Abstract
Phenylbiguanide (PBG) stimulates cardiopulmonary receptors and cardiovascular reflex responses, including decreases in blood pressure and heart rate mediated by the brain stem parasympathetic cardiac neurons in the nucleus ambiguus and nucleus tractus solitarius (NTS). Electroacupuncture (EA) at P5–6 stimulates sensory fibers in the median nerve and modulates these reflex responses. Stimulation of median nerves reverses bradycardia through action of γ-aminobutyric acid (GABA) in the nucleus ambiguus, important in the regulation of heart rate. We do not know whether the NTS or the neurotransmitter mechanisms in this nucleus participate in these modulatory actions by acupuncture. We hypothesized that somatic nerve stimulation during EA (P5–6) modulates cardiopulmonary inhibitory responses through a GABAergic mechanism in the NTS. Anesthetized and ventilated cats were examined during either PBG or direct vagal afferent stimulation while 30 min of EA was applied at P5–6. Reflex heart rate and blood pressure responses and NTS-evoked discharge were recorded. EA reduced the PBG-induced depressor and bradycardia reflexes by 67% and 60%, respectively. Blockade of GABAA receptors in the NTS reversed EA modulation of bradycardia but not the depressor response. During EA, gabazine reversed the vagally evoked discharge activity of cardiovascular NTS neurons. EA modulated the vagal-evoked cardiovascular NTS cellular activity for 60 min. Immunohistochemistry using triple labeling showed GABA immunoreactive fibers juxtaposed to glutamatergic nucleus ambiguus-projecting NTS neurons in rats. These glutamatergic neurons expressed GABAA receptors. These findings suggest that EA inhibits PBG-evoked bradycardia and vagally evoked NTS activity through a GABAergic mechanism, likely involving glutamatergic nucleus ambiguus-projecting NTS neurons.
Keywords: vagal excitation, phenylbiguanide, glutamic acid
clinical and experimental studies suggest that acupuncture reduces hypertension, hypotension, and myocardial ischemia (4, 11, 37, 41, 42, 51). Modulation of cardiovascular function and elevated sympathetic activity is effective with low frequency and voltage stimulation of electroacupuncture (EA) at specific acupoints P5–6 positioned to activate the underlying median nerves (44–46). Bilateral stimulation of P5–6 acupoints activates thinly myelinated and unmyelinated sensory fibers in the median nerve and neural pathways that project to cardiovascular-related regions in the central nervous system, which regulate autonomic activity and hence hemodynamic responses (21, 23, 39). Nuclei in the hypothalamus, midbrain, and medulla process this somatic input during EA to modulate sympathoexcitatory reflex responses (26, 28, 43). Several neurotransmitter systems, including opioids and γ-aminobutyric acid (GABA), participate in the inhibitory influence of acupuncture on excitatory cardiovascular responses (13, 17, 43, 46). The activity of presympathetic neurons in the rostral ventrolateral medulla (rVLM) and nucleus raphé pallidus (NRP) are modulated by EA applied to P5–6 acupoints (27, 28). Thus stimulation at P5–6 exerts strong cardiovascular actions by reducing reflex elevations in blood pressure (41, 44). Despite this large body of evidence indicating that EA modulates elevated blood pressure through its inhibitory action on sympathetic activity (4, 9, 21–28, 39, 41, 43–46, 52–54), there is less information available about its actions on the parasympathetic nervous system (38, 42, 48, 49).
EA modulates cardiovascular depressor reflexes that originate from the gastrointestinal tract in the setting of hypercapnia acidosis (38, 40). Under these conditions both spinal and vagal afferent pathways are stimulated to lower blood pressure through sympathetic withdrawal and increased parasympathetic outflow (40). Several medullary nuclei, including the caudal ventrolateral medulla (cVLM), rVLM, and nucleus ambiguus contribute to the modulatory actions of EA through GABA mechanisms (38).
Activation of cardiopulmonary vagal afferents by prostaglandin E22, veratrum alkaloids, serotonin (5-HT), capsaicin, or intravenous phenylbiguanide (PBG) elicits decreases in heart rate and blood pressure through the Bezold-Jarisch reflex (6, 8, 20). Intravenous PBG stimulates cardiopulmonary serotonin (5-HT3) afferent endings projecting to supraspinal regions to evoke hypotension and bradycardia. The nucleus tractus solitarius (NTS) and nucleus ambiguus innervated by these vagal afferents process the reflex response. Previous studies show that EA acts at the level of the nucleus ambiguus to modulate vagal preganglionic outflow to the heart (42). However, the influence of somatic nerve stimulation during EA on the NTS which, in turn, interacts with the nucleus ambiguus during stimulation of cardiopulmonary reflex responses in the NTS is uncertain. The purpose of the present study therefore was to evaluate the role of the NTS in EA modulation of the cardiopulmonary cardioinhibitory reflex.
Neurons in the intermediate NTS are involved in cardiovascular regulation. GABAergic neurons interspersed throughout the NTS coordinate autonomic motor outflow through the nucleus ambiguus to the heart (19, 42). Activation of GABA receptors in the NTS increases heart rate (32). Sved et al. reported that GABAergic interneurons in the NTS modulate baroreflex function (3, 35). Degtyarenko and Kaufman (10) showed that barosensitive neurons in the NTS receive input from somatic afferents. Thus the current study investigated the role of GABA receptors in the intermediate NTS in neurons receiving convergent input from vagal and somatic afferents as well as baroreceptor input. We hypothesized that somatic stimulation during EA (P5–6) modulates the cardiopulmonary inhibitory responses through a GABAergic mechanism in the NTS.
MATERIALS AND METHODS
Anatomical Studies
Retrograde tracing.
All procedures were carried out in accordance with the Society for Neuroscience and the National Institutes of Health guidelines and animal use and care committee at the University of California, Irvine. The minimum possible number of rats (n = 5) was used to obtain reproducible results in this study. In addition, every effort was made to minimize discomfort and suffering. Surgical and experimental protocols were approved by the Animal Use and Care Committee at the University of California, Irvine. Adult male Sprague-Dawley rats (350–500 g) were used for microinjection of a retrogradely transported microsphere tracer in the nucleus ambiguus to evaluate direct projections from the NTS to the nucleus ambiguus, as described in detail in our previous studies (23). Ketamine-xylazine (80/12 mg/ml, Sigma, St. Louis, MO) was used to induce (0.3–0.4 ml im) and maintain (0.1–0.2 ml im) anesthesia in the animals. Body temperature was monitored with a rectal probe and was maintained at 37°C. Heart rate and oxygen saturation were monitored using a pulse oximeter (Nonin Medical, Plymouth, MN). After induction, rats were positioned in a stereotaxic apparatus (David Kopf Instruments). Under aseptic conditions, a 1-in. incision was made to expose the skull. A burr hole (4 mm diameter) was made in the bone so that a glass micropipette could be inserted using the following coordinates: 13.20–14.28 mm caudal from the bregma, 1.8–2.2 mm from the midline, and 6.2–6.5 mm deep from the dural surface (30). One hundred nanoliters of a retrogradely transported tracer, rhodamine-labeled fluorescent microspheres in suspension (0.04 μm, Molecular Probes, Eugene, OR), were injected into the nucleus ambiguus through a glass micropipette. The wound was sutured shut. Buprenorphine (0.5 mg/kg im) and penicillin (7,500 U/kg im) were administered before recovery. Microspheres were transported during a 7- to 10-day recovery and maintenance period.
Terminal procedures occurred 7 to 10 days after administration of the retrograde tracer. Rats were reanesthetized with ketamine-xylazine, as described above. After tracheotomy and intubation, the cannulation and monitoring of vital signs were similar to the procedures described below for cats. Animals were stabilized for 2 h. They then were anesthetized deeply with a large dose of the ketamine-xylazine (0.5–0.7 ml im). Transcardial perfusion was performed using 500 ml of 0.9% saline solution followed by 500 ml of 4% paraformaldehyde. The medulla oblongata was harvested and sliced in coronal sections (30 μm) using a cryostat microtome (Leica CM1850 Heidelberger Strasse, Nussloch, Germany). Brain sections were placed serially in cold cryoprotectant solution and were used for immunohistochemical labeling as described below and for identifying sites of microsphere tracer injection. In this study, free-floating sections were used for labeling.
Immunohistochemical staining.
We conducted double-fluorescent immunohistochemical labeling for vesicular glutamate transporter 3 (VGLUT3, a potential marker for glutamatergic neurons) plus glutamic acid decarboxylase isoform 67 (GAD67, a marker for GABAergic neurons) or GABAA receptors. After being washed for 30 min (10 min × 3 times) in phosphate-buffered saline containing 0.3% Triton X-100 (pH = 7.4), brain sections were placed for 1 h in 1% normal donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA). The sections then were incubated with two primary antibodies, including a guinea pig anti-VGLUT3 antibody (1:500 dilution) and a mouse anti-GAD67 (1:500) or a goat anti-GABAA receptors (1:200) for 48 h at 4°C. We used three different antibodies for the immunochemical reactions. The characterizations of all three primary antibodies were provided by the manufacturers: 1) Guinea pig anti-VGLUT3 antibody (catalog no. AB5421, lot no. 0603024959, Chemicon International, Temecula, CA), single band on Western blots (∼65 kDa), preabsorption of this antibody with the immunogen peptide (catalog no. AG320) eliminates all immunostaining. 2) Mouse anti-GAD67 antibody (catalog no. MAB5406; lot no. 2042787, Millipore, Temecula, CA). No detectable cross reactivity with GAD65 by Western blot on rat brain lysate. 3) Goat anti-GABAA receptor antibody (catalog no. sc-7348; lot no. G2611; Santa Cruz Biotechnology), single band on Western blots (43∼55 kDa). The immunostaining is eliminated after preabsorption of this antibody with the immunogen peptide (catalog no, sc-7348 P). Sections then were incubated with a coumarin-conjugated donkey anti-guinea pig antibody and a fluorescein-conjugated donkey anti-mouse or anti-goat antibody (all 1:100; Jackson Immunoresearch Laboratories) at 4°C for 24 h. The sections were mounted on slides and coverslipped with mounting medium (Vector Laboratories). In the immunohistochemical control studies, no stain was detected when the primary or secondary antibody was omitted.
Brain sections were scanned and examined with a standard fluorescent microscope (Nikon, E400, Melville, NY). Three epifluorescence filters (B-2A, G-2A, or UV-2A) in a fluorescent microscope were used to identify single stains appearing as green (fluorescein), red (rhodamine), or blue (coumarin) in brain sections. Sections containing the NTS were identified according to their best-matched standard stereotaxic plane, as shown in Paxinos and Watson's atlas for the rat (30). After examination with the fluorescent microscope, selected sections were further evaluated with a laser scanning confocal microscope (Zeiss LSM 510, Meta System, Thornwood, NY) to confirm colocalization of two or three labels. This apparatus was equipped with argon and HeNe lasers and allowed operation of multiple channels. Lasers of 488- and 543-nm wavelengths were used to excite fluorescein (green) and rhodamine (red), respectively. A 790-nm laser was applied for two-photon excitation of coumarin (blue). Digital fluorescent images were captured and analyzed with software (Zeiss LSM) provided with the confocal microscope. Each confocal section analyzed was limited to 0.5 μm thickness in the Z-plane. Images containing two or three colors in the same plane were merged to reveal the relationship between two and/or three labels (see Figs. 1 and 2). Single-, double-, and triple-labeled neurons were evaluated.
Fig. 1.
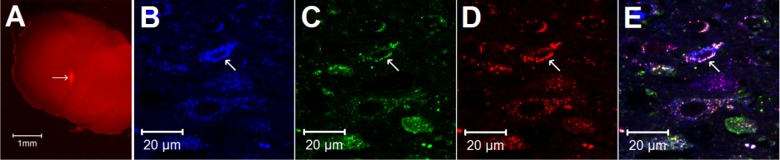
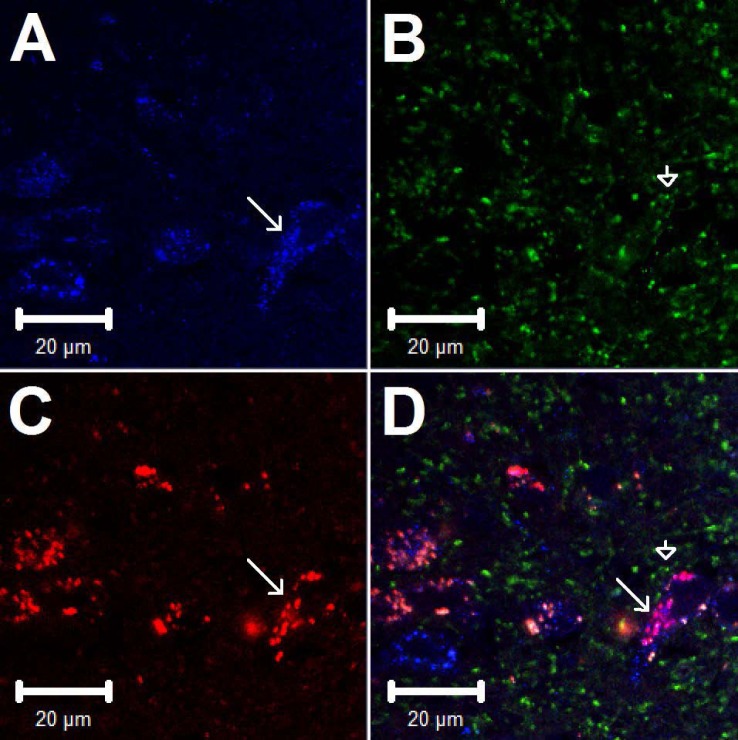
A: site of retrograde tracer injection (red spot) of an original brain of the rat medulla (bregma −13.32 mm) according to Paxinos and Watson's rat brain atlas (30). The bright red area indicated by the arrow demonstrates the injection site of rhodamine-labeled fluorescent microspheres in the nucleus ambiguus. Scale bar in A represents 1 mm. B–E: confocal microscopic images demonstrate triple-fluorescent labeling in the nucleus tractus solitarius (NTS) (bregma −13.56 mm) of a rat. Arrows indicate neurons stained with vesicular glutamate transporter 3 (VGLUT3), GABAA receptors, retrograde tracer originating from nucleus ambiguus, and colabeling of VGLUT3, GABAA, and tracer, respectively. Scale bars represent 20 μm.
Fig. 2.
Confocal microscopic images showing triple-fluorescent labeling in the NTS (bregma −13.56 mm) of a rat. Arrows in A, C, and D indicate neurons containing VGLUT3, the retrograde tracer originating from nucleus ambiguus, and colabeling of VGLUT3 and tracer, respectively. Arrowheads in B and D indicate neural processes containing GAD67. In D, the neural processes labeled with GAD67 (green) are in close proximity to the perikarya stained with VGLUT3 and the retrograde tracer indicated with an arrow. Scale bars represent 20 μm.
Physiological Studies
Surgical procedures.
All procedures were carried out in accordance with the guidelines set by the US Society for Neuroscience and the National Institutes of Health and University of California, Irvine. The minimal possible number of cats (n = 45) was used to obtain reproducible and statistically significant results. Cats (4–5 kg) of both sexes were preanesthetized with ketamine (40 mg/kg sc) followed with intravenous injection of α-chloralose (50 mg/kg). Then a femoral vein and artery were cannulated for administration of drugs and fluids and measurement of arterial blood pressure (Statham P23 ID, Oxnard, CA). To maintain adequate depth of anesthesia, supplemental α-chloralose (5–10 mg/kg iv) was given if the animals exhibited a corneal reflex, withdrew a limb in response to a noxious stimulus during the experiment, or displayed an unstable respiratory pattern or blood pressure. Heart rate was derived from the arterial blood pressure pulse by a biotech (Gould Instrument, Cleveland, OH). Blood pressures and heart rates were recorded and analyzed offline with a computer and CED Spike 2 Windows software. Intubation of the trachea facilitated artificial respiration of room air enriched with oxygen (Harvard pump, model 662, Ealing, South Natick, MA). Arterial blood gases were examined frequently (model ABL-3, Radiometer, Westlake, OH) and were maintained within the normal physiological range (Po2, 100–150 mmHg; Pco2, 35–40 mmHg; pH 7.35–7.45) by intravenous administration of 8% sodium bicarbonate or by adjusting the ventilator. Body temperature was maintained between 36°C and 37.5°C with a heating pad and an external heat lamp.
The other femoral vein was cannulated to position the tip of the cannula close to the right ventricle for administration of PBG (14). To quantify cardiac vagal input to the NTS, a lateral thoracotomy on the right was performed between the fourth and fifth ribs. Ribs were cut to access the cardiac branch of the vagus nerve. To confirm isolation of the cardiac branch a bipolar flexible platinum electrode was placed around the nerve, held in place with polyvinyldimethylsiloxone dental impression material (Pentron, Wallington, CT), and transiently stimulated to demonstrate a decrease in heart rate. To allow quantification of neuronal activity in the NTS, the stimulating electrode was connected to an isolation unit and a stimulator (model S88, Grass). The thoracic wall was closed to prevent desiccation and heat loss. In other animals the cervical vagus was isolated and stimulated to elicit decreases in heart rate and afferent input to the NTS. A craniotomy was performed after animals were stabilized with a Kopf stereotaxic head frame to expose the dorsal surface of the medulla to access the NTS.
A microinjection probe consisting of a stainless steel guide tube with an outer diameter of 0.75 mm and an injection cannula with an inner diameter of 0.4 mm was inserted into the NTS to examine the inhibitory cardiovascular responses. To determine the site of the NTS sensitive to PBG, the probe was inserted at 1, 2, or 3 mm lateral to the obex or midline according to coordinates taken from Berman's atlas (2). Unilateral insertion of the electrode allowed maintenance of a more optimal physiological condition compared with bilateral electrode insertion. A one- or three-barrel glass pipette electrode was used to evaluate neuronal activity or evaluate neuronal activity and iontophorese the antagonist. One barrel of the glass pipette electrode was filled either with saline or the GABAA receptor antagonist (gabazine). The other two barrels contained a platinum recording electrode in either 0.5 M sodium acetate containing 2% Chicago sky blue (Sigma Chemical) or 4 M NaCl to balance the current. A one- or three-barrel glass pipette or microinjection electrode was positioned perpendicularly to the dorsal surface of the medulla using visual approximation, 0 to 0.5 mm rostral to the obex and advanced ventrally ∼0.8 mm to reach the NTS. A stimulating electrode was positioned at an angle of 36 degrees to the dorsal surface of the medulla, 3.5 mm lateral to the midline, and 0.5 mm rostral to the obex and advanced to depth of ∼4 mm to the nucleus ambiguus to evaluate antidromically projection of the NTS neuron to the nucleus ambiguus. At end of experiment, the recording and microinjection sites were marked with Chicago blue dye for later histological confirmation following administration of drugs into the NTS.
Acupuncture needles were inserted to a depth of about 4 mm, bilaterally, at the Neiguan-Jianshi acupoints (P5–6). Needles at these acupoints were located 2–3 cm proximal to the flexor crease on the cat's wrist and were separated by 5–7 mm. They were connected to an isolation unit and stimulator (model S88, Grass) to deliver bipolar stimuli at 2 Hz, 0.5 ms, and 2–4 mA.
Methods of blockade.
The importance of the NTS in the PBG response was determined by microinjecting kainic acid (KA, 1 mM, 50 nl) (41) into three sites in this region. The role of GABAA receptors in the NTS during EA were evaluated by microinjecting gabazine (27 mM, 50 nl, SR-95331, Sigma Aldrich) (43) at a time when the cardiovascular effects of EA were still present. Iontophoresis (Neuro Phore BH-2 system, Medical System, Greenvale, NY) of the saline control or gabazine into the NTS was applied for ∼2 min following EA stimulation. The antagonist also was iontophoresed during repeated stimulation of the vagus nerve in the absence of EA. A current of 120–130 nA was used for iontophoresis.
Stimulating methods.
Repeated stimulation (every 10 min) of cardiopulmonary serotonin receptors with PBG (40 μg·ml−1·kg−1 iv) or the vagus nerve (2 Hz, 0.4–1 mA, 0.5 ms), respectively, induced decreases in blood pressure and heart rate or increases in NTS neuronal activity. The median nerves beneath P5–6 acupoints were stimulated bilaterally with EA at a frequency of 2 Hz, an intensity of 2 to 4 mA using 0.5-ms pulses (46). Confirmation of median nerve stimulation was achieved by noting slight paw twitches. We applied 30 min of continuous low-frequency, low-intensity EA to simulate clinical use of this procedure (21, 25). To elicit a decrease in heart rate, the cardiac vagal branch was transiently stimulated with 0.4 mA, 10 Hz, and 0.5 ms while the cervical vagus was stimulated with 0.7 to 1 mA, 10 Hz, and 0.5 ms. To support anatomical studies on NTS-nucleus ambiguus projection, 15 NTS neurons were activated antidromically from the nucleus ambiguus with 2 Hz, ∼10 μA, and 0.5 ms.
Extracellular NTS recordings.
Single-unit activity of NTS neurons was recorded with a platinum electrode inserted in a three-barrel pipette positioned in the NTS. Action potentials were amplified with a preamplifier (Neuroprobe Amplifier model 1600, A-M Systems) attached to a Nerve Traffic Analysis System 662C-3 (Bioengineering, College of Medicine, University of Iowa), and then filtered (3–10 kHz) and monitored with an oscilloscope (Tektronix 2201). Action potentials, blood pressures, and heart rates were digitized and analyzed online with a Pentium IV computer and a four-channel data acquisition system program (SHMU; Shanghai Medical College of Fudan University, China) that uses wave-shape recognition algorithms to allow detection of similar wave shapes, heights, and latencies of response (23, 24). Peristimulus time histograms were constructed for each neuron to assess evoked responses to stimulation of the vagal or median nerves. The relationship between NTS neuronal activity and blood pressure was assessed by both time and frequency domain analyses using arterial pulse triggered averaging and coherence analysis (23, 44–46). Examination of the responses to baroreceptor afferent input with either nitroprusside (50 μg/kg) or phenylephrine (2.5 μg/kg) provided additional characterization of NTS neurons. Each NTS neuron studied that received convergent input from P5–6 (median nerves), vagal (afferent) nerves, and baroreceptors and displayed cardiac rhythmicity was stimulated with EA for 30 min.
Experimental Protocols
Role of NTS in cardiopulmonary reflex.
Reflex decreases in blood pressure and heart rate were elicited by intravenous injection of PBG (40 μg·ml−1·kg−1 iv) every 10 min. KA (1 mM, 50 nl) was microinjected unilaterally into the NTS 1, 2, or 3 mm lateral to the obex after observing two repeatable decreases in blood pressure and heart rate in four animals. The principal site in the NTS responsible processing the Bezold-Jarisch reflex response then was used for all subsequent studies.
Effects of EA on PBG evoked reflexes.
Maximal decreases in blood pressure and heart rate were evaluated as the difference between mean arterial blood pressure (MAP) and heart rate before application of PBG and the lowest hemodynamic values during induction of the Bezold-Jarisch reflex. We first examined for consistency of hemodynamic responses to PBG injected every 10 min in a group of five animals. In eight other subjects, after obtaining two consistent responses, eight additional PBG reflex responses were evaluated during and after 30 min of EA at P5–6. In addition as a control for receptor blockade studies, 50 nl of saline were microinjected unilaterally into the NTS following stimulation of EA in a subgroup of seven animals.
NTS GABA systems in EA cardiopulmonary reflex modulation.
GABAA receptors in the NTS were blocked to evaluate their role in mediating the action of EA on the PBG-evoked hypotension and bradycardia. Similar to studies used to evaluate the influence of EA on PBG-evoked inhibitory reflex responses, gabazine (27 mM, 50 nl) was microinjected unilaterally into the NTS of five animals immediately after application of 30 min of EA. The effect of gabazine on the cardiopulmonary reflex responses in the absence of EA was evaluated with repeated PBG administration in four animals.
Electrophysiological studies in NTS.
Neurons in the NTS were activated every 10 min by stimulating the cardiac or cervical vagus nerves. Peristimulus histograms were constructed with histogram bars representing evoked activity over and above the basal discharge rate. Each neuron was characterized by assessing input during brief (30 to 60 s) stimulation of median nerve at the P5–6 acupoints. We examined only the neurons that received input from baroreceptors, identified as neurons responsive to nitroprusside or phenylephrine. We also evaluated the NTS response to intravenous PBG. Each neuron displayed a cardiac rhythmicity as determined by arterial pulse-triggered activity averaging over a period of 5 min through analysis of the time and frequency domain relationships between blood pressure and cellular activity (pulse triggered activity and coherence, respectively). In some cases, we examined antidromically evoked activity in NTS neurons to identify direct NTS-nucleus ambiguus projections. These neurons were evaluated for collision of spikes during median nerve afferent-evoked orthodromic activity and nucleus ambiguus evoked antidromic action potentials in 15 subjects. NTS neurons that responded to stimulation of the nucleus ambiguus with a constant latency were further examined for evidence of faithful responses at 200 Hz and for a stable threshold of the all-or-none evoked activity. The refractory period and the critical time interval (latency plus refractory period) were determined during the collision of the orthodromic and antidromic spikes (23).
Consistency of evoked responses in the absence of EA first was established during repeated vagal stimulation at 10-min intervals in five neurons. Then to evaluate the influence of EA on NTS neurons, cardiac or cervical vagal afferent-evoked activity was determined repeatedly (every 10 min) before, during, and after 30 min of EA in seven animals. In a subgroup of five of these animals, saline as a control was iontophoresed at end of EA. The influence of GABAA receptor blockade was assessed by iontophoresing gabazine (−120 mA, 2 min) immediately following termination of EA in five other animals.
Statistical Analysis
The number of neurons was tabulated (in %) to determine NTS-nucleus ambiguus projection in relation to GABAA receptor and/or VGLUT3. Data are presented as means ± SE. Evoked activity during stimulation of vagal or median nerves was measured as the increase in number of spikes above baseline. Reflex changes in MAP and heart rate are presented as bar histograms. Data were plotted and analyzed with the Kolmogorov-Smirnov test for normal data distribution and normalized when necessary with Sigma plot (Jandel Scientific). The increase in cellular activity and decreases in blood pressure and heart rate before and after delivery of experimental drugs or saline or application of EA were compared using a one-way ANOVA followed post hoc with the Student-Newman Keuls test. Additionally, the saline group was compared with gabazine treatment group using a two-way ANOVA. All statistical analyses were performed with Sigma Stat (Jandel Scientific). The P < 0.05 level was used to detect significant differences.
We also evaluated time and frequency relationships between NTS neuronal activity and arterial blood pressure using pulse-triggered averaging as well as coherence analysis, as we have described previously (23, 24). Time domain analyses involved ECG or arterial pulse-triggered averaging. A threshold was set at the peak of the ECG wave or the systolic phase of the arterial pulse while another threshold was used for spike height discrimination and waveform recognition to sort action potentials during the 300-s evaluation period. Averages of the arterial pulse and histograms of NTS neuronal activity were constructed (27, 28). Frequency domain analysis involved assessment of the coherence between NTS activity and arterial blood pressure using a Fast Fourier Transform (FFT) algorithm. We recorded data using a sampling rate of 10,000 Hz. Reconstructed data utilized every tenth sample, including assessment of the mean and peak amplitudes and the maximum and minimum slopes of the original spike to preserve the action potentials. The spikes were sorted and identified with a window discriminator to construct histograms before coherence analysis. The number of data sections (15–20 each lasting for 12.8 s) was chosen to determine the average histogram. Autospectra of NTS discharge and arterial blood pressure were generated with a FFT algorithm. Thus, coherence was generated with seven overlapping windows, each with a length of 12.8 s, consisting of 256 bins, with bin widths of 50 ms. The autospectral analysis was adopted from Shin et al. (34) in 1995 using contiguous segments of 256 beats with 50% overlap between the segments. The frequency resolution was 1/12 s or 0.08 Hz. The coherence function (normalized cross-spectrum) provided a measure of the strength of linear correlation of NTS neuronal activity and blood pressure at each frequency. Coherence values of ≥0.5 were chosen to reflect a statistically significant relationship between NTS spikes and arterial blood pressure (46).
Histology
At the end of each experiment, animals were euthanized under deep α-chloralose anesthesia followed by injection of saturated KCl. Recording and/or microinjection sites were marked by either iontophoresis and/or microinjection of 2% Chicago blue dye. Thereafter, the brain was removed and fixed in 10% paraformaldehyde for at least 2 days. Brain stems were sliced with a microtome cryostat in 60-μm coronal sections. Recording and microinjection sites were reconstructed from the dye spots with the aid of a microscope (Nikon) and software (Corel presentation). The sites were plotted at to 0.6 mm rostral to the obex (2).
RESULTS
Anatomical Studies
Two animals were eliminated from this study since the sites for microinjection were found to be outside the nucleus ambiguus. Thus three rats in which the microinjection site of the retrograde tracer was found inside the nucleus ambiguus were included in this study. The location of injected tracer (100 nl) in the medulla closely matched the coordinates of the nucleus ambiguus as defined by Paxinos and Watson's atlas for the rat (30). This microinjection site was within 1.6–2.2 mm lateral from the midline and 1.0–1.6 mm ventral to surface of the medulla and was observed ventrally adjacent to the rostral-ventral-respiratory-group. The tracer did not spread to caudal-ventrolateral-reticular-nucleus, containing C1 epinephrine cells and/or A1 norepinephrine cells (30). The area of distribution of the tracer in dorso-ventral planes ranged approximately 0.16 by 0.19 mm and at rostral-caudal extension from 0.48 to 0.56 mm (Fig. 1A).
We consistently observed that neurons labeled with the retrograde microsphere tracer were distributed rostrally and caudally throughout the NTS when the tracer was deposited in the nucleus ambiguus of the three rats. The labeled neurons were located in commissural, medial, ventral, and lateral subdivisions of NTS in the rat, mainly at levels from Bregma −12.6 to −15.0 mm (30). Approximately two-thirds of the neurons labeled with microspheres in the NTS were found to be located ipsilateral to the injection site in the nucleus ambiguus.
In all three rats, VGLUT3, GAD67, and GABAA receptors were distributed bilaterally throughout the caudal and rostral NTS. Cell bodies were stained with tracer, VGLUT3, or GABAA receptors, while neuronal processes were labeled with GAD67.
We evaluated the relationship between VGLUT3 + tracer-labeled NTS neurons and GABAA receptors. More than half of the retrograde tracer-labeled NTS neurons were double labeled with either VGLUT3 or GABAA receptors (Table 1). The majority of neurons double stained with the retrograde tracer + VGLUT3 (71%) also were labeled with GABAA receptors (Table 1 and Fig. 1, B-E). Neural processes labeled with GAD67 were in close apposition to the majority of NTS neurons that contained the retrograde tracer alone as well as neurons containing both the retrograde tracer and VGLUT3 (Fig. 2).
Table 1.
Neurons labeled with the retrograde tracer, VGLUT3, and/or GABAA receptors in the NTS of rats
| Labeling | Bregma −13.56 mm (n = 3) |
|---|---|
| Tracer# | 138 ± 14 |
| VGLUT3# | 179 ± 11 |
| GABAA# | 97 ± 7 |
| Tracer + VGLUT3# | 80 ± 12 |
| Tracer + GABAA# | 70 ± 7 |
| Tracer + VGLUT3 + GABAA# | 57 ± 7 |
| (Tracer + VGLUT3)/Tracer% | 58 ± 3 |
| (Tracer + GABAA)/Tracer% | 51 ± 2 |
| (Tracer + VGLUT3 + GABAA)/(Tracer + VGLUT3)% | 71 ± 2 |
Values are means ± SE. Average number (#) of neurons labeled with retrograde microsphere tracer (Tracer) injected into nucleus ambiguus, vesicular glutamate transporter 3 (VGLUT3), and/or γ-aminobutyric acid A (GABAA) receptors in the nucleus of tractus solitarius (NTS). Percentages of neurons (%) stained with tracer and labeled with VGLUT3 or GABAA relative to Tracer. Percentages of neurons (%) stained with tracer and labeled with VGLUT3 and GABAA relative to Tracer and VGLUT3.
Physiological Studies
Role of NTS in cardiopulmonary reflex.
KA microinjected 0–0.5 mm rostral to the obex and between 1.6 and 2.2 mm lateral to midline (intermediate NTS) reduced the Bezold-Jarisch responses by 12 mmHg and 16 beats/min (Fig. 3). In contrast, KA microinjected 0.5 to 1.0 mm and 3 to 3.4 mm lateral to the midline did not alter the cardiopulmonary reflex responses. Blockade with KA did not influence baseline blood pressure and heart rate. The PBG-induced blood pressure and heart rate responses were not affected by KA microinjection ventral to the NTS.
Fig. 3.
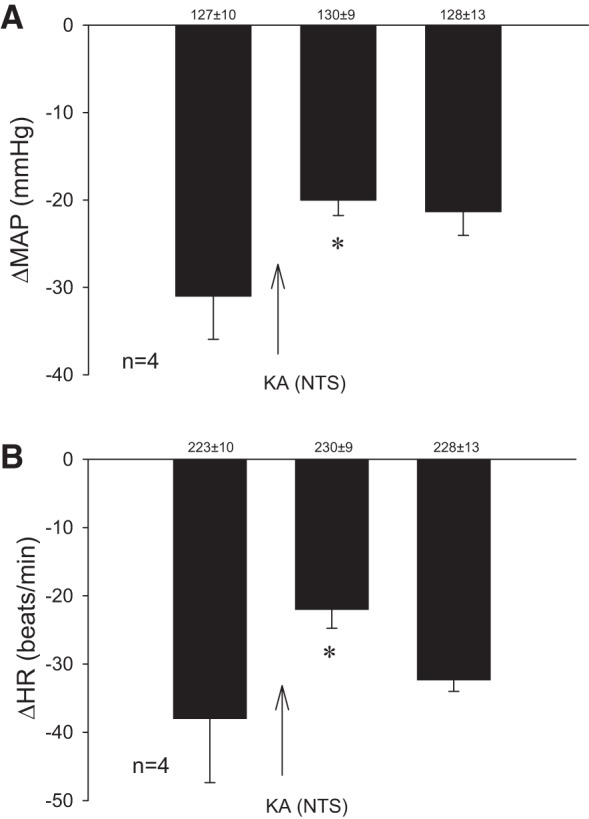
Bar histograms display decreases in mean blood pressure (ΔMAP) and heart rate (ΔHR) to phenylbiguanide (PBG) before and after microinjection of kainic acid (KA) in the intermediate NTS. KA transiently reduced parasympathoexcitatory hemodynamic and negative chronotropic reflex responses. Baseline blood pressure and heart rate are shown above each bar as means ± SE. *Significant difference compared with control PBG responses.
Effects of EA on PBG-evoked reflexes.
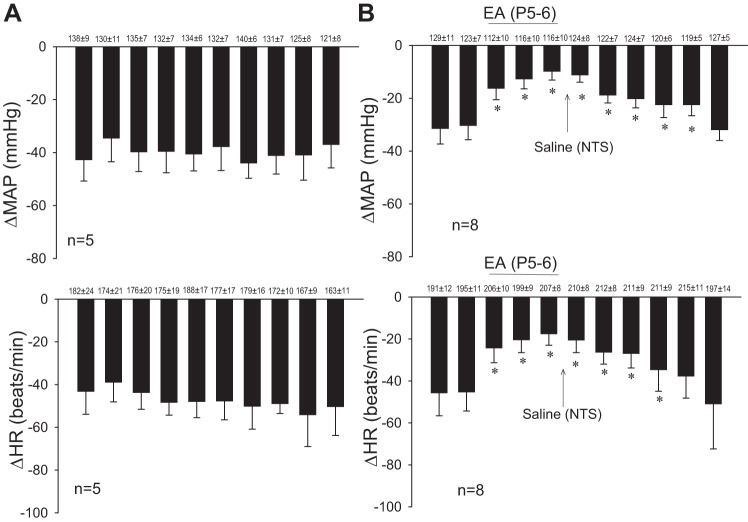
We observed consistent decreases in blood pressure and heart rate with repeated stimulation of cardiopulmonary serotonin receptors with PBG every 10 min (Fig. 4A). The baseline blood pressures and heart rates before each Bezold-Jarisch reflex were not significantly different throughout the experiment. The inhibitory cardiovascular responses were reduced by EA applied for 30 min at the P5–6 acupoints. The modulatory effect of EA on the PBG-induced decrease in blood pressure lasted for 80 min, whereas the bradycardia persisted for 70 min (Fig. 4B). EA did not influence baseline blood pressure or heart rate (42). Gallamine triethiodide, used to inhibit muscle movement during stimulation of median nerve with EA at P5–6 as in previous studies, did not influence the response to EA (42).
Fig. 4.
Decreases in mean arterial pressure (MAP) and heart rate (HR) were reduced with electroacupuncture (EA). Bar histograms display consistent responses to repeated PBG (every 10 min). EA reduced the decrease in MAP and HR for at least 70 min. Microinjection of saline into the NTS did not influence the inhibitory cardiovascular reflex responses. Baseline blood pressure and HR are shown above each bar as means ± SE. *Significant difference compared with control PBG responses.
NTS GABA mechanisms in EA cardiopulmonary reflex modulation.
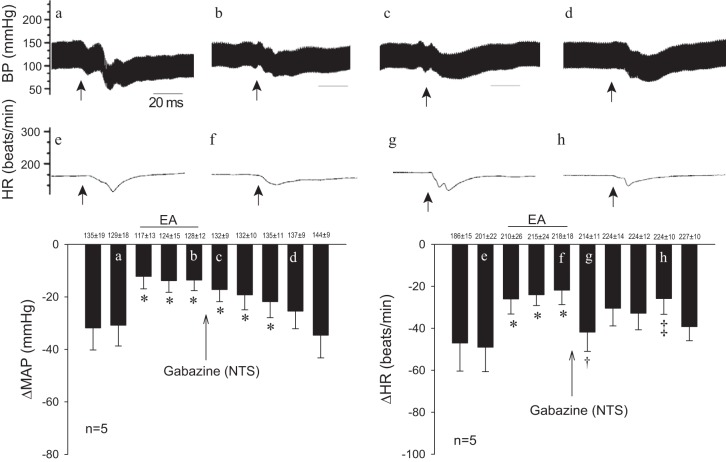
EA modulation of the PBG-evoked bradycardia responses was reversed for at least 10 min by GABAA receptor blockade in the intermediate NTS (compare Fig. 4B and Fig. 5). The acupuncture effect outlasted the action of gabazine for at least 10 min. Gabazine did not influence the action of EA on the blood pressure response (Fig. 5).
Fig. 5.
EA modulation of the PBG-induced bradycardia (right) but not hypotension (left) is transiently reversed by gabazine. *Significant difference compared with baseline PBG responses; †significant difference from preceding EA PBG-EA response. ‡End of gabazine's activity and the effect of EA. Letters in a-h shown in the bars correspond to the original tracings above showing decreases in MAP and HR. Baseline MAP and HR are shown above bars as means ± SE.
Microinjection of gabazine into the NTS transiently increased baseline heart rate from 206 ± 9 to 211 ± 9 beats/min, while baseline blood pressure tended to increase from 123 ± 7 to 129 ± 5 mmHg in nine subjects. Baseline heart rate and blood pressure were restored to normal levels before the next administration of PBG. Blockade of GABAA receptors in the NTS did not influence the Bezold-Jarisch reflex blood pressure (−16 ± 4 vs. −14 ± 3 mmHg) or heart rate response (−20 ± 7 vs. −22 ± 10 beats/min) in four subjects.
NTS neuronal activity during EA.
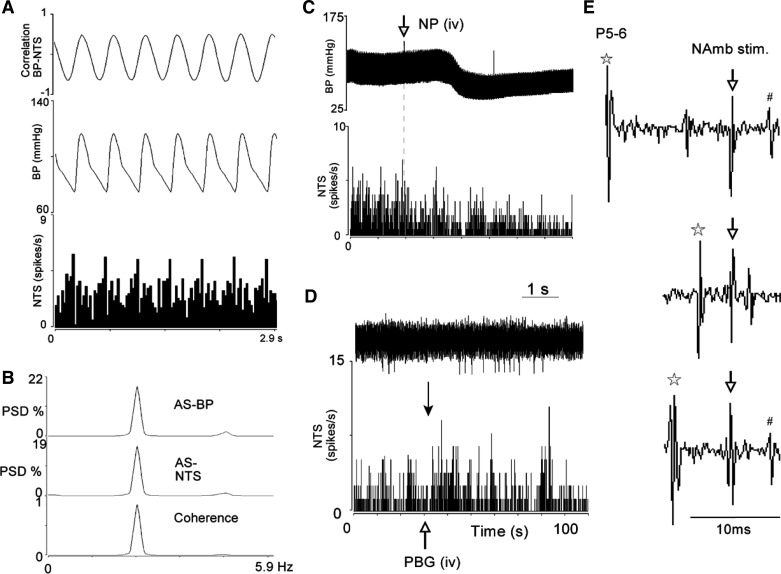
Before evaluation of their responses to repeated activation of cardiac vagal afferents and superimposition of EA, NTS neurons were characterized using time and frequency domain analysis. Baseline discharge activity of the NTS neurons was 3.8 ± 0.6 spikes/s. Forty-five neurons displayed cardiac rhythmicity (Fig. 6, A and B), while a subset of 17 cells also were shown to integrate baroreceptor input (Fig. 6C) and receive convergence from vagus and median nerve afferents. All 17 NTS neurons also were activated following intravenous PBG administration (Fig. 6D). Two of 15 neurons responsive to EA projected directly to the nucleus ambiguus since they could be driven antidromically from the nucleus ambiguus, were faithful to high-frequency stimulation, and displayed a constant latency (Fig. 6E).
Fig. 6.
Characterization of NTS neurons. A and B, respectively, display time and frequency (coherence of 0.87 and frequency 2.4) domain analyses to show cardiac rhythmicity. C: decreased NTS activity during a nitroprusside-evoked decrease in BP, demonstrating that it was barosensitive. Discharge activity of the NTS neuron also was increased in response to intravenous PBG (D, closed arrow indicates time of NTS activity shown with neurogram). Antidromic stimulations show that the neuron projects to the nucleus ambiguus (E). The NTS neuron, activated antidromically by 2 Hz, 11 μA, and 0.5 ms NAmb stimulation (↓), collided with the orthodromically median nerve (MN, P5–6, *) evoked spike (E, middle). #Antidromic spike that is absent during collision (middle). Critical interval was 13.5 ms and refractory period was 4.86 ms. PSD, power spectral density.
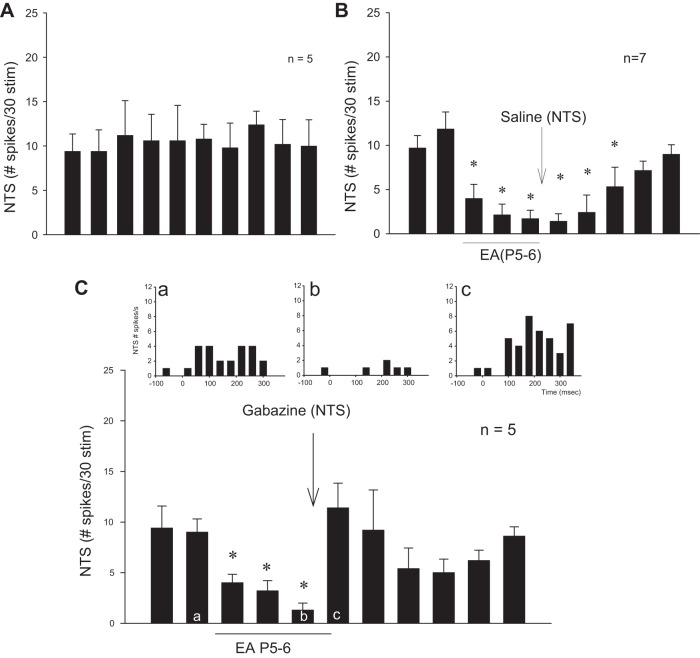
In the absence of EA, five NTS cells examined for repeated stimulation of cardiac vagal afferents induced consistent activity (Fig. 7A). However, 30 min of EA stimulation at P5–6 reduced NTS vagally evoked activity for at least 60 min in seven neurons (Fig. 7B). Modulation by EA was reversed by blockade of GABAA receptors (n = 5) (Fig. 7C).
Fig. 7.
Bar histograms display NTS neuronal-evoked activity during repeated stimulation of vagal afferents every 10 min. A: consistent evoked activity with stimulation of vagal afferents. B and C: EA reduced evoked activity for at least 60 min through GABA mechanism. Blockade with gabazine (C) reversed the effect of EA (c) compared with preblockade (b) in contrast to saline (B). Letters a-c in bars correspond with peristimulus histograms (C).
Histology.
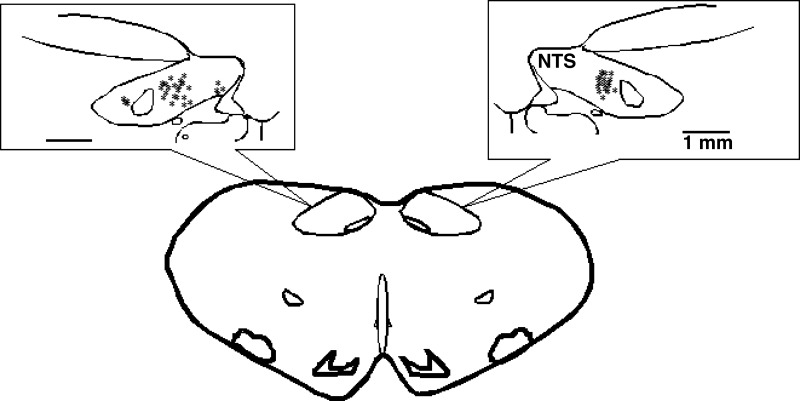
The microinjection and recording sites in the NTS were confirmed histologically to be 1.6–2.2 mm lateral to the midline and 0.7–1.4 mm from the surface and at 0.6–0 mm rostral to the obex (Fig. 8) as shown in Berman's atlas (2). The sites were determined by the locations of microinjection tracks and dye spots.
Fig. 8.
Composite map displays the sites of microinjections, iontophoresis, and extracellular recordings in the intermediate NTS of cats. For ease of representation, all recording sites are displayed on the right and microinjections on the left. Microinjections with KA at 1 and 3 mm lateral to midline also are shown on the left NTS. *Sites located within NTS. ○, control site outside intermediate NTS. Coronal section is 0 to 0.6 mm rostral to obex.
DISCUSSION
EA applied at the P5–6 acupoints inhibits sympathoexcitatory activity and reflex responses through long-loop neuronal pathways that involve the hypothalamic arcuate nucleus, midbrain ventrolateral periaqueductal gray, medullary raphé pallidus, and rostral ventrolateral medulla. The present study extends these observations by demonstrating that EA at the same acupoints modulates parasympathoexcitatory (inhibitory cardiovascular) responses in the NTS that projects to the nucleus ambiguus to regulate heart rate. In this regard, prolonged activation of sensory fibers in the median nerves for 30 min using low-frequency and low-voltage modulates PBG-related bradycardia responses through a GABAergic system in the NTS. Combined physiological and immunohistochemical studies demonstrate that GABA contributes to the EA-related modulation of the negative chronotropic response through activation of GABAA receptors in NTS neurons that ultimately project to the nucleus ambiguus.
We used rats rather than cats to demonstrate the presence of nucleus ambiguus projecting NTS neurons that are juxtaposed to GABAergic fibers and expressing GABA receptors. Our laboratory has shown that the anatomic circuitry in the cardiovascular responses in rats and cats are virtually identical (9, 23, 28, 38, 42). Therefore, we believe that our data obtained in rats likely also apply to cats.
We anatomically showed the presence of NTS-nucleus ambiguus projections with microinjection of microsphere tracer in the nucleus ambiguus. Although unlikely, the tracer potentially could stain both cardiovascular and respiratory neurons. To determine whether nucleus ambiguus projecting NTS neurons participate in the PBG-evoked and cardiovascular responses, we further examined the projections with extracellular recording and antidromic stimulation. We showed that NTS-nucleus ambiguus neurons displayed cardiac rhythmicity and were responsive to baroreceptor stimulation and PBG application. Thus, while the retrograde tracing provided anatomical evidence for a direct projection, we functionally supported this finding with characterization of cardiovascular NTS-nucleus ambiguus neuronal projection displaying cardiac rhythmicity and neuronal responses to PBG.
VGLUTs transport glutamate into vesicles of neurons and thus offer a unique marker to distinctively identify neurons that use glutamate as a neurotransmitter (12). Although there has been discussion about the specificity of VGLUT3 for glutamatergic neurons, the VGLUT3 labeling represents a valuable way to visualize glutamatergic neurons in the brain (12, 15, 16, 33). VGLUT3-labeled NTS neurons projecting to nucleus ambiguus are colocalized with GABAA receptors or juxtaposed to GABAergic nerve fibers. The apposition of the two neuronal structures suggests that GABAergic fibers may synapse on a glutamatergic NTS-nucleus ambiguus neuron. Although confocal microscopic images display juxtaposed positioning, it is unclear if synaptic transmission occurs between the neuronal processes. In this regard, our physiological data demonstrated activation of GABAA receptors in cardiovascular PGB-responsive NTS neurons in support of a GABA synaptic transmission. Thus the current findings show the potential for GABA to influence glutamatergic NTS-nucleus ambiguus projections.
Neuroanatomical and electrophysiological studies have shown that neurons in the NTS process sensory input from vagal afferents with endings originating from the gastrointestinal tract, baroreceptors, and cardiopulmonary region. Injection of PBG into the right atrium activates cardiopulmonary vagal afferent C fibers (7, 8, 29) and the NTS (18). The present experiments demonstrate that cardiovascular NTS neurons activated by PBG and vagal afferent stimulation are influenced by somatic afferent input. Thus NTS vagal-evoked activity is reduced by 30 min of median nerve stimulation (EA). Moreover, the vagal-evoked NTS neurons are inhibited by EA for a prolonged period of time during and after 30 min of median nerve stimulation, a hallmark of EA. Thus the NTS processes convergent input from both visceral and somatic afferent fibers and their interaction influences cardiopulmonary-related vagal reflex bradycardia.
In the present study, we demonstrated that GABA in the NTS contributes importantly to EA modulation of the profound bradycardia consequent to activation of cardiopulmonary serotonin receptors. Blockade of GABAA receptors in the NTS transiently increased baseline heart rate confirming that GABA tonically inhibits premotor parasympathoexcitatory NTS neurons (32). However, baseline heart rates and blood pressures returned to preblockade levels before subsequent administration of PBG, suggesting that the responses to gabazine were not influenced by the transient baseline changes. EA modulation of NTS-evoked activity through a GABAA mechanism is similar to the prolonged actions by EA in the nucleus ambiguus and the rostral ventrolateral medulla (38, 42).
The NTS processes sympathetic and parasympathetic activity differentially and possibly controls these pathways independently (31, 55). We have observed that KA in the NTS partially reduces both the bradycardia and depressor responses, whereas action of EA through GABA in NTS partially influences heart rate but not blood pressure changes. A similar discrepancy has been reported in the nucleus ambiguus (42) demonstrating that both the NTS and the nucleus ambiguus regulate EA modulatory action on heart rate. Moreover, the NTS processes the initial cardiopulmonary reflex inhibitory responses and projects to the caudal and rostral VLM (47) that could regulate vasomotor tone and the effects of EA on inhibitory hemodynamic responses (38). Thus the restoration of blood pressure with EA during the Bezold-Jarisch reflex likely occurs in vasomotor centers such as the rVLM (28, 46) suggesting unique roles of various brain stem nuclei in EA mechanisms of regulation of reflex lowering of heart rate and blood pressure.
Electroacupuncture appears to be capable of normalizing blood pressure by lowering increased blood pressure and elevating decreased blood pressure. The somatic sensory nerve evoked input during acupuncture through specific neurotransmitters decreases the extent of neuronal excitation associated with increased sympathetic outflow and lowers elevated blood pressure (25, 46). On the other hand, if acupuncture is applied in the presence of reflex sympathetic withdrawal and/or increased parasympathetic outflow, the somatic sensory input activates modulatory neurotransmitter systems to reduce the extent of hypotension and bradycardia (38, 42). Thus acupuncture normalizes blood pressure by modulating elevated neuronal activity in various brain stem regions known to be important in regulating autonomic function, including both sympathetic and parasympathetic nerves.
Perspectives and Significance
Acupuncture can profoundly influence cardiovascular function and may serve a role in medical conditions associated with low blood pressure. This condition occurs, for example, during hemorrhage. Syuu et al. (36) reported that EA reverses hemorrhage-induced hypotension, possibly by increasing venous return through enhanced vasomotor tone and the preload. In a more clinically related study, acupuncture has been shown to elevate blood pressure in patients with shock (5). With the exception of our previous study that acupuncture modulates cardiopulmonary inhibitory heart rate responses through GABAergic inhibition of parasympathetic preganglionic neurons in nucleus ambiguus (42), the underlying central neural mechanisms associated with action of EA in hypotension have not been evaluated previously.
Activation of cardiopulmonary vagal afferents to lower heart rate shares many features of vasovagal syncope. Vasovagal syncope is the most common cause of transient unconsciousness (50) and is thought to be mediated by activation of a cardiopulmonary-mediated reflex (1). EA may serve as a therapeutic option for subjects at risk for recurrent vasovagal syncope.
In conclusion, through the GABAA receptor mechanism 30 min of electroacupuncture reduces augmented activity of parasympathetic premotor NTS neurons and reverses heart rate during PBG stimulation of the cardiopulmonary afferents.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-72125 and HL-63313.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.C.T.-AL. and J.C.L. conception and design of research; S.C.T.-AL. and Z.-L.G. performed experiments; S.C.T.-AL. and Z.-L.G. analyzed data; S.C.T.-AL., Z.-L.G., and J.C.L. interpreted results of experiments; S.C.T.-AL. and Z.-L.G. prepared figures; S.C.T.-AL. drafted manuscript; S.C.T.-AL., Z.-L.G., and J.C.L. edited and revised manuscript; S.C.T.-AL., Z.-L.G., and J.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for the technical input of Jesse Ho and Ai-Thuan Nguyen.
REFERENCES
- 1.Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann NY Acad Sci 940: 48–58, 2001. [PubMed] [Google Scholar]
- 2.Berman AL. The Brainstem of the Cat: A Cytoarchitectonic Atlas With Stereotaxic Coordinates. Madison, WI: The University of Wisconsin Press, 1968. [Google Scholar]
- 3.Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371–387, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao DM, Shen LL, Tjen-ALooi SC, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Cheung L, Li P, Wong C. The Mechanism of Acupuncture Therapy and Clinical Case Studies. New York: Taylor and Francis, 2001. [Google Scholar]
- 6.Coleridge H, Coleridge J. Cardiovascular afferents involved in regulation of peripheral vessels. Annu Rev Physiol 42: 413–427, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Coleridge H, Coleridge J, Kidd C. Cardiac receptors in the dog with particular reference to two types of afferent ending in the ventricular wall. J Physiol 174: 323–339, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleridge H, Coleridge J, Luck J. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol 179: 248–262, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisostomo M, Li P, Tjen-ALooi SC, Longhurst JC. Nociceptin in rVLM mediates electroacupuncture inhibition of cardiovascular reflex excitatory response in rats. J Appl Physiol 98: 2056–2063, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Degtyarenko AM, Kaufman MP. Barosensory cells in the nucleus tractus solitarius receive convergent input from group III muscle afferents and central command. Neuroscience 140: 1041–1050, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Flachskampf FA, Gallasch J, Gefeller O, Gan J, Mao J, Pfahlberg AB, Wortmann A, Klinghammer L, Pflederer W, Daniel WG. Randomized trial of acupuncture to lower blood pressure. Circulation 115: 3121–3129, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Fremeau RJ, Jr, Burman J, Qureshi T, Tran C, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen D, Storm-Mathisen J, Reimer R, Chaudry F, Edwards R. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA 99: 14488–14493, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu LW, Longhurst C. Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptor. J Appl Physiol 106: 1800–1809, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LW, Longhurst JC. Reflex pressor response to arterial phenylbiguanide; role of abdominal sympathetic visceral afferents. Am J Physiol Heart Circ Physiol 275: H2025–H2035, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143: 1051–1064, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo ZL, Longhurst JC. Activation of reciprocal pathways between arcuate nucleus and ventrolateral periaqueductal gray during electroacupuncture: involvement of VGLUT3. Brain Res 1360: 77–88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo ZL, Moazzami A, Tjen-ALooi S, Longhurst J. Responses of opioid and serotonin containing medullary raphe neurons to electroacupuncture. Brain Res 1229: 125–136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circ Res 74: 1188–1196, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Izzo PN, Sykes RM, Spyer KM. γ-Aminobutyric acid immunoreactive structures in the nucleus tractus solitarius: a light and electron microscopic study. Brain Res 591: 69–78, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Jeggo R, Kellett D, Wang Y, Ramage A, Jordan D. The role of central 5-HT3 receptors in vagal reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J Physiol 566: 939–953, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Pitsillides KF, Rendig SV, Pan HL, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Rowshan K, Crisostomo M, Tjen-ALooi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distention. Am J Physiol Regul Integr Comp Physiol 283: R1335–R1345, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Tjen-ALooi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Tjen-ALooi SC, Guo ZL, Longhurst JC. An arcuate-ventrolateral periaqueductal gray reciprocal circuit participates in electroacupuncture cardiovascular inhibition. Autonomic Neurosci 158: 13–23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Tjen-ALooi SC, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Autonomic Neurosci 89: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Tjen-ALooi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Tjen-ALooi SC, Longhurst JC. Nucleus raphé pallidus participates in midbrain-medullary cardiovascular sympathoinhibition during electroacupuncture. Am J Physiol Regul Integr Comp Physiol 299: R1369–R1376, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moazzami A, Tjen-ALooi SC, Guo ZL, Longhurst JC. Serotonergic projection from nucleus raphe pallidus to rostral ventrolateral medulla modulates cardiovascular reflex responses during acupuncture. J Appl Physiol 108: 1336–1346, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Pickering AE, Boscan P, Paton JF. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol 551: 589–599, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potts JT, Paton JF, Mitchell JH, Garry MG, Kline G, Anguelov PT, Lee SM. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signaling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience 119: 201–214, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462: 651–655, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Jpn J Physiol 45: 1053–1069, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Sved AF, Tsukamoto K. Tonic stimulation of GABAB receptors in the nucleus tractus solitarius modulates the baroreceptor reflex. Brain Res 592: 37–43, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Syuu Y, Matsubara H, Hosogi S, Suga H. Pressor effect of electroacupuncture on hemorrhagic hypotension. Am J Physiol Regul Integr Comp Physiol 285: R1446–R1452, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Tam KC, Yiu HH. The effect of acupuncture on essential hypertension. Am J Chin Med 3: 369–375, 1975. [DOI] [PubMed] [Google Scholar]
- 38.Tjen-ALooi SC, Guo ZL, Li M, Longhurst JC. Medullary GABAergic mechanisms contribute to electroacupuncture modulation of cardiovascular depressor responses during gastric distention in rats. Am J Physiol Regul Integr Comp Physiol 304: R321–R332, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tjen-ALooi SC, Fu LW, Zhou W, Longhurst JC. Role of unmyelinated fibers in electroacupuncture cardiovascular responses. Auton Neurosci 118: 43–50, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Tjen-ALooi SC, Hsiao AF, Longhurst JC. Central and peripheral mechanisms underlying gastric distention inhibitory reflex responses in hypercapnic-acidotic rats. Am J Physiol Heart Circ Physiol 300: H1003–H1012, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tjen-ALooi SC, Li P, Longhurst JC. Midbrain vIPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol Heart Circ Physiol 290: H2543–H2553, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Tjen-ALooi SC, Li P, Li M, Longhurst JC. Modulation of cardiopulmonary depressor reflex in nucleus ambiguus by electroacupuncture: roles of opioids and gamma aminobutyric acid. Am J Physiol Regul Integr Comp Physiol 302: R833–R844, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjen-ALooi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J Appl Physiol 106: 1793–1799, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjen-ALooi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119–131, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Tjen-ALooi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852–R862, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Tjen-ALooi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627–H3635, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Verberne AJM, Guyenet PG. Medullary pathway of the Bezold-Jarisch relfex in the rat. Am J Physiol Regul Integr Comp Physiol 263: R1195–R1202, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Guo X, Li P. The inhibitory effect of somatic inputs on the excitatory responses of vagal cardiomotor neurons to stimulation of the nucleus tractus solitarii in rabbits. Brain Res 439: 350–356, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Li P. A GABAergic mechanism in the inhibition of cardiac vagal reflexes. Brain Res 457: 367–370, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Wieling W, Rozenberg J, Schon I, Karemaker J, Westerhof B, Jardine DL. Prolonged post-faint hypotension can be reversed by dynamic tension. Clin Auton Res 21: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang CL. Clinical investigation of acupuncture therapy. Clin J Med 42: 514–517, 1956. [Google Scholar]
- 52.Zhou W, Fu LW, Tjen-ALooi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol 98: 872–880, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Zhou W, Tjen-ALooi S, Longhurst JC. Brain stem mechanisms underlying acupuncture modality-related modulation of cardiovascular responses in rats. J Appl Physiol 99: 851–860, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Zhou W, Fu LW, Tjen-ALooi SC, Guo ZL, Longhurst JC. Role of glutamate in a visceral sympathoexcitatory reflex in rostral ventrolateral medulla of cats. Am J Physiol Heart Circ Physiol 291: H1309–H1318, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol 95: 909–918, 2010. [DOI] [PubMed] [Google Scholar]