Abstract
Mitochondrial dysfunction has been implicated in many neurological disorders that only develop or are much more severe in adults, yet no methodology exists that allows for medium-throughput functional mitochondrial analysis of brain sections from adult animals. We developed a technique for quantifying mitochondrial respiration in acutely isolated adult rat brain sections with the Seahorse XF Analyzer. Evaluating a range of conditions made quantifying mitochondrial function from acutely derived adult brain sections from the cortex, cerebellum, and trigeminal nucleus caudalis possible. Optimization of this technique demonstrated that the ideal section size was 1 mm wide. We found that sectioning brains at physiological temperatures was necessary for consistent metabolic analysis of trigeminal nucleus caudalis sections. Oxygen consumption in these sections was highly coupled to ATP synthesis, had robust spare respiratory capacities, and had limited nonmitochondrial respiration, all indicative of healthy tissue. We demonstrate the effectiveness of this technique by identifying a decreased spare respiratory capacity in the trigeminal nucleus caudalis of a rat model of chronic migraine, a neurological disorder that has been associated with mitochondrial dysfunction. This technique allows for 24 acutely isolated sections from multiple brain regions of a single adult rat to be analyzed simultaneously with four sequential drug treatments, greatly advancing the ability to study mitochondrial physiology in adult neurological disorders.
Keywords: mitochondria, adult brain sections, neuron-glial interactions, neurological disorders, brain energy metabolism
no reliable technique currently exists to allow for medium-throughput functional mitochondrial analysis of acutely derived brain sections from adult animals. A technique of this nature, however, is critical because numerous neurological disorders that have been associated with mitochondrial dysfunction, such as Alzheimer's disease (60), Parkinson's disease (15), amyotrophic lateral sclerosis (28), Huntington's disease (23), Friedreich's ataxia (23), and migraine (50), rely on adult animal models that traditionally do not yield healthy brain sections and are impossible to use in organotypic cultures (18, 30, 57). These manifestations of mitochondrial dysfunction likely involve disruptions in the complex metabolic interplay between multiple neuronal and glial subtypes, such as the astrocyte-neuron lactate shuttle (2, 26). Analyzing the bioenergetics of this metabolic unit within an intact section is, therefore, essential for understanding how mitochondrial dysfunction correlates to these neurological disorders. This is not possible with dissociated cells, cell compartments such as synaptosomes, or isolated mitochondria. To meet these challenges, we developed a technique that allows for the analysis of multiple metabolic parameters from acutely isolated brain sections of adult male rats (33, 38, 42, 58).
For many years, the Clark electrode and other O2 microelectrodes were the primary means to measure mitochondrial respiration. These methods are insufficient for simultaneous measurement of several samples and often require tissue amounts that are larger than discrete brain regions of experimental animals (11, 20, 59, 61). The Seahorse XF Analyzer overcomes these challenges by utilizing a plate-based, fluorescence technology to measure oxygen consumption with a very high signal-to-noise ratio while only requiring small amounts of cells or mitochondria per well (4, 27, 43, 46, 52). The technology has been validated in isolated mitochondria (43) and has been widely adopted for the study of mitochondrial function in numerous cell types and cellular compartments, including neurons and synaptosomes. Use of this instrument to study intact tissue is emerging but has proven to be challenging (45, 49). Although limited progress has been made with use of this technology in organotypic hippocampal sections from mice (45), until this study, it has not been successful in acutely derived sections in adult animals (45).
We present here a technique to facilitate the study of mitochondria in animal models of adult neurological disorders, allowing for stable metabolic recordings of acute sections simultaneously from multiple brain regions of an adult rat. Together with substrate-utilization studies, five key parameters of mitochondrial function are assessed within each section: basal respiration, ATP turnover, proton leak, maximal respiration, and nonmitochondrial respiration. Sectioning adult rat brains at physiological temperatures (37°C) as opposed to the more commonly used ice-cold artificial cerebral spinal fluid (aCSF) results in stable metabolic measurements for over 6 h of experimental analysis. This is in agreement with recent findings by Huang et al. (18) that sectioning adult brains at warmer temperatures results in enhanced section quality without negatively affecting intrinsic electrophysiological properties of neurons. Bourne et al. (6) also showed that sectioning at warmer temperatures maintains the structural quality of the section and prevents an increase in synapse density, which could obscure investigation of long-term potentiation.
Using a tissue punch to obtain a consistent amount of brain tissue after sectioning greatly reduces the variability of the metabolic rate measurements between sections. Evaluating a range of section sizes, we find that 1-mm-wide sections had basal oxygen consumption rates (OCRs) within an optimal range for the Seahorse XF Analyzer and allow for penetration of diffusing mitochondria-affecting compounds, resulting in robust responses similar to those reported in dissociated neuronal cultures (12) and isolated mitochondria from neurons (10). Utilizing metabolic and cellular parameters, we show the viability of these sections throughout the duration of the experiment. These sections are well coupled to ATP synthesis, as shown by a 50% reduction of basal OCRs upon injection of the ATP synthase inhibitor, oligomycin. The mitochondrial uncoupler, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), increases OCRs to 250% of basal respiration, reflecting a strong spare respiratory capacity. Addition of antimycin A, the electron transport chain (ETC) complex III inhibitor, decreases OCRs to 10% of basal respiration, demonstrating that nearly all of the oxygen consumed was attributable to mitochondrial respiration.
Over 40 years of clinical evidence has suggested a mitochondrial etiology for migraine, yet no experimental studies have ever been performed to test these hypotheses because of the previous lack of animal models of migraine and efficient technologies to perform functional mitochondrial analyses on adults. Histological studies have revealed ragged-red fibers and cytochrome-c-oxidase-negative fibers in skeletal muscle of patients with migraines with prolonged aura (7, 53). Biochemical studies and imaging studies have demonstrated decreased activity in the ETC complexes and metabolic abnormalities of patients with migraines (7, 44, 55). Increased plasma lactate levels and impaired brain oxidative energy metabolism have been observed in migraineurs during and between migraine attacks (36, 47). In 2007, we developed a model of chronic migraine that is produced by the infusion of an inflammatory soup (IS) onto the dura of awake rats three times per week for a month (38). The infusion process produces permanent trigeminal sensitivity that outlasts the final infusion, representing a sustained state change within the neuronal systems responsible for trigeminal sensory information processing. This sensitivity in these “transitioned” rats is represented behaviorally by an increased nociceptive response to mechanical stimuli, phonophobia, and sensitivity to known human migraine triggers and treatments. These rats feature greater increases of glutamate in the trigeminal nucleus caudalis (TNC), a brainstem nucleus vital for central processing of trigeminal sensory information, following exposure to the migraine trigger, glyceryl trinitrate (38). Because this model of chronic migraine relies on the use of adult rats, it was critical to develop a technique to allow for the use of acute sections for mitochondrial analysis.
We focused our optimization of this technique on areas in the forebrain and hindbrain that contain brain regions that are involved in a number of neurological disorders, such as Alzheimer's disease, Parkinson's, Huntington's, stroke, epilepsy, and migraine (3, 5, 15, 17, 24, 60). This method represents the first time mitochondria of intact brain sections from adult rats are reliably analyzed, allowing for the investigation of the impact of mitochondrial dysfunction on adult neurological disorders.
MATERIALS AND METHODS
Male Sprague Dawley rats (400–550 g, 16–20 wk old; Charles River Laboratories, Wilmington, MA; n = 43) were housed individually in a temperature-controlled (21–22°C) environment under a 12-h:12-h light/dark cycle and given food and water ad libitum.
Ethics Statement
All procedures performed on the animals were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee, animal welfare assurance number A3085-01. Efforts were made to minimize animal number and suffering.
Rat Model of Chronic Migraine
Implantation of the cannula.
After 2 wk of habitation and training, rats were put under isoflurane anesthesia (3% induction, 1.5% maintenance) mixed with compressed air. A 3-mm-wide craniotomy was performed just above the junction of the superior sagittal and transverse sinuses to expose the dura. A plastic cap and stainless steel cannula were affixed to the skull with a combination of small screws and dental cement and then sealed with an obdurator that was custom cut to extend just beyond the internal end of the cannula above the dura (26 gauge; Plastics One, Roanoke, VA). The obdurator prevented the formation of scar tissue, allowing for unobstructed flow of the IS onto the dura. Rats were allowed 1 wk of recovery to ensure that trigeminal pressure thresholds remained at their presurgery baseline.
Infusion of IS.
Rats were infused through the cannula within their home cages to allow for free movement during the infusion period. The IS contained 1 mM histamine, serotonin, bradykinin, and 0.1 mM prostaglandin E2 in 0.9% sterile saline (Sigma Aldrich, St. Louis, MO). IS (25 μl) was delivered at a steady flow rate via polyethylene tubing (PE50) that was connected to a microinfusion pump (WPI, Sarasota, FL) and the exposed cannula. Infusions were made on Monday, Wednesday, and Friday of each week for 1 mo for a total of 12 infusions.
Tactile sensory testing.
Rats were trained and acclimated to a plastic tube restraint before and after cannula implantation and entered uncoaxed. This atraumatic restrainer was necessary to prevent the rats from walking away during sensory testing. Periorbital pressure thresholds were determined each morning before the infusion by applying von Frey monofilaments (Stoelting, Wood Dale, IL) to both the left and right sides of the face over the medial portion of the face near the eye. These von Frey hairs are calibrated to reproducibly buckle at specific assigned force values (10, 8, 6, 4, 2, 1.4, 1, 0.6, 0.4, and 0.07 g). The higher the value, the stiffer and more difficult the monofilament is to bend. Both left and right sides of the face were measured, but the lesser of both is reported as the threshold of that day. The von Frey stimuli were presented in sequential descending order to determine the threshold of response. Vigorous stroking of the face with the ipsilateral forepaw, quick recoiling of the head away from the stimulus, or vocalization was considered a positive response. The threshold was considered the lowest force value that produced positive response for two of three trials. Results are presented as the threshold in g ± SE. Rats that did not respond to the 10-g stimulus were assigned 10 g as their threshold. Rats receiving the IS featured thresholds that were less than 2 g, whereas naive rats had thresholds of no less than 8–10 g. The group of rats that received the IS infusions and developed trigeminal hypersensitivity are known as transitioned rats.
Preparation of Acute Brain Sections
Acute sections were prepared from male Sprague Dawley rats (400–550 g). Animals were briefly anesthetized with 3% isoflurane and then decapitated. Brains were immediately dissected with intact spinal cord from obex to C7. Dura mater was removed from the cortex (CTX), brainstem, and spinal cord, and brains were placed into a 50-ml beaker with 37°C aCSF (120 mM NaCl, 3.5 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2 hexahydrate, 0.4 mM KH2PO4, and 5 mM HEPES with 10 mM glucose added day of experiment, pH to 7.4 using 10 N NaOH). Brains were incubated at 37°C throughout the entire experimental period (sectioning, incubation, and when in the Seahorse XF Analyzer) except for those experiments where we tested the effect of sectioning on ice. For these experiments, the brains were quickly removed and placed in ice-cold aCSF for the isolation of specific brain regions and throughout the sectioning procedure.
The cerebellum (CB), brainstem, and CTX were then quickly dissected and incubated in a beaker containing aCSF. When one of the brain regions was ready to be sectioned, it was removed from the aCSF and placed into a 1.5 ml Eppendorf tube containing 4% low-melting-point agarose (V2111; Promega, Madison, WI) with 10 mM glucose incubating at 37°C. To solidify agarose, the tube was briefly placed under running tap water. The tissue-containing agar was then removed and super-glued to the fixed tissue holder of the vibratome for sectioning (752M Vibraslice; Campden Instruments, Loughborough, UK). Ice-cold or 37°C aCSF was used to fill the vibratome tissue bath.
Obtaining Sections of a Consistent Diameter
To obtain consistent sections for analysis, we used 3-mm, 2-mm, 1-mm, and 0.5-mm stainless steel biopsy punches (Miltex, York, PA). The tissue punch was carefully lowered into the bath and pressed gently overtop the area of interest on the section. With the use of a nylon bristled brush, the remaining tissue was removed, and the tissue punch was lifted away, leaving a circular section of tissue with diameter of 3 mm, 2 mm, 1 mm, or 0.5 mm.
Loading Sections onto the XF Islet Capture Microplate
The XF Islet Capture Microplate (101174-100; Seahorse Bioscience, Billerica, MA) was floated on a 37°C water bath while each section was loaded onto the plate. The mesh capture screens were lowered into the section-containing dish, and the sections were carefully placed onto the mesh side of the capture screen. The mesh insert was then lifted from the aCSF, and excess moisture was removed, securing the slice to the mesh insert. With the moisture removed, the tissue became sticky, which allowed the section to secure itself to the mesh insert. These sections remain secured to the screen throughout the entire experimental process. The mesh insert was then placed into a well of the incubating capture microplate with the section facing down. aCSF-media (700 μl, aCSF with 4 mg/ml BSA) (9048-46-8, Sigma Aldrich) was immediately put into the well, avoiding air bubbles. Every effort was made to quickly remove the brain, obtain brain regions, and carefully perform sectioning, punching, and loading of the sections onto the plate.
Because the biosensors are positioned in the center of the well, it is vital that air bubbles are not present and the tissue is properly centered. Air bubbles can be removed and tissue centered using a micropipette tip. The microplate was then incubated at 37°C for 1 h, per standard Seahorse Biosciences protocol, to allow for temperature and pH equilibration. For sections processed in ice-cold aCSF, the sections were loaded onto the XF Islet Capture Microplate at room temperature to allow them to slowly increase their temperature to the physiological range used during the respiratory assay. These sections were allowed to acclimate to this temperature for 30 min before being incubated at 37°C for 1 h. During this time, each drug of interest was diluted in 37°C media (aCSF + 4 mg/ml BSA) to the desired concentration so that, when injected into the well, containing 700 μl of media, the drug concentration was at experimental levels. The injection volume was 75 μl. Drugs were then preloaded into the reagent delivery chambers A, B, C, and D of the sensor cartridge that had been hydrating in Seahorse calibration solution overnight at 37°C.
Seahorse XF Analyzer Respiratory Assay
While the microplate was incubating for 1 h, the drug-filled sensor cartridge could be loaded into the Seahorse XF Analyzer for calibration. This takes ∼30 min. Assay protocols contained a combination of 3-min mix, 3-min wait, and 2-min measure sequences. First, the probe array was lowered to create a minimally oxygen-impermeable microchamber for 2 min. The OCR was calculated from 10 readings of the Po2 during this time. The probe array was then raised, after which the solution in each well was mixed for 3 min (by gently moving the probe array up and down) to remove O2 and metabolite gradients, followed by a 3-min waiting period before the next measurement phase (i.e., lowering of the probe). Our experiments included five OCR measurements to create a baseline, followed by the injection of pyruvate (113-24-6, Sigma Aldrich). Five OCR measurements would then be made, followed by injection of oligomycin from Streptomyces diastatochromogenes (1404-19-9, Sigma Aldrich). Ten to fifteen OCR measurements were then made, followed by injection of FCCP (370-86-5, Sigma Aldrich). Five OCR measurements would then be made, followed by injection of antimycin A from Streptomyces sp. (1397-94-0, Sigma Aldrich). Fifteen to twenty OCR measurements were then made. The experimental period takes a total of 360 min. Combinations of other substrates, inhibitors, uncouplers, and ionophores can also be used to identify other mitochondrial parameters. For comparisons, inhibitor and uncoupler rates (or responses to other environmental or chemical challenges) can be assessed as a percent of the basal rate. Temperature correction wells were excluded because temperature was found not to vary during the experiments, increasing the functional use of the plate by 20%.
We noted that, when we used the Seahorse XF Analyzer Islet Microplates and performed more than five OCR measurements without any injection, there was a significant drop in the Po2. To prevent this, the distance the probe head lowers had to be increased from the standard baseline levels before the start of the experiment. To do this, the load, measure, and calibration distances were changed from 26,600 to 27,800. This was done in the hardware settings under instrument settings. The instrument settings are password protected, and the password was obtained from Seahorse Biosciences.
Propidium Iodide Fluorescence Imaging
After gross sectioning of the brain region of interest, the first five sections were discarded to ensure that sections selected for analysis were not affected by the initial brain-removal procedure. Brain sections were incubated with 10 mM propidium iodide (PI) for 10 min to assess cell death similar to that of previously published protocols (9, 12, 32, 34, 51). After this incubation, sections were rinsed twice with media, and PI fluorescence was imaged using ×10 objectives of a Nikon Ni-U upright fluorescence microscope (Nikon, Tokyo, Japan). The filter sets for excitation/emission were 540 nm/620 nm. The time points for these experiments represent the amount of time following the 1-h incubation at 37°C after the sectioning procedure. Total fluorescence was quantified with ImageJ (v. 1.47; NIH, Bethesda, MD) for three regions of each section to obtain an average fluorescence value for each section. The same three regions were used across all sections analyzed. Data were represented in arbitrary fluorescence units or as a percent change from the average of the 0-h time point basal levels.
Statistical Analysis
Raw data were compiled with the XF24 Analyzer Software (v. 1.7.0.74) and analyzed with Microsoft Excel and SPSS (v. 22; IBM, Armonk, NY). OCRs and PI fluorescence intensity of acute rat brain sections were compared using one-way ANOVA with Tukey's test for post hoc analysis. Each OCR is obtained from the slope of 10 readings of the Po2 over a 2-min measuring period. The baseline of data represented as percent baseline is the mean of the last three OCRs calculated before the first injection. The oligomycin response is the minimum value obtained for a particular section after oligomycin injection. The FCCP response is the maximum value obtained for a particular section after FCCP injection. The antimycin response is the mean of the first three OCRs after it has reached its minimum following antimycin injection. The antimycin response is interpreted as nonmitochondrial respiration and is subtracted from the oligomycin and basal OCR to determine the percentage of mitochondrial-only oxygen consumption coupled to ATP synthesis. The effect of temperature on OCR stability was compared in sections from three rats sectioned at 37°C and two rats sectioned on ice. The effect of temperature on basal OCRs was compared in sections from 24 rats prepared at 37°C and three rats prepared on ice. The effect of temperature on the amount of respiration coupled to ATP production was compared in sections from five rats prepared at 37°C and four rats prepared on ice. For analysis of basal OCRs, 3-mm sections were obtained from two rats, 2-mm sections were obtained from seven rats, 1-mm sections were obtained from 24 rats, and 0.5-mm sections were obtained from two rats. Analysis of mitochondrial parameters was performed on sections from two rats for 3-mm sections, five rats for 2-mm sections, and seven rats for 1-mm sections. For analysis of OCR response to various drug concentrations, sections were obtained from eight rats. For PI analysis, sections were obtained from two rats sectioned at 37°C and two rats sectioned on ice. For mitochondrial analysis comparisons between transitioned and naive rats, sections were obtained from six transitioned rats and seven naive rats. Data are expressed as means, and P < 0.05 was considered significant. Because OCR and PI fluorescence measurements were an average of a number of mean measurements, we reported the error bars as SE.
RESULTS
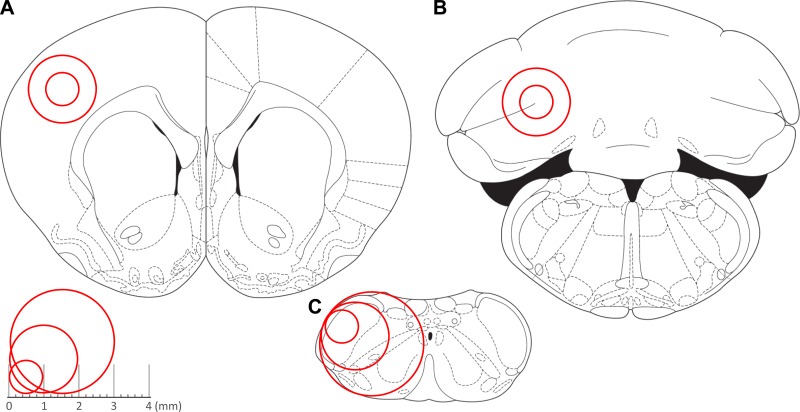
We calculated OCRs from 267 acute brain sections from 43 adult rats (16–20 wk old), in response to pharmacological manipulation to quantify basal respiration, the amount of respiration linked to ATP production, proton leak, maximum respiratory rate, spare respiratory capacity, and nonmitochondrial respiration in three distinct brain regions: the CTX (Fig. 1A), CB (Fig. 1B), and TNC (Fig. 1C).
Fig. 1.
Brain regions analyzed and punch sizes. Representative tissue punch sizes (1 mm, 2 mm, and 3 mm) for cortex (A), cerebellum (B), and trigeminal nucleus caudalis (TNC) (C). Red circles represent the areas that were obtained for analysis for each of the brain regions. [Adapted from Paxinos and Watson (41), with permission from Elsevier.]
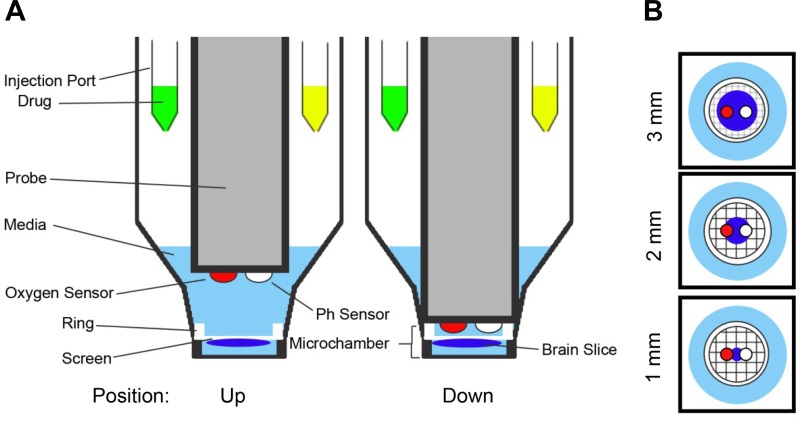
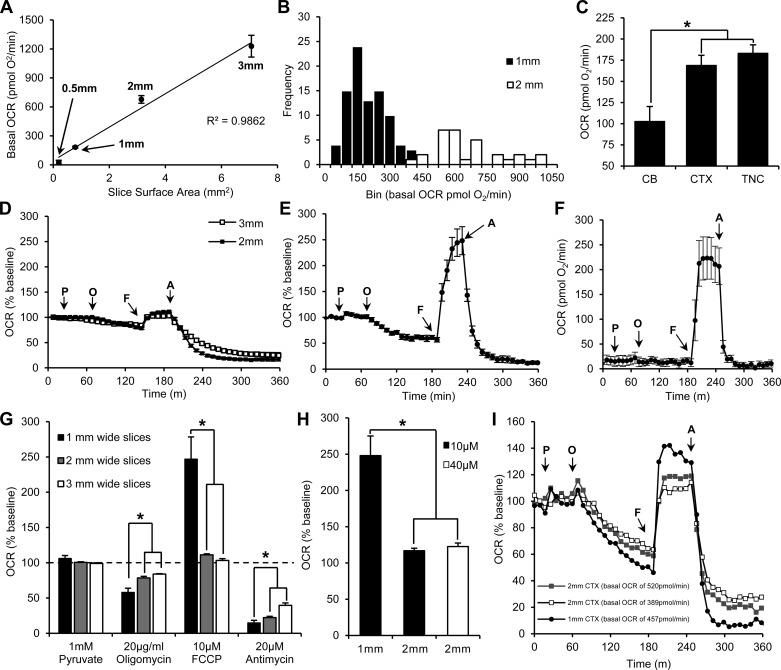
Sections (250 μm thick) of various diameters (0.5 mm, 1 mm, 2 mm, and 3 mm) were mounted on nylon mesh inserts and placed at the bottom of each well of a 24-well XF Islet Capture Microplate containing aCSF-media (Fig. 2). An array of 24 retractable probes, each containing two biosensors (one of which monitors the Po2 to derive OCRs and the other to measure H+ concentration), was lowered into each well simultaneously, creating a minimally oxygen-impermeable 3-mm-diameter, 1-mm-deep, 17-μl microchamber (Fig. 2A). The probe array remained in the lowered position for only a short duration (e.g., 2 min) to measure O2 depletion that reflects cellular reduction of oxygen within the microchamber. aCSF O2 levels were 20 ± 1% (155 ± 9 mmHg) before the probe lowering, creating the microchamber, and never fell below 18 ± 1% (138 ± 9 mmHg) with the lowest O2 level of any well at 118 mmHg only after respiration rates were maximally increased with the addition of FCCP. The 3-mm sections spanned the entire diameter of the microchamber formed by the mesh insert. The 2-mm sections had 0.5 mm of space around the section, spanning the entire biosensor area. The 1-mm sections were positioned directly between the two biosensors and had 1 mm of space around the section (Fig. 2B). The 0.5-mm sections had 1.25 mm of space around the tissue.
Fig. 2.
Plate configuration. A: side-view schematic of 2 wells of the 24-well XF Islet Microplate. The probe head containing an oxygen and pH biosensor is shown in the up and down positions. When in the down position, a transient microchamber (1 mm deep × 3 mm diameter) is formed to allow for measurement of oxygen consumption rates (OCRs) of a brain section. B: top-view schematic of the 3 section sizes (1 mm, 2 mm, and 3 mm) in relation to the microchamber and the biosensors on the probe head.
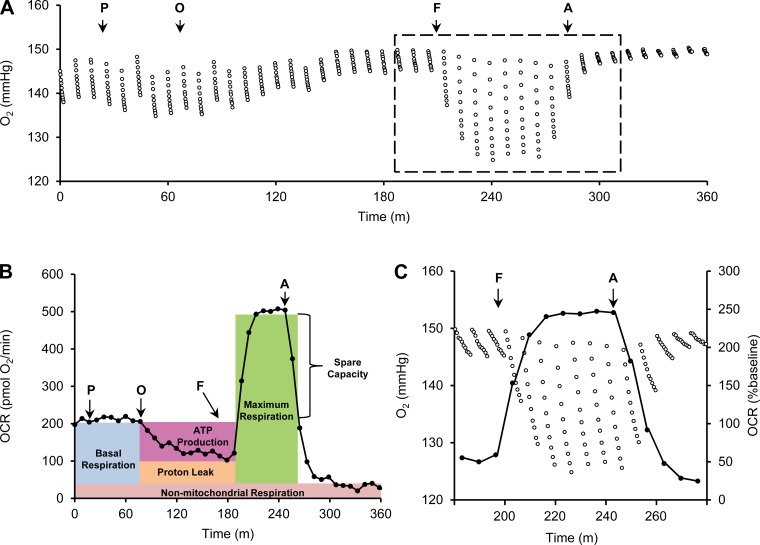
The cartridge that houses the probes has four injection ports for each well. Pyruvate was injected first as a supplemental substrate in addition to glucose already within the aCSF for mitochondrial respiration. As seen with previous groups, pyruvate was needed to prevent any substrate-limiting constraints of substrate supply upon injection of other mitochondria-targeting compounds (45). Without it, the FCCP response was not as robust, slower to peak, and would feature a decrease in the plateaued response following FCCP injection that was indicative of limited substrate supply, obscuring accurate quantification of the spare respiratory capacity (data not shown). The ongoing reduction of oxygen in a 1-mm-wide TNC section, mainly by mitochondrial respiration, is shown as a continual drop of the Po2 during each measurement phase (Fig. 3A). An acceleration of cellular O2 reduction is seen as a greater drop in Po2 over time (Fig. 3A; compare traces outside and inside the dashed box). This can be expressed as a function of time, either to provide a snapshot of a series of responses to challenges, or to evaluate kinetics (Fig. 3B). Fundamental bioenergetics experiments require that the following parameters be evaluated (Fig. 3B): 1) total basal mitochondrial OCR; 2) nonmitochondrial OCR (achieved by inhibiting the ETC at complex III with antimycin A); 3) maximal mitochondrial capacity using the chemical uncoupler FCCP; 4) maximal nonphosphorylating (leak-dependent) OCR using oligomycin to inhibit ATP synthase, which also provides a measure of the amount of respiration coupled to ATP production. These parameters were obtained by sequential injection of pyruvate, oligomycin, FCCP, and antimycin A. O2 is consumed faster by the section following FCCP injection, translating to an increase in the OCR, and slower following antimycin A injection, translating to a decrease in the OCR (Fig. 3C). Injection of 20 μg/ml oligomycin reduced the OCR to 102 pmol O2/min (51% of baseline). Injection of 10 μM FCCP increased respiration to 504 pmol O2/min (254% of baseline), illustrating a high respiratory capacity. Antimycin (20 μM) attenuated respiration to 35 pmol O2/min (16% of baseline), showing that the majority of respiration is mitochondrial in nature. Subtracting the antimycin response from both the basal OCR and oligomycin response to obtain a measure of only mitochondrial respiration showed that 61% of the mitochondrial respiration of this section was coupled to ATP synthesis, illustrating that this section featured good mitochondrial integrity. Data from the second biosensor can be used to monitor pH changes that are useful for interpretation of glycolytic rates throughout the experimental process. The pH of the aCSF-containing sections from the CTX, CB, and TNC did not fluctuate, remaining at 7.4 ± 0.2 pH. Glycolytic analysis can be performed by injection of an alternative sequence of drugs (i.e., glucose, oligomycin, and 2-deoxy-d-glucose), but, because this treatment sequence is different than that used for mitochondrial analysis, we did not perform analysis on the data from this sensor.
Fig. 3.
OCRs from O2 readings of a single acute brain section. A: O2 readings from a single respiring 1-mm-wide, 250-μm-thick TNC section. 10 sequential O2 measurements are made after the probe head has lowered, forming the minimally oxygen-impermeable microchamber. O2 levels rise back to atmospheric levels after the probe head is lifted. This is then followed by another set of 10 O2 readings after the probe head lowers again. Injection of 1 mM pyruvate (P), 20 μg/ml oligomycin (O), 10 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (F), and 20 μM antimycin (A) are marked by arrows, modifying mitochondrial respiration, and leading to changes in the amount of oxygen consumed by the section. B: OCRs calculated from the individual O2 readings from A. Drug responses can be used to interpret basal mitochondrial respiration, ATP production, proton leak, max respiration, spare capacity, and nonmitochondrial respiration. C: this is an expanded version of the portion from A that is marked by the dashed lines. It shows the translation of the 10 O2 measurements (left y-axis) to the OCRs (right y-axis) for 3 OCR measurements before FCCP injection, 7 after FCCP injection, and 5 after antimycin injection.
Optimizing the Seahorse XF Analyzer for Use With Tissue Sections
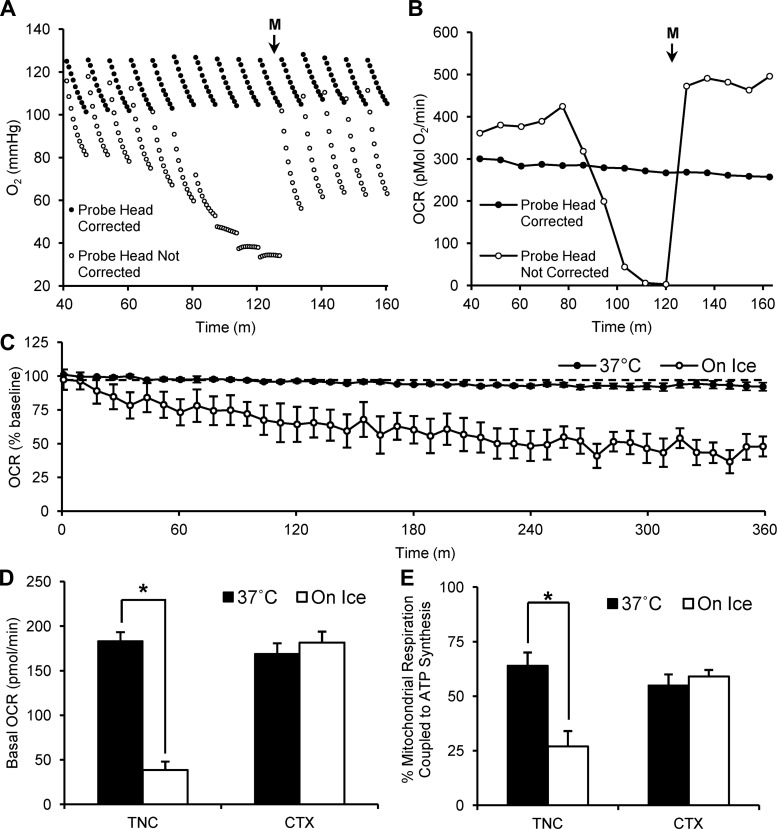
The amount of time for the substrates, inhibitors, and the uncoupler compounds to reach their maximum effect on OCRs was similar to that of pancreatic islets in the Seahorse XF Analyzer but was longer than that reported for dissociated neurons and isolated mitochondria (10, 56). Allowing for relatively long measurement periods between injections revealed that the initial Po2 during a measurement phase dramatically declined after approximately five consecutive OCR measurements (Fig. 4A; probe head not corrected). This led to a sharp decrease in Po2 and consequentially OCRs (Fig. 4B; probe head not corrected). The initial Po2 and corresponding OCRs returned to baseline only after a subsequent injection was made (in this case, media only). This decline was prevented by increasing the distance the probe array was lowered (Fig. 4, A and B; probe head corrected). This problem is only seen after five OCR measurements, but, because our experiments required greater than five OCR measurements after each drug injection, we increased the number of steps the probe head lowered from 26,600 to 27,800 (Fig. 4, A and B; probe head corrected).
Fig. 4.
Probe head adjustment and sectioning temperature essential for stability of acute sections. A: O2 readings of 2 TNC sections representing measurements from reads when the probe head height was corrected (lowered from 26,600 steps to 27,900 steps) and when the probe head height was not corrected. B: absolute OCRs derived from O2 readings from A. C: 6 h of OCR measurements from 250-μm-thick, 1-mm-wide TNC sections processed at 37°C (n = 7 sections) and on ice (n = 9 sections). D: basal OCR (pmol/min) of 250-μm-thick, 1-mm-wide TNC sections processed at 37°C (n = 81 sections) and on ice (n = 13 sections) compared with cortex (CTX) sections processed at 37°C (n = 47 sections) and on ice (n = 11 sections). E: percentage of mitochondrial respiration coupled to ATP synthesis (calculated from maximum response to 20 μg/ml oligomycin) of 250-μm-thick, 1-mm-wide TNC sections processed at 37°C (n = 12 sections) and on ice (n = 7 sections) compared with CTX processed at 37°C (n = 9 sections) and on ice (n = 8 sections). Media injection is represented by M; *P < 0.05.
Sectioning Temperature is Important for Metabolic Stability
We found that 1-mm-wide adult brain sections prepared at 37°C had greater drug responses and were more stable than if they were sectioned in ice-cold aCSF, which is the traditional method. Brains were removed from adult rats and immediately placed in either 37°C or ice-cold aCSF. The sectioning procedure was then performed in aCSF of the respective temperature. Once sections were mounted onto the XF Islet Plate, both sets of sections were incubated at 37°C for 1 h, after which, O2 levels were recorded at 37°C within the Seahorse XF Analyzer.
OCRs from TNC sections obtained on ice dramatically decreased after only 180 min of analysis (Fig. 4C). Basal OCRs of TNC sections were significantly reduced if prepared on ice (38 ± 9 pmol/min) compared with sections prepared at 37°C (183 ± 10 pmol/min) (P < 0.05, ANOVA). CTX sections showed no significant difference in basal OCR when prepared on ice (181 ± 12 pmol/min) compared with sections prepared at 37°C (169 ± 12 pmol/min) (Fig. 4D). TNC sections obtained on ice also had significantly less mitochondrial respiration coupled to ATP synthesis, 27 ± 7% of basal OCRs, than did TNC sections obtained at 37°C, 64 ± 6% of basal OCRs (P < 0.05, ANOVA). CTX sections obtained on ice had similar levels of mitochondrial respiration coupled to ATP synthesis, 59 ± 3% of basal OCRs, to sections obtained at 37°C, 55 ± 5% of basal OCRs (Fig. 4E). Sections obtained at 37°C featured a large spare respiratory capacity (Fig. 5E); thus the higher basal rates likely do not reflect elevated ATP demand caused by increased stress. These data showed that different brain regions may be more sensitive to sectioning temperature than others but that sectioning at 37°C allows for greater metabolic stability in acute adult brain sections than does sectioning on ice.
Fig. 5.
Optimal section diameter for basal OCR and drug responses. A: basal OCRs vs. section surface area for 3-mm (n = 4 slice), 2-mm (n = 32 sections), 1-mm (n = 81 sections), and 0.5-mm (n = 7 sections) TNC sections are linearly correlated. B: histogram of basal OCR for 1-mm (n = 81 sections) and 2-mm (n = 32 sections) TNC sections (bin = 50 pmol/min). C: mean basal OCRs for 1-mm sections from cerebellum (CB) (n = 10 sections), CTX (n = 47 sections), and TNC (n = 81 sections). D: baseline-normalized OCRs from 3-mm TNC (n = 4 sections) and 2-mm TNC (n = 13 sections). E: baseline-normalized OCRs from 1-mm TNC sections (n = 12 sections). F: absolute OCRs from 0.5-mm TNC sections (n = 7 sections). G: quantification of drug effects (% baseline) for each section diameter from D and E. H: maximum respiration following addition of 10 μM FCCP in 1-mm (n = 12 sections) and 2-mm TNC sections (n = 5 sections) and 40 μM in 2-mm TNC sections (n = 5 sections). I: 3 separate sections from the CTX with similar starting basal OCRs. Injection of 1 mM pyruvate, 20 μg/ml oligomycin, 10 μM FCCP, and 20 μM antimycin are marked by arrows, *P < 0.05.
Optimizing Section Diameter
We normalized the amount of tissue per well by measuring OCRs in sections from similar areas of the brain with the same thickness, diameter, and weight. Seahorse Bioscience reports that the upper detectable limit of OCRs is 1,500 pmol/min with optimal basal OCRs between 100–300 pmol/min. In our experience, basal OCRs <50 pmol/min resulted in erratic O2 readings, which led to unreliable OCRs. We, therefore, optimized section diameter to have basal OCRs between 100–300 pmol/min without the potential for extending beyond the reliable limit of 1,500 pmol/min when treated with the uncoupler, FCCP. In TNC sections, basal OCRs were linearly correlated to section surface area (Fig. 5A). Moreover, 1-mm TNC sections had the least variability in basal OCR and the highest probability of the basal OCR falling within the optimal range (183 ± 10 pmol O2/min) (Fig. 5B). The 3-mm TNC sections had basal OCRs of 1,228 ± 113 pmol/min, which risk extending beyond the upper detectable limit after FCCP was applied. The 2-mm TNC sections had basal OCRs of 677 ± 10 pmol/min. The 0.5-mm TNC sections had basal OCRs of 27 ± 21 pmol/min, which were below the lower detectable limit (Fig. 5A). Using the optimized section diameter of 1 mm, we subsequently found that basal OCRs for TNC sections were similar to that of 1-mm CTX sections (169 ± 12 pmol/min), and both were significantly higher than 1-mm CB sections (103 ± 18 pmol/min) (P < 0.05, ANOVA) (Fig. 5C).
The 1-mm TNC sections had the most robust drug responses compared with 2-mm and 3-mm sections (P < 0.05, ANOVA). Injection of 20 μg/ml oligomycin reduced OCRs to 57 ± 4% of baseline in 1-mm sections (Fig. 5, E and G). Increasing the number of OCR measurements after oligomycin injection from 10 (80 min postinjection) to 15 (120 min postinjection) confirmed that the full drug effect was observed as seen by the plateau of the trace (Fig. 5E). Injection of 10 μM FCCP increased OCRs to 248 ± 27% of baseline in 1-mm sections (Fig. 5, E and G). Injection of 20 μM antimycin reduced OCRs to 6 ± 2% of baseline in 1-mm sections (Fig. 5, E and G). Using the absolute OCR values for each 1-mm TNC section to subtract the antimycin response from the basal OCR and oligomycin response revealed that 64 ± 6% of mitochondrial respiration was coupled to ATP synthesis.
Responses in 3-mm or 2-mm sections were not as robust; injection of 20 μg/ml oligomycin reduced OCRs to 84 ± 1% of baseline in 3-mm sections and 78 ± 2% of baseline in 2-mm sections (Fig. 5, D and G). Injection of 10 μM FCCP increased OCRs to 103 ± 3% of baseline in 3-mm sections and 111 ± 1% of baseline in 2-mm sections (Fig. 5, D and G). Injection of 20 μM antimycin reduced OCRs to 25 ± 6% of baseline in 3-mm sections and 16 ± 3% of baseline in 2-mm-wide sections (Fig. 5, D and G). Injection of 1 mM pyruvate significantly, but minimally, increased OCRs in 1-mm and 2-mm TNC sections to 108 ± 3% and 102 ± 1% of baseline, respectively, but did not significantly increase OCRs in 3-mm TNC sections (100 ± 1% baseline) (Fig. 5, D and G). These responses to pyruvate, however, were not significantly different than that of a media-only injection control (0 mM pyruvate) in 1-mm TNC sections (103 ± 4% baseline) and, thus, are likely an injection artifact (Fig. 6A).
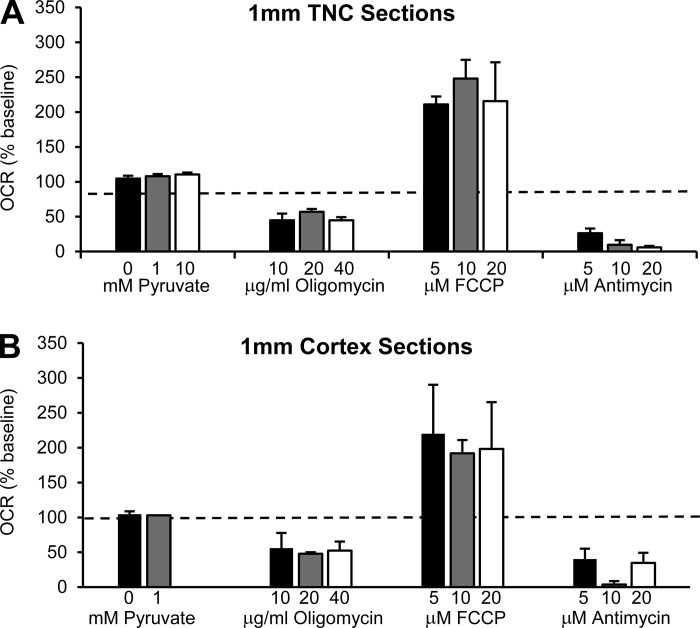
Fig. 6.
1-mm TNC and CTX section responses to various drug concentrations. A: pyruvate, oligomycin, FCCP, and antimycin drug response in TNC sections: 0 mM (n = 3 sections), 1 mM (n = 5 sections), and 10 mM (n = 5 sections) pyruvate; 10 μg/ml (n = 5 sections), 20 μg/ml (n = 5 sections), and 40 μg/ml (n = 5 sections) oligomycin; 5 μM (n = 4 sections), 10 μM (n = 5 sections), and 20 μM (n = 5 sections) FCCP; 5 μM (n = 4 sections), 10 μM (n = 5 sections), and 20 μM (n = 5 sections) antimycin. B: pyruvate, oligomycin, FCCP, and antimycin response in CTX sections: 0 mM (n = 5 sections) and 1 mM (n = 5 sections) pyruvate; 10 μg/ml (n = 6 sections), 20 μg/ml (n = 4 sections), and 40 μg/ml (n = 5 sections) oligomycin; 5 μM (n = 5 sections), 10 μM (n = 4 sections), and 20 μM (n = 5 sections) FCCP; 5 μM (n = 6 sections), 10 μM (n = 4 sections), and 20 μM (n = 3 sections) antimycin.
Because 0.5-mm sections had basal OCRs that were below the lower detectable limit of 50 pmol/min, we were not able to measure their oligomycin or antimycin responses. The FCCP response, however, increased OCRs to 223 ± 42 pmol/min (Fig. 5F). Although the increase seemed robust, the basal OCRs are below the detectable range for the equipment, falsely amplifying the measurement of spare capacity in terms of percent change from baseline.
Although 2-mm sections were not beyond the upper detectable limit for the Seahorse XF Analyzer, their responses to treatments were limited, so we tested to see whether this limited response was attributable to a lower concentration of treatment compounds to total tissue ratio by increasing the FCCP concentration proportionally to the surface area of 2-mm sections (40 μM FCCP). This did not, however, increase the FCCP response in 2-mm sections (123 ± 5% basal OCRs) to that seen in 1-mm sections (248 ± 27% basal OCRs), nor did it significantly increase the response over that of 2-mm sections receiving 10 μM FCCP (111 ± 1% basal OCRs) (Fig. 5H). When comparing a 1-mm section respiring on the higher end of the distribution (457 pmol/min) to two 2-mm sections respiring at similar levels (389 and 520 pmol/min), we found that the 1-mm section still had greater responses to all three drugs compared with the two 2-mm sections respiring slightly above or below the 1-mm section (Fig. 5I).
Limited Variability in Response to Multiple Concentrations of Drugs/Inhibitors in 1-mm TNC and CTX Sections
We tested the OCR response of 1-mm TNC and CTX sections to multiple concentrations of pyruvate, oligomycin, FCCP, and antimycin. In TNC sections, we observed a maximum response with the least variability to the following concentrations: 10 mM pyruvate (111 ± 3% basal OCRs), 20 μg/ml oligomycin (45 ± 6% basal OCRs), 10 μM FCCP (258 ± 49% basal OCRs), and 10 μM antimycin (10 ± 7% basal OCRs) (Fig. 6A). In CTX sections, we observed a maximum response with the least amount of variability to the following concentrations: 1 mM pyruvate (103 ± 2% basal OCRs), 20 μg/ml oligomycin (48 ± 2% basal OCRs), 10 μM FCCP (192 ± 19% basal OCRs), and 10 μM antimycin (4 ± 5% basal OCRs) (Fig. 6B). No statistical significance was seen, however, among the drug concentrations in 1-mm CTX and TNC sections (Fig. 6).
Cell Viability Analysis Using PI Staining
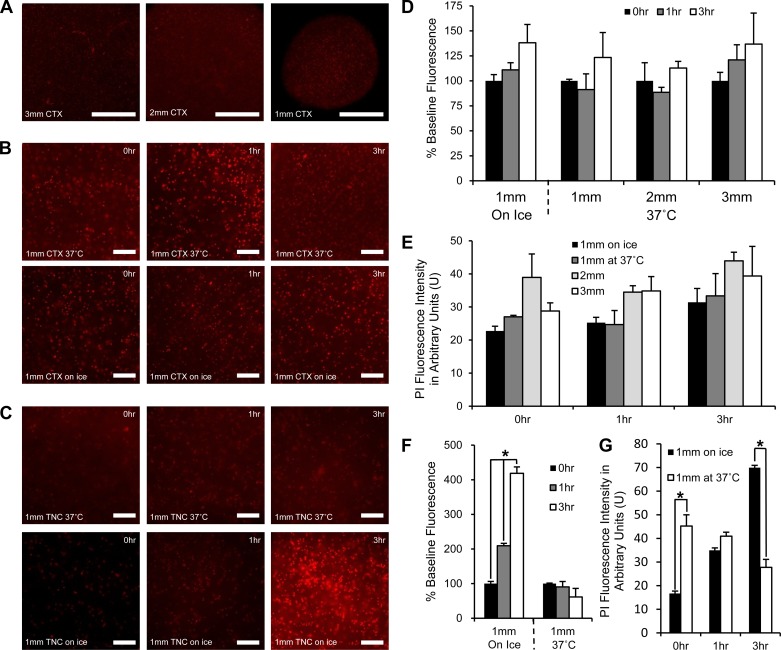
To confirm cellular viability in acute brain sections from adult rats over time for each section size, we quantified the level of PI uptake, a nuclear stain not permeable to living cells, via fluorescence imaging (Fig. 7). We quantified time points representing critical moments within our experimental timeline (e.g., 0 h representing the time point during baseline OCR measurements, 1 h representing the time point during oligomycin injection, and 3 h representing the time point just before FCCP injection). To identify any differences in cell viability caused by sectioning temperature, we used both 1-mm TNC and CTX sections. To identify any differences in cell viability attributable to section diameter, we used CTX sections because they represent a more homogenous cellular environment than TNC sections.
Fig. 7.
Propidium iodide (PI) fluorescence in CTX and TNC sections obtained at 37°C and on ice. A: representative images of PI staining of a 3-mm CTX, 2-mm CTX, and 1-mm CTX at 0 h (×4 magnification) (bar = 500 μm). B: representative images of PI staining of 1-mm CTX sections obtained at 37°C or on ice during 0-h, 1-h, and 3-h time points (×10 magnification) (bar = 10 μm). C: representative images of PI staining of 1-mm TNC sections obtained at 37°C or on ice during 0-h, 1-h, and 3-h time points (×10 magnification) (bar = 10 μm). D: PI fluorescence quantified as percent change from 0-h time point (baseline) for 1-mm, 2-mm, and 3-mm CTX sections over 3 h of incubation at 37°C following sectioning at 37°C or on ice. E: PI fluorescence intensity in arbitrary units (U) for comparison of section size and sectioning temperature on CTX sections sectioned at 37°C or on ice at 0-h, 1-h, and 3-h time points. F: PI fluorescence quantified as percent change from 0-h time point (baseline) for 1-mm TNC sections over 3 h of incubation at 37°C following sectioning at 37°C or on ice. G: PI fluorescence intensity in arbitrary units for comparison of sectioning temperature on TNC sections at 0-h, 1-h, and 3-h time points (n = 5 for sections obtained at 37°C and n = 6 for those obtained on ice). *P < 0.01.
To compare changes in cellular death over time for each section type, we quantified the percent change from baseline in CTX and TNC sections obtained at 37°C and on ice. No significant changes were seen in the level of PI staining over time for 1-mm, 2-mm, and 3-mm CTX sections obtained at 37°C or 1-mm CTX sections obtained on ice (Fig. 7D). Significant changes were seen, however, in 1-mm TNC sections cut on ice with PI fluorescence increasing to 209 ± 10% baseline fluorescence after 1 h and increasing further to 419 ± 20% baseline fluorescence after 3 h of incubation (P < 0.01, ANOVA) (Fig. 7F). No significant changes were seen in 1-mm TNC sections cut at 37°C (Fig. 7F).
To compare section viability between section sizes obtained at 37°C or on ice for each time point, we quantified absolute PI fluorescence intensity in arbitrary units. There were no significant differences between PI fluorescence at each time point between 1-mm, 2-mm, and 3-mm CTX sections obtained at 37°C or 1-mm CTX sections obtained on ice (Fig. 7E). When comparing 1-mm TNC sections obtained on ice and 37°C at each time point, we found that 1-mm TNC sections obtained on ice initially had less PI fluorescence than those obtained at 37°C, but, by the 3-h time point, PI fluorescence was significantly greater in sections obtained on ice (P < 0.01, ANOVA). The 1-mm TNC sections obtained on ice had significantly lower levels of PI fluorescence (17 ± 3 U) than did 1-mm sections obtained at 37°C (45 ± 6 U) during the 0-h time point (P < 0.01, ANOVA) (Fig. 7G). During the 1-h time point, 1-mm TNC sections obtained on ice (35 ± 2 U) were not significantly different than 1-mm TNC sections obtained at 37°C (41 ± 6 U) (Fig. 7G). During the 3-h time point, 1-mm TNC sections obtained on ice (70 ± 3 U) were significantly greater than 1-mm TNC sections obtained at 37°C (28 ± 7 U) (P < 0.01, ANOVA) (Fig. 7G).
Deficits in Spare Respiratory Capacity in the TNC of a Rat Model of Chronic Migraine
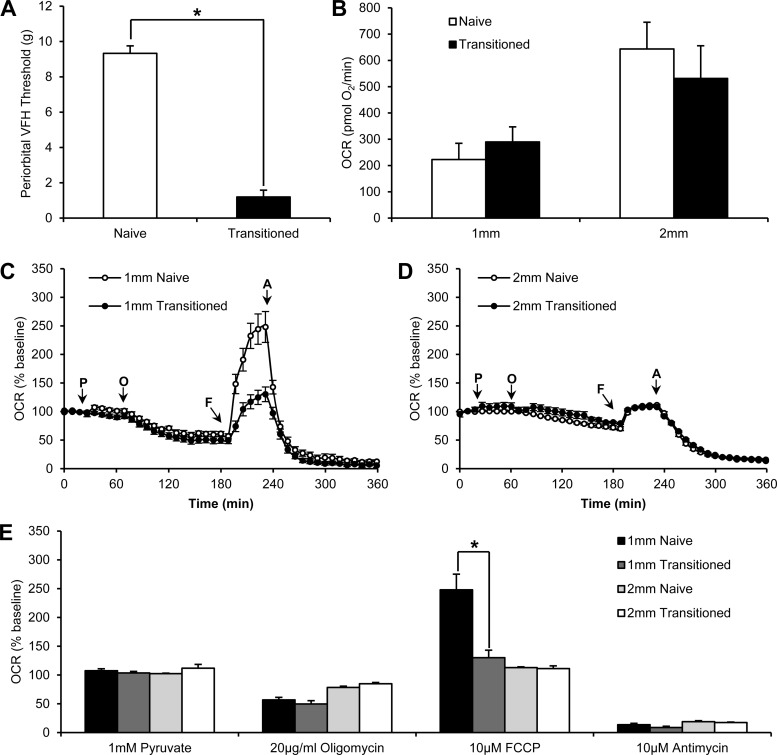
Using the above parameters, we demonstrated a decreased spare respiratory capacity within TNC sections from a rat model of chronic migraine. Transitioned rats that had received a total of 12 IS infusions had significantly lower periorbital thresholds (1.2 ± 0.4 g) than naive rats (9.3 ± 0.4 g) (P < 0.01, ANOVA) (Fig. 8A). Spare respiratory capacity, measured as percent baseline after injection of 10 μM FCCP, was significantly decreased in 1-mm TNC sections from transitioned rats (130 ± 13% basal OCRs) compared with 1-mm TNC sections from naive rats (248 ± 27% basal OCRs) (P < 0.01, ANOVA) (Fig. 8, C and E). There was no other significant difference between the transitioned and naive groups in any of the other mitochondrial parameters (as measured by injection of 1 mM pyruvate, 20 μg/ml oligomycin, and 10 μM antimycin). This difference was only apparent when using 1-mm sections, illustrating the importance of using the optimal section width of 1 mm over 2 mm. In 2-mm TNC sections, the FCCP response in transitioned rats (111 ± 5% basal OCRs) was not significantly different than naive rats (111 ± 1% basal OCRs). No significant difference was seen between transitioned and naive rats in the response of 2-mm TNC sections to 1 mM pyruvate, 20 μg/ml oligomycin, or 20 μM antimycin (Fig. 8, D and E). When we compared these sections, basal mitochondrial respiration was not significantly different between 1-mm TNC sections from naive (223 ± 62 pmol O2/min) or transitioned rats (285 ± 57 pmol O2/min) or 2-mm TNC sections from naive (643 ± 102 pmol O2/min) or transitioned rats (532 ± 124 pmol O2/min) (Fig. 8B).
Fig. 8.
Decreased spare respiratory capacity in 1-mm TNC sections from a rat model of chronic migraine. A: periorbital Von Frey (VFH) thresholds of naive and transitioned rats, rats that have received 12 inflammatory soup infusions onto their dura (n = 6 rats for naive and 6 rats for transitioned groups). B: basal OCRs of 1-mm TNC sections from naive rats (n = 12 sections), 2-mm TNC sections from naive rats (n = 13 sections), 1-mm TNC sections from transitioned rats (n = 11 sections), and 2-mm TNC sections from transitioned rats (n = 9 sections). C: baseline-normalized OCRs from 1-mm TNC sections from naive rats (n = 12 sections) and 1-mm TNC sections from transitioned rats (n = 11 sections). D: baseline-normalized OCRs from 2-mm TNC sections from naive rats (n = 13 sections) and 2-mm TNC sections from transitioned rats (n = 9 sections). E: quantification of drug effects (% baseline) from C and D. (*P < 0.01). Injections of 1 mM pyruvate, 20 μg/ml oligomycin, 10 μM FCCP, and 20 μM antimycin are marked by arrows (*P < 0.05).
DISCUSSION
The lack of techniques to efficiently analyze the bioenergetics of brain tissue with intact cytoarchitecture from adult animals has been a barrier to the study of mitochondrial dysfunction in local or systems-level adult neurological disorders. The method described here allows for a detailed simultaneous analysis of multiple metabolic parameters of 24 acutely isolated brain sections from several brain regions of the same adult rat. We show that acutely isolated 1-mm brain sections have well-coupled mitochondrial respiration, robust spare respiratory capacities, and stable mitochondrial respiration rates, all indicative of healthy, viable brain sections. Our discovery that a decreased spare respiratory capacity exists within the TNC of a rat model of chronic migraine is the first example of the power of this technique.
Forty years of clinical evidence has suggested that migraine might be attributable to mitochondrial dysfunction (7, 31, 44, 47, 48, 53, 55). Montagna et al. and Sangiorgi et al. (31, 44) both found reduced activity levels of key mitochondrial enzymes in migraineurs. Phosphorus magnetic resonance spectroscopy, a method to quantify ATP production in a patient's brain energy, revealed that there was a low availability of free cellular energy in patients with migraine with aura (55). These clinical observations suggest that decreased mitochondrial output exists in migraineurs, which may be explained by the observed decrease in spare respiratory capacity identified in our rat model of chronic migraine. Neuronal tissue is incredibly energy demanding, and oxygen consumption levels of a firing neuron are thought to represent as much as 80% of the maximum respiration of a particular cell; thus, minimal changes to the spare capacity of the mitochondria of a cell could have vastly profound effects on neuronal function (13, 35). No changes are seen in basal OCRs, indicating that TNC sections from either naive or transitioned rats likely have similar levels of healthy, respiring neurons and glia; thus, the observed decrease in spare respiratory capacity is likely not due to differences in cell viability of the TNC between naive and transitioned rats. Both groups also feature mitochondrial oxygen consumption that is well coupled to ATP synthesis, as seen by a strong response to oligomycin, indicating good mitochondrial integrity that would be representative of healthy sections. Furthermore, sections from both groups have similar low levels of nonmitochondrial oxygen consumption, indicating high mitochondria:nonmitochondria ratios, which further illustrates that both groups of sections had healthy cells with functioning mitochondria. Future studies will characterize the factors and cell-type-specific contributions to this decrease in spare respiratory capacity with the hope of developing treatment options that directly target mitochondrial function for patients with chronic migraine.
TNC sections had higher basal OCRs, sustained basal metabolic levels for 6 h, and had a greater amount of mitochondrial respiration coupled to ATP synthesis, indicating good mitochondrial integrity, if the entire experimental process was kept at 37°C. TNC sections prepared on ice, as is the standard sectioning methodology in the field, resulted in sections that did not have stable metabolic levels, featured low basal OCRs, and had mitochondrial oxygen consumption that was minimally coupled to ATP production (Fig. 4) (37). These multiple metabolic parameters indicate that TNC sections obtained on ice had poor mitochondrial integrity. We do note, however, that CTX sections were not affected by sectioning at cold temperatures, suggesting that different brain regions may have differential sensitivities to temperature. Similarly, Huang et al. (18) found that prepared rat cerebellar brain sections at physiological temperatures for electrophysiological recordings were just as stable as sections prepared on ice. The belief is that sectioning tissue at lower temperatures slows cellular metabolism to support cell survival (19). We believe this process may negatively affect adult tissue, which is more sensitive to low temperatures, than tissue from young animals (1). We used PI staining to quantify the impact of sectioning temperature on section viability as previously performed by numerous groups on organotypic slice cultures (9, 12, 32, 34, 51). Overall, PI fluorescence of a section is linearly correlated with the number of dead cells within a particular section (34); thus, fluorescence intensity is an effective measure of section viability. Corroborating our metabolic analyses, we found no difference in PI fluorescence intensity over time in 1-mm CTX and TNC sections obtained 37°C, nor did we find changes in 1-mm CTX sections prepared on ice. We did find, however, a significant increase in PI fluorescence over time in 1-mm TNC sections obtained on ice, suggesting increased cell death. Although 1-mm TNC sections cut on ice had significantly less PI fluorescence than did 1-mm TNC sections cut at 37°C during the 0-h time point, fluorescence intensity increased over fourfold at the 3-h time point in sections cut on ice, whereas sections cut at 37°C did not. This suggests that, although colder temperatures may be beneficial to prevent cellular death initially, it may be detrimental to long-term cellular viability and mitochondrial function over time, likely being the reason why we found limited metabolic stability in these sections. These findings corroborate, with another physiological and cellular measure, the studies by other groups that provide structural and electrophysiological evidence that sectioning adult brains at warmer temperatures results in enhanced section viability (6, 18, 21, 22).
Importantly, we noted that 1-mm sections had basal OCRs within the optimal range of the Seahorse and featured the most robust treatment effects compared with 2-mm and 3-mm sections. We believe that this is most likely attributable to the geometry of the section within the well in relation to the probe head and injection ports because increasing the concentration of FCCP proportionally with the surface area of the section did not increase the FCCP response. Sections of different widths may experience variable absorption and diffusion rates of reagents across their surface, which could differentially impact the responses. Analysis of PI staining within these sections shows that section size does not modulate section viability at three critical time points throughout the Seahorse experimental timeline (0, 1, and 3 h postsectioning/incubation) and is likely not responsible for the differences in drug efficacy. We propose that the 1-mm sections are simply the optimal size for the Seahorse device. These observations demonstrate the importance of standardizing the size of any tissue used with these plates. Testing multiple concentrations of oligomycin, FCCP, and antimycin resulted in little variation in the OCRs of TNC and CTX sections, but we found that 20 μg/ml oligomycin, 10 μM FCCP, and 20 μM antimycin resulted in the least amount of variability between sections, validating the use of these concentrations in future experiments.
Schuh et al. (45) measured OCRs of organotypic hippocampal sections from mice but did not reliably adapt these Islet Capture Microplates for use with organotypic or acute hippocampal sections from young or old rats. Their difficulty could be explained by our observation that success depends on the width of the section. The mouse hippocampus is ∼1.5 mm wide, close to our most successful section width, whereas the rat hippocampus is ∼2.5 mm wide, larger than the 2-mm sections that did not have robust substrate, inhibitor, uncoupler, and ionophore responses (Figs. 1 and 5). Further supporting this is our observation that the difference in spare respiratory capacity between naive and transitioned rats could only be quantifiable when using 1-mm TNC sections, as opposed to 2-mm TNC sections. Reinforcing the novelty of our method, our data provide vital information about the capabilities of the Seahorse XF Islet Microplates for use with neuronal tissue to produce meaningful and reliable data. Future analyses may provide an even more detailed investigation of the exact size constraints of the Islet Microplates for optimal performance or even the development of new plates with microchambers specifically designed for varying tissue sizes.
The 2-min reading period in which a semi-oxygen-impermeable microchamber is created does not produce transient hypoxia in these sections because, although O2 diffusion into the section core may be limited, our aCSF O2 levels approximate levels used with organotypic section cultures (20–21% O2), which results in both section surface and core O2 levels similar to and above physiological levels. Previous research has shown that the O2 tension at the surface of a 210-μm-thick section is 19% O2 with the section core being 7%, which is higher than values measured in vivo in the rat CTX (2–5% O2) (14, 25, 40). We found that aCSF O2 levels, which can be used to approximate O2 levels of the section surface, were 20 ± 1% (155 ± 9 mmHg) before the creation of the microchamber. Even after we uncoupled these sections with FCCP and increased respiration, aCSF O2 never fell below 18 ± 1% (138 ± 9 mmHg), with the lowest level of any section being 118 mmHg. Although it is not possible to measure O2 levels at the section core with the Seahorse XF Analyzer, these high O2 levels should support O2 diffusion within the section core at physiological levels throughout the entire experimental procedure. This further supports the use of 1-mm sections because basal OCRs of 2- and 3-mm sections are much higher and may result in an increased chance of transient hypoxia within the section core.
Although it has been common to use isolated cell types in culture, such as neurons, astrocytes, and microglia, or isolated cellular compartments, such as synaptosomes, these preparations fail to account for the complex interplay that exists between these cell types in situ. Mitochondrial analysis of isolated cells from the brain does not consider critical neuron-glial metabolic coupling, such as the neuron-astrocyte lactate shuttle and the two-compartment model of cerebral acetate consumption, two examples of metabolic brain physiology that would not be present in isolated cell types (29, 40). Furthermore, the complex interplay between these cell types functionally affects one another in that mitochondrial dysfunction in astrocytes can have profound effects on neuronal stability (8, 54). It is possible, however, that the OCRs for each section may be mainly attributable to glial respiration because it is thought that a large glia-to-neuron ratio exists within the brain (16, 39). Our technique, therefore, may provide a powerful estimate of glial metabolic function while still considering its coupling to neurons, which could be a more appropriate analysis of the mitochondrial component of neurological disorders that are not solely the result of neuronal dysfunction.
This method significantly advances the capabilities for studying the bioenergetics of neuronal systems in a number of ways. The possibility to obtain data from 24 sections from the same animal greatly increases experimental sensitivity by reducing noise through the use of multiple sections of the same region of the brain in one animal. This was not previously possible with other methods that required the pooling of separate animals to obtain sufficient amounts of tissue for analysis. Our method also provides the possibility of studying multiple brain regions of the same animal in a single experiment or multiple animals on a single plate. Discrete brain nuclei can easily be isolated, allowing metabolic questions to be answered about specific regions involved in a neurological disorder. Furthermore, utilizing acute sections, as opposed to organotypic sections, provides a representation of the cellular environment without the complicating factor of long-term adaptation to a new cellular environment, which may occur when cultured for days to weeks.
This method will be ideal for analysis of discrete brain regions, such as the numerous nuclei within the brainstem that serve multiple and differential processes in a vast number of sensory input and motor output systems. Drugs, small molecules, or natural compounds that target mitochondria can also be easily screened for their effect on specific brain regions. Furthermore, our method provides a foundation for adaptation of this technique with other tissues, such as the liver, heart, or lung. We believe this method will greatly increase the options for the neuroscience community to study the impacts of mitochondrial dysfunction in neurological disorders expressed in adult animal models.
GRANTS
This study was funded by a grant from NIH/NINDS R01-NS061571 (M. Oshinsky) and NIAAA K05-AA017261 (N. Fried).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.T.F., C.M., E.L.S., and M.L.O. conception and design of research; N.T.F., C.M., and M.L.O. performed experiments; N.T.F. and M.L.O. analyzed data; N.T.F., C.M., E.L.S., and M.L.O. interpreted results of experiments; N.T.F. and M.L.O. prepared figures; N.T.F. and M.L.O. drafted manuscript; N.T.F., C.M., E.L.S., and M.L.O. edited and revised manuscript; N.T.F., C.M., E.L.S., and M.L.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Leyla Murphy, Marnie Cooper, Eric Kostuk, and Purnika Selvan for technical assistance. We thank Harumitsu Hirata and Jan Hoek for reviewing the manuscript and Dr. Robert Sterling for statistical expertise.
REFERENCES
- 1.Abend NS, Mani R, Tschuda TN, Chang T, Topjian AA, Donnelly M, LaFalce D, Krauss MC, Schmitt SE, Levine JM. EEG monitoring during therapeutic hypothermia in neonates, children, and adults. Am J Electroneurodiagnostic Technol 51: 141–164, 2011. [PMC free article] [PubMed] [Google Scholar]
- 2.Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci 34: 76–87, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: Perspective and implications to migraine pathophysiology. J Clin Neurol 8: 89–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blättler SM, Verdeguer F, Liesa M, Cunningham JT, Vogel RO, Chim H, Liu H, Romanino K, Shirihai OS, Vazquez F, Rüegg MA, Shi Y, Puigserver P. Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle. Mol Cell Biol 32: 3333–3346, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogousslavsky J, Meienberg O. EYe-movement disorders in brain-stem and cerebellar stroke. Arch Neurol 44: 141–148, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Bourne JN, Kirov SA, Sorra KE, Harris KM. Warmer preparation of hippocampal slices prevents synapse proliferation that might obscure LTP-related structural plasticity. Neuropharmacology 52: 55–59, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bresolin N, Martinelli P, Barbiroli B, Zaniol P, Ausenda C, Montagna P, Gallanti A, Comi GP, Scarlato G, Lugaresi E. Muscle mitochondrial DNA deletion and 31P-NMR spectroscopy alterations in a migraine patient. J Neurol Sci 104: 182–189, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: Prevention by mitochondrial-targeted antioxidants. J Neurosci 28: 4115–4122, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S, Liu D, Fairman D, Li P, Jenkins L, McGonigle P, Wood A. Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochem Int 45: 117–127, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Choi SW, Gerencser AA, Ng R, Flynn JM, Melov S, Danielson SR, Gibson BW, Nicholls DG, Bredesen DE, Brand MD. No consistent bioenergetic defects in presynaptic nerve terminals isolated from mouse models of Alzheimer's disease. J Neurosci 32: 16775–16784, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark LC, Jr., Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol 6: 189–193, 1953. [DOI] [PubMed] [Google Scholar]
- 12.Clerc P, Polster BM. Investigation of mitochondrial dysfunction by sequential microplate-based respiration measurements from intact and permeabilized neurons. PLoS One 7: e34465, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel Rasmussen L. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res 2012: e192503, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 128: 263–276, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson's disease: Molecular mechanisms and pathophysiological consequences. EMBO J 31: 3038–3062, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilgetag C, Barbas H. Are there ten times more glia than neurons in the brain?. Brain Struct Funct 213: 4–5, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Horner J, Buoyer FG, Alberts MJ, Helms MJ. Dysphagia following brain-stem stroke: Clinical correlates and outcome. Arch Neurol 48: 1170–1173, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Uusisaari MY. Physiological temperature during brain slicing enhances the quality of acute slice preparations. Front Cell Neurosci 7: 48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov A, Zilberter Y. Critical state of energy metabolism in brain slices: The principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics 3: 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. “Mild Uncoupling” does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem 101: 1619–1631, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience 127: 69–80, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci 19: 2876–2886, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolesnikova EÉ. Mitochondrial dysfunction and molecular bases of neurodegenerative diseases. Neurophysiology 45: 89–102, 2013. [Google Scholar]
- 24.Kunz WS, Bimpong-Buta NYB, Kudin AP, Elger CE. The role of mitochondria in epilepsy: Implications for neurodegenerative diseases. Toxicol Mech Methods 14: 19–23, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Liu KJ, Bacic G, Hoopes PJ, Jiang J, Du H, Ou LC, Dunn JF, Swartz HM. Assessment of cerebral Po2 by EPR oximetry in rodents: Effects of anesthesia, ischemia, and breathing gas. Brain Res 685: 91–98, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol 209: 2304–2311, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 286: 21865–21875, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion 5: 77–87, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: Evidence from modeling of measured lactate levels during visual stimulation. J Neurochem 109, Suppl 1: 55–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathis DM, Furman JL, Norris CM. Preparation of acute hippocampal slices from rats and transgenic mice for the study of synaptic alterations during aging and amyloid pathology. J Vis Exp 49: 2330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagna P, Sacquegna T, Martinelli P, Cortelli P, Bresolin N, Moggio M, Baldrati A, Riva R, Lugaresi E. Mitochondrial abnormalities in migraine. Preliminary findings. Headache 28: 477–480, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Müller M, Ballanyi K. Dynamic recording of cell death in the in vitro dorsal vagal nucleus of rats in response to metabolic arrest. J Neurophysiol 89: 551–561, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–1169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci 15: 7702–7711, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol 34: 1372–1381, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Araga S, Takeshima T, Nakashima K. Plasma lactic acid and pyruvic acid levels in migraine and tension-type headache. Headache 38: 39–42, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Opitz-Araya X, Barria A. Organotypic hippocampal slice cultures. J Vis Exp 48: 2462, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47: 1026–1036, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakkenberg B, Gundersen HJG. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc 150: 1–20, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Patel AB, de Graaf RA, Rothman DL, Behar KL, Mason GF. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab 30: 1200–1213, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates, 4th ed Waltham, MA: Academic, 1998. [Google Scholar]
- 42.Phillips W, Michell A, Pruess H, Barker RA. Animal models of neurodegenerative diseases. Methods Mol Biol 549: 137–155, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, Murphy AN. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One 6: e21746, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangiorgi S, Mochi M, Riva R, Cortelli P, Monari L, Pierangeli G, Montagna P. Abnormal platelet mitochondrial function in patients affected by migraine with and without aura. Cephalalgia Int J Headache 14: 21–23, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Schuh RA, Clerc P, Hwang H, Mehrabian Z, Bittman K, Chen H, Polster BM. Adaptation of microplate-based respirometry for hippocampal slices and analysis of respiratory capacity. J Neurosci Res 89: 1979–1988, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert EL, Caron AZ, Morin K, Coulombe J, He XH, Jardine K, Dewar-Darch D, Boekelheide K, Harper ME, McBurney MW. SirT1 catalytic activity is required for male fertility and metabolic homeostasis in mice. FASEB J 26: 555–566, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Skinhoj E. Hemodynamic studies within the brain during migraine. Arch Neurol 29: 95–98, 1973. [DOI] [PubMed] [Google Scholar]
- 48.Sparaco M, Feleppa M, Lipton RB, Rapoport AM, Bigal ME. Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia Int J Headache 26: 361–372, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Stackley KD, Beeson CC, Rahn JJ, Chan SSL. Bioenergetic profiling of zebrafish embryonic development. PLoS One 6: e25652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuart S, Griffiths LR. A possible role for mitochondrial dysfunction in migraine. Mol Genet Genomics 287: 837–844, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Su T, Paradiso B, Long YS, Liao WP, Simonato M. Evaluation of cell damage in organotypic hippocampal slice culture from adult mouse: A potential model system to study neuroprotection. Brain Res 1385: 68–76, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uncini A, Lodi R, Di Muzio A, Silvestri G, Servidei S, Lugaresi A, Iotti S, Zaniol P, Barbiroli B. Abnormal brain and muscle energy metabolism shown by 31P-MRS in familial hemiplegic migraine. J Neurol Sci 129: 214–222, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: Implications for neuroprotection. J Neurochem 102: 1383–1394, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch KM, Levine SR, D'Andrea G, Schultz LR, Helpern JA. Preliminary observations on brain energy metabolism in migraine studied by in vivo phosphorus 31 NMR spectroscopy. Neurology 39: 538–541, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Wikstrom JD, Sereda SB, Stiles L, Elorza A, Allister EM, Neilson A, Ferrick DA, Wheeler MB, Shirihai OS. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS One 7: e33023, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van der Worp HB, de Haan P, Morrema E, Kalkman CJ. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol 252: 1108–1114, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci 14: 128–142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27: 7310–7317, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 106: 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Nuebel E, Wisidagama DRR, Setoguchi K, Hong JS, Van Horn CM, Imam SS, Vergnes L, Malone CS, Koehler CM, Teitell MA. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc 7: 1068–1085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]