Abstract
The proportion of pregnant women who are obese at conception continues to rise. Compelling evidence suggests the intrauterine environment is an important determinant of offspring health. Maternal obesity and unhealthy diets are shown to promote metabolic programming in the offspring. Mitochondria are maternally inherited, and we have previously shown impaired mitochondrial function in rat offspring exposed to maternal obesity in utero. Mitochondrial health is maintained by mitochondrial dynamics, or the processes of fusion and fission, which serve to repair damaged mitochondria, remove irreparable mitochondria, and maintain mitochondrial morphology. An imbalance between fusion and fission has been associated with obesity, insulin resistance, and reproduction complications. In the present study, we examined the influence of maternal obesity and postweaning high-fat diet (HFD) on key regulators of mitochondrial fusion and fission in rat offspring at important developmental milestones which included postnatal day (PND)35 (2 wk HFD) and PND130 (∼16 wk HFD). Our results indicate HFD-fed offspring had reduced mRNA expression of presenilin-associated rhomboid-like (PARL), optic atrophy (OPA)1, mitofusin (Mfn)1, Mfn2, fission (Fis)1, and nuclear respiratory factor (Nrf)1 at PND35, while OPA1 and Mfn2 remained decreased at PND130. Putative transcriptional regulators of mitochondrial dynamics were reduced in rat placenta and offspring liver and skeletal muscle [peroxisome proliferator-activated receptor gamma coactivator (PGC1)α, PGC1β, and estrogen-related receptor (ERR)α], consistent with indirect calorimetry findings revealing reduced energy expenditure and impaired fat utilization. Overall, maternal obesity detrimentally alters mitochondrial targets that may contribute to impaired mitochondrial health and increased obesity susceptibility in later life.
Keywords: mitochondrial dynamics, developmental programming, pregnancy, PGC1α/β, mitofusin
childhood obesity is a growing health concern as it is estimated that 60 million children will be overweight or obese by 2020 worldwide (16). A recent study revealed that children who were overweight at 5 yr of age were four times more likely to be obese at age 14 yr compared with normal-weight children (15). Moreover, the prevalence of obesity is evident at a much earlier age (0–2 yr) (28, 40), suggesting early life events such as the intrauterine environment may influence obesity development. Exposure to maternal obesity in utero, as well as accelerated growth during infancy, contribute to obesity and development of obesity comorbidities such as Type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease in later life (8, 11, 12, 32, 49). Previous reports, including from our group, show that maternal obesity at conception and during gestation leads to detrimental metabolic outcomes in offspring as shown in animal models (1, 4, 6, 7, 32, 45, 46) and clinical studies (12, 35, 38, 50).
In the present study, we used a rat model of gestational obesity that employs a precisely controlled feeding system where female dams are intragastrically overfed a liquid diet to induce obesity prepregnancy; in addition, this obese phenotype is maintained throughout gestation (3, 6, 44, 45). At birth, there were no differences in offspring birth weight between lean and obese dams (45). However, offspring of obese dams develop a metabolic syndrome phenotype when challenged with a high-fat diet (HFD) that includes excess adiposity, insulin resistance, NAFLD, increased adipogenesis, and disrupted circadian rhythm (4, 6, 45, 46) in the absence of differences in food intake between HFD-fed lean and obese dam offspring (45). In addition, at weaning, and prior to the development of obesity, offspring of obese dams demonstrated transcriptomic profiles in the liver and white adipose tissue that favored obesity development (6, 46). These changes were associated with impaired whole body metabolism and fatty acid utilization that may, in part, be driven by changes in mitochondria (5).
Mitochondria are organelles critical for several biological processes such as energy metabolism, embryonic development, redox signaling, and apoptosis (13, 14, 30, 57) and are maternally inherited. Mitochondrial dynamics (fusion and fission) regulate the health and fate of mitochondria. A balance between mitochondrial fusion and fission is necessary for maintenance of appropriate morphology, number, and function (13, 20, 57). Fusion and fission targets are known to be regulated by peroxisome proliferator-activated receptor gamma coactivator (PGC)1α, PGC1β, and other coactivators such as estrogen-related receptor (ERR)α (27, 51). PGC1α, PCG1β, and ERRα also regulate mitochondrial biogenesis and energy metabolism via control of downstream targets (43, 48). There is evidence that the intrauterine environment can alter mtDNA number and mitochondrial function in a variety of instances (5, 7, 9, 22).
Here we examine the hypotheses that maternal obesity impairs mitochondrial dynamics as demonstrated by changes in mitochondrial number and expression of key targets in the placenta and in metabolically active tissues of offspring during early development [postnatal day (PND)35] and in later life (PND130). Mitochondrial targets were examined in rat placenta at days postcoitum (dpc) 18.5 and in rat offspring liver and skeletal muscle in response to short- and long-term HFD (2 wk and ∼16 wk). Our studies reveal that putative transcriptional regulators (PGC1α, PGC1β, and ERRα) of mitochondrial dynamics and mitofusins (Mfn1 and Mfn2) were reduced in obese rat placenta and offspring liver and skeletal muscle.
MATERIALS AND METHODS
Animals and chemicals.
Female Sprague-Dawley rats (150–175 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility in a temperature- and light-controlled room (12 h light-12 h dark cycle). All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Unless specified, all chemicals were obtained from Sigma-Aldrich Chemical (St. Louis, MO).
Experimental animal protocol.
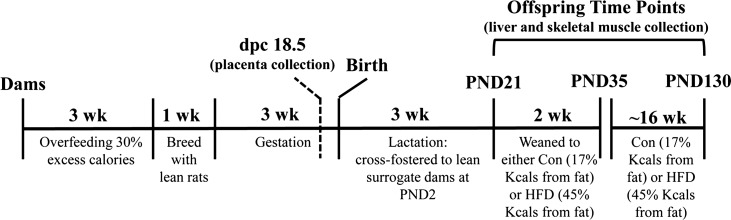
A timeline of the experimental design is shown in Fig. 1. Virgin female Sprague-Dawley rats were individually housed in metabolism cages and were intragastrically cannulated to receive food via total enteral nutrition (TEN) at age 8 wk and allowed to recover for 10 days as previously described (2, 3, 6, 44). Sample sizes for each study are provided below. Rats dams were fed liquid TEN diets at either 155 kcal/kg3/4·day (referred to as lean dams) or at 220 kcal/kg3/4·day (40% excess calories, referred to as obese dams) 23 h per day for 3 wk, which is sufficient to induce an obese prepregnancy phenotype that is maintained throughout gestation. We have previously reported the body weights and body compositions of these lean and obese dams (45). TEN diets met National Research Council (NRC) nutrient recommendations as used previously by our group (6, 45, 46) and consisted of 20% protein (casein), 75% carbohydrate (dextrose and maltodextrin), and 5% fat (corn oil). The dietary composition of the TEN was exactly the same between lean and obese dams; only the volume of infused diet differed. Infusion of diets was carried out for 3 wk, allowing for precise control of both diet composition and caloric intake in a low-stress sedentary environment. Animals had ad libitum access to drinking water, and body weights were measured three times per week. Following 3 wk of diets, lean and obese rats (n = 15/group) were allowed to mate for 1 wk. Each female rat was housed with one lean breeder male and allowed ad libitum access to AIN-93G diet during this period. After mating, all female rats (lean and obese) received TEN diets at 220 kcal/kg3/4·day (NRC recommended caloric intake for pregnancy in rats), and overfeeding of obese dams was discontinued, but obesity was maintained throughout gestation. A separate set of dams were used to collect placenta. Mating was confirmed by the presence of sperm in the vaginal lavage and designated as dpc 0.5. These dams were euthanized, and placentas and offspring tissues collected at dpc 18.5 from individual fetuses and immediately frozen for later analyses. The normal gestational length for this strain of rats is 22 days. Sex was determined for all placentas via PCR amplification of the Sry gene in genomic DNA. The placentas were then pooled together by litter according to sex (lean n = 7, obese n = 8). Fetal body weights were not different between lean and obese dam offspring at dpc 18.5. All other dams were allowed to give birth naturally. Numbers and sex of pups, birth weight, and crown-to-rump and anogenital distance were measured for each pup on PND1 as previously described (45). On PND2, four male and four female pups from each litter were cross-fostered to a separate set of lean dams that had been previously time-impregnated to give birth on the same day as the lean and obese dams receiving infusion diets. Cross-fostered dams were not cannulated and had ad libitum access to AIN-93G pelleted diets throughout lactation ensuring that offspring's exposure to any effects of maternal obesity was limited exclusively to the intrauterine environment (2, 3, 6, 44, 45, 45, 46). Female offspring of lean and obese dams were used for separate experiments, and only data from male offspring are reported here. At PND21 male offspring were weaned onto either an AIN-93G [17% kcals fat, control (con)] or semipurified high-fat diet (45% kcals fat, HFD) (Harlan Diets) for 2 wk (n = 12 animal/group) or ∼16 wk (n = 7 lean-con, n = 8 obese-con, n = 11 lean-HFD, and n = 9 obese-HFD). Dietary composition of postweaning diets is provided in Table 1. Offspring were weighed and euthanized via exsanguination following asphyxiation with carbon dioxide, in the fed condition at PND35 and PND130. Each offspring group represented four to eight distinct biological litters. Following euthanization of the rats, livers were weighed and immediately frozen in liquid nitrogen and stored at −70°C for later analyses. Serum was obtained by centrifugation of blood samples and stored at −20°C. Maternal prepregnancy and gestational body weights are provided in Fig. 2, A and B. Offspring characteristics and serum parameters of at PND35 and PND130 are shown in Table 2, Table 3, and Fig. 2C.
Fig. 1.
Schematic showing experimental design and important time points at which analyses were conducted in dams and offspring. dpc, days postcoitum; PND, postnatal day; HFD, high-fat diet.
Table 1.
Composition of postweaning diets
| Component | AIN93-G, g/kg | HFD, g/kg |
|---|---|---|
| Casein | 200.0 | 195.0 |
| L-Cystine | 3.0 | 3.0 |
| Corn starch | 397.5 | 150.0 |
| Maltodextrin | 132.0 | |
| Sucrose | 100.0 | 341.46 |
| Corn oil | 70.0 | — |
| Anhydrous milk fat | — | 210.0 |
| Cellulose | 50.0 | 51.54 |
| Mineral mix | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 |
Dietary composition of each respective diet offspring were weaned onto at postnatal day (PND)21 until the time of euthanization at PND35 or PND130. Control diet was 3.8 Kcal/g deriving 19 % kcal from protein, 64% from carbohydrate, and 17% from fat. High-fat diet (HFD) was 4.5 Kcal/g deriving 15 % kcal from protein, 40% from carbohydrate, and 45% from fat.
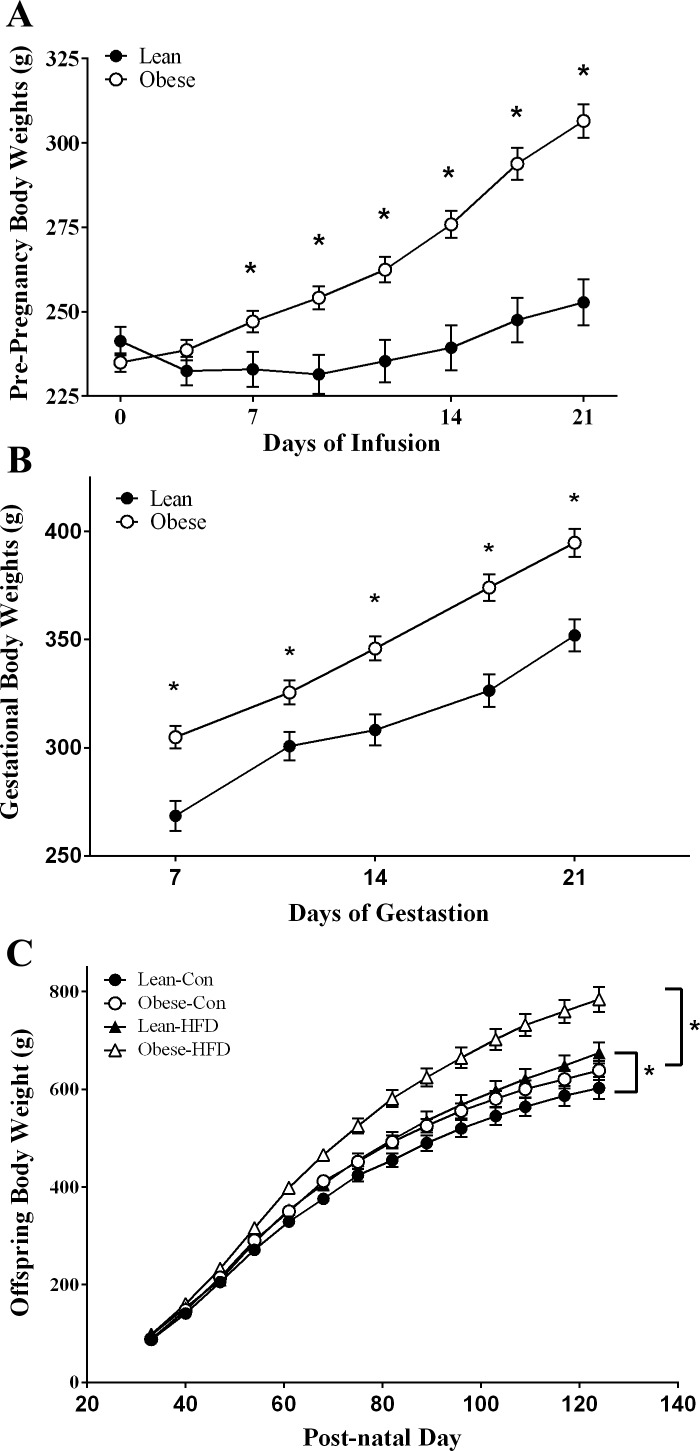
Fig. 2.
A: body weights of female rats fed diets via total enteral nutrition (TEN) for 21 days prior to mating (lean n = 7 and obese n = 15). Infusion of diets was carried out for 23 h/day via computer-controlled syringe pumps. Data are expressed as means ± SE. Statistical differences were determined using Student's t-test. *Significance P < 0.05. B: body weights of female rats fed diets via TEN (220 kcal/kg3/4) from gestation (GD)8 to GD21. Lean and obese dams (lean n = 7 and obese n = 15) were mated with ad libitum-fed lean male rats for a period of 1 wk. Data are expressed as means ± SE. Statistical differences were determined by Student's t-test. *Significance P < 0.05. C: body weights of male offspring of lean and obese dams from weaning through PND130. At PND21 offspring were weaned to either control (Con; lean n = 7, obese n = 11) AIN-93G diets (17% kcal from fat) or HFD (45% kcal from fat; lean n = 8, obese n = 9). Data are expressed as means ± SE. Statistical differences were determined by 2-way ANOVA for the effects of maternal obesity and postweaning HFD, followed by Student-Newman-Keuls post hoc analyses, *P < 0.05. This figure is adapted from previously published results using the same animals (45).
Table 2.
Animal characteristics and serum parameters of lean and obese dam offspring at PND35 (2 wk HFD)
| Offspring of Lean Dams |
Offspring of Obese Dams |
P Values |
|||||
|---|---|---|---|---|---|---|---|
| Parameter | Control | HFD | Control | HFD | Maternal Obesity × Postweaning Diet | Effect of Maternal Obesity | Effect of Postweaning HFD |
| Body weight, g | 198 ± 6 | 222 ± 10 | 192 ± 9 | 199 ± 9 | 0.450 | 0.127 | 0.045 |
| Liver weight, g | 10.8 ± 0.3 | 12.4 ± 0.8 | 10.8 ± 0.7 | 10.2 ± 0.5 | 0.074 | 0.070 | 0.411 |
| % Liver weight | 5.5 ± 0.1 | 5.5 ± 0.5 | 5.6 ± 0.1 | 5.1 ± 0.4 | 0.222 | 0.522 | 0.403 |
| Fat pad weight, g | 2.4 ± 0.2 | 5.0 ± 0.4 | 2.3 ± 0.3 | 3.7 ± 0.4 | 0.151 | 0.052 | <0.001 |
| % Fat weight | 1.2 ± 0.1 | 2.2 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.2 | 0.279 | 0.122 | <0.001 |
| Insulin, ng/ml | 4.1 ± 0.6 | 5.2 ± 0.6 | 4.6 ± 0.9 | 4.9 ± 0.5 | 0.540 | 0.861 | 0.261 |
| Glucose, mg/dl | 142.5 ± 2.5a | 145.8 ± 4.3a,b | 135.7 ± 3.2a | 153.7 ± 3.2b | 0.037 | 0.823 | 0.002 |
| Leptin, ng/ml | 7.0 ± 0.7 | 13.0 ± 1.2 | 6.7 ± 1.1 | 11.9 ± 1.1 | 0.686 | 0.528 | <0.001 |
| Triglycerides, mg/dl | 168 ± 18 | 455 ± 50 | 180 ± 20 | 389 ± 40 | 0.268 | 0.439 | <0.001 |
| NEFA, mmol/l | 0.5 ± 0.02 | 0.9 ± 0.05 | 0.4 ± 0.04 | 0.6 ± 0.06 | 0.068 | <0.001 | <0.001 |
| Adiponectin, μg/ml | 17.2 ± 1.5 | 16.9 ± 1.3 | 17.5 ± 0.9 | 17.7 ± 1.4 | 0.815 | 0.686 | 0.965 |
Data were obtained from male offspring of lean and obese dams at PND35 (n = 12 animals/group). Dams were fed diets via total enteral nutrition (TEN) to induce obesity as described in materials and methods. Male offspring were cross-fostered at birth to unsurgerized dams fed AIN-93G diet ad libitum. At PND21 offspring were weaned onto ad libitum HFD (45% of kcal from fat) for a duration of 2 wk as a metabolic challenge. Weights of liver and fat pads (retroperitoneal plus gonadal fat depots) were assessed at the time of sacrifice. Percent liver and fat weight were calculated as tissue weight in grams divided by total body weight in grams. Data are expressed as means ± SE. Differences between offspring of lean and obese dams were analyzed by 2-way ANOVA, significance was set at P < 0.05 as indicated by boldface P values. Significant interactions identified by 2-way ANOVA were followed by a 1-way ANOVA and all pair-wise comparisons by Student-Newman-Keuls.
Table 3.
Animal characteristics and serum parameters of lean and obese dam offspring at PND130 (∼16 wk HFD)
| Offspring of Lean Dams |
Offspring of Obese Dams |
P Values |
||||
|---|---|---|---|---|---|---|
| Parameter | Control | HFD | Control | HFD | Effect of Maternal Obesity | Effect of Postweaning HFD |
| Body Weight, g | 607 ± 25 | 692 ± 23 | 650 ± 20 | 803 ± 24 | 0.003 | <0.001 |
| % Liver Weight | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.6 ± 0.1 | 0.016 | 0.009 |
| % Fat Weight | 4.4 ± 0.6 | 8.2 ± 0.3 | 5.8 ± 0.3 | 9.95 ± 0.3 | 0.003 | <0.001 |
| Insulin, ng/ml | 3.9 ± 1.0 | 9.4 ± 1.7 | 8.7 ± 2.8 | 13.3 ± 3.1 | 0.08 | 0.04 |
| Glucose, mg/dl | 134 ± 5.7 | 154 ± 7.4 | 130 ± 2.3 | 153 ± 12.6 | 0.78 | 0.01 |
| Leptin, ng/ml | 9.5 ± 4.6 | 36.2 ± 7.7 | 22.1 ± 3.8 | 53.6 ± 12.9 | 0.08 | 0.002 |
| TAG, mg/dl | 581 ± 162 | 1498 ± 228 | 504 ± 125 | 979 ± 365 | 0.21 | 0.006 |
| NEFA, mmol/l | 1.3 ± 0.16 | 2.0 ± 0.16 | 1.3 ± 0.08 | 1.6 ± 0.18 | 0.16 | 0.004 |
| Adiponectin, μg/ml | 6.4 ± 1.2 | 9.8 ± 2.0 | 8.6 ± 1.2 | 9.0 ± 2.3 | 0.69 | 0.28 |
Female rats in lean (155 Kcal/kg3/4/day) or obese (220 Kcal/kg3/4/day) groups were fed via TEN for 3 wk prior to conception. Data were obtained from male offspring at PND130 fed control (lean n = 7, obese n = 11) or HFD (45% of kcal from fat) for ∼16 wk (lean n = 8, obese n = 9). Data are expressed as means ± SE. Weights of liver and fat pads (retroperitoneal, gonadal, and perirenal fat depots) were assessed at the time of sacrifice. Percent liver and fat weight were calculated as tissue weight in grams divided by total body weight in grams. Differences between offspring of lean and obese dams were analyzed by 2-way ANOVA, significance was set at P < 0.05 as indicated by boldface P values. Table adapted from Shankar et al. (45).
Real-time RT-PCR.
Total RNA was isolated from rat placenta at dpc 18.5 (n = 7 lean, n = 8 obese), from rat offspring liver at PND35 (n = 12 animals/group), and from rat offspring liver and gastrocnemius muscle at PND130 (n = 7 lean-con, n = 8 obese-con, n = 11 lean-HFD, and n = 9 obese-HFD) using RNeasy mini columns (QIAGEN, Valencia, CA) including on-column DNase digestion. We reverse-transcribed 1 μg of total RNA with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR analysis was performed as described previously on an ABI Prism 7500 Fast instrument (Carlsbad, CA) (5, 6). Gene-specific primers were designed with Primer Express software (Table 4). Relative amounts of mRNA were quantified with a standard curve and normalized to the mRNA expression of SRP14 and cyclophilin A (Table 4).
Table 4.
Primer sequences for real-time RT-PCR analyses
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Cyclophilin A | AAGCATACAGGTCCTGGCATCT | TGCCATCCAGCCACTCAGT |
| ERRα | AAAGTCCTGGCCCATTTCTATG | CCCTTGCCTCAGTCCATCAT |
| Fis1 | GTGCCTGGTTCGAAGCAAATAC | CATAATCCCGCTGCTCCTCTT |
| Mfn1 | ATCTTCGGCCAGTTACTGGAGTT | AGATCATCCTCGGTTGCTATCC |
| Mfn2 | CCTTGAAGACACCCACAGGAATA | CGCTGATTCCCCTGACCTT |
| Nrf1 | CTCTGCATCTCACCCTCCAAAC | TCTTCCAGGATCATGCTCTTGTAC |
| PARL | GCTTGGACGCAGGTTTAACCTA | CTCCACTTGACCCTGTGTCTGA |
| PGC1α | CTACAATGAATGCAGCGGTCTT | TGCTCCATGAATTCTCGGTCTT |
| PGC1β | TCGGTGAAGGTCGTGTGGTATAC | GCACTCGACTATCTCACCAAACA |
| OPA1 | AAAAGCCCTTCCCAGTTCAGA | TACCCGCAGTGAAGAAATCCTT |
| SIRT1 | CTGTTTCCTGTGGGATACCTGACT | ATCGAACATGGCTTGAGGATCT |
| SRP14 | GAACAAGTTTCAGATGGCCTATTCA | GTGCTGGTTTGCTCTTCTTACTCTT |
Gene-specific primers were designed using Primer Express Software (Applied Biosystems, Foster City, CA). Real-time PCR reactions were carried out according to manufacturer's instructions for fast SYBR green master mix and monitored on an ABI Prism 7500 sequence detection system (Applied Biosystems) as described under materials and methods.
Indirect calorimetry.
Offspring from lean and obese rat dams (n = 5/group, representing four to five distinct biological litters) were housed under conditions of 12:12 h light-dark cycle in metabolic chambers using the Complete Lab Animal Monitoring system (CLAMS) to assess energy expenditure (EE), respiratory exchange ratio (RER), and total cage activity (Columbus Instruments, Columbus, OH) as previously described (5). Briefly, offspring were housed in the CLAMS chambers between PND87 and PND102, where five separate runs (n = 1 animal/group) were performed for a duration of 3 days each. Rats had ad libitum access to the AIN-93G or HFD (45% kcal from fat) throughout the CLAMS measurement period. Data from three consecutive 24 h cycles for both AIN-93G and HFD were converted into percent relative cumulative frequency (PRCF) values (39). Expressing indirect calorimetry data as PRCF has been shown to discern small changes in EE and RER values that may be missed by averaging values over 24-h periods. EE was calculated by a modified Weir equation (23, 54): EE = calorific value (CV)·V̇o2subject, CV = 3.815 + 1.232·RER. PRCF was used to analyze EE and RER values as previously described (39). EC50 values were derived following nonlinear regression with a four-parameter Hill plot.
mtDNA RT-PCR.
Genomic DNA was extracted from rat liver at PND35 and PND130 with the Promega Wizard SV Genomic DNA Purification System (Madison, WI). mtDNA content for liver was determined by rat D-loop and β-actin Taqman primers and probes using previously published sequences (33). PCR amplification was performed using Taqman Fast Universal PCR Master Mix 2X (Applied Biosystems/Life Technologies, Grand Island, NY). ΔΔCT was used to quantify mtDNA content relative to β-actin for rat tissue (Taqman Gene Expression Assay, Applied Biosystems/Life Technologies).
Statistical analysis.
Data are expressed as means ± SE; significance was set at P < 0.05. Differences in mRNA expression between offspring of lean and obese dams at PND35 and PND130 fed AIN-93G or HFD were analyzed by two-way analysis of variance (ANOVA). Significant interactions identified by two-way ANOVA were followed by a one-way ANOVA, and all pair-wise comparisons by Student-Newman-Keuls. Statistical analyses were performed using SigmaPlot 12.0 software (Systat Software, San Jose, CA).
RESULTS
HFD-induced reductions in hepatic mRNA expression of regulators of mitochondrial dynamics in lean and obese dam offspring at PND35 and PND130.
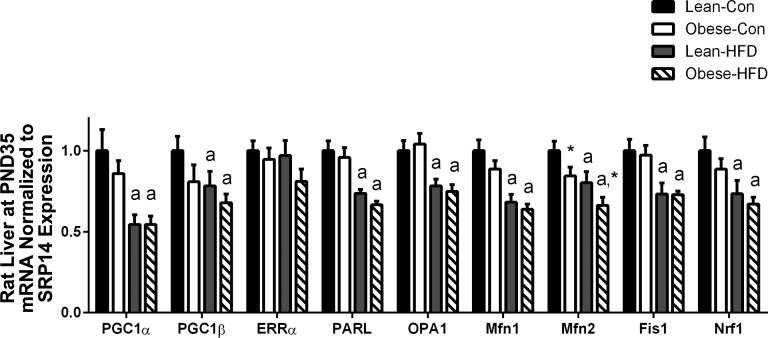
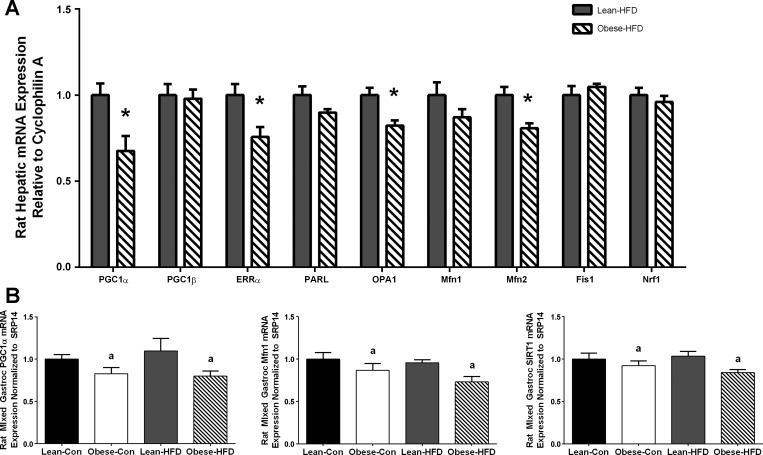
At PND35, except for ERRα, HFD reduced mRNA expression of every fusion and fission target in both lean and obese dam offspring, which included PGC1α (P < 0.001), PGC1β (P = 0.048), presenilin-associated rhomboid-like (PARL) (P < 0.001), optic atrophy (OPA)1 (P < 0.001), Mfn1 (P < 0.001), Mfn2 (P = 0.002), fission (Fis)1 (P < 0.001), and nuclear respiratory factor (Nrf)1 (P < 0.001) as shown in Fig. 3. Maternal obesity further decreased mRNA expression of only Mfn2 (Fig. 3). However, at PND130, although there was no difference due to maternal obesity on the control diet (data not shown), offspring exposed to both gestational obesity and postweaning HFD for ∼16 wk showed decreased mRNA expression of PCG1α (P = 0.014), ERRα (P = 0.018), OPA1 (P = 0.007), and Mfn2 (P = 0.006), and PARL was nearly reduced (P = 0.093) as shown in Fig. 4A. We also examined mRNA expression of these same targets in mixed gastrocnemius skeletal muscle in the offspring of lean and obese dams at PND130 fed either control or HFD (Fig. 4B). In this metabolically active and mitochondrially rich tissue we found that maternal obesity reduced mRNA expression of PGC1α (P = 0.015), Mfn1 (P = 0.018), and SIRT1 (P = 0.020) in both control- and HFD-fed offspring.
Fig. 3.
Hepatic mRNA expression of mitochondrial targets of rat offspring from lean (n = 12) and obese (n = 12) rat dams following a 2 wk HFD challenge (PND35). Gene expression was assessed via real-time RT-PCR. Statistical differences were determined by 2-way ANOVA examining the effects of maternal obesity and postweaning HFD. Data are expressed as means ± SE, significance was set at P < 0.05 (amain effect of postweaning HFD; *main effect of maternal obesity).
Fig. 4.
A: hepatic mRNA expression of mitochondrial targets of offspring from lean (n = 8) and obese (n = 9) rat dams fed HFD for ∼16 wk (PND130) in liver and mixed gastrocnemius muscle. Statistical differences were determined using Student's t-test. *Significance P < 0.05. B: mRNA expression of mitochondrial targets of offspring from lean and obese rat dams fed control (lean n = 7, obese n = 11) or HFD (lean n = 8, obese n = 9) for ∼16 wk (PND130) in mixed gastrocnemius muscle. Statistical differences were determined by 2-way ANOVA examining the effects of maternal obesity and postweaning HFD. Data are expressed as means ± SE, significance was set at P < 0.05 (amain effect of maternal obesity).
RER, EE, and total activity in lean and obese rat dam offspring at PND130.
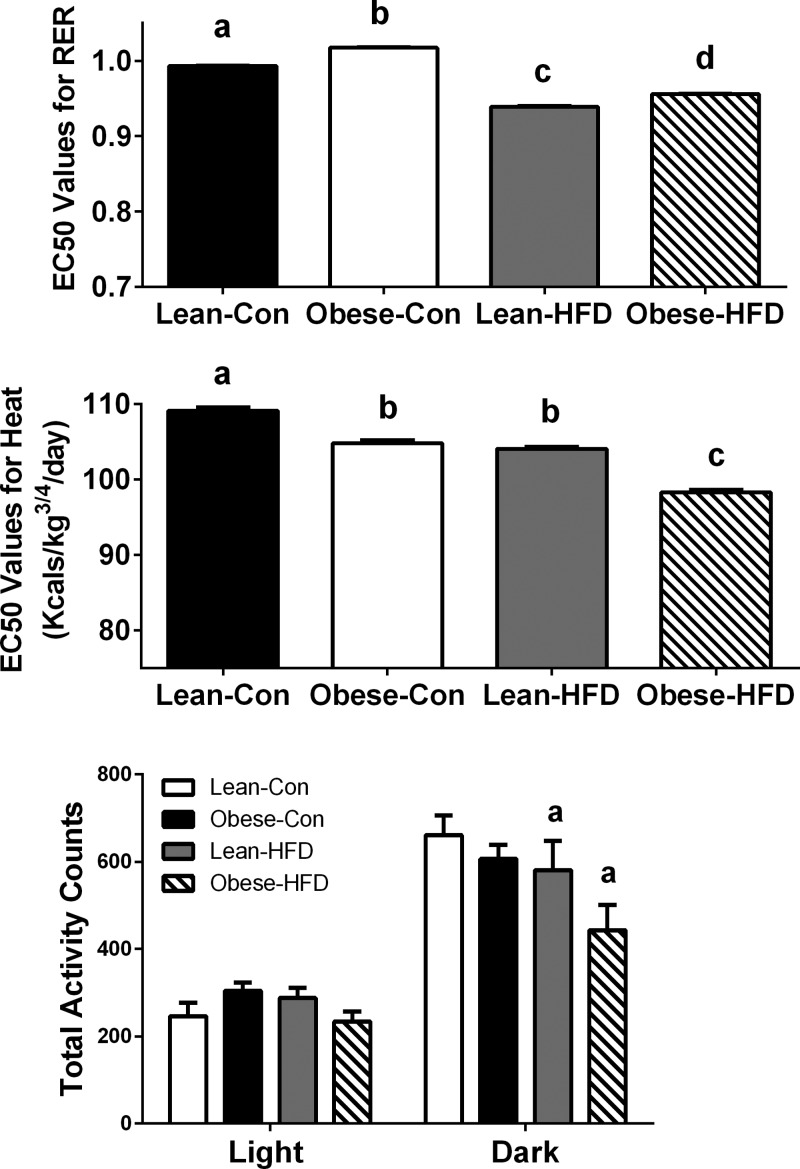
Using indirect calorimetry, we assessed RER, EE, and total cage activity in lean and obese dam offspring at PND87–102 fed control diet or HFD that began at weaning. RER values range from 0.7 to 1.0, where a value of 0.7 represents fatty acids as the sole source of energy and 1.0 represents carbohydrate as the sole substrate source. As expected, HFD led to decreased RER values in both lean and obese dam offspring as shown in Fig. 5A. However, RER values in obese-HFD were higher than lean-HFD (P < 0.001), which broadly represents an increased reliance on glucose and an impaired capacity to utilize fatty acids for energy when challenged by long-term HFD. An inability to substrate switch is indicative of metabolic inflexibility, or a reduced ability to adapt to HFD. RER values were also higher in obese dam offspring fed control diet. EE was assessed by heat (Kcal/kg3/4/day) and was lower in obese dam offspring (P < 0.001) compared with lean dam offspring on control diet (Fig. 5B). Obese-HFD offspring elicited the lowest EE among all offspring groups (P < 0.001), indicating an increased propensity for weight gain. Moreover, during the dark cycle, lean-HFD and obese-HFD offspring had reduced total cage activity (P = 0.033), and a maternal obesity-induced reduction in total cage activity nearly reached significance (P = 0.084) in offspring fed control diet or HFD over the 3-day period as shown in Fig. 5C.
Fig. 5.
Indirect calorimetry and PRCF analysis was used to assess EC50 values for respiratory exchange ratio (RER, A), energy expenditure (EE, kcal/kg3/4/day; B), and total cage activity counts (C) in the offspring of lean and obese rat dams (n = 5 animals per group) fed either an AIN-93G or HFD (45% kcal from fat) ad libitum at PND87-PND102. EC50 values are presented as means ± SE. Different letter superscripts (a, b, c, d) indicate statistical significance (P < 0.05). Statistical differences for total cage activity counts in the light and dark cycles were determined by 2-way ANOVA examining the effects of maternal obesity and postweaning HFD. Data are expressed as means ± SE; significance was set at P < 0.05 (amain effect of postweaning HFD).
Mitochondrial dynamics-related targets are decreased in rat placenta of obese dams.
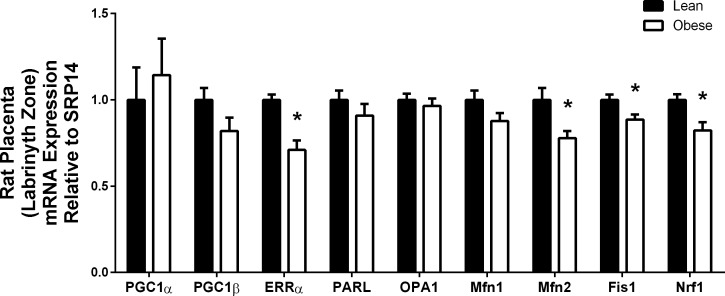
mRNA expression of key mitochondrial targets were measured in the labyrinth zone of male placenta from lean or obese rat dams. Maternal under- and overnutrition is known to alter placental function and lead to fetal programming that may be detrimental to fetal development (24, 26). Placentas from obese dams had significantly decreased mRNA expression of key mitochondrial transcription factors that included ERRα (P < 0.001) and Nrf1 (P = 0.011). PGC1β (P = 0.083) mRNA was nearly significantly reduced (Fig. 6). Moreover, critical targets associated with the processes of fusion and fission, which included Mfn2 (P = 0.011) and Fis1 (P = 0.015), were decreased, while Mfn1 (P = 0.084) trended toward decreased expression but did not reach statistical significance, as shown in Fig. 6. Moreover, placentas from obese dams were heavier (lean 0.39 ± 0.01 g vs. obese 0.47 ± 0.02 g, P = 0.003) and had reduced placental efficiency, which represents fetal weight (g)/placenta weight (g) (lean 3.84 ± 0.24 vs. obese 2.94 ± 0.11, P = 0.004), suggesting maladaptation in placenta of obese dams.
Fig. 6.
Male rat placental mRNA expression of mitochondrial targets from lean (n = 7) and obese (n = 8) dams at dpc 18.5. Gene expression was assessed via real-time RT-PCR. Data are expressed as means ± SE. Statistical differences were determined by Student's t-test. *Significance P < 0.05.
Effect of maternal obesity and HFD on hepatic mtDNA content in rat offspring.
To determine mitochondrial copy number, we used a Taqman assay to quantify mtDNA content in rat liver at PND35 and PND130. While maternal obesity by itself did not affect mtDNA content at either time point in offspring liver, HFD led to increased mtDNA in both lean and obese dam offspring at PND35 (P < 0.001, Fig. 7A) and PND130 (P < 0.001, Fig. 7B).
Fig. 7.
mtDNA content in rat liver assessed by Taqman assay from offspring of lean and obese dams at PND35 fed control or HFD (n = 12/group) (A) and PND130 fed control (lean n = 7, obese n = 11) or HFD (lean n = 8, obese n = 9) (B). Statistical differences were determined by 2-way ANOVA examining the effects of maternal obesity and postweaning HFD. Data are expressed as means ± SE, significance was set at P < 0.05 (amain effect of postweaning HFD).
DISCUSSION
Mitochondrial dynamics encompasses mitochondrial fusion and fission and is essential for optimal mitochondrial function, which, in turn, influences numerous metabolic and developmental processes (13, 14, 30, 57). The present study provides significant new insight into the premise that mitochondrial dynamics is susceptible to in utero programming following exposure to maternal obesity. Our findings reveal that maternal obesity and postweaning HFD in rats lead to tissue- and developmental stage-specific effects on regulators of mitochondrial plasticity. Two overarching findings consistently emerged from these studies: 1) reductions in transcriptional regulators (PGC1α, PGC1β, and ERRα) and 2) decreased mRNA expression of mitofusins, namely Mfn2.
PGC1α, PGC1β, and ERRα are known pleiotropic transcriptional regulators of energy metabolism and mitochondrial biogenesis (17, 41, 42), as well as mitochondrial fusion targets (27, 51, 55). PGC1α, PGC1β, and ERRα were reduced in association with maternal obesity and/or HFD in the rat liver (PND35 and PND130), skeletal muscle (PND130), and placenta (dpc 18.5). However, despite the documented roles of these genes in mitochondrial biogenesis, there was no change in mitochondrial copy number in rat liver with maternal obesity. In fact, hepatic mtDNA content in both lean and obese dam offspring was increased with HFD. These findings are nevertheless consistent with previous reports, perhaps representing a compensatory increase associated with mitochondrial dysfunction. Igosheva et al. (22) reported increased mtDNA copy number, TFAM, and Nrf1 in oocytes from diet-induced obese mice, which the authors suggested was indicative of mitochondrial dysfunction. Furthermore, a study investigating mtDNA content in placenta exposed to intrauterine growth restriction (IUGR) found that the most severe cases of IUGR had the highest mtDNA levels and speculated increased mtDNA may be a causative mechanism in IUGR (25). Our findings support the view of increased mtDNA content's being associated with impaired mitochondrial function. Importantly, higher mtDNA content did not translate into an increase in mitochondrial capacity, as the apparent increase in mtDNA content was insufficient to prevent obesity and metabolic dysfunction in the obese dam offspring as shown previously (5, 45, 46). Collectively, our findings suggest that altered mitochondrial number was not the driving factor influencing obesity development in rat offspring, which is in agreement with previous reports by others.
A much stronger case can be made for diminished mitochondrial function as an important contributor to impaired metabolism in offspring of obese rats. In previous studies we unequivocally demonstrated that maternal obesity per se was associated with impaired fatty acid oxidation, decreased OXPHOS proteins in the offspring at weaning, and reduced TFAM and Nrf1 in blastocysts from obese dams (5, 46, 47). Others have also reported impaired mitochondrial function in offspring and oocytes due to maternal HFD in various mouse models (7, 22, 52). Collectively, these studies indicate programming of mitochondria might begin early (oocyte quality or embryogenesis) and persist into adulthood, which we speculate may be due to disruptions in mitochondrial fusion and fission. In most cases, consumption of HFD exacerbates developmentally programmed effects, so it is plausible that modest defects in fusion/fission of mitochondria contribute to diminished mitochondrial function (such as fatty acid oxidation), in the face of postnatal HFD challenge. While we did not directly measure mitochondrial function (such as mitochondrial respiration) in situ, these studies are highly warranted based on the preponderance of evidence linking impaired mitochondrial and altered metabolism in offspring of obese dams.
Indeed, maternal obesity led to impaired whole body energy metabolism as shown by decreased ability to utilize fatty acids for energy and decreased EE in offspring fed either control or HFD. An inability to oxidize additional fatty acids for energy when challenged by HFD suggests metabolic inflexibility (19). Moreover, obese dam offspring fed HFD had the lowest EE values among all groups at PND130 and lower cage activity levels compared with control-fed offspring. These findings are consistent with our previous report demonstrating similar results in obese dam offspring in the early postweaning period (5). The present report establishes that in concert with impaired mitochondrial gene expression, decreased EE and metabolic inflexibility not only were apparent at PND21, an age prior to obesity and increased adiposity development, but also persisted into adulthood. Consequently, abated energy metabolism and metabolic inflexibility are likely to have strong contributions to increased adiposity, NAFLD, and insulin levels evident in obese dam offspring at PND130 (45).
A salient finding of the current report is the effect of maternal obesity on mitofusins. Mitofusins (Mfn1 and Mfn2) are mitochondrial GTPases that are critical in repairing damaged mitochondria through the process of complementation where healthy mitochondria fuse with damaged mitochondria to allow mixing and thus repair (13, 57). As mentioned previously, PGC1α, PGC1β, and ERRα are transcriptional regulators of mitochondrial fusion targets (27, 51, 55). As expected, mRNA expression of Mfn1 and Mfn2 mirrored that of PGC1α, PGC1β, and ERRα and was reduced in placenta, liver, and skeletal muscle because of maternal obesity and/or HFD in rat offspring. Low levels of Mfn2 in skeletal muscle have been previously reported in both subjects with insulin resistance and obese Zucker rats (37, 56). Mfn2 has also been shown to play an important role in the placenta as decreased Mfn2 has been related to increased mitochondrial fragmentation and cristae remodeling during syncytialization in the human placenta (53). Furthermore, mice lacking Mfn2 die in midgestation from a severe disruption of placental trophoblast giant cells (14). Fragmented mitochondria are known to be impaired and are more likely to undergo apoptosis (14, 18, 31).
Our findings are the first to show decreased Mfn2 in the rat placenta labyrinth zone of obese dams. Reduced protein levels of Mfn2 and Bcl-2, decreased ATP levels, and increased Bax have been shown in women with unexplained miscarriages compared with women with normal pregnancies, suggesting an important role of placental mitochondria, and specifically Mfn2, for healthy pregnancy and fetal development (34). Furthermore, a recent study by Hastie and Lappas (21) reported impaired placental mitochondrial activity of electron chain transport complex I in obese women with normal glucose tolerance. Another group recently demonstrated impaired placental mitochondrial function with increasing prepregnancy body mass index (29). Moreover, physical activity has been shown to increase Mfn2 expression, reinforcing the idea that Mfn2 involvement is important to energy metabolism (10). Although we did not directly assess mitochondrial fusion and fission in a quantitative manner as previously reported using a model of photo-activatable mitochondria (36), our results clearly demonstrate consistent and persistent repression of mitofusins which could contribute to impaired metabolism.
It is important to note the limitations of the current study. First, only male placenta and offspring samples were examined as part of our ongoing studies. In previous studies, we found increased susceptibility of male offspring from obese dams to HFD-induced obesity. Hence, mechanistic studies in rats were limited to male offspring. Similar sexual dimorphism in fetal programming has been reported in other models, and in the majority of cases the precise mechanisms remain intriguing but unknown. A second limitation relates to direct measures of mitochondrial function, which were not available as the majority of tissues available were frozen. The present studies provide strong, but associative evidence linking specific transcription factors (PGC1α, PGC1β, and ERRα) to downstream targets (Mfn1 and Mfn2). Direct recruitment of and regulation via these factors on important targets certainly warrants future investigation. A third limitation includes a lack of inflammatory markers as obesity is known to induce inflammation in metabolic tissues like the liver, skeletal muscle, and placenta. Recent findings from our group using the same rat gestation obesity model (currently under consideration) demonstrated greater inflammation (Egr-1 and TNF-α) in the placenta labyrinth zone of male pups due to exposure to maternal obesity, which could also be contributing to mitochondrial alterations in our animal model. Finally, we have recently begun to collect samples to investigate mitochondrial morphology using transmission electron microscopy from animal tissues.
In summary, the present report demonstrates dysregulation of putative regulators of mitochondrial health (PGC1α, PGC1β, ERRα, and mitofusins) in male rat offspring following intrauterine exposure to maternal obesity and/or postweaning HFD. This was associated with suppressed EE and metabolic inflexibility at PND130 in obese dam rat offspring. Since there is an expanding body of literature implicating the role of mitochondria as organelles directing metabolic health outcomes, interventions to reverse mitochondrial programming and prevent obesity development in early and later life certainly warrant future investigations.
GRANTS
These studies were supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-084225 (to K. Shankar) and USDA-Agriculture Research Service CRIS 6251-51000-007-04S. The funding agencies had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.B. and K.S. conception and design of research; S.J.B., J.F., and P.K. performed experiments; S.J.B., J.F., P.K., M.L.B., and K.S. analyzed data; S.J.B. and K.S. interpreted results of experiments; S.J.B. prepared figures; S.J.B. drafted manuscript; S.J.B., M.L.B., T.M.B., and K.S. edited and revised manuscript; S.J.B. and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Matt Ferguson, Trae Pittman, Bobby Fay, and other members of the Arkansas Children's Nutrition Center Animal Research Core Facility for assistance with animal studies.
REFERENCES
- 1.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 35: 325–335, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Badger TM, Crouch J, Irby D, Hakkak R, Shahare M. Episodic excretion of ethanol during chronic intragastric ethanol infusion in the male rat: continuous vs. cyclic ethanol and nutrient infusions. J Pharmacol Exp Ther 264: 938–943, 1993. [PubMed] [Google Scholar]
- 3.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol 294: G27–G38, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Borengasser SJ, Kang P, Faske J, Gomez-Acevedo H, Blackburn ML, Badger TM, Shankar K. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One 9: e84209, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One 6: e24068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez-Acevedo H, Shankar K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 154: 4113–4125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50: 1796–1808, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res 75: 140–147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgueno AL, Cabrerizo R, Gonzales MN, Sookoian S, Pirola CJ. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J Nutr Biochem 24: 6–13, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM. Obesity and pregnancy–the propagation of a viscous cycle? J Clin Endocrinol Metab 88: 3505–3506, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32: 1076–1080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 370: 403–411, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 92: 1257–1264, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol 17: 292–301, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–E1017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway CA, Yoon Y. Perspectives on: SGP symposium on mitochondrial physiology and medicine: what comes first, misshape or dysfunction? The view from metabolic excess. J Gen Physiol 139: 455–463, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastie R, Lappas M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 35: 673–683, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 5: e10074, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 299: R1097–R1105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol 56: 591–601, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, Cetin I. Higher mitochondrial DNA content in human IUGR placenta. Placenta 29: 1029–1033, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lewis RM, Demmelmair H, Gaillard R, Godfrey KM, Hauguel-de Mouzon S, Huppertz B, Larque E, Saffery R, Symonds ME, Desoye G. The placental exposome: placental determinants of fetal adiposity and postnatal body composition. Ann Nutr Metab 63: 208–215, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One 3: e3613, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei Z, Grummer-Strawn LM, Scanlon KS. Does overweight in infancy persist through the preschool years? An analysis of CDC Pediatric Nutrition Surveillance System data. Soz Praventivmed 48: 161–167, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab 307: E419–E425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel S, Wanet A, De PA, Rommelaere G, Arnould T, Renard P. Crosstalk between mitochondrial (dys)function and mitochondrial abundance. J Cell Physiol 227: 2297–2310, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58: 2303–2315, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol 34: 515–526, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas JA, Brooks EM, Hunter TC, Single R, Branda RF. Development of a quantitative PCR (TaqMan) assay for relative mitochondrial DNA copy number and the common mitochondrial DNA deletion in the rat. Environ Mol Mutagen 44: 313–320, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Pang W, Zhang Y, Zhao N, Darwiche SS, Fu X, Xiang W. Low expression of Mfn2 is associated with mitochondrial damage and apoptosis in the placental villi of early unexplained miscarriage. Placenta 34: 613–618, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Pereira-da-Silva L, Cabo C, Moreira AC, Virella D, Guerra T, Camoes T, Silva AR, Neves R, Ferreira GC. The adjusted effect of maternal body mass index, energy and macronutrient intakes during pregnancy, and gestational weight gain on body composition of full-term neonates. Am J Perinatol 31: 875–882, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Pham AH, McCaffery JM, Chan DC. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis 50: 833–843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14: 1405–1415, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Bhattacharya S, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347: f4539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol 82: 1075–1083, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Ritchie LD, Ivey SL, Woodward-Lopez G, Crawford PB. Alarming trends in pediatric overweight in the United States. Soz Praventivmed 48: 168–177, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett 582: 46–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813: 1269–1278, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar K, Harrell A, Kang P, Singhal R, Ronis MJ, Badger TM. Carbohydrate-responsive gene expression in the adipose tissue of rats. Endocrinology 151: 153–164, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Shankar K, Kang P, Harrell A, Zhong Y, Marecki JC, Ronis MJ, Badger TM. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology 151: 2577–2589, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 152: 4158–4170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao D, Liu Y, Liu X, Zhu L, Cui Y, Cui A, Qiao A, Kong X, Liu Y, Chen Q, Gupta N, Fang F, Chang Y. PGC-1 beta-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR alpha. Mitochondrion 10: 516–527, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 363: 1642–1645, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Biertho L, Simard S, Kral JG, Marceau P. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 94: 4275–4283, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55: 1783–1791, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Turdi S, Ge W, Hu N, Bradley KM, Wang X, Ren J. Interaction between maternal and postnatal high fat diet leads to a greater risk of myocardial dysfunction in offspring via enhanced lipotoxicity, IRS-1 serine phosphorylation and mitochondrial defects. J Mol Cell Cardiol 55: 117–129, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Wasilewski M, Semenzato M, Rafelski SM, Robbins J, Bakardjiev AI, Scorrano L. Optic atrophy 1-dependent mitochondrial remodeling controls steroidogenesis in trophoblasts. Curr Biol 22: 1228–1234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zorzano A. Regulation of mitofusin-2 expression in skeletal muscle. Appl Physiol Nutr Metab 34: 433–439, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Zorzano A, Hernandez-Alvarez MI, Palacin M, Mingrone G. Alterations in the mitochondrial regulatory pathways constituted by the nuclear co-factors PGC-1alpha or PGC-1beta and mitofusin 2 in skeletal muscle in type 2 diabetes. Biochim Biophys Acta 1797: 1028–1033, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol 21: 566–574, 2010. [DOI] [PubMed] [Google Scholar]