because they can be shaped to mimic the geometry and size of any organ. However, the larger the scaffold becomes, the more difficult it is to seed cells within the interior core due to limitations associated with porosity and diffusion. One strategy many groups have explored to overcome these limitations is the bottom-up assembly of tissue substitutes from smaller, modular components. This approach is scalable, can potentially be used to create tissues with much higher cellular densities, and can incorporate a wide variety of cells. Collagen gel beads[1], gelatin microcarriers[2], toroidal shaped units of controlled shape[3], stereolithography and photopolymerization to generate complex 3-D shapes[4], DNA template assembly[5], PEG microgels[6] and the self-assembly of PEG-based, cell-laden microgels using multiphase liquid-liquid systems and biphasic reactors[7–9] have all been used to generate modular components and related structures.
Our group has pioneered the use of rod-shaped modular components that permit the use of multiple cell types and blood perfusion after assembly[10–13]. Specifically, endothelial cell (EC)-covered, submillimeter-sized cylindrical collagen rods (modules) are randomly assembled into a larger container to form a construct (Figure 1a). The interstices created by randomly packing the modules within the container form an interconnected network of perfusion channels through which medium or blood can flow. Because blood contacts the EC-covered module surfaces, a blood-contacting surface is created that is similar to the EC-lined lumen of native blood vessels[13]. Furthermore, when cells are embedded within the modules, the tissue engineered construct is expected to carry out therapeutic functions (e.g., liver metabolism or replacement cardiac muscle tissue). Because the constructs are porous and capable of perfusion, they avoid the internal mass transfer limitations that are often observed in solid constructs[14]. However, the current method for producing cylindrical modules is not amenable to high-speed production because each module must be individually cut in a sequential manner[13], albeit using a semi-automated cutter. This process is reasonable and robust enough to generate 0.1 mL implants for rodent experiments but the lack of speed is a significant hindrance when a large number of modules is required, as is the case when assembling large 1:1 scale tissue substitutes containing several mL of modules.
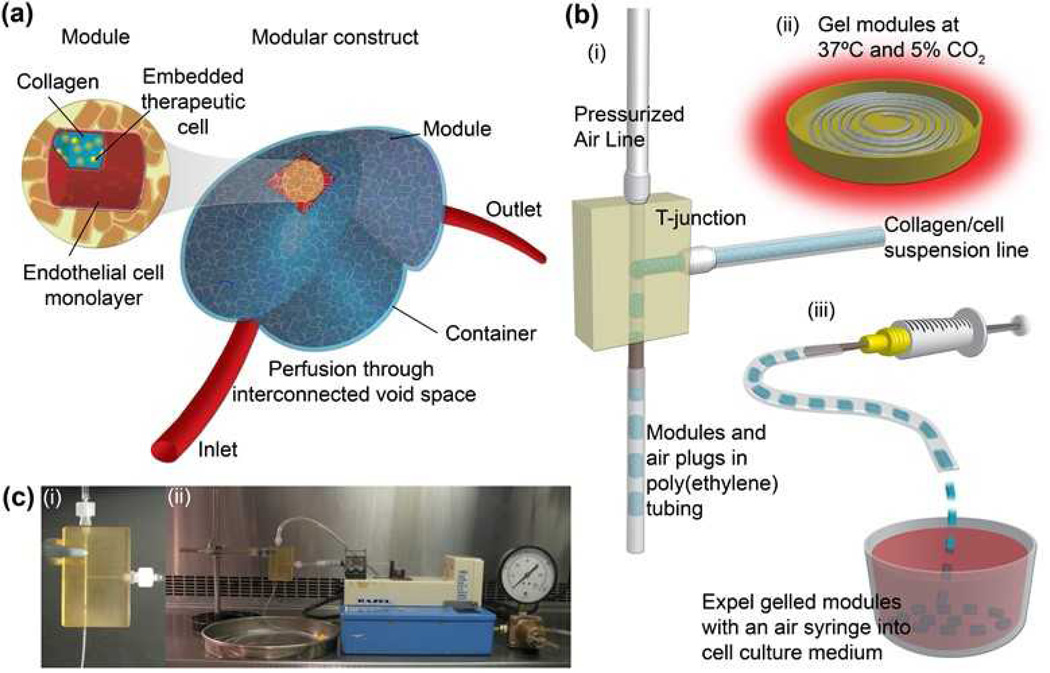
Figure 1.
(a) Sub-millimeter-sized collagen modules (highlighted inset) containing embedded therapeutic cells and surface-seeded ECs are randomly packed into a larger container to form a construct. The void space created by random packing and the aspect ratio of the modules creates an interconnected network through which blood or medium perfusion occurs. (b) Plug flow reactor-inspired high-speed module production method. (i) Neutralized liquid collagen solution containing cells was pumped into a T-junction whereupon a stream of air sheared off droplets to form modules. The modules were separated by a slug of air and fill the attached poly(ethylene) tubing.(ii) Tubing filled with modules was gelled in an incubator. (iii) Gelled modules were expelled into culture medium. (c) Experimental setup in a biological safety cabinet: (i) T-junction (ii) experimental assembly
The primary objective of this work was to create a high speed method of producing the modular components required for the bottom-up assembly of much larger tissues that are, in turn, capable of perfusion. This process was based on a combination of air-induced shearing and a plug flow reactor (PFR). The secondary objective was to qualitatively demonstrate the assembly of large, “full-sized” tissue-like constructs using the rapidly-produced modules. Specifically, the shape and scale of an adult rat liver was recapitulated in a tissue-like construct, while the use of perfusion and electrical stimulation was explored for the generation of primitive cardiomyocyte-laden tissues.
2. Results and Discussion
2.1. Plug Flow Produced Modules
Collagen or a collagen/Matrigel mixture with or without suspended cells was pumped via syringe pump (Figure 1c(ii)) into the side inlet of a custom-designed T-junction fabricated from polysulfone (illustrated in Figures 1b(i) and c(i)). Compressed air at a pressure of 47.5 kPa was directed into the upper inlet of the T-junction (Figures 1b(i) and 1c(i)). The collagen solution accumulated at the intersection region of the T-junction and slugs of collagen separated by air were propelled out of the T-junction’s lower outlet into the attached 3meter long poly(ethylene) (PE) tubing (Figure 1b (i)). Due to plug flow within the PE tubing, the air and collagen did not mix or intersperse.
When the PE tubing was filled, the air and collagen flows were stopped. The tubing was removed from the T-junction outlet and placed within a humidified incubator for 45 minutes at 37°C and 5% CO2 to gel the collagen (Figure 1b(ii)). To recover modules, an air-filled syringe was connected to the PE tubing and modules were expelled into a non-tissue culture treated dish containing the appropriate cell culture medium (Figure 1b(iii)). The module shapes were cylindrical, conforming to the inner lumen of the PE tubing (diameter = 0.76 mm). This resulted in modules with an initial radius of 0.76 mm, which then shrink to approximately 0.4 mm in diameter. The cells within are uniformly distributed and without apparent loss of viability even in the interior; making larger modules leads to loss of viability but this depends on cell density[14]. When a collagen solution containing suspended NIH 3T3 cells was used, all cells were uniformly distributed throughout the modules (Figure 2a). More than 95% of embedded cells were viable (assessed by calcein-AM and ethidium homodimer-1 live/dead assay) after production and one day later.
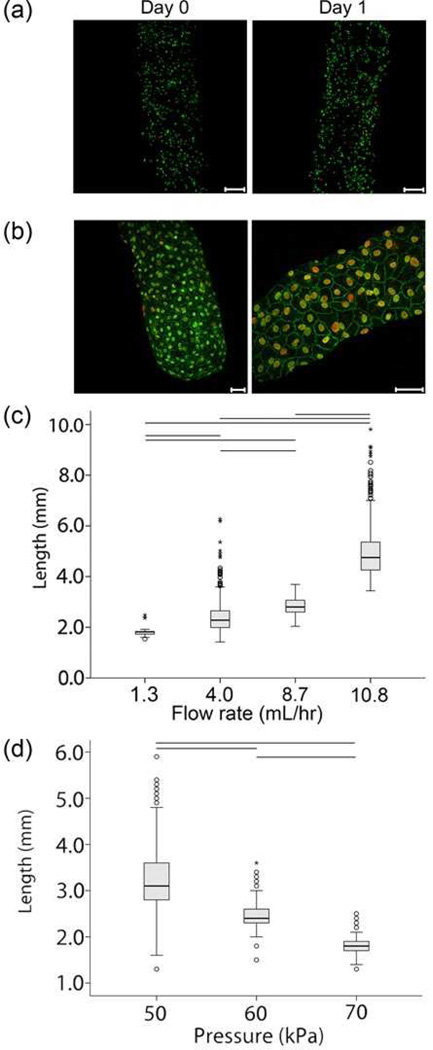
Figure 2.
(a) Live/Dead images of 3T3 cell-embedded modules produced with T-junction. 3T3 cells were suspended in neutralized collagen. The nuclei of live cells appear green while those of dead cells appear red. Cells appeared uniformly distributed within the modules and did not appear to settle to either side under gravity. Images are projections of confocal microcopy images from a z-stack series. Scale bars = 200 µm. (b) Intact endothelial cell monolayers were observed 7 days after seeding module surfaces with human umbilical vein endothelial cells, as evidenced by excellent VE-cadherin expression (green). Nuclei are counterstained in red. Scale bars = 50 µm. (c) Box plot showing the ability to increase module length by fixing air pressure and increasing the volumetric flow rate of the cell-containing collagen solution. The long module outliers were the result of drops in shearing pressure caused by fluctuations in the compressed air source. Connecting lines indicate a statistically significant difference (p<0.05) in mean value by ANOVA. n = 162 for 1.3 mL/hr., n = 374 for 4.0 mL/hr., n = 377 for 8.7 mL/hr., and n = 393 for 10.8 mL/hr. Pressure = 47.5 kPa. (d) Box plot showing the ability to increase module length by fixing volumetric flow rate and decreasing shearing pressure. Connecting lines indicate a statistically significant difference (p<0.05) in mean value by ANOVA. n = 305 for 50 kPa, n = 426 for 60 kPa and n = 390 for 70 kPa. Collagen volumetric flow rate = 5.85 mL/hr.
In separate experiments, endothelial cells (EC) were seeded on the surfaces of cell-free modules immediately after gelation. As evidenced by the excellent VE-cadherin staining shown in Figure 2b, EC were able to attach to the surfaces of the modules, and achieve confluence and normal morphology. Thus the procedure, including the use of pressurized air, did not impede the attachment and growth of EC. The phenotype was very similar to those produced with the low-speed automatic cutter method[13], as both showed continuous VE-cadherin expression around the perimeter of the cells and no gaps in the monolayer.
Module length was controlled by the air shearing pressure and syringe pump outflow rate. Figure 2c shows the effect of the collagen flow rate on module length at a constant air pressure of 47.5 kPa. Increasing syringe outflow increased mean module length as more collagen built up in the time required to generate sufficient pressure for shearing. Figure 2d also shows the effect of air pressure on module length at a constant outflow rate of 5.85 mL/hr. The module production rate was dependent on the relative syringe pump rate and air pressure. Increasing air pressure caused the collagen in the intersection region to be sheared off sooner, thus resulting in a decreased mean module length.
When the pressure was maintained at 47.5 kPa, modules and air slugs were approximately equal in size. To assess the reproducibility of the system, three separate batches of modules were produced using a fixed pressure of 47.5 kPa and a collagen flow rate of 9 mL/hr. A batch was a run in which 3m of the PE tubing was filled with modules. Between each batch, the system was shut down, dismantled and cleaned. The average module size of each batch was compared (Table 1) and no statistically significant differences were found by ANOVA analysis (p = 0.166), thus indicating a high degree of reproducibility.
Table 1.
Module length reproducibility. Three batches of modules were produced and, no statistically significant differences in the average batch length of the modules was found (ANOVA, p=0.166). Additionally, the standard deviation among batches was low and within reasonable tolerances. Operating conditions were 3 m meter of PE60 tubing, air pressure at 47.5 kPa, a 3 mL syringe of collagen and a flow rate of 9 mL/hr.. The standard deviation among batches (i.e. the variability among the average lengths of each individual batch) was 0.04 mm.
| Batch | N | Average module length (mm) |
Standard deviation within batch (mm) |
|---|---|---|---|
| 1 | 142 | 2.80 | 0.28 |
| 2 | 82 | 2.89 | 0.29 |
| 3 | 153 | 2.85 | 0.39 |
2.2. Liver Shaped Construct Assembly and Perfusion
Previously, this group produced small scale modular assemblies in short lengths of cylindrical tubing held in place by glass wool (volume 38–75 µL)[13] and within microfluidic remodeling chambers (volume 0.1 mL)[10, 11]. Embedded cells remained viable in these small-scale perfuseable constructs. To qualitatively demonstrate what is achievable with an excess of modules, a large modular construct containing embedded cells was assembled within a 1:1 scale adult rat liver-shaped bioreactor. The construction and use of this bioreactor are illustrated in Figure 3a. The volume of the liver mold was nearly 5 mL and held approximately 3.5 mL of modules. The 1.5 mL difference in volume was void space that became occupied by flowing cell culture medium. By changing the aspect ratio of the cylindrical modules, it is possible to optimize the amount of void space or specific experiments. Module diameter is changed by using tubing with a different internal diameter and the length is changed by either changing the air pressure or collagen flow rate.
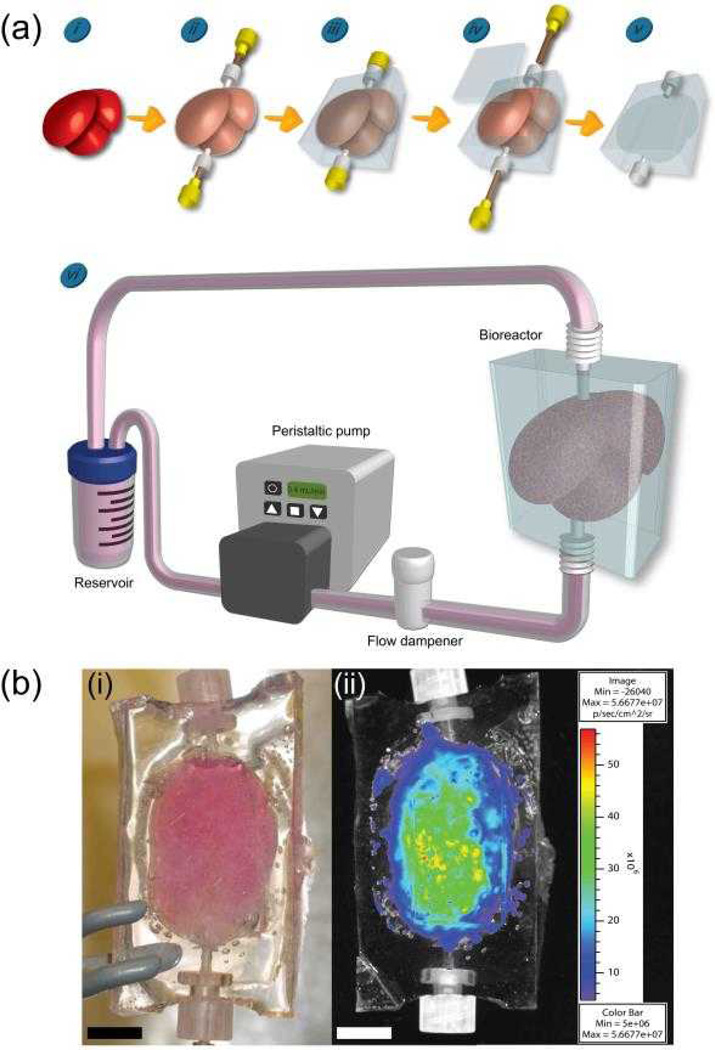
Figure 3.
Creation of a 1:1 scale rat liver-shaped modular assembly using a PDMS bioreactor. (i) A rat liver was fixed in buffered formalin overnight and then air dried overnight. (ii) The liver was sheathed in plastic wrap which served as a barrier against PDMS infiltration. Female Luer threads were inserted into the top and bottom of the liver to serve as the inlet and outlet ports of the bioreactor. 20 gauge needles were threaded through the Luer threads to anchor them to the liver. (iii) The liver was immersed in PDMS, which was degassed to remove any air bubbles and cured. (iv) After curing, the needles were removed, a window was cut into the cast and the sheathed liver was removed, leaving the embedded Luer threads (inlet and outlet ports) behind. (v) The window was re-sealed with additional PDMS, cured and sterilized through steam autoclaving. The internal volume of the liver-shaped void space was 5 mL. (vi) The bioreactor was filled with modules (3.5 mL) and connected to a closed loop flow circuit. With a peristaltic pump, medium was drawn from a reservoir, through a flow dampener to remove air bubbles and eliminate backflow, and pumped into the bottom inlet of the bioreactor. Perfusing the reactor from the bottom ensured the entire volume was filled with medium (i.e. limited channeling). The out flow was returned to the reservoir. (b) A completed bioreactor filled with modules. (i) A 1:1 scale adult rat liver-shaped bioreactor with a total internal volume of 5 mL was filled with modules containing embedded luciferase-expressing L929 cells. The bioreactor was perfused for 3 days at a flow rate of 0.4 mL/min. Scale bar = 1 cm. (ii) Luminescence of L929 modular construct after 3 days of perfusion. Luciferin was perfused through the modular construct and the embedded L929 cells displayed luminescence, illustrating their viability. Size scale bar = 1 cm and colour bar is in the units of photon flux (photons/s/cm2/sr).
The modules produced for this liver-shaped bioreactor contained embedded L929 cells that were stably transfected to express luciferase for use as a bioluminescence marker of viability[15]. This was done for the purpose of detecting viability after high speed production, reactor loading and short-term perfusion. As seen in Figure 3b, the cells within the modules continued to show bioluminescent signal 3 days after rapid production and loading, while perfused at 0.4 mL/min.
The rapidly-formed modules were capable of withstanding perfusion at an equivalent rate of 0.11 mL/min/g of tissue construct. By contrast, in vivo, hepatic blood flow in normal rats is an order of magnitude higher (2.0 ± 0.9 mL/min/g liver[16]). A higher flow rate was not used in these initial experiments because higher flows caused modules to be washed out of the bioreactor through the outlet port. While we can recapitulate the shape and size of an adult rat liver, future studies will require a more sophisticated bioreactor that is filled with hepatocyte-laden modules and which overcomes these perfusion limitations.
The rapid and reproducible production of modules allows one to reach volumes that may one day enable entire human tissues to be replaced. Using a single T-junction, modules are produced at a rate of 10 mL/min (10 mL/min collagen/cell suspension flow rate and an air pressure of 47.5 kPa). 500 mL of modules can thus be produced in 50 minutes, followed by approximately two hours of module gelation and endothelial cell seeding. Thus, within 3 hours, a large bioreactor can be filled with 500 mL of modules (ignoring the problem and cost of collecting the required hundreds of mL of cells). If using several T-junctions in parallel, this method becomes even more efficient at making large volume tissues. Reaching this scale, however, requires a more efficient means of rapidly gelling the collagen cell suspension. A continuous process with a long residence time for gelation and/or a different biomaterial (amenable to say photopolymerization[17,18]) could be a way around the slow step of collagen gelation.
2.3. Cardiac Tissue
To demonstrate the potential to create functional tissues, a large-scale cardiac-like tissue was assembled using this system. In general, it was observed that large-scale assemblies containing multiple cell types underwent remodeling, illustrating a central challenge in creating large, vascularized constructs. Using the availability of a larger number of modules, approximately 3.5 mL of modules containing cardiomyocyte-enriched cell isolates were loaded into bioreactors that were constructed to mimic a cardiac microenvironment while experiencing perfusion (Figure 4a). Four culture conditions were used to highlight the effect of electrical stimulation and/or endothelial cells: cardiac cell embedded modules (CM) with or without 3V electrical conditioning and CM-embedded modules with rat aortic endothelial cell (RAEC) surface coating, with and without 3V electrical conditioning.
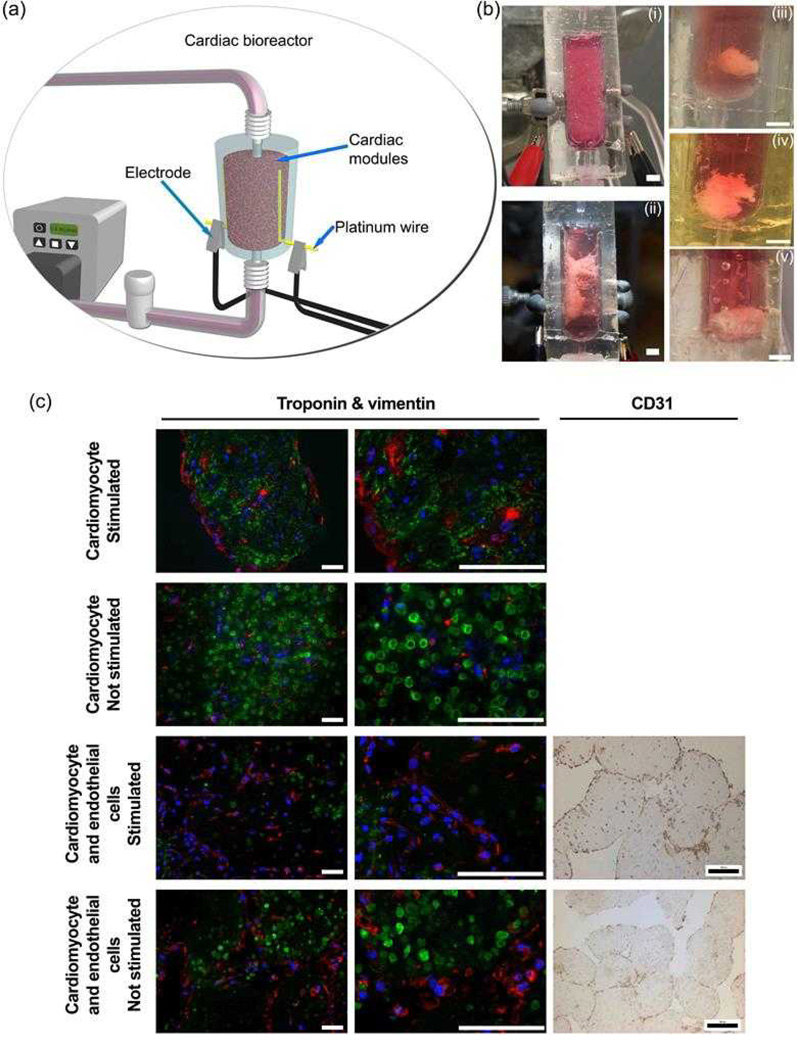
Figure 4.
(a) Cardiac bioreactor connection to a flow circuit. Two independent platinum wires lined the inner chamber of the bioreactor and acted as electrodes when attached to an electrical stimulator. The modules within the bioreactor experienced perfusion and electrical stimulation simultaneously. (b) Macroscopic remodeling of cardiac bioreactor tissues. Cardiac modules with embedded rat neonatal cardiomyocytes at an initial density of 2×107 cell/mL were fabricated. When used, endothelial cells were seeded on the outer surfaces of the modules to form a confluent layer. Modules were loaded into the cardiac bioreactor, perfused at 0.4 mL/min and were electrically conditioned with a 3V, 2 ms biphasic pulse at a rate of 1 pulse per second. (i) Cardiac bioreactor immediately after module loading. (ii) Cardiomyocyte-only modules on day 12. Module assembly appeared anchored between the internal electrodes and showed a reduction in volume. When electrical stimulation was removed, the assembly spontaneously contracted (i.e., the cells were beating). (iii) Without electrical stimulation, cardiomyocyte-only modules formed a globular structure after 12 days. (iv) Non-stimulated cardiomyocyte and endothelial cell modules also formed a globular structure at day 12. (v) Electrically stimulated cardiomyocyte and endothelial cell modules formed a globular structure, some of which were anchored to the platinum electrodes. Three replicates were tested for each condition. Images are representative of the shape of all replicates for that condition. All scale bars = 0.5 cm.
(c) Basic characterization of cardiac tissues under different operational and co-culture conditions after 12 days. All modularly-constructed tissues were triple stained for troponin I (green), vimentin (red) and the nucleus (blue), and imaged with confocal microscopy. Additionally, a brown immunohistochemical CD31 stain with a blue nuclear counter stain was used to confirm the presence of endothelial cells in co-culture tissues using light microscopy. After 12 days, the troponin I in stimulated cardiomyocyte-only tissues was organized as fibers throughout the tissue. In contrast, troponin I in unstimulated control and EC coated modules (stimulated and unstimulated) did not form similar structures and the cardiomyocytes appeared round. Moreover, some CD31-positive cells were still seen around the perimeter of the modules, demonstrating the endothelial cells’ longevity. All scale bars = 200 µm
Over 12 days, module fusion and remodeling occurred such that the overall volume of the assembled tissues were much smaller than they were initially, regardless of whether they were electrically conditioned or not, as shown in Figure 4b. In general, the tissue assemblies shrank as they remodeled, though they still remained anchored to the internal electrodes that lined the inner walls of the bioreactor. After 12 days of culture, electrically conditioned CM-embedded tissues (without RAEC) were found to beat spontaneously in culture (Table 2); this was not observed for CM-embedded tissues with RAEC or for tissues cultured without electrical stimulation. Moreover, this implied that the EC coating attenuated the electrically mediated maturation of the embedded cardiomyocyte modules in the shrunken, remodeled tissue, an effect noted earlier, manifesting as increased excitation potential and reduced maximum capture rate[12]. Troponin I staining (Figure 4c) showed the presence of striated muscle fibers for stimulated CM-embedded modules (without RAEC) distributed throughout the entire tissue, which had adopted a sheet-like morphology that spanned the gap between the electrodes. In all other conditions, cells remained spherical and did not form elongated muscle fibers. In the case of the EC-coated modules, CD31 staining indicated sparse coverage after 12 days of culture, with or without electrical stimulation (Figure 4c).
Table 2.
Maximum Capture Rate and Excitation Threshold Measurements for Stimulated (3V), non-EC Coated CM-embedded tissues on day 12. Three replicates were tested for this condition.
| UNIT | MCR (Hz) | ET (V/cm) |
|---|---|---|
| 1 | 2 | 3.8 |
| 2 | 3 | 8 |
| 3 | 2 | 6 |
| mean ± SD | 2.3 ± 0.58 | 5.9 ± 2.10 |
Thus to maintain tissue construct volume, a repeated addition of modules is necessary to compensate for the initial shrinkage. Here, high-speed, on-demand production of modules may prove to be a great advantage because it facilitates periodic, large-volume bioreactor refills. This may also be applicable to other tissue types that have great difficulty with maintaining large volumes, such as engineered adipose tissue[19–21].
3. Conclusions
A new method for the rapid and reproducible production of modules for the bottom-up construction of large-scale tissue-engineered constructs was created. This was done by combining air shearing and a plug flow reactor-style design. This system was capable of generating modules with tunable diameters and lengths. With this technique, many cell types can be embedded within the module without loss of viability. Additional complexity is also possible by having a mixture of cells within a single module or by producing a variety of modules with different embedded and surface-seeded cells. To illustrate what is achievable when a surplus of modules is available, a tissue-like construct that recapitulated the shape and size of an adult rat liver was produced. Moreover, the use of electrical stimulation was demonstrated as a first step towards more sophisticated multiple cell constructs. Besides creating large-volume constructs with defined shape, the bottom-up modular assemblies may also have applications in drug screening, especially considering the high speed production technique discussed herein.
4. Experimental Section
4.1. Module Length Measurement
Once produced and gelled, the modules were imaged while still within the tubing. Images were then scale calibrated in ImageJ (NIH, version 1.42) and their size was measured. Module length was varied by fixing air pressure at 47.5 kPa and varying collagen flow rate. In another experiment, collagen flow rate was fixed at 5.85 mL/hr. and the air pressure was varied.
4.2. Cell Culture and Seeding
Several different cell types were used in different aspects of the project. These, along with media, are listed in Table 3. For modules with embedded cells, cardiomyocyte-enriched cell isolates from neonatal rat pups were suspended at 2×107 cells/mL in collagen/Matrigel™ (80/20 v/v; 3 mg/mL collgen, BD) as before[22] while all other cell types were embedded at a concentration of 2×106 cells/mL in collagen. Primary endothelial cells (EC, below passage 6) were seeded onto module surfaces as previously outlined[13]. Briefly, EC between passage 2 and 6 at a concentration of 1.5–2.0 × 106 cells per mL of settled modules were added to modules in a 15 mL centrifuge tube and incubated under sterile conditions at 37°C for 60 min with gentle shaking every 10 min.
Table 3.
Cell types and culture media
| Cell | Medium |
|---|---|
| Human umbilical vein EC (HUVEC, Lonza, Basel, Switzerland) | EGM-2 medium (Lonza) |
| Rat aortic endothelial cells (RAEC, VEC Technologies, Rensselaer, NY) | Serum supplemented MCDB-131-based EC medium (VEC Technologies) |
| L929 cells (ATCC), transduced to produce luciferase, as described elsewhere[15] | Serum supplemented RPMI 1640 (Invitrogen, Burlington, ON) |
| NIH 3T3 cells (ATCC, Manassas, VA) | DMEM, (Invitrogen) supplemented with 10% calf serum (Sigma) |
| Cardiac cells, primary isolate from neonatal Sprague-Dawley rats according to a published protocol[22] | If no EC: DMEM (25 mM glucose and 0 mM sodium pyruvate, Gibco) with 10% bovine serum (Sigma) and 1% Hepes (Gibco). With EC: a co-culture medium composed of DMEM with 5.5 mM glucose and 1.28 mM sodium pyruvate supplemented with 10% bovine serum (Sigma) |
All medium were also supplemented with 1% penicillin/streptomycin (Lonza).
4.3. Plug Flow Module Production
As illustrated in Figure 1, neutralized and bicarbonate buffered collagen (3 mg/mL, Sigma, Mississauga, ON) or a collagen/Matrigel mixture with or without suspended cells was pumped via syringe pump (Razel Scientific Instruments, Inc., Stamford, CT) into the side inlet of the T-junction fabricated from polysulfone. Masterflex Tygon L/S 13 lab tubing (Cole-Parmer Instrument Co., Vernon Hills, IL) connected the syringe to the T-junction via Luer locks (Cole-Parmer Instrument Co.). Compressed air from a house line was directed into the upper inlet also using Masterflex tubing and Luer locks. 3 meter long strands of poly(ethylene) tubing (PE60, 0.76 mm ID, Intramedic, Becton Dickinson, Franklin Lakes, NJ) fit tightly over the T-junction outlet but could be removed after filling and replaced with empty tubing (different lengths can be used).
4.4. Liver Shaped Bioreactor Fabrication and Assembly
As illustrated in Figure 3a rat liver was obtained from a euthanized Sprague-Dawley rat and was first fixed in 5% buffered formalin (Sigma, Oakville, ON) overnight. After air-drying, the liver was wrapped in plastic wrap to prevent PDMS penetration into the liver tissue. 20-gauge needles were threaded through two female Luer threads (Cole Parmer Instrument Co.) and inserted into the top and bottom of the liver. To create the mold, the liver was cast in poly(dimethyl siloxane) (PDMS, Sylgard® 184 silicone elastomer (Dow Corning Corporation, Midland, MI), which was degassed under vacuum and cured at 75 °C for 2 hours. After curing, the needles were removed, the PDMS cast was cut open and the plastic wrapped liver was removed, leaving behind the liver shaped space and embedded Luer threads which acted as inlet and outlet. The cast was re-sealed with cured PDMS and sterilized through steam autoclaving prior to use. A 5 mL syringe and 16 gauge needle was used to inject modules into the bioreactor. Modules (3.5 mL; ~1000) embedded with luciferase expressing L929 cells at a concentration of 2×106 cells/mL were loaded into the bioreactor through the Luer lock port. During loading, the bottom Luer lock was closed with Luer locking caps to avoid leakage. The loaded bioreactor was then connected to a multichannel peristaltic pump (Cole-Parmer Instrument Co.). Culture medium was perfused at 0.4 mL/min and the reservoir was filled with 12 mL of fresh medium after 2 days of culture. Flow dampeners were constructed to eliminate pulsatile flow and backflow, as described elsewhere[23].
4.5 Cardiac Bioreactor Fabrication and Assembly
For cardiac cell studies, bioreactors were designed to mimic the cardiac environment and constructed using the same method described above; however, the plastic-wrapped organ was replaced with a 5 mL round bottom polypropylene tube (Falcon). When the PDMS cast was cut open to remove the polypropylene tube, platinum wires (Surepure Chemetals Inc, Florham Park, NJ) were positioned on opposite sides of the window to run lengthwise along the inside of the bioreactors (Figure 4a). The opening was then resealed in its original position with PDMS. The inner diameter and length of the flow chambers were 10 mm and 45 mm respectively (volume ~ 3.5 mL). Approximately 3.5 mL of modules embedded with cardiomyocytes at a concentration of 2×107 cells/mL were loaded in each bioreactor immediately after gelation. Cardiomyoctye-containing modules with EC coating were seeded (as above) prior to loading into bioreactor.. A collagen/Matrigel blend (80/20 percent by volume) was used for this set of experiments.
Modules were cultured for 12 days under 0.4 mL/min media flow at 3V electrical stimulation in the flow circuit described above. In this case fresh medium was exchanged each day. A signal generator (Grass Technologies) connected to the platinum electrodes provided the desired electrical stimulation (1Hz biphasic, 2 ms square pulse). Stimulation tests were carried out on day 12 of culture to test for maximum capture rate (MCR) and excitation threshold (ET). ET was defined as the minimum voltage required for synchronous contraction at 1 Hz. MCR was defined as the maximum rate of synchronous contraction under an electric field equivalent to twice the ET.
4.6 Immunohistochemical and Immunofluorescent Imaging
Cell viability was estimated by live/dead imaging using a LIVE/DEAD® viability/cytotoxicity kit (Life Technologies, Grand Island, NY). Modules were imaged at the Advanced Optical Microscope Facility (University Health Network, Toronto, Ontario) using a Zeiss LSM 510 META NLO confocal microscope. Modules were imaged using the same confocal microscope settings for objective, gain and offset, all optimized to prevent saturation. For live/dead imaging, ImageJ macros were written to split the live (green) and dead (red) channels, convert to 8-bit threshold images and run the Analyze Particles function to count the number of nuclei. Viability was calculated as the ratio of live cells to the total number of cells. To assess EC coverage, EC-seeded modules were fixed with 4% paraformaldehyde (EMS, Fort Washington, PA) and stained for VE-cadherin as described elsewhere[23].
As previously described[12], cardiac cell containing modules were stained for cardiac troponin I, a protein subunit associated with the troponin–tropomyosin complex in cardiac muscle. Contaminating fibroblasts from cardiomyocyte-rich suspension were identified by staining for vimentin. EC-containing cardiac modules were stained for the endothelial cell marker CD31 as described previously[10].
4.7 Bioluminescent Imaging
With luciferase transduced L929 cells, a Xenogen IVIS Imaging System (Xenogen, Alameda, CA) at the Advanced Optical Microscope Facility was used to assess cell viability[15]. D-luciferin (Promega, Madison, WI) was diluted in 5 mL of medium, added to the bioreactor flow reservoir (final concentration of 2.5 mg/mL), and circulated for 15 minutes. After circulation, the bioreactor was removed and sealed with Luer locking caps (Cole-Parmer Instrument Co.) in a sterile field and transported to the Xenongen imager. The bioluminescence (photon flux intensity) was imaged and recorded using the Xenogen software. After imaging, bioreactors were returned to their flow circuits to continue perfusion.
4.8 Statistical Analysis
Under either constant collagen flow rate or constant air pressure, module lengths were normally distributed (as determined by Q-Q plots) and independent sample means were compared using the student ANOVA with the Tukey HSD multiple-comparison correction. Differences were considered statistically significant if p < 0.05. For box plots, the bottom and top of the box were the lower (Q1) and upper (Q3) quartile measurements, respectively. The interquartile range, IQR, was Q3-Q1. The bottom whisker was Q1-1.5(IQR) and the top whisker was Q3+1.5(IQR). The center line was the median measurement.
Acknowledgements
The authors acknowledge the financial support of the Natural Sciences and Engineering Research Council, the Canadian Institutes of Health Research and the US National Institutes of Health (EB 006903). O.F. Khan and D.N. Voice contributed equally to this work. O.F. Khan acknowledges scholarship support from the Ontario Graduate Scholarship Program. D.N. Voice acknowledges scholarship support from the Microfluidic Applications and Training in Cardiovascular Health Program distributed through the NSERC CREATE program. B.M. Leung acknowledges financial support from the Training Program in Regenerative Medicine (PI: G. A. Levy).
Contributor Information
Omar F. Khan, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, M5S 3G9, Canada; Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, M5S 3E5, Canada.
Derek N. Voice, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, M5S 3G9, Canada.
Brendan M. Leung, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, M5S 3G9, Canada
Michael V. Sefton, Email: michael.sefton@utoronto.ca, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, M5S 3G9, Canada; Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, M5S 3E5, Canada.
References
- 1.Matsunaga YT, Morimoto Y, Takeuchi S. Advanced Materials. 2011;23:H90. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- 2.Palmiero C, Imparato G, Urciuolo F, Netti P. Acta Biomaterialia. 2010;6:2548. doi: 10.1016/j.actbio.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Livoti CM, Morgan JR. Tissue Eng. Part A. 2010;16:2051. doi: 10.1089/ten.tea.2009.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan V, Zorlutuna P, Jeong JH, Kong H, Bashir R. Lab on a Chip. 2010;10:2062. doi: 10.1039/c004285d. [DOI] [PubMed] [Google Scholar]
- 5.Li CY, Wood DK, Hsu CM, Bhatia SN. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2011;11:2967. [Google Scholar]
- 6.Scott EA, Nichols MD, Kuntz-Willits R, Elbert DL. Acta Biomaterialia. 2010;6:29. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, Ghodousi M, Qi H, Haas N, Xiao W, Khademhosseini A. Biotechnology and Bioengineering. 2011;108:1693. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du YA, Ghodousi M, Lo E, Vidula MK, Emiroglu O, Khademhosseini A. Biotechnology and Bioengineering. 2010;105:655. doi: 10.1002/bit.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du YA, Lo E, Ali S, Khademhosseini A. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9522. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan OF, Chamberlain MD, Sefton MV. Tissue Eng Part A. 2012;18:744. doi: 10.1089/ten.tea.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan OF, Sefton MV. Biomaterials. 2010;31:8254. doi: 10.1016/j.biomaterials.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung BM, Sefton MV. Tissue Engineering - Part A. 2010;16:3207. doi: 10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuigan AP, Sefton MV. Proc.Natl.Acad.Sci.U.S.A. 2006;103:11461. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corstorphine L, Sefton MV. Journal of Tissue Engineering and Regenerative Medicine. 2011;5:119. doi: 10.1002/term.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng D, Lo C, Sefton MV. J Biomed Mater.Res.A. 2008;87:321. doi: 10.1002/jbm.a.31716. [DOI] [PubMed] [Google Scholar]
- 16.Ossenberg FW, Denis P, Benhamou JP. Journal of Applied Physiology. 1974;37:806. doi: 10.1152/jappl.1974.37.6.806. [DOI] [PubMed] [Google Scholar]
- 17.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2008;8:1056. [Google Scholar]
- 18.Young CJ, Poole-Warren LA, Martens PJ. Biotechnology and Bioengineering. 2012;109:1561. doi: 10.1002/bit.24430. [DOI] [PubMed] [Google Scholar]
- 19.Butler MJ, Sefton MV. Tissue Eng. Part A. 2012;18:1628. doi: 10.1089/ten.tea.2011.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick CW, Zheng B, Johnston C, Reece GP. Tissue Engineering. 2002;8:283. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 21.von Heimburg D, Zachariah S, Heschel I, Kuhling H, Schoof H, Hafemann B, Pallua N. Biomaterials. 2001;22:429. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 22.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan OF, Sefton MV. Biomedical Microdevices. 2011;13:69. doi: 10.1007/s10544-010-9472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]