Abstract
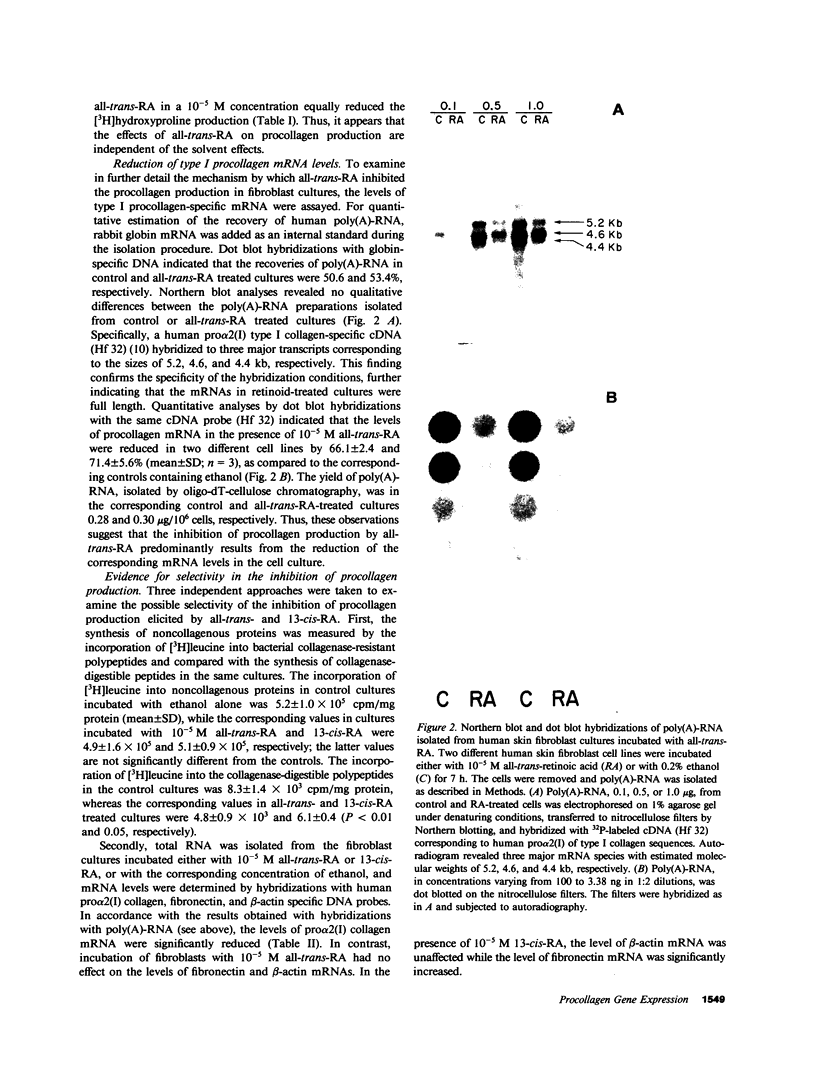
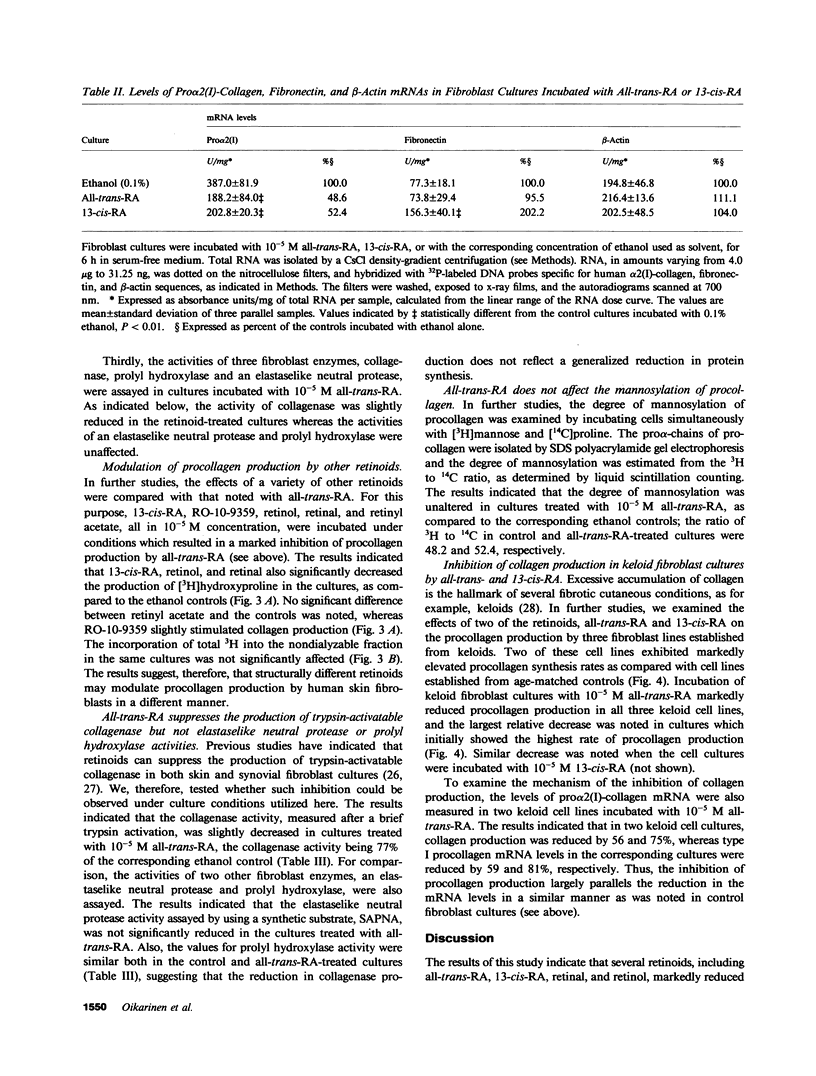
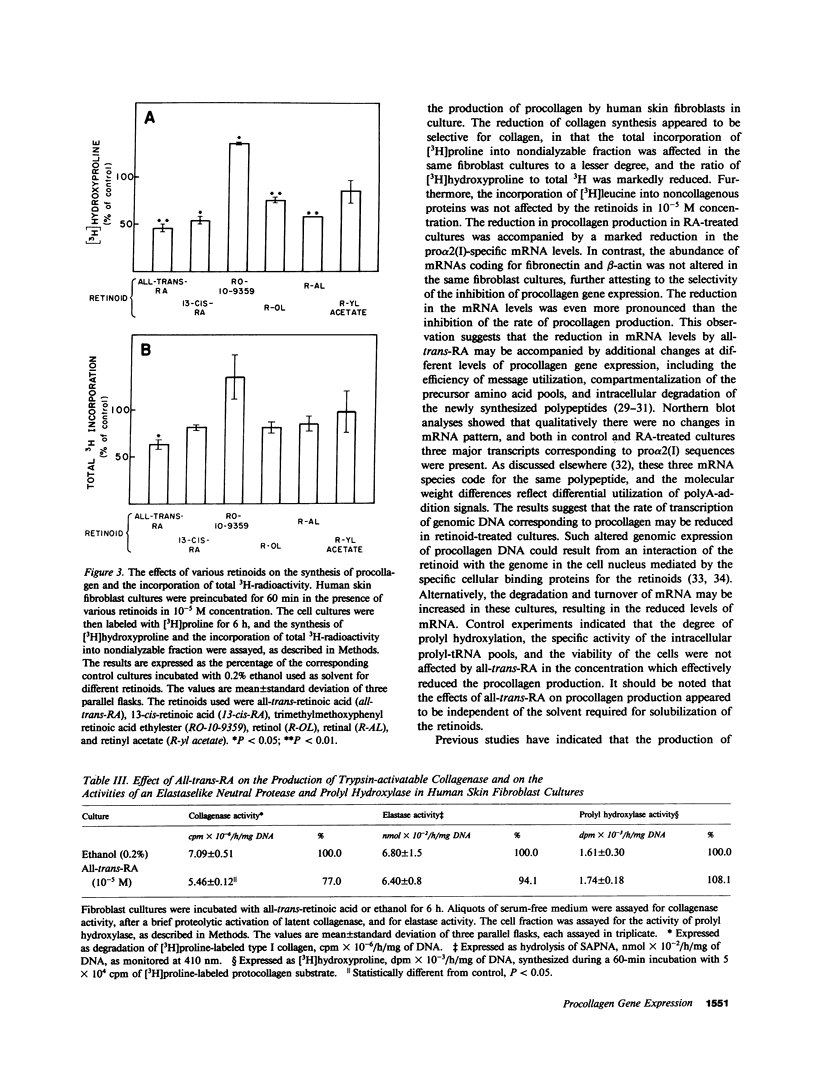
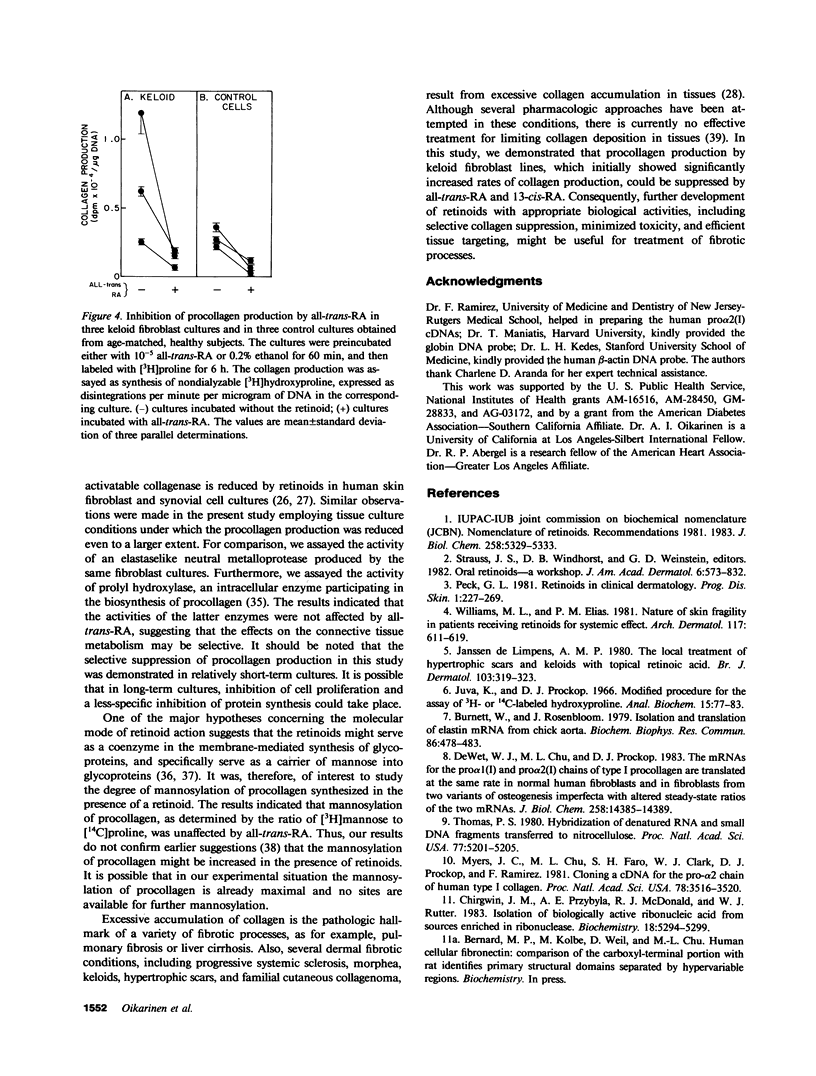
Recent clinical observations have suggested that retinoids, which are in frequent use in dermatology, can affect the connective tissue metabolism in skin and other tissues. In this study, we examined the effects of several retinoids on the metabolism of collagen by human skin fibroblasts in culture. Incubation of cultured fibroblasts with all-trans-retinoic acid or 13-cis-retinoic acid, in 10(-5) M or higher concentrations, markedly reduced the procollagen production, as measured by synthesis of radioactive hydroxyproline. The effect was selective in that little, if any, inhibition was noted in the incorporation of [3H]leucine into the noncollagenous proteins, when the cells were incubated with the retinoids in 10(-5) M concentration. Similar reduction in procollagen production was noted with retinol and retinal, whereas an aromatic analogue of retinoic acid ethyl ester (RO-10-9359) resulted in a slight increase in procollagen production in these cultures. The reduction in procollagen production by all-trans-retinoic acid was accompanied by a similar reduction in pro alpha 2(I) of type I procollagen specific messenger RNA (mRNA), as detected by dot blot and Northern blot hybridizations. Hybridizations with human fibronectin and beta-actin specific DNA probes indicated that the levels of the corresponding mRNAs were not affected by the retinoids, further suggesting selectivity in the inhibition of procollagen gene expression. Further control experiments indicated that all-trans-retinoic acid, under the culture conditions employed, did not affect the posttranslational hydroxylation of prolyl residues, the mannosylation of newly synthesized procollagen, the specific radioactivity of the intracellular prolyltransfer RNA pool, or DNA replication. All-trans-retinoic acid also elicited a reduction in trypsin-activatable collagenase, but not in the activity of prolyl hydroxylase or an elastaselike neutral protease in the fibroblast cultures. Incubation of three fibroblast lines established from human keloids with all-trans-retinoic acid or 13-cis-retinoic acid also resulted in a marked reduction in procollagen production. The results, therefore, suggest that further development of retinoids might provide a novel means of modulating collagen gene expression in patients with various diseases affecting the connective tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Seltzer J. L., Eisen A. Z. Inhibition of collagen degradative enzymes by retinoic acid in vitro. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):603–607. doi: 10.1016/s0190-9622(82)70049-0. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Purification of (14C) protocollagen and its hydroxylation by prolyl-hydroxylase. Biochemistry. 1973 Aug 28;12(18):3395–3401. doi: 10.1021/bi00742a005. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Uitto J. Collagen biosynthesis by human skin fibroblasts. III. The effects of ascorbic acid on procollagen production and prolyl hydroxylase activity. Biochim Biophys Acta. 1981 Jun 11;675(1):117–122. doi: 10.1016/0304-4165(81)90076-3. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Nagase H., Nagel J. E., Harris E. D., Jr Effects of all-trans-retinoic acid (retinoic acid) and 4-hydroxyphenylretinamide on synovial cells and articular cartilage. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):591–602. doi: 10.1016/s0190-9622(82)70048-9. [DOI] [PubMed] [Google Scholar]
- Burnett W., Rosenbloom J. Isolation and translation of elastin mRNA from chick aorta. Biochem Biophys Res Commun. 1979 Feb 14;86(3):478–484. doi: 10.1016/0006-291x(79)91739-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ding J. F., Morabito M., Myers J., Williams C., Ramirez F. Human pro alpha 1(I) collagen gene structure reveals evolutionary conservation of a pattern of introns and exons. 1984 Jul 26-Aug 1Nature. 310(5975):337–340. doi: 10.1038/310337a0. [DOI] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- De Luca L. M. Studies on mannosyl carrier function of retinol and retinoic acid in epithelial and mesenchymal tissues. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):611–619. doi: 10.1016/s0190-9622(82)80043-1. [DOI] [PubMed] [Google Scholar]
- Dion L. D., De Luca L. M., Colburn N. H. Phorbol ester-induced anchorage independence and its antagonism by retinoic acid correlates with altered expression of specific glycoproteins. Carcinogenesis. 1981;2(10):951–958. doi: 10.1093/carcin/2.10.951. [DOI] [PubMed] [Google Scholar]
- Goodman D. S. Retinoid-binding proteins. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):583–590. doi: 10.1016/s0190-9622(82)70065-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen de Limpens A. M. The local treatment of hypertrophic scars and keloids with topical retinoic acid. Br J Dermatol. 1980 Sep;103(3):319–323. doi: 10.1111/j.1365-2133.1980.tb07251.x. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Moen R. C., Rowe D. W., Palmiter R. D. Regulation of procollagen synthesis during the development of chick embryo calvaria. Correlation with procollagen mRNA content. J Biol Chem. 1979 May 10;254(9):3526–3530. [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori M., Chytil F. Mechanism of vitamin A action. Gene expression in retinol-deficient rats. J Biol Chem. 1982 Dec 10;257(23):14370–14374. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Russell S. B., Russell J. D., Trupin J. S. Alteration of amino acid transport by hydrocortisone. Different effects in human fibroblasts derived from normal skin and keloid. J Biol Chem. 1982 Aug 25;257(16):9525–9531. [PubMed] [Google Scholar]
- Ryhänen L., Rantala-Ryhänen S., Tan E. M., Uitto J. Assay of collagenase activity by a rapid, sensitive, and specific method. Coll Relat Res. 1982 Mar;2(2):117–130. doi: 10.1016/s0174-173x(82)80028-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Uitto J., Ryhänen L., Tan E. M., Oikarinen A. I., Zaragoza E. J. Pharmacological inhibition of excessive collagen deposition in fibrotic diseases. Fed Proc. 1984 Oct;43(13):2815–2820. [PubMed] [Google Scholar]
- Uitto J., Santa Cruz D. J., Eisen A. Z. Connective tissue nevi of the skin. Clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980 Nov;3(5):441–461. [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981 Oct;117(10):611–619. [PubMed] [Google Scholar]
- de Luca L. M., Bhat P. V., Sasak W., Adamo S. Biosynthesis of phosphoryl and glycosyl phosphoryl derivatives of vitamin A in biological membranes. Fed Proc. 1979 Oct;38(11):2535–2539. [PubMed] [Google Scholar]
- de Wet W. J., Chu M. L., Prockop D. J. The mRNAs for the pro-alpha 1(I) and pro-alpha 2(I) chains of type I procollagen are translated at the same rate in normal human fibroblasts and in fibroblasts from two variants of osteogenesis imperfecta with altered steady state ratios of the two mRNAs. J Biol Chem. 1983 Dec 10;258(23):14385–14389. [PubMed] [Google Scholar]