Abstract
Despite preeclampsia being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for its pathogenesis have yet to be fully elucidated. Growing evidence indicates that reduced uteroplacental perfusion and the resulting placental ischemia triggers the cascade of events leading to this maternal disorder. While the well-established rat model of reduced uterine perfusion pressure (RUPP) is providing invaluable insight into the etiology of preeclampsia, the aim of this study was to develop a mouse model of reduced uterine perfusion to expand mechanistic investigation by incorporation with novel gene-targeted mice. To accomplish this aim, a sham surgical procedure or a restriction of blood flow at the abdominal aorta and the ovarian arteries was initiated at day 13 of gestation in C57BL/6J mice. Mean arterial pressure measured in conscious, chronically instrumented mice was significantly elevated in the RUPP (120 ± 4 mmHg) compared with the sham (104 ± 4 mmHg) mice at day 18 of gestation (P < 0.01). Placental ischemia reduced fetal weights (0.95 ± 0.04 and 0.80 ± 0.02 g; RUPP vs. Sham, respectively; P < 0.02) and increased circulating levels of antiangiogenic soluble fms-related tyrosine kinases (sFlt)-1 (P < 0.05) in the RUPP at day 18 of gestation. Plasma concentrations of sFlt-1 are increased in preeclamptic patients and in response to reduced uterine perfusion in the rat. Thus, these results suggest that the mouse model of reduced uterine perfusion is applicable to facilitate novel mechanistic investigation into the etiology of hypertension that results from placental ischemia during pregnancy.
Keywords: mouse, hypertension, pregnancy, intrauterine growth restriction, placental ischemia
preeclampsia is one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity (29). However, the mechanisms responsible for the pathogenesis of hypertension during pregnancy or preeclampsia are not known. Preeclampsia is a complex disorder involving multiple organ systems (24), yet numerous studies indicate that the placenta plays a central role in the pathogenesis of preeclampsia and that reduced uteroplacental perfusion, which develops as a result of abnormal cytotrophoblast invasion of the spiral arterioles, triggers the cascade of events leading to this maternal disorder (24). Placental ischemia leads to the release of soluble placental factors, including the soluble form of the vascular endothelial growth factor (VEGF) receptor type 1 and the soluble fms-related tyrosine kinases (sFlt-1) (19). Plasma concentrations of sFlt-1 are increased in preeclamptic patients (28), and exogenous administration of sFlt-1 into the pregnant rat results in an increase in arterial pressure associated with a decrease in plasma-free VEGF and impaired endothelial function (5). The rat model of reduced uterine perfusion is associated with a marked increase in arterial pressure (3), variable proteinuria (3), and increased sFlt-1 (7), all characteristics of the human condition of preeclampsia. The development of a mouse model of reduced uterine perfusion pressure would greatly expand the ability to investigate the etiology of preeclampsia through the use of genetically manipulated mice. Thus, the aim of this study was to develop a mouse model of reduced uterine perfusion to further address the mechanisms by which placental insufficiency leads to increases in blood pressure in the mother during pregnancy.
METHODS
Animal experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals with all animal protocols approved by the University of Mississippi Medical Center's Institutional Animal Care and Use Committee. Timed-pregnant C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA); gestation day (GD) 0 was defined as the day on which sperm plugs were detected. Mice arrived at our laboratory animal facilities at GD 11. Mice were housed individually at 23°C on a 12:12-h light-dark cycle, while being maintained on water and food (8640; Harlan Laboratories, Indianapolis, IN) ad libitum.
Reduced uterine perfusion pressure protocol in the mouse.
On GD 13, mice were subjected to a mechanical procedure to obtain reduced uterine perfusion pressure (RUPP). For this procedure, mice were anesthetized using isoflurane (Butler Schein Animal Health, Dublin, OH), which was administered via vaporizer (Ohmeda, BOC Health Care, Steeton, West Yorkshire, UK). Under aseptic conditions, an abdominal incision was made allowing for visualization of the abdominal aorta and exteriorization of the ovarian and utero-fetal-placental vascular beds. The region of the abdominal aorta near the iliac bifurcation was carefully cleaned of periadventitial fat using pointed-tipped cotton swabs and then separated from the vena cava using a cotton swab and a pair of angled fine-tipped forceps (Fine Science Tools, Foster City, CA). A 6–0 silk braided silk suture (Fine Science Tools) was placed underneath the isolated portion of the aorta using strict caution to avoid bleeding. The ligature was tied around the aorta and a metal rod (OD, 0.1 mm), which lay parallel to the aorta. The ligature was knotted only once. Once taut, the rod was carefully but swiftly removed from the knotted ligature allowing blood flow to return through the aorta. The ligature knot was immobilized with a tiny drop of Vetbond tissue adhesive (3M, St. Paul, MN). Preliminary studies showed that this procedure reduced iliac artery blood flow by 50%, (n = 3). To prevent the occurrence of compensatory blood flow from the ovarian-side of the uterine circulation in response to constriction of the abdominal aorta, two silver clips (width of 0.275 mm ± 5%) were each placed around the combined arterial and venous branches of the ovarian vascular arcade that lead to the first fetus; these vessels were cleaned just enough to allow proper placement of clips without causing bleeding. The clips were clamped around the blood vessels using a pair of needle holders manufactured to form a 0.6-mm gap when closing the silver clips, which resulted in an ID of 0.05 mm for each silver clip. Alongside the RUPP surgeries, a subset of mice were subjected to a sham operation, whereby an abdominal incision was made and blood vessels were prepared in a similar fashion as in the RUPP procedure, except without placement of the ligature or clips (Sham group). The abdominal incision was sutured and cleaned, and mice were provided with Carprofen analgesic (5 mg/kg sc). Measure of mean arterial pressure (MAP) and biochemical analyses were performed in n = 7 for the Sham control (normal pregnant, NP) group and n = 10 for the RUPP experimental group, as detailed below.
MAP measurements.
On GD 17, under isoflurane anesthesia, indwelling catheters were implanted in the right carotid artery and exposed at the nape of the neck. Catheters were composed of RenaPulse RPT-040 Tubing (Braintree Scientific, Braintree, MA), which were stretched over a heat gun to create an insert length of 1 cm with an approximate OD of 0.28 mm. Catheters were filled with sterile mixture of 30% heparin/saline solution to preserve patency. On GD 18, catheters were connected to pressure transducers (MLT0699; AD Instruments, Colorado Springs, CO) coupled to a computerized data acquisition system (PowerLab, AD Instruments), while mice were moving freely in cages in the conscious state. Once hemodynamic readings stabilized, blood pressure values were collected for a 20-min recording period.
Tissue harvest.
On GD 18, under isoflurane anesthesia, a midline incision was made, the uterine horns were exposed, and the fetuses were exteriorized. Blood was collected in a syringe via cardiac puncture and was centrifuged to obtain the serum. Urine was collected via syringe by tapping the bladder. Heart and kidneys were weighed and normalized to body weight. The number of viable and reabsorbed fetuses in each animal was recorded along with individual fetal and placental weights. Representative placentas were snap-frozen in liquid N2 and stored at −80°C until processed. Serum and urine were stored at −20°C until analyzed.
Tissue homogenization.
Representative placentas were selected, and whole placentas were homogenized by a FastPrep-24 Instrument for 2 min in 2-ml tubes containing Lysing Matrix D (MP Biomedicals, Santa Ana, CA) and 1 ml of radioimmunoprecipitation buffer supplemented with a protease inhibitor cocktail per manufacturer's instructions (Santa Cruz Biotechnology, Santa Cruz, CA). Homogenates were quickly centrifuged at room temperature at 13,200 rpm. Supernatants were stored at −80°C until analyzed. Total protein concentration in each sample was quantified using the BCA method (Thermo Scientific, Rockford, IL).
Biochemistry.
Serum sFlt-1 and VEGF were quantified by ELISA per the manufacturer's instructions (R&D Systems, Minneapolis, MN). Placental sFlt-1, VEGF, and tumor necrosis factor-α (TNF-α) levels were also quantified using ELISAs from R&D Systems followed by normalization to placental total protein. Proteinuria was assessed in the following manner: urinary total protein concentration was quantified by the BCA method, of which values were normalized to urinary creatinine concentration determined by a commercially available kit (The Creatinine Companion, Exocell, Philadelphia, PA).
Statistical analyses.
Data are presented as means ± SE. Data were graphed and analyzed by unpaired Student's t-test using GraphPad Prism (La Jolla, CA). A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of reduced uterine perfusion in the mouse on mean arterial pressure and proteinuria.
Blood pressure was significantly elevated in RUPP mice (∼16 mmHg) compared with sham mice at G18 (104 ± 4 vs. 120 ± 4 mmHg; Sham vs. RUPP, respectively, P < 0.01) (Fig. 1A), indicating that the reduced uterine perfusion procedure initiated at G13 of gestation induced hypertension during pregnancy. However, reduced uterine perfusion did not result in proteinuria with urine obtained from a spot measurement despite correction for creatinine [protein (mg/ml) creatinine (mg/ml) ratio; 684.6 ± 25.8 vs. 686.3 ± 30.1; Sham vs. RUPP, respectively] (Fig. 1B).
Fig. 1.
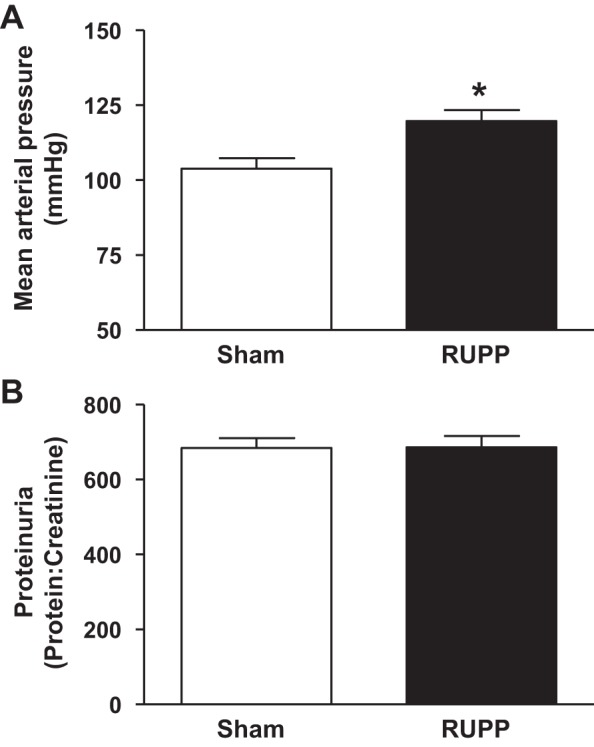
The effect of reduced uterine perfusion on mean arterial blood pressure (A) and proteinuria (B) at day 18 of gestation in pregnant mice that underwent either a sham or the reduced uterine perfusion procedure (RUPP) at day 13 of gestation. Mice were instrumented via carotid artery catheter at day 17 of gestation. Measure of mean arterial pressure was in the conscious state. The bar graph represents means ± SE. *P < 0.01 using unpaired Student's t-test. n = 7 for sham and n = 10 for RUPP.
The effect of reduced uterine perfusion in the mouse on body weight, pup weight, and placenta weight.
Body weight did not differ between the Sham (30.7 ± 1.3 g) and the RUPP (29.4 ± 1.4 g) groups at GD 18. The heart weight (g)-to-body weight (g) (0.105 ± 0.002 vs. 0.187 ± 0.085) and the kidney weight (g) -to-body weight (g) (0.275 ± 0.008 vs. 0.283 ± 0.004) ratios also did not differ upon comparison of Sham to RUPP mice, respectively, at GD 18. Litter size was not significantly different between the Sham (5.9 ± 0.7 pups) and the RUPP group (5.9 ± 1.9 pups) at GD 18. The percent of reabsorbed pups at GD 18 of gestation was slightly increased but not significantly different upon comparison to the Sham group (7.4 ± 3.7 vs. 28.3 ± 13.9%, respectively). Fetal weight at GD 18 was significantly reduced in the RUPP group (0.79 ± 0.02 g) relative to the Sham group (0.95 ± 0.04 g) at GD 18 (P < 0.01) (Fig. 2A), despite the fact that placenta weight (0.084 ± 0.006 vs. 0.078 ± 0.003 g; Sham vs. RUPP, respectively) (Fig. 2B), placenta efficiency, and fetal weight/placenta weight did not differ (11.6 ± 0.9 vs. 10.2 ± 0.4; Sham vs. RUPP group, respectively).
Fig. 2.
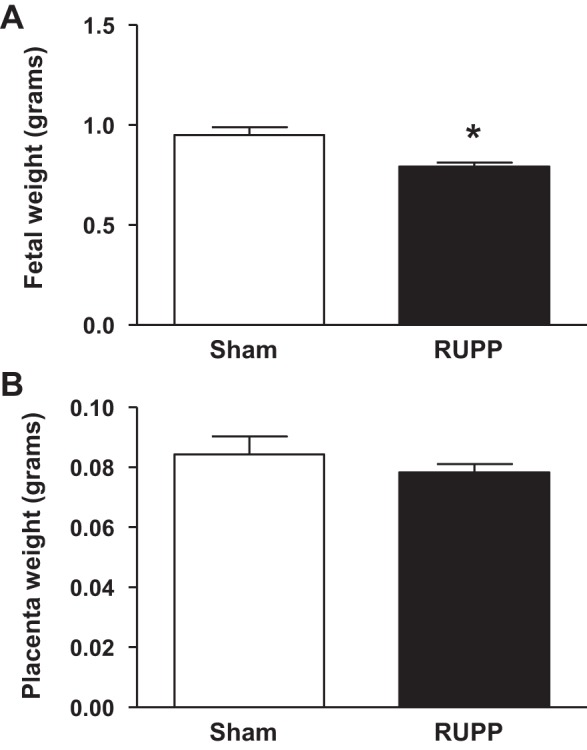
The effect of reduced uterine perfusion on fetal weight (A) and placenta weight (B) at day 18 of gestation in pregnant mice that underwent either a sham or the RUPP at day 13 of gestation. Bar graph represents means ± SE. *P < 0.01 using unpaired student t-test. n = 7 for sham and n = 10 for RUPP.
The effect of reduced uterine perfusion in the mouse on circulating and placental factors. We determined parameters that have been reported to play a role in the cascade related to the etiology of preeclampsia and parameters reported for the well-established model of RUPP in the rat (1, 3, 5, 8). Serum sFlt-1 was significantly increased in the RUPP group relative to the Sham group at GD 18 (54.9 ± 14.3 vs. 104.9 ± 14.1 ng/ml; Sham vs. RUPP, respectively, P < 0.05) (Fig. 3). However, levels of sFlt-1 were not elevated in the placenta of the RUPP group (683.3 ± 37.5 μg/mg tissue) relative to Sham group (784.4 ± 64.1 μg/mg tissue). Serum and placental levels of VEGF (serum: 824.8 ± 157.0 vs. 1,150.0 ± 182.0 pg/ml and placental: 423.3 ± 9.6 vs. 441.6 ± 10.6 pg/mg tissue; Sham vs RUPP, respectively) and TNF-α (serum: 25.63 ± 8.2 vs. 23.64 ± 4.2 ng/ml and placental: 1.35 ± 0.15 vs. 1.58 ± 0.21 ng/mg tissue; sham vs. RUPP, respectively) were also not significantly different between Sham and RUPP groups at GD 18.
Fig. 3.
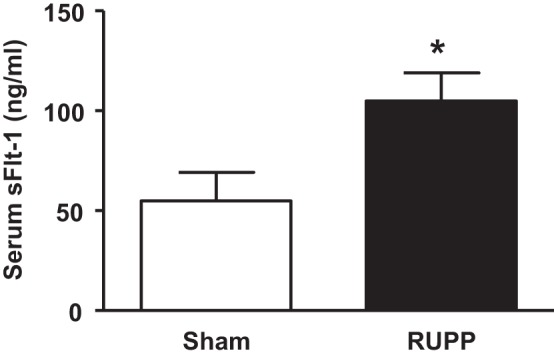
The effect of reduced uterine perfusion on erum sFlt-1 levels at day 18 of gestation in pregnant mice that underwent either a sham or the RUPP at day 13 of gestation. Bar graph represents means ± SE. *P < 0.05 using unpaired Student's t-test. n = 7 for sham and n = 10 for RUPP.
DISCUSSION
The prevailing hypothesis for the initiation of preeclampsia is reduced uteroplacental perfusion, thought to arise from incomplete remodeling of the maternal uterine spiral arteries (6, 11, 20). This reduced perfusion of the placenta results in placental ischemia. This study sought to develop and characterize a mouse model of placental ischemia. Like preeclampsia patients and the rat placental ischemia model, reduced uteroplacental perfusion in the mouse resulted in a significant elevation in blood pressure, intrauterine growth restriction, and increased circulating sFlt-1 levels compared with sham-operated pregnant mice. No differences in proteinuria, plasma, or placental TNF-α levels, or placental weights were observed in the RUPP mice relative to Sham controls; circulating free-VEGF was also not altered. The measure of free VEGF via ELISA in serum resulted in significant variability that may be due to inherent challenges related to the clipping of the small vessels in the mouse to induce placental insufficiency. Importantly, the consistent development of maternal hypertension following reduced uterine perfusion validates this model for study of mechanisms related to hypertension during pregnancy. In addition, our data also indicated that placental sFlt-1 was not increased following placental ischemia in the mouse. The lack of a difference in placental sFlt-1 protein may be due to the method of determination via ELISA, the limited amount of measurable placental protein, or the possible variation in reference protein expression between placentas collected from control pregnancies vs. pregnancies complicated by preeclampsia (14). Whether the placenta is the source for sFlt-1 in this model remains unclear; however, peripheral blood mononuclear cells may represent an extra-placental source of sFlt-1 (26). Although future studies will determine the mechanistic role of circulating factors like sFlt-1, the overall finding of increased blood pressure in the mice exposed to reduced uterine perfusion pressure represents an important advance toward a new model that can be used to better understand the mechanisms that contribute to the development of hypertension during pregnancy.
The American College of Obstetricians and Gynecologists recommends that a diagnosis of preeclampsia be made if a patient presents with blood pressure greater than 140/90 mmHg after the 20th wk of gestation, accompanied by proteinuria or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms (3). A significant increase in MAP is a characteristic of the rat model of reduced uteroplacental perfusion pressure (2, 10, 17), and this study demonstrated that our mouse model of placental ischemia was also associated with a significant increase in MAP. Intrauterine growth restriction is another common consequence reported for pregnancies complicated by hypertension (23, 27). These clinical reports of intrauterine growth restriction are confirmed in animal models of placental ischemia (1, 8, 19, 25), including this current mouse model of placental ischemia.
The requirement for the presence of proteinuria for the diagnosis of preeclampsia was recently questioned and is now an optional symptom for preeclampsia diagnosis (3). In patients, proteinuria is defined as the excretion of 300 mg or more protein in a 24-h urine collection (12) or a protein/creatinine ratio of 0.3 or more in a spot urinalysis (30). Our laboratory reports an increase in proteinuria in the rat model of placental ischemia (2). Additionally, a primate model of uteroplacental ischemia also reports significant proteinuria (19). Although we found no differences in proteinuria in our mice exposed to placental ischemia compared with Sham pregnant mice, we did not measure proteinuria from a 24-h urine collection. Determination of 24-h proteinuria may yield a significant difference. In addition, 24-h evaluation of albuminuria may also provide a better assessment of renal injury. However, 24-h proteinuria within the rat model of RUPP, like within the human population, is variable, and further studies are needed to validate these findings in the mouse.
We demonstrated in this study that mice exposed to placental ischemia had a higher plasma sFlt-1 concentration than Sham-operated pregnant mice. These results are in line with our previous report in rats exposed to reduced uterine perfusion (6) and in women with preeclampsia (15). Interestingly, chronic sFlt-1 infusion into normal pregnant rats increases MAP and decreases fetal weight (5, 22), and blockade of sFlt-1 with recombinant VEGF 121 lowers the blood pressure response in placental ischemic rats (9). sFlt-1-induced hypertension in pregnant rats is mediated by increases in oxidative stress (5) and endothelin-1 and decreases in nitric oxide (21, 22). Overexpression of sFlt-1 in the pregnant mouse also elicits the development of hypertension (4, 16, 18). Neutralization of increased sFlt-1 via coadministration of VEGF restores blood pressure back to baseline (4), and endothelin and nitric oxide are also implicated in the etiology of sFlt-1-induced hypertension in the pregnant mouse (16), suggesting similar mechanistic pathways within the rat and mouse sFLT-1-induced models or preeclampsia. Although circulating TNF-α levels are elevated in rats exposed to placental ischemia (13), we did not observe differences in TNF-α levels in mice exposed to placental ischemia. Therefore, involvement of these pathways in modulating the hypertensive and fetal growth restriction effects of placental ischemia in pregnant mice will be an important topic of future studies.
As discussed above, rodent models of reduced uteroplacental perfusion pressure mimic many features of human preeclampsia (17), serving as a good tool to study the mechanisms underlying hypertension and fetal growth restriction induced by placental ischemia. These models can also be used to study the developmental programming of cardiovascular diseases through follow-up of the offspring born to animals exposed to placental ischemia. Furthermore, rodent models provide the opportunity to study potential therapies for management of preeclampsia, which is difficult to explore in the human scenario in consideration of fetal health and chronic health later in life. However, the reduced uteroplacental perfusion pressure model has some limitations, including the fact that it only resembles the pathogenesis responsible for the hypertension in preeclampsia, downstream from the development of placental ischemia. Thus, this model is not useful to study early alterations in the immune system, trophoblastic invasion, and endothelial function. In addition, the use of the Sprague-Dawley rat or the C57BL/6J rodent strains, which are more resistant to renal injury relative to other strains, may limit investigation into the renal pathologies associated with preeclampsia. Difference in gestational length and time of exposure to placental ischemia in the rodent relative to the human may also alter the severity of the syndrome restricting usefulness of rodent models for some mechanistic investigations. The technical aspects associated with the procedure for induction of placental insufficiency in the mouse also result in variability in the quantitation of some factors, such as VEGF. Nonetheless, the characteristics of the reduced uteroplacental perfusion pressure model are relatively consistent among different animals (rodents, rabbit, guinea pig, dog, sheep, and nonhuman primates); the use of rodents has significant advantages, such as the short gestation period, the reduced cost, and easy maintenance, compared with the use of larger animals (17) and importantly, the mouse and rat models of placental ischemia induced in late gestation result in the development of consistent and significant maternal hypertension.
The development of this new model of placental ischemia in the mouse represents a major advance for the preeclampsia field because it allows for the investigation of specific mechanisms with the use of knockout and transgenic animals with the aim of linking placental ischemia to the development of hypertension during pregnancy. Use of a chronic reduction in uterine perfusion in genetically modified mouse models will permit investigation into the full spectrum of the preeclampsia phenotypes in humans.
In conclusion, this study demonstrated that placental ischemia in the mouse was associated with a significant increase in MAP and a decrease in fetal weight during late gestation, indicative of intrauterine growth restriction. Notably, these alterations were accompanied by increases in circulating sFlt-1 concentration. These data reinforce previous reports, describing placental ischemia as a critical event for the development of pregnancy-induced hypertension. Furthermore, these results support the use of a reduced uteroplacental perfusion pressure model in mice as an alternative approach to study preeclampsia and intrauterine growth restriction. This model will greatly expand the capacity to investigate the etiology of pregnancy-induced hypertension through the use of genetically manipulated mice.
GRANTS
Funding for this article was provided by American Heart Association (AHA) Grant 12POST1198002 (to S. Intapad), 13POST16240000 (to J. P. Warrington), 14POST18970005 (to A. C. Palei); National Institutes of Health (NIH) Grants 1T32HL105324 (to F. T. Spradley) and P20GM104357 (to J. P. Granger, B. T. Alexander, H. A. Drummond, and S. Intapad), HL51971 (to J. P. Granger, B. T. Alexander, and H. A. Drummond), HL109763 (to J. P. Granger and B. T. Alexander), HL121527 (J. P. Granger and B. T. Alexander); and AHA Grant 12060203 (to M. J. Ryan), NIH Grant HL074927 (B. T. Alexander); and AHA Grant 19900004 (to B. T. Alexander).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.I., J.P.W., F.T.S., H.A.D., M.J.R., J.P.G., and B.T.A. conception and design of research; S.I. performed experiments; S.I., J.P.W., F.T.S., and B.T.A. analyzed data; S.I., J.P.W., F.T.S., and B.T.A. interpreted results of experiments; S.I. and B.T.A. prepared figures; S.I., J.P.W., F.T.S., A.C.P., J.P.G., and B.T.A. drafted manuscript; S.I., J.P.W., F.T.S., A.C.P., H.A.D., M.J.R., J.P.G., and B.T.A. edited and revised manuscript; S.I., J.P.W., F.T.S., A.C.P., H.A.D., M.J.R., J.P.G., and B.T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Kathy Cockrell for her discussions related to the development of this model in the mouse and Marietta Arany for her help with tissue processing and ELISA procedures.
REFERENCES
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians, and Gynecologists and Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves ACh, Gröne HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14: 1857–1867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducray JF, Naicker T, Moodley J. Pilot study of comparative placental morphometry in pre-eclamptic and normotensive pregnancies suggests possible maladaptations of the fetal component of the placenta. Eur J Obstet Gynecol Reprod Biol 156: 29–34, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JS, Bauer AJ, Gingery A, Banek CT, Chasson S. Circulating and utero-placental adaptations to chronic placental ischemia in the rat. Placenta 33: 100–105, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension 55: 380–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Kharfi A, Giguère Y, Sapin V, Massé J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin Biochem 36: 323–331, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kuo VS, Koumantakis G, Gallery ED. Proteinuria and its assessment in normal and hypertensive pregnancy. Am J Obstet Gynecol 167: 723–728, 1992. [DOI] [PubMed] [Google Scholar]
- 13.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Lanoix D, St-Pierre J, Lacasse AA, Viau M, Lafond J, Vaillancourt C. Stability of reference proteins in human placenta: general protein stains are the benchmark. Placenta 33: 151–156, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol 23: 652–660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196: 396. e1–e7, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Morgan T, Craven C, Ward K. Human spiral artery renin-angiotensin system. Hypertension 32: 683–687, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Murphy SR, LaMarca B, Cockrell K, Arany M, Granger JP. l-arginine supplementation abolishes the blood pressure and endothelin response to chronic increases in plasma sFlt-1 in pregnant rats. Am J Physiol Regul Integr Comp Physiol 302: R259–R263, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page EW, Christianson R. Influence of blood pressure changes with and without proteinuria upon outcome of pregnancy. Am J Obstet Gynecol 126: 821–833, 1976. [DOI] [PubMed] [Google Scholar]
- 24.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 208: 224–233, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 42: 768–774, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26: 563–573, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Sadovsky E, Aboulafia Y, Milwidsky A, Weinstein D, Polishuk WZ. Preeclampsia and fetal well-being. Isr J Med Sci 12: 528–533, 1976. [PubMed] [Google Scholar]
- 28.Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res 102: 1566–1574, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler TL, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol 196: 465, e461–e464, 2007. [DOI] [PubMed] [Google Scholar]


