Abstract
IFITM3 restricts cellular infection by multiple important viral pathogens, and is particularly critical for the innate immune response against influenza virus. Expression of IFITM3 expands acidic endolysosomal compartments and prevents fusion of endocytosed viruses, leading to their degradation. This small, 133 amino acid, antiviral protein is controlled by at least four distinct post-translational modifications. Positive regulation of IFITM3 antiviral activity is provided by S-palmitoylation, while negative regulatory mechanisms include lysine ubiquitination, lysine methylation and tyrosine phosphorylation. Herein, we describe specific insights into IFITM3 trafficking and activity that were provided by studies of IFITM3 post-translational modifications, and discuss evidence suggesting that IFITM3 adopts multiple membrane topologies involving at least one intramembrane domain in its antivirally active conformation.
Keywords: antiviral, IFITM3, intramembrane domain, methylation, palmitoylation, phosphorylation, post-translational modification, ubiquitination
The IFITMs comprise a family of small, 10–15 kDa proteins [1, 2] that restrict cellular infection by a variety of significant viral pathogens of humans and animals [3–13]. IFITMs contain variable N- and C-termini but are characterized by two hydrophobic segments resembling transmembrane domains linked by a highly conserved region of approximately 30 amino acids [14]. Vertebrate IFITMs are thought to have derived from a horizontal gene transfer of bacterial origin to a unicellular ancestor of metazoans [2], and IFITMs from diverse species such as fish, chickens, mice and humans have conserved antiviral activity [3, 6, 15, 16]. Several of the most highly conserved residues in IFITMs are altered with post-translational modifications (PTMs; Table 1), suggesting that PTMs may have evolutionarily ancient functions in regulating IFITM activity [2, 17]. These PTMs include three palmitoylated cysteines [4], one methylated and four ubiquitinated lysines [18, 19] and a phosphorylated tyrosine [20, 21].
Table 1.
Summary of known post-translational modifications occurring on IFITM3.
| Modification | Amino acid | Position | Enzyme | Ref. |
|---|---|---|---|---|
| Palmitoylation | Cysteine | 71, 72, 105 | Unknown | [4] |
| Ubiquitination | Lysine | 24, 83, 88, 104 | Unknown | [18] |
| Methylation | Lysine | 88 | Set7 | [19] |
| Phosphorylation | Tyrosine | 20, 27† | Fyn | [20,21] |
Nonconserved, found in murine IFITM3.
Gene duplications in humans have resulted in the presence of IFITMs 1, 2, 3, 5 and 10, while mice encode at least seven homologous Ifitm genes for IFITMs 1, 2, 3, 5, 6, 7 and 10 [22]. The Ifitm4 gene is present in mice, but has lost the ability to produce a protein product and is considered a pseudogene [2]. The mouse and human IFITMs 1–3 and 5 show mostly overlapping abilities to inhibit different viruses in overexpression assays in vitro, though IFITM5 is not IFN inducible and some differences in their antiviral function have been observed (recently reviewed in [23]). Unique roles for specific IFITMs have also emerged from genetic studies and in vivo experimentation. For example, IFITM5 is expressed specifically in bone and has evolved to perform critical roles in osteoblast function and bone mineralization [17, 24–26]. On the other hand, IFITM3 is essential for restriction of influenza virus infections in both mice and humans. Ifitm3 knockout mice develop severe influenza virus infections, succumbing to doses of virus that are sublethal to wild-type mice and, interestingly, viral infections were not further exacerbated when comparing Ifitm3 knockout mice with mice deficient for all IFITMs [27, 28]. Importantly, a genetic polymorphism at a splice site in the human IFITM3 gene, which is thought to result in a truncated and less active protein, has been associated with severe cases of H1N1 and H7N9 influenza virus infections [27, 29–30]. Taken together, these data suggest that other IFITMs are not able to compensate for a loss of IFITM3 in restricting influenza virus infection in vivo. Thus, much recent work has focused on understanding the molecular mechanisms controlling IFITM3-mediated virus restriction. Herein, we provide a model for IFITM3 activity, incorporating the current knowledge of IFITM3 post-translational regulation by palmitoylation, ubiquitination, methylation and phosphorylation. Studying these PTMs has provided insights into IFITM3 trafficking, membrane topology and activity, and may ultimately provide entry points for manipulating IFITM3 for therapeutic purposes.
IFITM3 mechanism of antiviral action
IFITM3 has been shown to inhibit viral infections by preventing virus fusion with endolysosomal membranes [10, 31–33]. This is in accord with early studies using retroviruses pseudotyped with distinct viral surface proteins that showed IFITM3 inhibition of infection is dependent upon the viral glycoprotein used for entry and fusion [3]. Comprehensive lists of viruses shown to be inhibited by IFITMs have been recently compiled and confirm that the majority of viruses inhibited by IFITM3, including influenza virus, SARS coronavirus, dengue virus and West Nile virus, among many others, share a common ability to enter cells via endocytosis and utilize pH-dependent fusion mechanisms [34, 35]. Conversely, Sendai virus and Moloney leukemia virus, which fuse at the cell surface, are only modestly affected by IFITM3 [3, 36]. Additionally, treatment of SARS conronavirus with trypsin allows the virus to fuse at the cell surface by eliminating the need for its proteolytic activation by cathepsin L in lysosomes, resulting in virus evasion of IFITM3-mediated restriction [6].
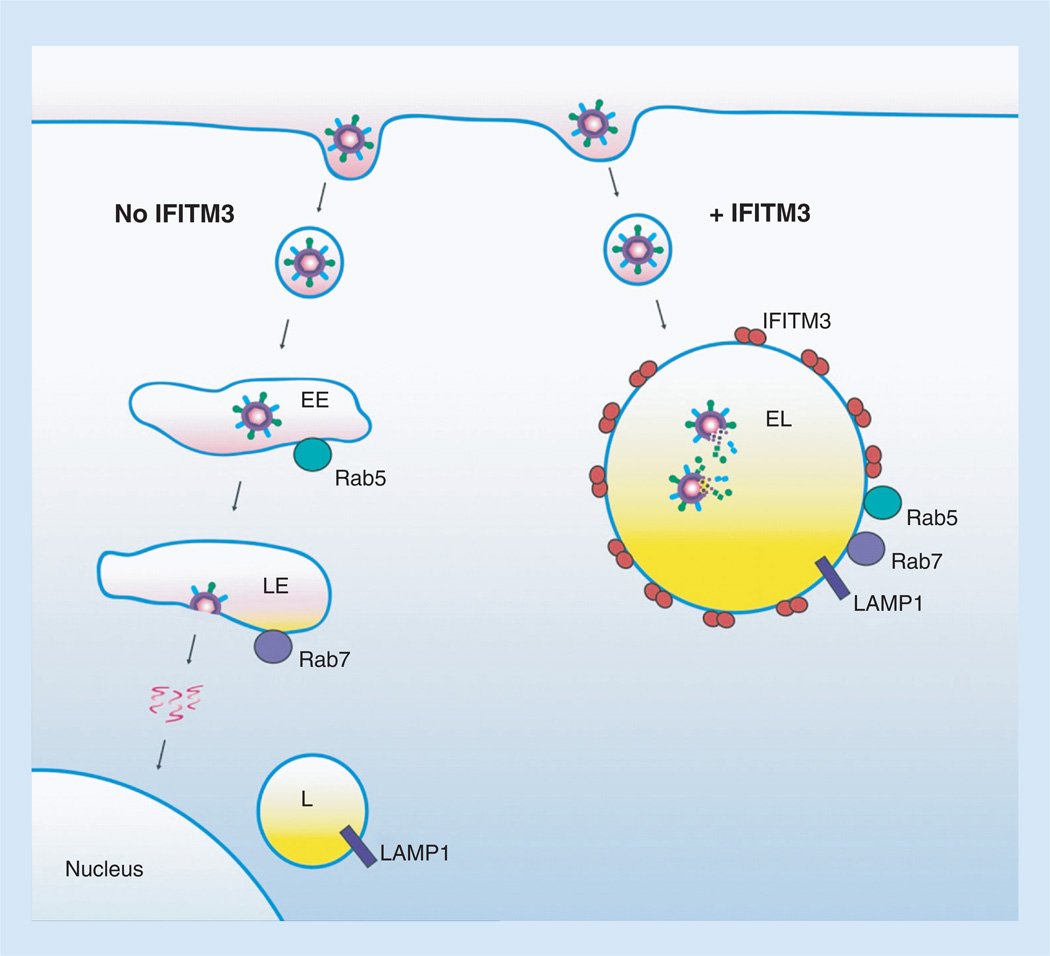
IFITM3 dimerizes [37] and localizes with markers of endosomes and lysosomes, and these compartments are characterized by increased size and decreased pH in cells expressing IFITM3 [6, 18, 31, 38]. Our work with a hyperactive IFITM3 mutant lacking ubiquitination sites suggested that Rab5- and Rab7-positive endosomes and acidic LAMP1-positive lysosomes are aberrantly merged upon IFITM3 expression, providing a compartment for viral degradation (and explaining why we refer to IFITM3-positive compartments as endolysosomes) [18]. This compartment also stains positive for the autophagosome marker LC3 [18, 31], and has characteristics of multivesicular bodies when visualized by electron microscopy [39], indicating that the effects of IFITM3 on the endocytic pathway are multifaceted. Indeed, influenza virus particles have been visualized to enter cells expressing IFITM3 but are eliminated by degradation prior to their fusion/escape from IFITM3-positive endolysosomes [31, 40]. The expansion and alteration of the endolysosomal compartment in IFITM3-expressing cells and inhibition of influenza virus were found to be independent of the canonical autophagy protein 5-dependent autophagy induction pathway [18], but may involve an interaction between IFITM3 and the vacuolar ATPase [38].
The confluence of data discussed above supports a model whereby viruses are degraded in acidic endolysosomes in IFITM3-expressing cells (Figure 1). However, this model contains a paradox in that influenza virus fusion is normally triggered by lowered pH in late endosomes and yet fusion is apparently prevented in low-pH endolysosomes when IFITM3 is present (Figure 1) [31–33, 40]. Two primary theories have emerged to explain inhibition of viral fusion by IFITM3:
Theory 1 stems from the observation that increased cholesterol levels are present within endolysosomes of cells expressing IFITM3 as compared with control cells [33, 39]. Changes in cholesterol were shown to be dependent upon an interaction of IFITM3 with VAPA that interrupts a homeostatic partnership between VAPA and a cholesterol trafficking protein [39]. Proponents of this theory posit that dysregulation of cholesterol homeostasis is responsible for virus inhibition. However, more recent publications have challenged this notion, showing minimal effects of either cholesterol depletion or modulation of VAPA levels on IFITM3 activity [33, 40]. Likewise, Niemann–Pick type C1 fibroblasts, which contain high levels of cholesterol in the late endosomal compartment, show little change in influenza virus infectivity when compared with control cells [39, 40]. Thus, though potentially complementary to other antiviral functions of IFITM3, it remains unclear whether cholesterol dysregulation is essential for the primary mechanism of action of IFITM3;
Theory 2 has arisen from two independent studies demonstrating decreases in membrane fluidity in cells expressing IFITMs [32, 40]. The necessity of membrane fluidity for efficient fusion of a multitude of viruses has been appreciated for decades [41], and though IFN-induced enzymes can influence membrane lipid composition [42], IFITM3 would be the first cellular antiviral effector that limits infection through directly altering the ‘rigidity’ of cell membranes [43]. Discovering the molecular details through which IFITM3 alters the structural features of endolysosomal membranes to inhibit virus fusion, and deciphering the potential role that cholesterol dysregulation may play in this process are exciting areas of future research.
Figure 1. Model of influenza virus entry and IFITM antiviral activity.
Influenza virus enters cells via receptor-mediated endocytosis and fuses in the acidic late endosomal compartment, depositing its genomic contents into the cytosol, thereby avoiding lysosomal hydrolases. In cells expressing IFITM3, viruses are localized in an enlarged acidic and degradative compartment staining positive for endosomal and lysosomal markers. Virus fusion is inhibited by alterations to the endolysosomal membrane imposed by IFITM3, and virions are subsequently degraded. IFITM3 is depicted as a dimer.
EE: Early endosome; EL: Endolysosome; L: Lysosome; LE: Late endosome.
IFITM3 palmitoylation
Palmitoylation is the enzymatic addition of a 16-carbon fatty acid to proteins, most often occurring on cysteine residues (known as S-palmitoylation in reference to the thioester linkage between the fatty acid and the sulfur-bearing side chain) [44]. This hydrophobic modification results in the membrane attachment of otherwise cytoplasmic proteins [45, 46]. Palmitoylation can also have a variety of effects on proteins that are already membrane-associated, including targeting to specific cellular compartments, modulation of protein interactions or stability and modulation of protein structure or positioning in membranes [47]. We identified IFITM3 in a chemical proteomics screen designed to detect immunityrelated proteins that are post-translationally palmitoylated [4, 48]. At the time, there were no reports of antiviral activity for IFITM3, yet its well-established induction by type I IFN [49, 50] prompted us to further investigate its PTM and potential antiviral function. We were able to confirm IFITM3 S-palmitoylation occurring on three cysteines within or adjacent to its two hydrophobic domains at positions 71, 72 and 105 [4, 18, 51]. A palmitoylation-deficient, triple cysteine-to-alanine IFITM3 mutant showed a more diffuse pattern of localization than wild-type IFITM3, indicating that S-palmitoylation promotes IFITM3 clustering, and also suggested that, despite its two hydrophobic stretches predicted to be transmembrane domains, a fraction of IFITM3 may not be associated with membranes [4]. Indeed, cellular fractionation experiments have indicated that a significant amount of endogenous IFITM3 is not strongly membrane-bound and that membrane association is increased by S-palmitoylation [18]. Importantly, we also found that overexpressed IFITM3 was able to inhibit influenza virus infection in a variety of cells types, and that this effect was largely lost upon mutation of its S-palmitoylated cysteines [4]. Concurrently, at least three additional groups also identified IFITM3 as an antiviral effector protein active against multiple viruses in large-scale screening studies, providing validation of the antiviral effects we observed for IFITM3, and suggesting that its palmitoylation is likely important for innate immunity to a variety of infections [3, 7, 52].
Alternative lipid modification sites added to the N- and C-termini of IFITM3 (myristoylation and prenylation, respectively) were able to rescue the membrane affinity and antiviral activity of the palmitoylation-deficient IFITM3 mutant, suggesting that S-palmitoylation does not act by inducing a structural change in the IFITM3 hydrophobic regions, but rather, acts by increasing membrane affinity [18]. Thus, studies of IFITM3 S-palmitoylation would indicate that proper membrane anchoring, and potentially also clustering of IFITM3, are the features mediated by S-palmitoylation that are essential for its antiviral function. S-palmitoylation is conserved on all IFITMs that have been tested to date (human IFITMs 1, 2, 3, 4 and 5 [53], and mouse IFITMs 1 [36] and 3 [4]), and indeed the palmitoylated cysteines are among the most conserved IFITM residues present throughout evolution [2]. Interestingly, palmitoylation of murine IFITM1 is essential for preventing its proteasomal degradation [36], and palmitoylation of human IFITM5 is necessary for its interaction with a factor regulating its ability to promote bone formation [53], suggesting that, though S-palmitoylation is a conserved modification, it may have distinct effects on different IFITMs. Palmitoyltransferase(s) responsible for modifying IFITM3 and other IFITMs have not yet been identified. These enzymes may themselves represent novel virus susceptibility factors if polymorphisms in the human population exist that render them unable to modify IFITM3, thus warranting their identification and study.
IFITM3 ubiquitination
Ubiquitination is the addition of the 9 kDa ubiquitin polypeptide to proteins usually on lysines. Upon overexposure of IFITM3 western blots, specific bands above the 15 kDa expected molecular weight can be observed at approximately 24 and 33 kDa [18, 21], corresponding to shifts that would be predicted for mono- and di-ubiquitination, respectively. By immune-precipitating IFITM3 and examining these upper bands by MS/MS ubiquitin peptides and lysine-containing IFITM3 peptides that were covalently modified with a diglycine motif, which is the characteristic signature of ubiquitination that remains following trypsin digestion, were identified [18]. Several large-scale MS studies aiming to identify ubiquitinated proteins have also identified various IFITMs as substrates for ubiquitination [54–57]. MS results were confirmed by antiubiquitin blotting of immunoprecipitated IFITM3, which revealed that IFITM3 is also polyubiquitinated with both Lys-48 and Lys-63 linkages [18]. These polyubiquitin linkages, which often control protein turnover or trafficking, respectively [58], suggest that ubiquitination of IFITM3 has multiple effects.
IFITM3 contains four conserved lysines. IFITM3 constructs in which three of the four lysines were mutated to alanine allowed the demonstration that ubiquitination can occur at all four positions, though Lys-24 appeared to be the most strongly modified residue, particularly with Lys-63 poly-ubiquitin chains [18]. However, Lys-24 mutagenesis resulted in no overt changes in antiviral activity, localization, or abundance of IFITM3, indicating that other lysines may functionally compensate for the role of ubiquitination on this residue [18, 20, 37]. In fact, a novel phenotype was only observed for IFITM3 when all four lysines were mutated to alanine. Lysine-less IFITM3 showed increased stability in pulse-chase experiments, and localized entirely to endolysosomes, losing the overlap with endoplasmic reticulum (ER) markers that is observed for a portion of wild-type IFITM3 [18]. This may suggest that ubiquitinated IFITM3 is recruited to an ER-proximal site for degradation. Hyperactivity was also observed for the ubiquitination-deficient, lysine-less IFITM3 mutant in inhibiting influenza virus correlating with its complete localization to endolysosomes, thereby further supporting a model of antiviral activity in which endolysosomal localization is essential [18]. The specific roles of the different linkages of polyubiquitin chains on IFITM3 remain to be decrypted, and likewise, the ubiquitin ligase(s) responsible for modifying IFITM3 have yet to be identified. As the available data suggest that ubiquitination is a negative regulator of IFITM3 stability and activity, these enzymes, once identified, may represent possible therapeutic targets for improving IFITM3-based antiviral immune responses.
IFITM3 methylation
In addition to being ubiquitinated, Lys-88 of IFITM3 can also be monomethylated by Set7 [19], a lysine methyltransferase that was initially reported to methylate histone H3 [59]. Aside from the predominant methylation of chromatin and its primary localization in the nucleus, Set7 also methylates several cellular nonhistone proteins and the HIV protein Tat [60–64]. Thus, IFITM3 is one of a growing list of proteins reportedly regulated by Set7. Monomethylation of Lys-88 was discovered using MS and was confirmed upon development of a Lys-88-methylation-specific anti-IFITM3 monoclonal antibody [19]. In this study, methylation was increased by Set7 overexpression correlating with a loss of antiviral activity. Conversely, knockdown of Set7 decreased IFITM3 methylation and resulted in enhanced IFITM3-mediated restriction of influenza virus and vesicular stomatitis virus. Interestingly, infection with either of these viruses increased IFITM3 methylation measured as early as 4 h post infection, while IFN-α treatment decreased methylation. Since the net result of virus infection is increased methylation, despite the presumed induction of type I IFNs, this may suggest that these viruses actively promote IFITM3 methylation as an immune evasion strategy.
The clear importance of IFITM3 methylation, as established by experiments modulating levels of Set7 [19], make it surprising that mutation of Lys-88 does not result in major changes to IFITM3 activity against influenza virus, dengue virus or vesicular stomatitis virus [19, 37]. As Lys-88 can also be ubiquitinated [18], the prevention of these two negative regulatory modifications through Lys-88 mutation might have been expected to increase antiviral activity. However, analogously to the ability of multiple IFITM3 lysines to be ubiquitinated, compensatory methylation may occur at another lysine in the absence of Lys-88, which will require further investigation. Continued study to determine what effect methylation has on IFITM3 protein–protein interactions, stability or modification with other PTMs will aid in mechanistically characterizing the effects of this modification. Despite the withstanding questions, Set7 is capable of affecting IFITM3 antiviral activity, and this knowledge broadens the repertoire of IFITM3-modulating enzymes that represent potential targets for antiviral therapy.
IFITM3 phosphorylation
Phosphorylation of IFITM3 was identified on Tyr-20 of both mouse and human IFITM3, and is mediated by the Src-family protein-tyrosine kinase Fyn [20, 21]. Tyr-20 is largely conserved throughout evolution, and recent work identified its involvement in a YxxΦ sorting motif (where Y denotes a tyrosine, x is any amino acid, and Φ denotes valine, leucine or isoleucine) that is critical for IFITM3 endocytosis [21, 65]. Disruption of this motif by mutagenesis led to accumulation of IFITM3 at the plasma membrane and loss of antiviral activity [20, 21, 37, 65]. Increasing the phosphorylation of this motif through Fyn overexpression led to a similar accumulation of IFITM3 at the plasma membrane, suggesting that phosphorylation acts as a negative regulator of activity by blocking the YxxΦ motif from interaction with endocytic adaptor complexes [21]. In fact, work by Jia, et al. identified the AP-2 complex as essential for IFITM3 trafficking to endolysosomes through recognition of the YxxΦ tetrapeptide [65]. It has been noted that IFITMs lacking this motif, such as human IFITM1, show increased localization at the plasma membrane, which may partially explain differences in activity observed when comparing IFITM1 and IFITM3 in overexpression assays, and perhaps the inability of IFITM1 to compensate for IFITM3 at endogenous levels [40]. Similarly, the human IFITM3 gene polymorphism, that has been associated with increased severity of influenza virus infections, is predicted to result in a truncation of the first 21 amino acids of the IFITM3 N-terminus [27]. Thus, this protein’s defect in antiviral activity may be explained, at least in part, by a loss of the YxxΦ motif and thus its proper trafficking to endolysosomes.
We initially predicted that phosphorylation of Tyr-20 and retention of IFITM3 at the plasma membrane might also allow for the recruitment of E3 ubiquitin ligases to mark IFITM3 for degradation since phosphorylation often serves as a cue for this process [66]. On the contrary, we found that increasing IFITM3 phosphorylation by over-expressing Fyn, and that mutating Tyr-20, both significantly decreased ubiquitination of IFITM3, suggesting that unmodified Tyr-20 might also be a critical component of a ubiquitin ligase recognition motif [21]. Indeed, the 17-PPNY-20 motif of IFITM3 has been noted to conform to the PPxY pattern recognized by HECT domain-containing E3 ubiquitin ligases, though to date, no function has been ascribed to this sequence [20].
Studies of IFITM3 phosphorylation have revealed important details regarding IFITM3 localization and trafficking, as they provided evidence that IFITM3 localizes to the plasma membrane prior to trafficking to endolysosomes [21, 65]. Fyn is cotranslationally myristoylated and postranslationally palmitoylated, rapidly targeting and anchoring it to the cytoplasmic leaflet of the plasma membrane [67, 68], and its direct interaction with IFITM3 further supports the notion that IFITM3 naturally traffics to the plasma membrane [20]. Fyn plays a critical role in several biological processes, including T-cell signaling [69], cell survival [70] and cell adhesion-mediated signaling [70]. It is kept in an inactive state through phosphorylation of its C-terminal tyrosine by C-Src kinase [71], and can be activated through dephosphorylation by protein tyrosine phosphatases [72]. Interestingly, tyrosine phosphorylation of IFITM3 is difficult to detect without pretreatment of cells with protein tyrosine phosphatase inhibitors, which suggests that phosphorylation of IFITM3 is also a reversible and dynamic process [20, 21]. The precise natures of the phosphatases involved in regulating Fyn and IFITM3 remain to be identified, and this knowledge may aid in understanding the sequence of events involved in phosphorylation and dephosphorylation of IFITM3, as well as the physiological triggers of the addition and removal of this PTM.
IFITMs adopt multiple membrane topologies
The membrane topology of IFITM3 is a subject of controversy, and evidence suggests that it adopts multiple conformations, with the anti-virally active topology being a topic of debate. Understanding this characteristic of active IFITM3 is essential for understanding its biophysical interactions with cellular membranes, its interactions with other cellular proteins and ultimately its mechanism of antiviral action. However, IFITM3’s presence at multiple cellular compartments including the plasma membrane, ER, autophagosomes and multivesicular endolysosomes, along with its potential for multiple topologies as discussed below, has made traditional topology mapping with protease digestion and protection assays difficult to interpret (unpublished data). Thus, other methods, including mapping of PTMs, have been used to infer its membrane topology.
IFITM3 is predicted to be a dual-pass transmembrane protein with its N- and C-termini facing into the lumen of cellular organelles such as the ER and endolysosomes, or into the extracellular space (Figure 2) [14]. Indeed, epitope tags at both termini can be detected extracellularly by antibody staining of nonpermeabilized cells [3, 5, 73]. Additionally, antibodies that bind the region linking the two hydrophobic domains require detergent permeabilization to stain IFITM3-expressing cells, indicating that this region is entirely intracellular [14]. Likewise, the positioning of the three conserved S-palmitoylated cysteines is consistent with this dual transmembrane topology [4]. Overall, these data would suggest that some portion of IFITM3 indeed exists as a dual-pass transmembrane protein (Figure 2). However, fractionation of cellular cytoplasmic and membrane compartments indicated that a significant portion of IFITM3 is present in the cytoplasm, in other words, it is not strongly membrane-associated [18]. The lack of an ER signal sequence at the N-terminus also makes it unclear precisely how efficiently IFITM3 membrane insertion might occur. These fractionation results were reminiscent of those obtained for the palmitoylated protein caveolin-1, in which its hydrophobic segment does not fully traverse the membrane, but rather forms a proposed hairpin-like structure within the membrane known as an intramembrane domain [74].
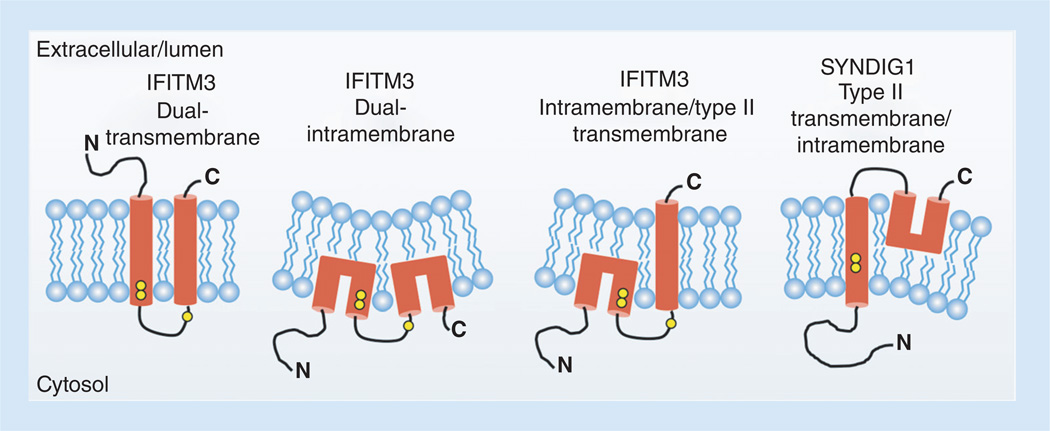
Figure 2. Membrane topologies for IFITM3 and SYNDIG1 that are supported by experimental evidence.
Data have been described supporting each of the shown topology models. Hydrophobic segments are shown in red. The IFITM3 dual transmembrane topology is predicted based on hydropathy plotting. However, IFITM3 in which its first hydrophobic segment adopts an intramembrane conformation is required for N-terminal access to cytoplasmic components necessary for its proper localization and activity. Whether the second hydrophobic segment in active IFITM3 traverses both membrane leaflets remains unclear. Yellow circles represent conserved cysteines that are known to be palmitoylated on IFITM3. Note that the cysteines in the first hydrophobic segment of SYNDIG1 appear earlier within the segment than IFITM3 cysteines making them more deeply embedded in the membrane and less likely to be palmitoylated. The third cysteine in SYNDIG1 is absent, agreeing with its experimentally determined topology. Potential changes in membrane properties induced by intramembrane domains are graphically depicted by altered membrane curvature.
An evolutionary study of IFITMs identified ten additional human genes that are likely related to IFITMs [2]. We examined whether topology mapping of any of the protein products for these genes had been previously performed. Membrane topology data for one of the IFITM relatives known as SYNDIG1 indicated that the second hydrophobic stretch in this protein is an intramembrane domain (Figure 2) [75]. Topology mapping for the additional nine relatives of the IFITMs remains to be performed. Nonetheless, this work provided a precedent for the presence of intramembrane domains within this family of proteins, and is also in accord with a previous prediction that the second hydrophobic domain of IFITMs may not be a true transmembrane domain due to the lack of sufficient anchoring residues at their C-termini, and because IFITMs from some species are truncated in this region [14].
Could active IFITM3 possess intramembrane domains rather than transmembrane domains? As discussed above, we proposed this possibility based first on the observations that a portion of IFITM3 is not strongly membrane-associated, and that IFITM3 palmitoylation increases its membrane affinity [18]. Additionally, analysis of PTMs supported the notion that the N-terminus of active IFITM3 is oriented toward the cytoplasm rather than extracellularly as predicted. First, ubiquitination of LyS-24 likely occurs in the cytoplasm since this is dogmatically the location of ubiquitin ligases [18]. Second, the conserved Tyr-20 residue is phosphorylated by the cytoplasmic kinase Fyn [20, 21]. Importantly, Tyr-20, as part of a YxxΦ motif, interacts with the cytoplasmic AP-2 complex that is essential for IFITM3 endocytosis and localization to endolysosomes [65]. Thus, the cytoplasmic orientation of the N-terminus is essential for proper IFITM3 localization and activity. It might also be concluded from these data that IFITM3 stained with N-terminus-directed antibodies at the cell surface of nonpermeabilized cells does not represent antivirally active IFITM3. It was also demonstrated that the N-terminus of an IFITM3 construct appended with a myristoylation motif is modified by cytoplasmic myristoylation machinery, and that this modification supported IFITM3 membrane association and antiviral activity [18]. In the same vein, a naturally occurring N-linked glycosylation consensus sequence in the N-terminus, 2-NHT-4, is only minimally modified by ER-lumenal glycosylation machinery [18, 73]. Likewise, additional glycosylation sites engineered within both the N- and C-terminal regions were mostly unmodified, agreeing with a cytoplasm-facing orientation of these domains for the majority of this protein [18]. Thus, the overwhelming data in support of active IFITM3 having a cytoplasmically oriented N-terminus has resulted in a general consensus that the first hydrophobic stretch within active IFITM3 is likely acting as an intramembrane domain (Figure 2) [18, 34, 37, 65, 73].
Conflicting evidence exists concerning whether the second hydrophobic stretch within active IFITM3 fully traverses the membrane bilayer. IFITM3 staining at the cell surface is stronger when staining for C-terminal epitope tags as compared with N-terminal tags, suggesting that a more significant fraction of the second hydrophobic domain fully spans the plasma membrane [65, 73]. It has also been reported that extensions of the C-terminus are partially cleaved in lysosomes [73]. This work suggested that a significant fraction of cellular IFITM3 exists as a type II transmembrane protein (Figure 2). Supporting this topology model, untagged human IFITM5 was recently demonstrated to adopt a type II transmembrane topology [76]. As IFITM5 has a significantly extended C-terminal domain compared with IFITM3 and localizes primarily to the cell surface, the relevance of this topology determination in relation to IFITM3 is not clear. Likewise, the importance of the type II transmembrane conformation in the antiviral activity of IFITM3 remains to be demonstrated, particularly since the percentage of cellular IFITM3 that adopts this topology has not been determined, and because this model was developed using IFITM3 constructs with C-terminal epitope tags that more than double the length of the C-terminal domain, potentially altering its ability to anchor a transmembrane region [65, 73]. Conversely, IFITM3 appended with a C-terminal CaaX box is prenylated by cytoplasmic enzymes, and is active against influenza virus [18]. Likewise, the murine IFITM1 protein has a nonconserved cysteine present within its C-terminus that is cytoplasmically S-palmitoylated and contributes to antiviral activity [36]. Overall, additional experiments involving endogenous IFITM3 or creatively engineered constructs that report on both IFITM3 topology and activity are required to conclusively determine the antivirally active IFITM3 membrane topology.
Conclusion & future perspective
PTM analysis of IFITM3 has revealed that it is dynamically trafficked to several compartments within the cell. Identification of the Tyr-20 phosphorylation site led to the discoveries that this residue is part of an endocytic motif and also plays a role in the control of IFITM3 ubiquitination. This work suggests that by default, IFITM3 traffics to the plasma membrane where it is then sorted in at least three distinct ways (Figure 3): IFITM3 can be phosphorylated by Fyn, thereby blocking its endocytic motif causing retention at the plasma membrane [21, 65]; IFITM3 can be ubiquitinated and trafficked away from the plasma membrane and endolysosomes to an ER-proximal site for degradation [18, 21]; or IFITM3 traffics via the AP-2 complex to endolysosomes, where it exerts its antiviral function through alteration of the organelle’s membrane properties such that endosomes and lysosomes are aberrantly merged and viruses are unable to fuse [65]. Intramembrane domains, though rarely found in proteins, inf luence membrane fluidity and curvature through what is known as the ‘bilayer couple effect’, in other words, lipids of the two membrane leaflets stay coupled to one another even as intramembrane domains wedge into only one side of the lipid bilayer [77]. Consistent with a role for IFITM3 specifically perturbing the outer leaflet of the endolysosomal membrane, a recent study found that hemifusion indicated by mixing of viral lipids with the inner leaflet of the endolysosome occurs, but that a fusion pore through both leaflets of the endolysosome bilayer is not achieved [33]. Thus, it is exciting to speculate that IFITM3 intramembrane domains are necessary components in its perturbation of the endolysosomal membrane. Future experiments will interrogate the role of intramembrane domains in IFITM3 antiviral activity and determine the precise effects IFITM3 has on membrane properties.
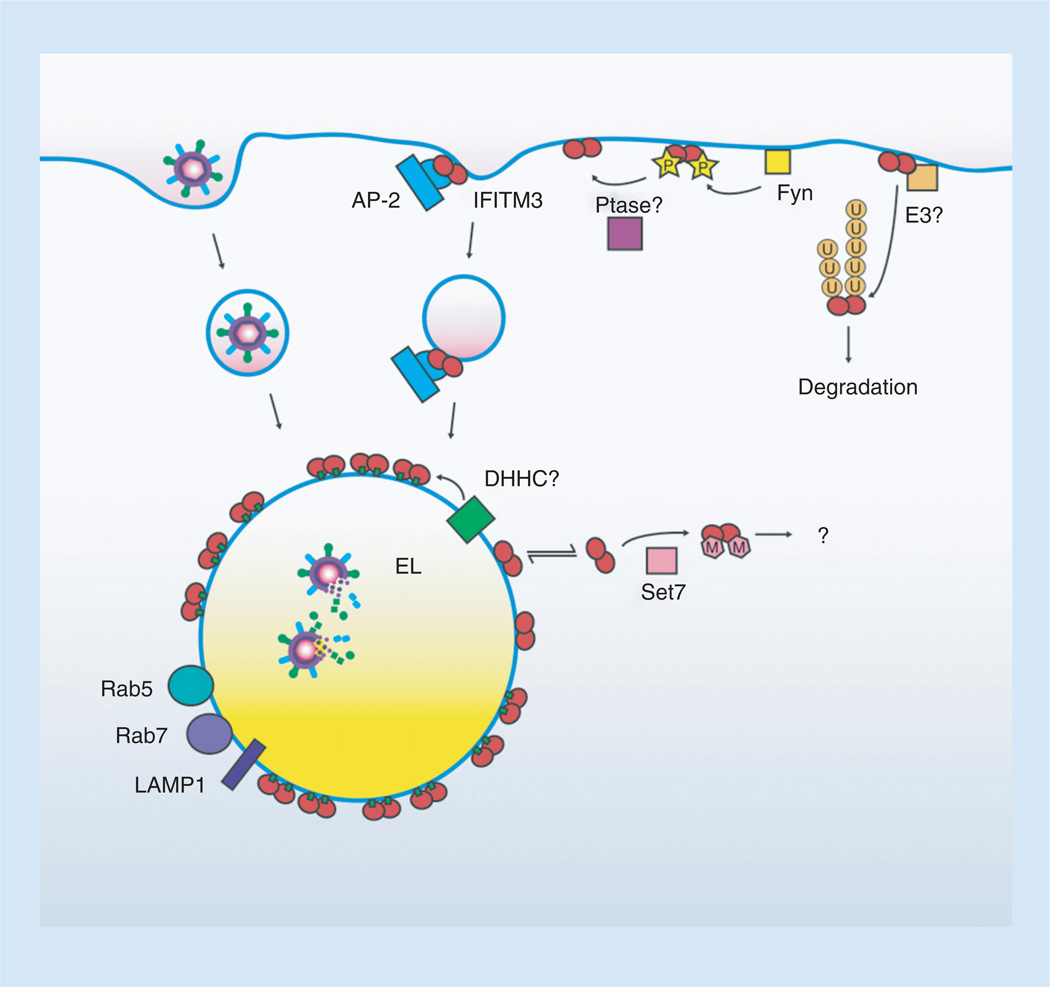
Figure 3. IFITM3 is controlled by multiple post-translational modifications.
IFITM3, depicted as a dimer, is initially localized to the plasma membrane. Tyrosine P (yellow stars) by Fyn retains IFITM3 at the plasma membrane by preventing its interaction with the AP-2 endocytic adaptor complex. Unknown Ptase dynamically remove phosphorylation allowing its endocytosis and targeting to endolysosomes where virus is degraded. Alternatively, unphosphorylated IFITM3 can be targeted for ubiquitination (U, orange circles) by an as yet unidentified E3 ubiquitin ligase resulting in its mislocalization and degradation. Palmitoylation (small green boxes) of IFITM3 by a currently unidentified DHHCanchors IFITM3 to membranes, promotes its clustering, and is necessary for IFITM3 antiviral activity. The location of this palmitoyltransferase activity is currently unknown but is depicted at the endolysosomes. A portion of nonpalmitoylated IFITM3 is cytoplasmic and may be the fraction of IFITM3 that encounters cytoplasmic Set7. IFITM3 methylation (M, pink polygons) by Set7 decreases antiviral activity through an unknown mechanism.
EL: Endolysosome; P: Phosphorylation; Ptase: Phosphatase.
The cellular compartment that facilitates IFITM3 palmitoylation, and whether or not IFITM3 associates and disassociates from membranes in an equilibrium in the absence of palmitoylation, both remain to be determined (Figure 3). Likewise, it remains to be fully determined where Set7 methylation of IFITM3 occurs, and ultimately what effect this has during the course of infection in vivo (Figure 3). Similarly, the crosstalk existing between all of these modifications has yet to be fully examined, and will likely shed further light on the trafficking and antiviral activity of IFITM3. There are also likely additional, yet to be discovered modes of post-translational regulation occurring on IFITM3, such as serine/threonine phosphorylation or various forms of oxidation, that should continue to inform our understanding of IFITM3 stability and trafficking, and the differences between the IFITM isoforms. An intriguing finding that will also require further investigation is that IFITMs are capable of inhibiting HIV replication through mechanisms distinct from their inhibition of most other viruses [10, 20, 78]. Finally, despite the effect of IFITM3 on the endolysosomal compartment, which is often co-opted by intracellular bacteria, a role for IFITM3 in inhibiting bacteria was not observed upon examination of multiple bacterial pathogens [79]. It will be interesting to determine whether bacteria actively evade IFITM3-positive endolysosomal compartments or perhaps alter IFITM3 PTMs for their own benefit, and whether or not this affects concurrent viral infections. Given the broad-ranging importance of IFITM3 in the innate response to endocytic viruses, answering these open questions should bring us closer to controlling and utilizing IFITM3 for preventative or therapeutic antiviral strategies.
Executive summary.
Background on IFITM3
IFITM3 inhibits cellular infection by influenza virus and many other endocytic viruses.
IFITM3 knockout mice are more susceptible than wild-type mice to low-dose infections with influenza virus.
A polymorphism in the human IFITM3 gene has been associated with an increase in risk for severe versus mild influenza virus infection.
IFITM3 mechanism of antiviral action
Expression of IFITM3 induces an enlarged, acidic endolysosomal compartment to which endocytosed viruses are localized to and degraded. IFITM3 prevents fusion pore formation between the virus and the endolysosome.
IFITM3 affects cholesterol homeostasis and decreases membrane fluidity potentially explaining its ability to inhibit virus fusion.
IFITM3 palmitoylation
S-palmitoylation of IFITM3 occurs on three membrane-proximal cysteines.
S-palmitoylation promotes IFITM3 clustering on membranes and is required for full antiviral activity making this modification a positive regulator of antiviral activity.
IFITM3 ubiquitination
IFITM3 mono- and poly-ubiquitination can occur on any of four lysines. LyS-48 and LyS-63 ubiquitin linkages have been observed.
Ubiquitination results in IFITM3 localization to an endoplasmic reticulum-proximal compartment and degradation.
A lysine-less mutant of IFITM3 possesses enhanced antiviral activity suggesting that ubiquitination is a negative regulator of activity.
IFITM3 methylation
Set7 methylates LyS-88 of IFITM3.
Overexpression of Set7 decreases IFITM3 antiviral activity while Set7 knockdown increases IFITM3 activity, indicating that methylation of IFITM3 plays a negative regulatory role.
IFITM3 phosphorylation
IFITM3 is phosphorylated on Tyr-20 by the kinase Fyn.
Tyrosine phosphorylation blocks a YxxΦ motif necessary for IFITM3 interaction with the AP-2 complex and endocytosis, and thus its localization to endolysosomes and antiviral activity. Therefore, phosphorylation of IFITM3 is a negative regulator of activity.
IFITM3 membrane topology
While IFITM3 is predicted to be a dual-pass transmembrane protein with both of its termini facing extracellularly, the N-terminus must be cytoplasmically located in order for proper localization and activity of IFITM3. This, along with the presence of PTMs installed by cytoplasmic enzymes on the IFITM3 N-terminus, suggests that the first hydrophobic region of IFITM3 acts as an intramembrane domain that does not fully span the lipid bilayer.
Whether the second hydrophobic domain within antivirally active IFITM3 acts as a transmembrane or intramembrane domain has not been fully elucidated.
Acknowledgments
Research in the Yount laboratory is supported by the NIH/NIAID (grant R00AI095348) and the Ohio State University Public Health Preparedness for Infectious Diseases program. NM Chesarino is supported by the Ohio State University Systems and Integrative Biology Training Program (NIH/NIGMS grant T32GM068412) and TM McMichael is supported by the American Society for Microbiology Robert D. Watkins Graduate Research Fellowship.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J. Interferon Cytokine Res. 2011;31(1):183–197. doi: 10.1089/jir.2010.0112. [DOI] [PubMed] [Google Scholar]
- 2.Sallman Almen M, Bringeland N, Fredriksson R, Schioth HB. The dispanins: a novel gene family of ancient origin that contains 14 human members. PLoS ONE. 2012;7(2):e31961. doi: 10.1371/journal.pone.0031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass AL, Huang IC, Benita Y, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139(7):1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yount JS, Moltedo B, Yang YY, et al. Palmitoylome profiling reveals S -palmitoylation-dependent antiviral activity of IFITM3. Nat. Chem. Biol. 2010;6(8):610–614. doi: 10.1038/nchembio.405. • Initial description of IFITM3 palmitoylation and palmitoylation-dependent antiviral activity.
- 5.Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010;84(24):12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang IC, Bailey CC, Weyer JL, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7(1):e1001258. doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoggins JW, Wilson SJ, Panis M, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoggins JW, MacDuff DA, Imanaka N, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anafu AA, Bowen CH, Chin CR, Brass AL, Holm GH. Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry. J. Biol. Chem. 2013;288(24):17261–17271. doi: 10.1074/jbc.M112.438515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Pan Q, Rong L, He W, Liu SL, Liang C. The IFITM proteins inhibit HIV-1 infection. J. Virol. 2011;85(5):2126–2137. doi: 10.1128/JVI.01531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raychoudhuri A, Shrivastava S, Steele R, Kim H, Ray R, Ray RB. ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J. Virol. 2011;85(24):12881–12889. doi: 10.1128/JVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkins C, Woodward J, Lau DTY, et al. IFITM1 is a tight junction protein that inhibits hepatitis virus entry. Hepatology. 2013;57(2):461–469. doi: 10.1002/hep.26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudhasani R, Tran JP, Retterer C, et al. IFITM-2 and IFITM-3 but not IFITM-1 restrict Rift Valley fever virus. J. Virol. 2013;87(15):8451–8464. doi: 10.1128/JVI.03382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith RA, Young J, Weis JJ, Weis JH. Expression of the mouse fragilis gene products in immune cells and association with receptor signaling complexes. Genes Immun. 2006;7(2):113–121. doi: 10.1038/sj.gene.6364278. [DOI] [PubMed] [Google Scholar]
- 15.Smith SE, Gibson MS, Wash RS, et al. Chicken interferon-inducible transmembrane protein 3 restricts influenza viruses and lyssaviruses in vitro. J. Virol. 2013;87(23):12957–12966. doi: 10.1128/JVI.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu R, Wang J, Lei XY, Gui JF, Zhang QY. Evidence for Paralichthys olivaceus IFITM1 antiviral effect by impeding viral entry into target cells. Fish Shellfish Immunol. 2013;35(3):918–926. doi: 10.1016/j.fsi.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Liu J, Li M, Yang H, Zhang CY. Evolutionary dynamics of the interferon-induced transmembrane gene family in vertebrates. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yount JS, Karssemeijer RA, Hang HC. S -palmitoylation and ubiquitination differentially regulate interferon-induced transmembrane protein 3 (IFITM3)-mediated resistance to influenza virus. J. Biol. Chem. 2012;287:19631–19641. doi: 10.1074/jbc.M112.362095. • Identifies ubiquitination of IFITM3 and proposes an intramembrane topology model.
- 19. Shan Z, Han Q, Nie J, et al. Negative regulation of interferon-induced transmembrane protein 3 by SET7-mediated lysine monomethylation. J. Biol. Chem. 2013;288(49):35093–35103. doi: 10.1074/jbc.M113.511949. • Discovers the negative effects of Set7 methylation on IFITM3 antiviral activity.
- 20. Jia R, Pan Q, Ding S, et al. The N-terminal region of IFITM3 modulates its antiviral activity by regulating IFITM3 cellular localization. J. Virol. 2012;86(24):13697–13707. doi: 10.1128/JVI.01828-12. • Provides the first description of IFITM3 phosphorylation by Fyn.
- 21. Chesarino NM, McMichael TM, Hach JC, Yount JS. Phosphorylation of the antiviral protein IFITM3 dually regulates its endocytosis and ubiquitination. J. Biol. Chem. 2014;289(17):11986–11992. doi: 10.1074/jbc.M114.557694. • Shows that the endocytic YxxΦ motif of IFITM3 can be blocked by phosphorylation.
- 22.Hickford D, Frankenberg S, Shaw G, Renfree MB. Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genom. 2012;13:155. doi: 10.1186/1471-2164-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013;13(1):46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffatt P, Gaumond MH, Salois P, et al. Bril: a novel bone-specific modulator of mineralization. J. Bone Miner Res. 2008;23(9):1497–1508. doi: 10.1359/jbmr.080412. [DOI] [PubMed] [Google Scholar]
- 25.Semler O, Garbes L, Keupp K, et al. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type with hyperplastic callus. Am. J. Hum. Genet. 2012;91(2):349–357. doi: 10.1016/j.ajhg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanagata N, Li XL, Morita H, Takemura T, Li J, Minowa T. Characterization of the osteoblast-specific transmembrane protein IFITM5 and analysis of IFITM5-deficient mice. J. Bone Miner. Metab. 2011;29(3):279–290. doi: 10.1007/s00774-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 27. Everitt AR, Clare S, Pertel T, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484(7395):519–523. doi: 10.1038/nature10921. •• Demonstrates that IFITM3 is essential for restriction of influenza virus infections in mice and humans.
- 28.Bailey CC, Huang IC, Kam C, Farzan M. Ifitm3 limits the severity of acute influenza in mice. PLoS Pathog. 2012;8(9):e1002909. doi: 10.1371/journal.ppat.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Zhang A, Wan Y, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc. Natl Acad. Sci. USA. 2013;111(2):769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YH, Zhao Y, Li N, et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat. Commun. 2013;4:1418. doi: 10.1038/ncomms2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feeley EM, Sims JS, John SP, et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7(10):e1002337. doi: 10.1371/journal.ppat.1002337. • Provides the first evidence that IFITM3 acts by inhibiting virus fusion with endolysosomes.
- 32.Li K, Markosyan RM, Zheng YM, et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9(1):e1003124. doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai TM, Marin M, Chin CR, Savidis G, Brass AL, Melikyan GB. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following ViruS-endosome hemifusion. PLoS Pathog. 2014;10(4):e1004048. doi: 10.1371/journal.ppat.1004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perreira JM, Chin CR, Feeley EM, Brass AL. IFITMs restrict the replication of multiple pathogenic viruses. J. Mol. Biol. 2013;425(24):4937–4955. doi: 10.1016/j.jmb.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S, Weston S, Kellam P, Marsh M. IFITM proteinS-cellular inhibitors of viral entry. Curr. Opin. Virol. 2014;4C:71–77. doi: 10.1016/j.coviro.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hach JC, McMichael T, Chesarino NM, Yount JS. Palmitoylation on conserved and non-conserved cysteines of murine IFITM1 regulates its stability and anti-influenza A virus activity. J. Virol. 2013;87(17):9923–9927. doi: 10.1128/JVI.00621-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John SP, Chin CR, Perreira JM, et al. The CD225 domain of IFITM3 is required for both IFITM protein association and inhibition of influenza A virus and dengue virus replication. J. Virol. 2013;87(14):7837–7852. doi: 10.1128/JVI.00481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wee YS, Roundy KM, Weis JJ, Weis JH. Interferon-inducible transmembrane proteins of the innate immune response act as membrane organizers by influencing clathrin and v-ATPase localization and function. Innate Immunol. 2012;18(6):834–845. doi: 10.1177/1753425912443392. [DOI] [PubMed] [Google Scholar]
- 39.Amini-Bavil-Olyaee S, Choi YJ, Lee JH, et al. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe. 2013;13(4):452–464. doi: 10.1016/j.chom.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin TY, Chin CR, Everitt AR, et al. Amphotericin B increases influenza A virus infection by preventing IFITM3-mediated restriction. Cell Rep. 2013;5(4):895–908. doi: 10.1016/j.celrep.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohn A. Early interactions of viruses with cellular membranes. Adv. Virus Res. 1979;24:223–276. doi: 10.1016/s0065-3527(08)60395-4. [DOI] [PubMed] [Google Scholar]
- 42.Schoggins JW, Randall G. Lipids in innate antiviral defense. Cell Host Microbe. 2013;14(4):379–385. doi: 10.1016/j.chom.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu SY, Sanchez DJ, Cheng G. New developments in the induction and antiviral effectors of type I interferon. Curr. Opin. Immunol. 2011;23(1):57–64. doi: 10.1016/j.coi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tom CT, Martin BR. Fat chance! Getting a grip on a slippery modification. ACS Chem. Biol. 2013;8(1):46–57. doi: 10.1021/cb300607e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 46.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE. 2006;2006(359):re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 47.Blaskovic S, Blanc M, van der Goot FG. What does S -palmitoylation do to membrane proteins? FEBS J. 2013;280(12):2766–2774. doi: 10.1111/febs.12263. [DOI] [PubMed] [Google Scholar]
- 48.Yount JS, Zhang MM, Hang HC. Emerging roles for protein S -palmitoylation in immunity from chemical proteomics. Curr. Opin. Chem. Biol. 2013;17(1):27–33. doi: 10.1016/j.cbpa.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewin AR, Reid LE, McMahon M, Stark GR, Kerr IM. Molecular analysis of a human interferon-inducible gene family. Eur. J. Biochem. 1991;199(2):417–423. doi: 10.1111/j.1432-1033.1991.tb16139.x. [DOI] [PubMed] [Google Scholar]
- 50.Friedman RL, Manly SP, McMahon M, Kerr IM, Stark GR. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- 51.Yount JS, Charron G, Hang HC. Bioorthogonal proteomics of 15-hexadecynyloxyacetic acid chemical reporter reveals preferential targeting of fatty acid modified proteins and biosynthetic enzymes. Bioorg. Med. Chem. 2012;20(2):650–654. doi: 10.1016/j.bmc.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, Weidner JM, Qing M, et al. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J. Virol. 2010;84(16):8332–8341. doi: 10.1128/JVI.02199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsukamoto T, Li X, Morita H, et al. Role of S -palmitoylation on IFITM5 for the interaction with FKBP11 in osteoblast cells. PLoS ONE. 2013;8(9):e75831. doi: 10.1371/journal.pone.0075831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner SA, Beli P, Weinert BT, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol. Cell. Proteomics. 2011;10(10) doi: 10.1074/mcp.M111.013284. M111 013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner SA, Beli P, Weinert BT, et al. Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol. Cell. Proteomics. 2012;11(12):1578–1585. doi: 10.1074/mcp.M112.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim W, Bennett EJ, Huttlin EL, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44(2):325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T, Zhou T, He B, et al. mUbiSiDa: a comprehensive database for protein ubiquitination sites in mammals. PLoS ONE. 2014;9(1):e85744. doi: 10.1371/journal.pone.0085744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulathu Y, Komander D. Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 2012;13(8):508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Cao R, Xia L, et al. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol. Cell. 2001;8(6):1207–1217. doi: 10.1016/s1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 60.Kurash JK, Lei H, Shen Q, et al. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol. Cell. 2008;29(3):392–400. doi: 10.1016/j.molcel.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Munro S, Khaire N, Inche A, Carr S, La Thangue NB. Lysine methylation regulates the pRb tumour suppressor protein. Oncogene. 2010;29(16):2357–2367. doi: 10.1038/onc.2009.511. [DOI] [PubMed] [Google Scholar]
- 62.Pagans S, Sakane N, Schnolzer M, Ott M. Characterization of HIV Tat modifications using novel methyl-lysine-specific antibodies. Methods. 2011;53(1):91–96. doi: 10.1016/j.ymeth.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esteve PO, Chang Y, Samaranayake M, et al. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat. Struct. Mol. Biol. 2011;18(1):42–48. doi: 10.1038/nsmb.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Wang D, Zhao Y, et al. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1) Proc. Natl Acad. Sci. USA. 2011;108(5):1925–1930. doi: 10.1073/pnas.1019619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jia R, Xu F, Qian J, et al. Identification of an endocytic signal essential for the antiviral action of IFITM3. Cell. Microbiol. 2014;16(7):1080–1093. doi: 10.1111/cmi.12262. •• Identifies interaction of AP-2 and the YxxΦ motif of IFITM3 as essential for proper localization and activity of IFITM3.
- 66.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell. 2007;28(5):730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269(24):16701–16705. [PubMed] [Google Scholar]
- 68.van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J. Cell Biol. 1997;136(5):1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lancki DW, Qian D, Fields P, Gajewski T, Fitch FW. Differential requirement for protein tyrosine kinase Fyn in the functional activation of antigen-specific T lymphocyte clones through the TCR or Thy. J. Immunol. 1995;154(9):4363–4370. [PubMed] [Google Scholar]
- 70.Tang X, Feng Y, Ye K. SrC-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14(2):368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- 71.Peters DJ, McGrew BR, Perron DC, Liptak LM, Laudano AP. In vivo phosphorylation and membrane association of the fyn proto-oncogene product in IM-9 human lymphoblasts. Oncogene. 1990;5(9):1313–1319. [PubMed] [Google Scholar]
- 72.Mustelin T, Pessa-Morikawa T, Autero M, et al. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur. J. Immunol. 1992;22(5):1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- 73. Bailey CC, Kondur HR, Huang IC, Farzan M. Interferon-induced transmembrane protein 3 is a type II transmembrane protein. J. Biol. Chem. 2013;288(45):32184–32193. doi: 10.1074/jbc.M113.514356. • Provides evidence for an intramembrane/type II transmembrane topology for IFITM3.
- 74.Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J. Biol. Chem. 1998;273(11):6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- 75.Kalashnikova E, Lorca RA, Kaur I, et al. SynDIG1: an activity-regulated, AMPA-receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. 2010;65(1):80–93. doi: 10.1016/j.neuron.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patoine A, Gaumond MH, Jaiswal PK, Fassier F, Rauch F, Moffatt P. Topological mapping of BRIL reveals a type II orientation and effects of osteogenesis imperfecta mutations on its cellular destination. J. Bone Miner. Res. 2014 doi: 10.1002/jbmr.2243. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 77.Voeltz GK, Prinz WA. Sheets, ribbons and tubules - how organelles get their shape. Nat. Rev. Mol. Cell Biol. 2007;8(3):258–264. doi: 10.1038/nrm2119. [DOI] [PubMed] [Google Scholar]
- 78.Chutiwitoonchai N, Hiyoshi M, Hiyoshi-Yoshidomi Y, Hashimoto M, Tokunaga K, Suzu S. Characteristics of IFITM, the newly identified IFN-inducible anti-HIV-1 family proteins. Microbes Infect. 2013;15(4):280–290. doi: 10.1016/j.micinf.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Everitt AR, Clare S, McDonald JU, et al. Defining the range of pathogens susceptible to ifitm3 restriction using a knockout mouse model. PLoS ONE. 2013;8(11):e80723. doi: 10.1371/journal.pone.0080723. [DOI] [PMC free article] [PubMed] [Google Scholar]