Abstract
Leukotriene D4 (LTD4) is an important immune inflammatory mediator that is known to be elevated in the mucosa of chronically inflamed intestine and alter nutrient absorption. LTD4 inhibits Na-alanine cotransport in intestinal epithelial cells by decreasing the affinity of the cotransporter ASCT1. LTD4 is known to increase intracellular Ca++ and cAMP concentrations. However, the intracellular signaling mechanism of LTD4-mediated ASCT1 inhibition is unknown. In the present study, pretreatment with calcium chelator BAPTA-AM or inhibition of Ca++-dependent protein kinase C (PKC), specifically PKCα, resulted in the reversal of LTD4-mediated inhibition of ASCT1, revealing the involvement of the Ca++-activated PKC pathway. PKCα is known to phosphorylate Raf kinase inhibitor protein (RKIP), thus activating its downstream signaling pathway. Immunoblotting with anti-RKIP-Ser153 antibody showed an increase in phosphorylation levels of RKIP in LTD4-treated cells. Downregulation of endogenous RKIP showed no decrease in ASCT1 activity by LTD4, thus confirming its involvement in ASCT1 regulation. Phosphorylation of RKIP by PKC is known to activate different signaling pathways, and in this study it was found to activate cAMP-activated protein kinase A (PKA) pathway. Although protein abundance of ASCT1 was not altered in any of the experimental conditions, there was an increase in the levels of phosphothreonine in ASCT1 protein, thus showing that phosphorylation changes were responsible for the altered affinity of ASCT1 by LTD4. In conclusion, LTD4 inhibits ASCT1 through PKC-mediated phosphorylation of RKIP, leading to the subsequent activation of PKA pathway, possibly through β2-andrenergic receptor activation.
Keywords: Na-alanine cotransport; alanine, serine, and cysteine transporter 1; leukotriene D4; Raf kinase inhibitor protein; protein kinase C; chronic intestinal inflammation
leukotriene d4 (LTD4), an endogenously produced immune inflammatory mediator, is known to be elevated in the mucosa of the chronically inflamed intestine, altering a variety of cellular processes that eventually intensify and promote the inflammation process (20, 22, 27, 35, 38). One such essential cellular process that is affected during intestinal inflammation is the malabsorption of nutrients such as amino acids (e.g., alanine, glutamine).
Amino acids, the building blocks of protein synthesis, are efficiently absorbed from the intestinal luminal content through multiple Na-dependent cotransporter systems present in the brush border membrane (BBM) of enterocytes (4). Of these, Na-dependent neutral amino acid transporter systems such as alanine and glutamine cotransporters have been found to be significantly affected during chronic intestinal inflammation, leading to the malabsorption of these essential nutrients (29, 33). System ATB0 (SLC6A14) has been shown to be the predominant alanine cotransporter in the BBM of villus cells in a rabbit model of chronic intestinal inflammation, which has many features of human inflammatory bowel disease. In this model, Na-alanine cotransport is inhibited in the BBM of the villus cells, secondary to a decrease in the affinity of the cotransporter ATB0 for amino acid alanine, without a change in the number of BBM cotransporters. Furthermore, treatment with a broad spectrum immune modulator such as methylprednisolone reversed the inhibition of ATB0 activity by restoring its affinity. This indicated the involvement of immune inflammatory mediators in the regulation of this cotransporter during inflammation (33).
However, which of the wide variety of immune inflammatory mediators might be responsible for altering Na-alanine cotransporters during inflammation was not known till recently. LTD4, known to be elevated in the chronically inflamed intestine, was shown to inhibit Na-dependent alanine cotransport in an in vitro model of rat small intestine-derived epithelial cell line (IEC-18). In these cells, system alanine, serine, and cysteine transporter 1 (ASCT1; SLC1A4) is the most predominant Na-alanine cotransporter, and it was shown that LTD4 regulates BBM ASCT1 similarly to that seen in the rabbit model of chronic intestinal inflammation. LTD4 inhibited ASCT1 activity by decreasing its affinity for alanine without a change in the maximal rate of alanine uptake (35).
Previously published reports have shown that LTD4 increases intracellular Ca++ and cAMP levels (3, 6, 10, 12, 36). However, the intracellular signaling pathway and molecular mechanisms that are involved in the LTD4-mediated regulation of ASCT1 are unknown. Therefore, the aim of the present study was to investigate the intracellular pathways and molecular mechanisms that are responsible for the altered affinity of Na-alanine cotransporter ASCT1 by LTD4 in IEC-18 cells.
METHODS
Tissue culture and treatment.
Rat intestinal epithelial cells (IEC-18; American Type Culture Collection CRL-1589) were grown in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY) supplemented with 0.2 U/ml of insulin, 0.5 mM β-hydroxybutyrate (Sigma Chemical, St. Louis, MO), and 10% fetal calf serum (Atlanta Biologicals) and incubated at 37°C with 10% CO2. Cells grown to 10 days postconfluence on permeable membrane supports were used for all of the uptake experiments and molecular studies. Only cells between passages 5 and 25 were used for the experiments. They were pretreated for 1 h with each of the following specific inhibitors or antagonists to delineate the signaling pathway involved in the LTD4-mediated inhibition of ASCT1 activity: 1 μM calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM; Santa Cruz Biotechnology), 200 nM PKC inhibitor calphostin C (Calbiochem), 1 μM PKCα inhibitor Go-6976, 500 nM Raf-1 kinase inhibitor I (Calbiochem), and 50 μM PKA inhibitor Rp-cAMPS (Santa Cruz Biotechnology).
Na-alanine cotransport in IEC-18 cells.
[3H]alanine uptake experiments were performed at room temperature in IEC-18 cells, grown to 10 days postconfluence on permeable support (polyester membrane thickness 10 μm, pore size 0.4 μm). Briefly, cells were first washed and incubated for 10 min in a Na-free TMA buffer (130 mM trimethyl ammonium chloride, 4.7 mM KCl, 1.2 mM KH2PO4, 1 mM MgSO4, 1.25 mM CaCl2, and 20 mM HEPES). Uptakes were then performed at 2-min time intervals in reaction medium containing either 130 mM NaCl or 130 mM trimethyl ammonium chloride in HEPES medium with 10 μCi of [3H]alanine and 200 μM cold substrate. The reaction was stopped with ice cold Na-free medium. The cells were then digested with 1 N NaOH, and the digested cells from each reaction were placed in separate scintillation vials; 4 ml of scintillation fluid (Ecoscint A; National Diagnostics) was added. Radioactivity was determined in a Beckman Coulter scintillation counter (LS 6500).
RNA interference.
Small-interfering RNA (siRNA) against phosphatidylethanolamine-binding protein 1 alias Raf-1 kinase inhibitor protein (RKIP; ID s131669) and silencer predesigned negative control (catalog no. 4611), both from Ambion, were used for siRNA transfections. The siRNAs (1.5 μg of each) were suspended in nucleofector solution (pH 7.4, 7.1 mM ATP, 11.6 mM MgCl2.6H2O, 13.6 mM NaHCO3, 84 mM KH2PO4, and 2.1 mM glucose) and were individually nucleofected into IEC-18 cells using a Nucleofector II device (Amaxa) according to the manufacturer's protocol. The transfected cells grown to 7 days on permeable supports were used for transport studies and Western blotting analyses.
Metabolic labeling.
IEC-18 cell monolayers were incubated in phosphate-free DMEM for 1 h at 37°C; 1 mCi/ml carrier-free [32P]orthophosphate was then added to them and incubated further for 1 h to equilibrate the intracellular ATP pools with labeled phosphate. The adherent cells were then lysed by the addition of 400 μl/well ice-cold modified radioimmunoprecipitation (RIPA) buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 1% sodium deoxycholate, pH 7.4) containing protease (1 μM pepstatin A, 250 μM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml aprotinin) and phosphatase inhibitors (10 mM sodium fluoride, 50 mM sodium pyrophosphate, and 1 μM okadaic acid) and incubating for 1 h at 4°C with agitation. RIPA extracts were centrifuged at 20,000 g for 30 min at 4°C, and the supernatant was used for immunoprecipitation.
Preparation of BBM.
BBM was prepared by CaCl2 precipitation and differential centrifugation, as described previously (32). BBM protein was extracted from BBM preparation by suspending it in RIPA buffer, followed by incubation at 4°C for ≥1 h and centrifugation at 20,000 g for 30 min. The supernatant from this preparation was used for immunoprecipitation and Western blot analyses.
Immunoprecipitation.
IEC-18 protein extracts were precleared by the addition of 3 mg of protein A agarose beads for 1 h at 4°C. The ASCT1 protein was immunoprecipitated overnight at 4°C by the addition of ASCT1 antibody (raised in chicken and obtained through the custom antibody services provided by Invitrogen), followed by 1-h incubation with 3 mg of protein A agarose beads at 22°C. The immune adsorbents were washed three times with ice-cold RIPA buffer prior to the addition of 50 μl of protein sample buffer (62.5 mM Tris·HCl, pH 6.8, 20% glycerol, 2% SDS, and 5% β-mercaptoethanol, and 0.01% bromophenol blue) and incubated for 30 min at 22°C. The immunoprecipitated protein was then resolved by native gel electrophoresis, and radiolabeling was detected by autoradiography. The relative amounts of 32P incorporated into the protein were estimated with FluorChem SP (Alpha Innotech).
Protein determination.
Total protein for all of the samples in this study was measured by the using the Bio-Rad (Hercules, CA) protein assay kit with bovine serum albumin as standard.
Western blot.
Briefly, equal measurements of quantitated BBM protein preparations from different experimental conditions were individually mixed with sample buffer (100 mM Tris, 25% glycerol, 2% SDS, 0.01% bromophenol blue, and 10% β-mercaptoethanol, pH 6.8) and separated on an 8% polyacrylamide gel. The separated proteins were transferred to PVDF membranes (Immobilon-pSQ; Millipore) and probed with an ASCT1-specific antibody. Rabbit phosphorylated antibodies (phospho-serine, phospho-threonine) obtained from Abcam were used as primary antibodies to determine the phosphorylation levels of immunoprecipitated ASCT1 protein from control and LTD4-treated IEC-18 cells. RKIP (Cell Signaling Technology) and p-RKIP (rSer 153; Santa Cruz Biotechnology) antibodies were used to detect the RKIP protein levels from control and LTD4-treated cells. Incubation with specific secondary antibodies conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratories, West Grove, PA), followed by incubation with ECL Western Blotting Detection Reagent (GE Healthcare Bio-Sciences Corp, Piscataway, NJ), was done to detect the immobilized protein/antibody complex of interest. The resulting chemiluminescence was measured by autoradiography. The specific protein abundance was quantitated using a densitometric scanner (FlourChem SP; Alpha Innotech).
Statistical analysis.
All of the results are represented as means ± SE of experiments performed in triplicates. All the statistical analyses were done with the GraphPad InStat 4 (San Diego, CA) program as unpaired Student's t-test. A P value of <0.05 was considered significant.
RESULTS
Effect of Ca++ chelation on ASCT1 activity in LTD4-treated IEC-18 cells.
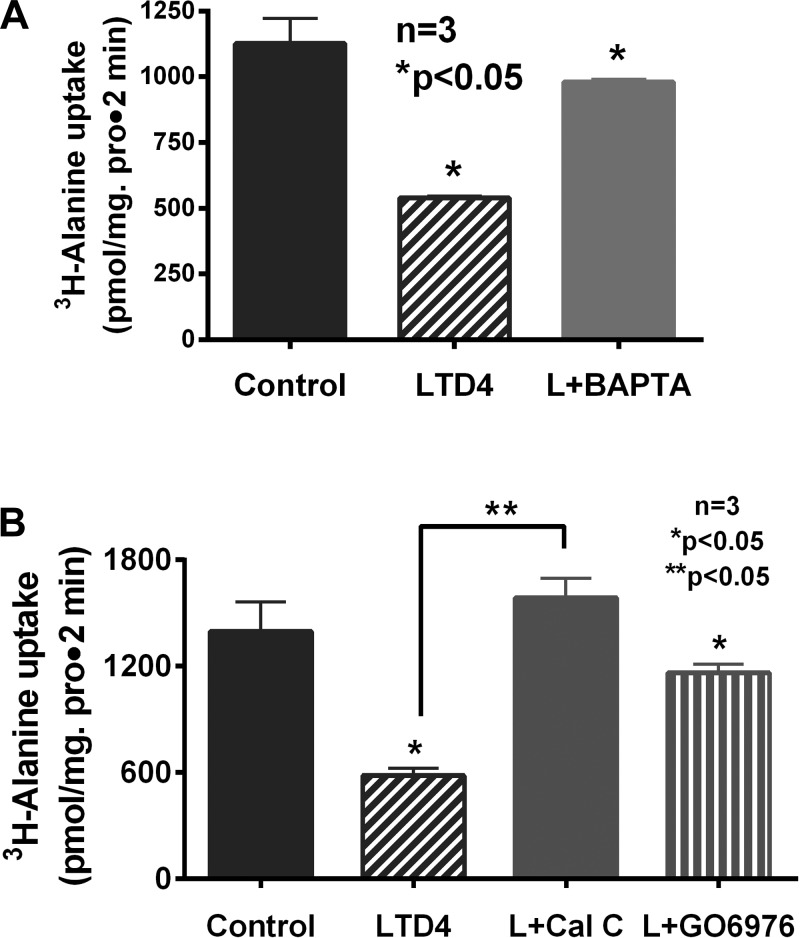
Pretreatment of IEC-18 cells with BAPTA-AM prevented the inhibition of LTD4-mediated inhibition of Na-alanine cotransport in IEC-18 cells (1,126 ± 96 pmol/mg protein for 2 min in control, 540 ± 6 in LTD4, 980 ± 11 in BAPTA-AM + LTD4, n = 3; Fig. 1A). These data indicated that an increase in intracellular Ca++ is responsible for LTD4-mediated inhibition of ASCT1 activity.
Fig. 1.
A: Ca++ chelation with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) prevented leukotriene D4 (LTD4)-mediated inhibition of Na-alanine cotransport in IEC-18 cells. B: inhibition of PKC pathway with calphostin C and specifically of PKCα with Go-6976 prevented the inhibition of Na-alanine cotransport by LTD4. These data indicated that the increase in intracellular Ca++ seen in LTD4-treated IEC-18 cells activates PKCα and its downstream signaling pathway.
Effect of PKC pathway inhibition on ASCT1 activity in LTD4-treated IEC-18 cells.
Calcium is known to conventionally activate PKC signal transduction pathway. To see whether this would be the case in the current study, the cells were pretreated with calphostin C before LTD4 and cellular uptake for [3H]alanine was performed. Pretreatment with calphostin C abolished LTD4-mediated inhibition of Na-alanine cotransport (1,396 ± 168 pmol/mg protein for 2 min in control, 582 ± 42 in LTD4, and 1,587 ± 109 in calphostin C + LTD4, n = 3; Fig. 1B). Conventional PKCs requiring Ca++ for their activation include the isoforms α, βI, βII, and γ. Preliminary studies with specific PKC inhibitors for these isoforms showed that pretreatment with inhibitors for β and γ did not have any effect on LTD4-mediated inhibition of Na-alanine cotransport (data not shown). But pretreatment with PKCα inhibitor Go-6976 abolished the inhibition of LTD4-mediated ASCT1 activity (1,164 ± 48 pmol/mg protein for 2 min; n = 3, Fig. 1B) compared with control. These data indicated that this specific isozyme of PKC family was responsible for mediating the inhibition of Na-alanine cotransport by LTD4 in intestinal epithelial cells.
Effect of RKIP silencing on ASCT1 activity in LTD4-treated IEC-18 cells.
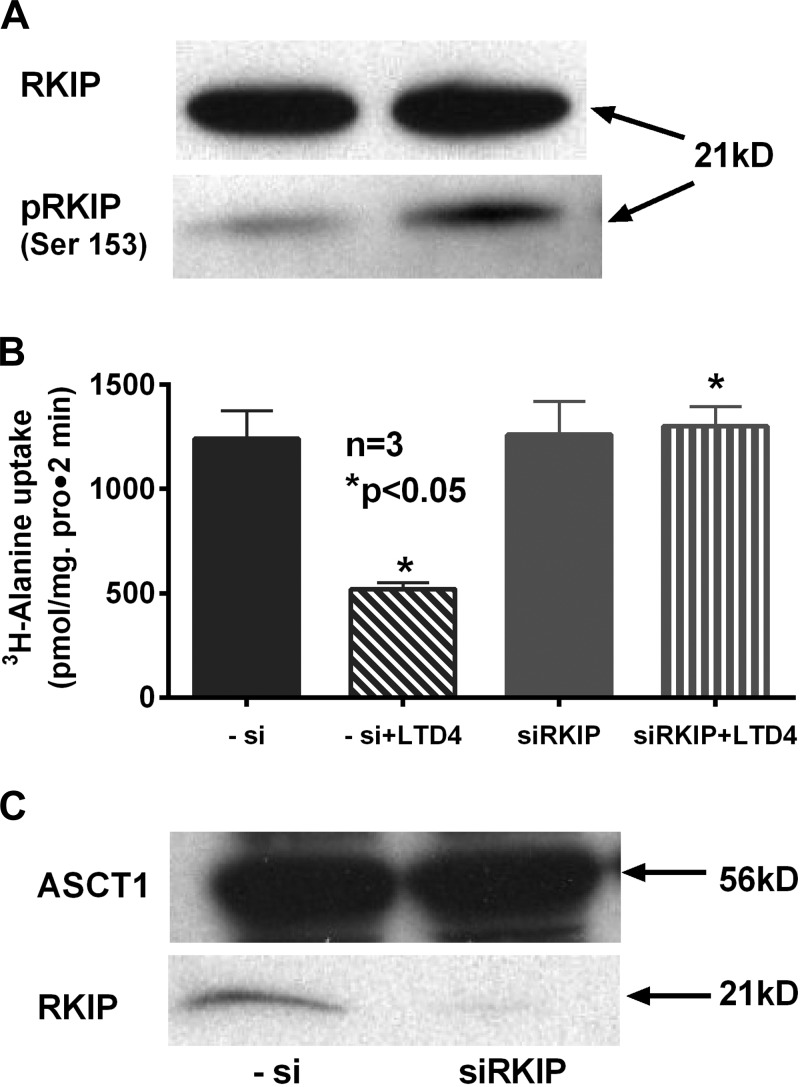
PKCα is known to phosphorylate and activate RKIP, a kinase modulator that is known to coordinate diverse signaling functions (8, 18). To determine the role of RKIP, the following experiments were conducted. Western blot analysis of RKIP protein (21 kDa) from the cell lysate of control and LTD4-treated IEC-18 cells showed that there was an increase in phosphorylated RKIP protein in LTD4-treated cells compared with control, whereas the level of native RKIP remained the same (Fig. 2A). Furthermore, to see whether RKIP might be involved in LTD4-mediated inhibition of Na-alanine cotransport, [3H]alanine uptake was performed in LTD4-treated cells that were transfected with siRNA to silence RKIP expression. In RKIP-silenced cells, LTD4 did not affect Na-alanine cotransport (1,242 ± 133 pmol/mg protein for 2 min in -ve si, 521 ± 30 in -ve si + LTD4 treatment, 1,260 ± 160 in siRKIP, 1,302 ± 94 in siRKIP + LTD4 n = 3; Fig. 2B). These data clearly established RKIP's role in LTD4-mediated inhibition of ASCT1.
Fig. 2.
Characterization of Raf kinase inhibitor protein (RKIP)-mediated regulation of Na-alanine cotransport in LTD4-treated IEC-18 cells. Representative blots of experiments done in triplicate are shown. A: immunoblotting with anti-RKIP-Ser153 antibody showed an increase in the phosphorylation levels of RKIP protein (pRKIP) in LTD4-treated cell extracts compared with control. B: downregulation of endogenous RKIP by expression of antisense siRNA followed by treatment with LTD4 showed no decrease in alanine, serine, and cysteine transporter 1 (ASCT1) activity compared with control. C: inhibition of endogenous RKIP expression did not alter the expression of brush border membrane (BBM) ASCT1 expression in IEC-18 cells. These data indicate that LTD4 induces phosphorylation of RKIP at Ser153 possibly by PKCα, thus activating its downstream signaling pathway, which regulates LTD4-mediated inhibition of ASCT1 in intestinal epithelial cells.
Effect of Raf-1 kinase inhibition on ASCT1 activity in LTD4-treated IEC-18 cells.
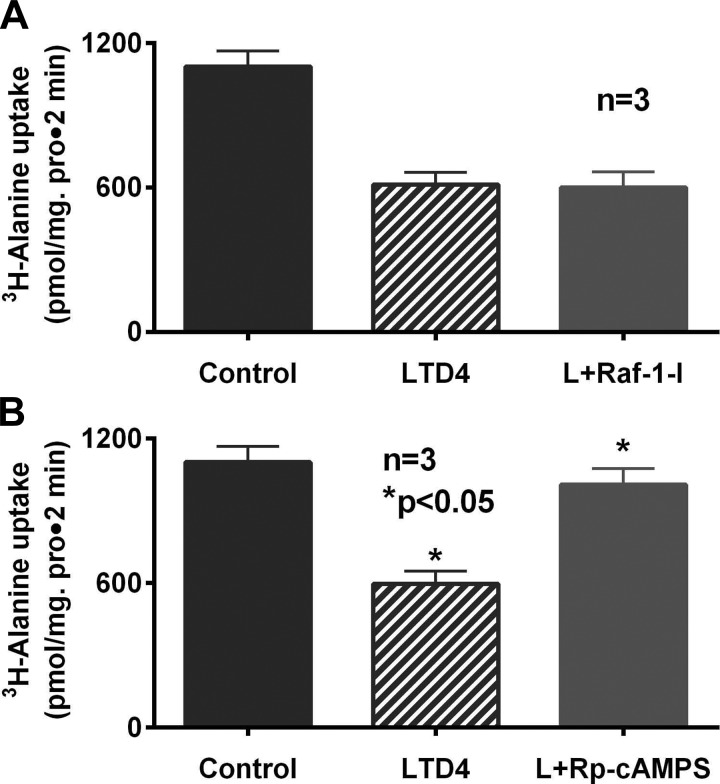
Phosphorylation of RKIP leads to its disassociation from Raf-1 kinase protein, thus activating Raf-1 kinase and its downstream signaling pathway, MAPK/ERK kinase (MEK1) (18). To determine whether Raf-1 kinase protein may mediate ASCT1 inhibition, 500 nM Raf-1 kinase inhibitor I (Raf-1-I) was used to pretreat the cells, and LTD4-mediated Na-alanine cotransport inhibition was studied. Pretreatment with Raf-1-I did not affect the inhibition of Na-alanine cotransport activity in LTD4-treated cells (1,112 ± 58 pmol/mg protein for 2 min in control, 612 ± 48 in LTD4 treatment, 602 ± 69 in Raf-1-I + LTD4, n = 3; Fig. 3A). Thus, Raf-1 kinase does not appear to have a role in the LTD4-mediated inhibition of ASCT1.
Fig. 3.
A: pretreatment of LTD4-treated cells with Raf-1 kinase inhibitor (Raf-1-I) did not affect LTD4-mediated inhibition of Na-alanine cotransport in IEC-18 cells. B: inhibition of PKA pathway with Rp-cAMPS prevented LTD4-mediated inhibition of ASCT1 activity in IEC-18 cells.
Effect of PKA pathway inhibition on ASCT1 activity in LTD4-treated IEC-18 cells.
RKIP phosphorylation, which mediates the inhibition of G protein-coupled receptor kinase, is known to increase cAMP. In fact, we have also observed an increase in intracellular cAMP levels in LTD4-treated cells (data not shown). Given this background, we determined whether the cAMP-PKA (protein kinase A) pathway may be activated downstream to RKIP phosphorylation. Thus, LTD4-treated IEC-18 cells were pretreated with Rp-cAMPS to inhibit PKA pathway activation, and [3H]alanine uptake was performed. As shown in Fig. 3B, inhibition of PKA pathway prevented LTD4-mediated inhibition of Na-alanine cotransport (1,102 ± 65 pmol/mg protein for 2 min in control, 598 ± 52 in LTD4 treatment, 1,009 ± 66 in Rp-cAMPS+LTD4, n = 3; Fig. 3B). This indicated that PKA pathway indeed plays a role in the regulation of ASCT1 activity in LTD4-treated IEC-18 cells.
Protein phosphorylation analyses.
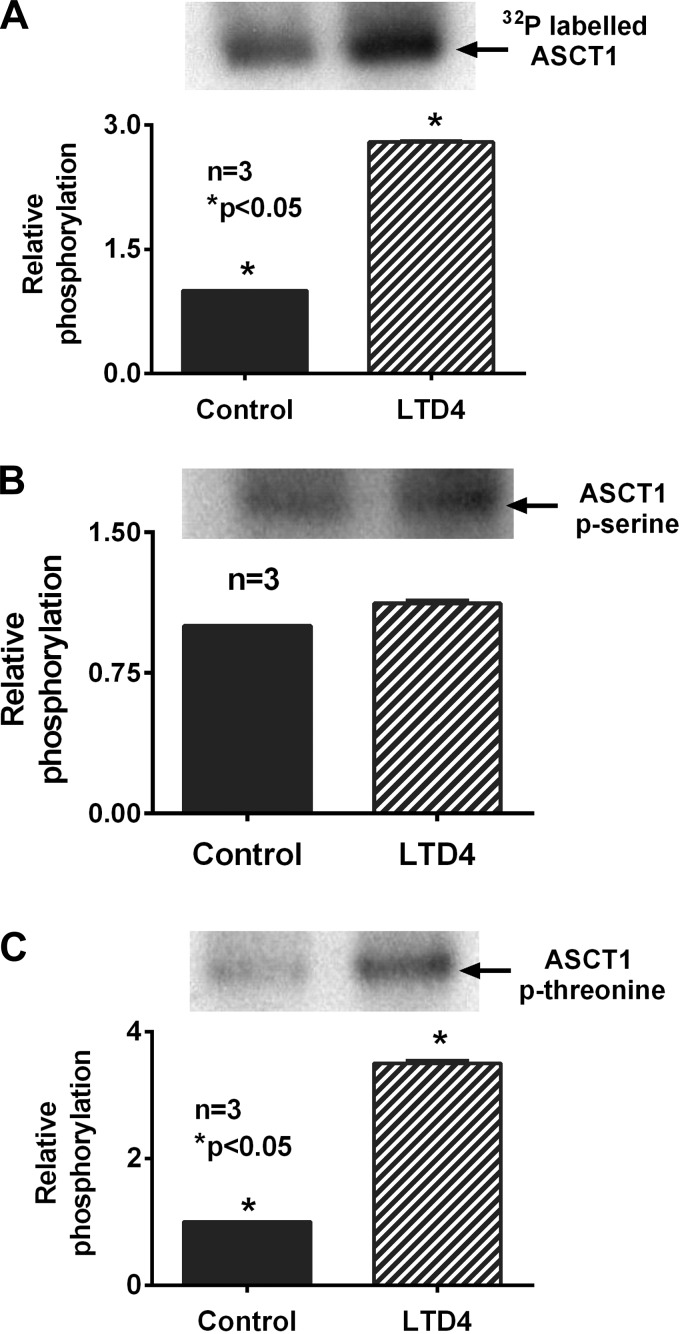
To examine whether LTD4 treatment would bring alterations in the phosphorylation levels of ASCT1 protein, thus affecting its affinity for its substrate alanine, a series of immunoprecipitation and Western blot experiments were performed. As shown in the representative blot and densitometric analysis in Fig. 4A, metabolic labeling of IEC-18 cells followed by LTD4 treatment produced a more than threefold increase in 32P incorporation in ASCT1 protein compared with control. Serine and threonine amino acid residues are the common targets of phosphorylation by PKC. Rat ASCT1 protein has 12 serine and seven threonine residues as possible targets of phosphorylation (NetPhos 2.0 prediction, Technical University of Denmark). To determine which residues might be the targets of phosphorylation changes mediated by LTD4 downstream pathways, ASCT1 protein from both control and LTD4-treated cells were immunoprecipitated from the BBM protein extract and probed with phosphorylated antibodies. As shown in the representative blots and densitometric analyses of Fig. 4, B and C, LTD4 treatment increased BBM ASCT1 phosphothreonine levels (>3-fold) with no significant changes in phosphoserine levels. These data confirmed that LTD4 mediates ASCT1 inhibition through PKC-mediated increase in its phosphorylation levels specifically via threonine sites of the protein.
Fig. 4.
Western blot characterization of LTD4-mediated increase in phosphorylation levels of ASCT1 protein. Representative blots of experiments done in triplicate are shown. A: LTD4 treatment showed a 3-fold increase in the level of carrier free [32P]orthophosphate incorporation in ASCT1 protein compared with control. B: the levels of phosphorylated serine residues in ASCT1 protein remained unchanged in the BBM of both control and LTD4-treated IEC-18 cells. C: phosphothreonine levels in BBM ASCT1 protein from LTD4-treated cells were significantly increased (>3-fold) compared with control ASCT1.
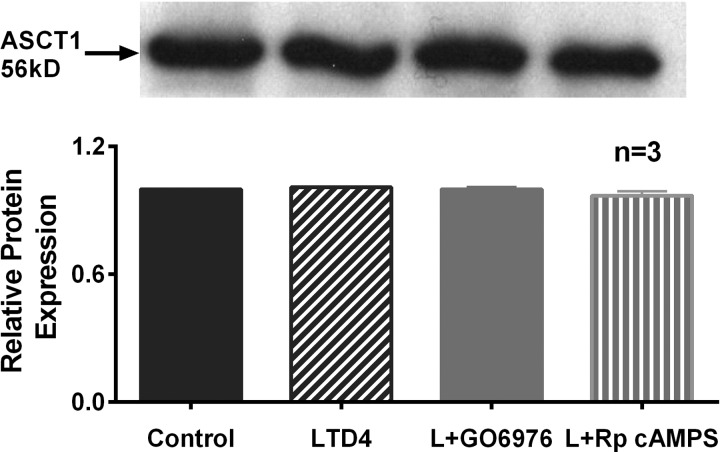
Western blot analyses.
Figure 5 shows a representative blot and densitometric analysis of BBM ASCT1 protein (56 kDa) expression in different experimental conditions. ASCT1 protein levels from BBM protein extract remained unchanged between control, LTD4, Go-6976, and Rp-cAMPS pretreated IEC-18 cells. These data show that the reversal of LTD4-mediated ASCT1 regulation by PKCα or PKA pathway inhibition is secondary to altered affinity of the cotransporter for alanine without a change in BBM cotransporter numbers.
Fig. 5.
Western blot characterization of ASCT1 protein expression in BBM of IEC-18 cells. Top: a representative blot of BBM ASCT1 protein expression is shown. Bottom: densitometric analysis showed that ASCT1 protein levels remained unchanged in all of the treatments used to determine either PKCα or PKA-mediated reversal of ASCT1 inhibition by LTD4.
DISCUSSION
In the chronically inflamed intestine, arachidonic acid metabolites produced by the cyclooxygenase (prostaglandins) and lipoxygenase (leukotrienes) pathways are known to be elevated. Leukotrienes (LTB4, LTC4, and LTD4) have been implicated in the pathogenesis of a variety of inflammatory diseases, including chronic intestinal inflammatory conditions such as inflammatory bowel disease (11, 30). LTD4 and LTB4 have also been shown to induce cell survival mechanisms in intestinal epithelial cells, implicating their possible participation in carcinogenesis (22, 24). Furthermore, LTD4 is known to activate a variety of signaling pathways, altering the activities of a wide range of target proteins in intestinal epithelial cells. (19, 28). Finally, LTD4 has been shown to alter the BBM Na-alanine cotransport in the intestinal epithelial cells secondary to altering the affinity of the cotransporter for alanine, with no change in its Vmax or maximal rate of uptake (35). This mechanism of alteration of Na-alanine cotransport was also observed in the chronically inflamed rabbit small intestine. With this background, the goal of the present investigation was to determine the underlying molecular mechanisms and intracellular signaling pathways that are involved in the LTD4-mediated regulation of the Na-alanine cotransporter ASCT1 in intestinal epithelial cells. This study demonstrates that the alteration in affinity of ASCT1 by LTD4 is due to changes in its phosphorylation levels mediated through the PKCα and PKA pathways, with RKIP as the intermediate regulator protein.
LTD4 has been demonstrated to increase intracellular Ca++ and cAMP levels, which in turn may activate dependent kinases and downstream signaling pathways (3, 6, 10, 12, 36). In the present study, the PKCα pathway was activated by LTD4. When PKCα was inhibited, LTD4-mediated inhibition of Na-alanine cotransporter activity was abolished, thus indicating an essential involvement of PKCα in the inhibitory effect of LTD4 on Na-alanine cotransport by ASCT1. There is considerable literature to indicate that PKC isoforms are significant regulators of intestinal transport processes. Depending on the cellular activator molecule, they have been shown to either up- or downregulate the activity of the intestinal transporters (13, 21, 25, 26, 37, 39). This study has shown for the first time that PKCα can regulate Na-alanine cotransporter ASCT1 in intestinal epithelial cells. The altered affinity of ASCT1 may be secondary to direct phosphorylation of the cotransporter or stimulation of a downstream signaling pathway that eventually alters the phosphorylation level, resulting in the cotransporter affinity change. Like PKC, cAMP-mediated PKA has also been shown to be an important regulator of intestinal transporters (2, 34). In the present study, inhibition of PKA pathway prevented LTD4-mediated inhibition of ASCT1 activity, thus also establishing its role as a regulator of Na-alanine cotransport in intestinal epithelial cells. Since both kinases PKC and PKA seem to regulate ASCT1 activity, it becomes important to understand whether they are activated in a sequential fashion and whether there may be a cellular intermediate involved in the activation of these kinases.
A multifaceted kinase modulator that has been increasingly recognized in modulating signal transduction recently is RKIP, also known as phoshaptidylethanolamine binding protein. This 21-kDa protein has been shown to modulate Raf/MEK/ERK, NF-κB, glycogen synthase kinase-3β, and GPCR-mediated signaling cascades affecting various cellular processes, such as cell differentiation, cell cycle, apoptosis, and cell migration. (1, 14, 17, 18, 40, 41). Emerging evidence suggests that RKIP is implicated in several human diseases or disorders, among them metastatic tumorigenesis and Alzheimer's disease (9, 17, 43). Overexpression of RKIP has also been shown to have a protective role against angiogenesis and metastatic capabilities of invasive tumors such as those in prostate, breast, and gastric cancer. Finally, RKIP has been suggested to be a candidate for a prognostic marker in prostate and colorectal cancers (16, 42, 44).
PKC phosphorylation of RKIP at Ser153 (7, 15, 18) is known coordinate many of its diverse cellular functions (8, 18). In the present study, RKIP was found to be phosphorylated at Ser153 in IEC-18 cells treated with LTD4. Furthermore, inhibition of endogenous RKIP production prevented LTD4-mediated inhibition of Na-alanine cotransport. This provides strong evidence of RKIP as the mediator of LTD4 inhibition of ASCT1. Phosphorylated RKIP can induce sensitization of GPCR signaling stimulated by different G-coupled receptors, such as β2-andrenergic receptor, that eventually activate the sequence of PKA signaling pathway (18). In the present study, inhibition of the PKA pathway at the level of kinase itself abolished LTD4-mediated inhibition of ASCT1 activity. This indicates this pathway's involvement in LTD4-mediated inhibition of ASCT1 activity downstream to RKIP phosphorylation.
Though the inhibition of Na-alanine cotransport activity was abolished or prevented when intracellular pathways were affected, the expression of ASCT1 in the BBM was unaffected in all conditions. This further confirmed that the level of regulation of ASCT1 by LTD4 was at its affinity for alanine. Phosphorylation changes by kinases are the most common posttranslational phenomena responsible for the altered affinity of a number of target proteins, including intestinal cotransporters (5, 23, 31). In this study, metabolic labeling, IP, and Western blot analysis of BBM ASCT1 protein showed an increase in its phosphorylation levels in LTD4-treated cells. More specifically, an increase in phosphothreonine levels appears to be responsible for altered affinity of ASCT1 in LTD4-treated IEC-18 cells.
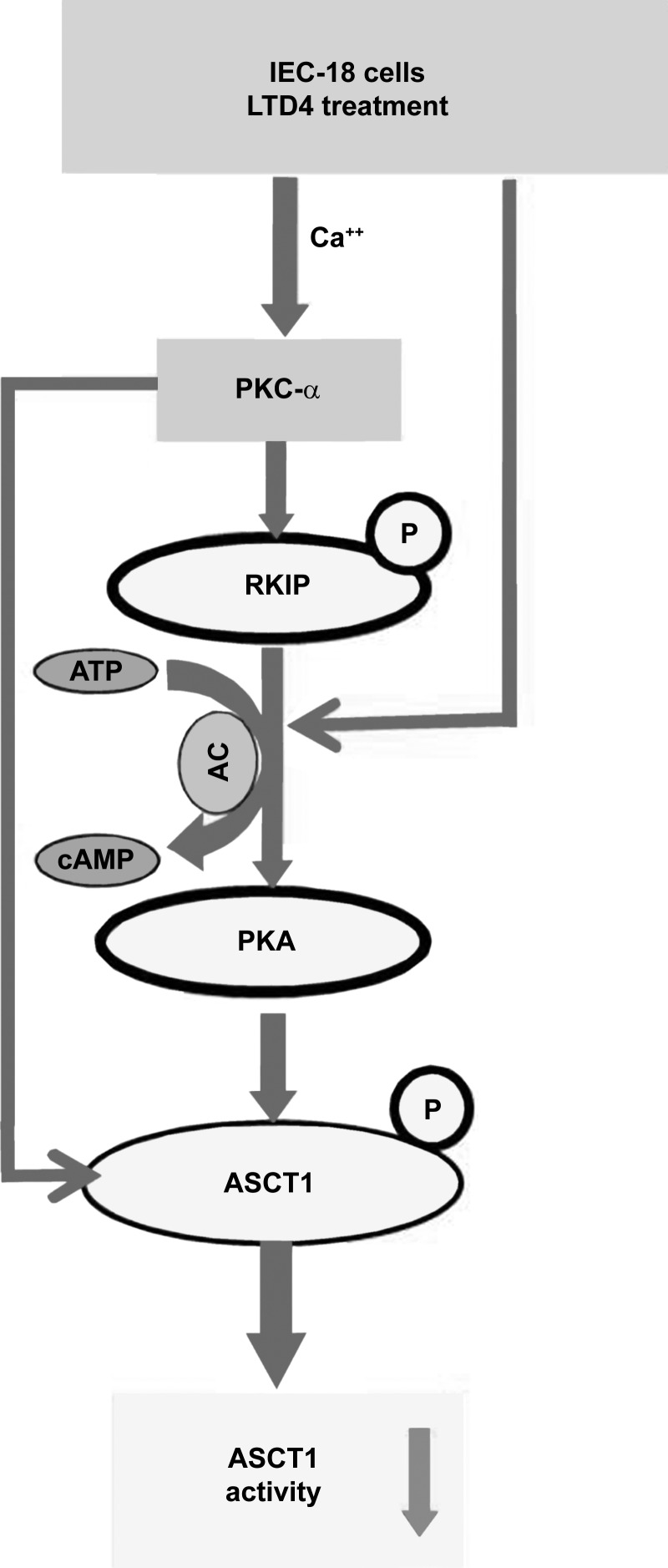
In summary, the proposed signaling pathways that are responsible for LTD4-mediated regulation of ASCT1 activity are shown in Fig. 6. In the LTD4-mediated inhibition of ASCT1 in intestinal epithelial cells, RKIP appears to be a significant intermediate regulator molecule in the signaling pathway. The final mechanism of inhibition, diminished affinity, of Na-alanine cotransport is identical whether in the chronically inflamed rabbit small intestine, where LTD4 is known to be elevated or by LTD4 in vitro in IEC-18 cells. In conclusion, the results suggest that RKIP could be important in regulating Na-dependent amino acid absorption during chronic intestinal inflammation. Thus, with more comprehensive future studies, RKIP may be a potential therapeutic target for the immune-based treatment of nutritional deficiencies of conditions characterized by chronic intestinal inflammation.
Fig. 6.
LTD4-mediated regulation of Na-alanine cotransport in intestinal epithelial cells. LTD4 regulates ASCT1 activity through 1) PKCα-mediated phosphorylation (P) of ASCT1, 2) cAMP/PKA-mediated phosphorylation of ASCT1, and 3) PKCα mediated phosphorylation of RKIP, leading to the activation of the PKA pathway with eventual regulation of ASCT1.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Research Grants DK-45062 and DK-58034 to U. Sundaram.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A. performed experiments; S.A. analyzed data; S.A. prepared figures; S.A. drafted manuscript; U.S. conception and design of research; U.S. interpreted results of experiments; U.S. edited and revised manuscript; U.S. approved final version of manuscript.
REFERENCES
- 1.Al-Mulla F, Bitar MS, Al-Maghrebi M, Behbehani AI, Al-Ali W, Rath O, Doyle B, Tan KY, Pitt A, Kolch W. Raf kinase inhibitor protein RKIP enhances signaling by glycogen synthase kinase-3beta. Cancer Res 71: 1334–1343, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1). J Cell Physiol 204: 604–613, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bouchelouche PN, Ahnfelt-Rønne I, Thomsen MK. LTD4 increases cytosolic free calcium and inositol phosphates in human neutrophils: inhibition by the novel LTD4 receptor antagonist, SR2640, and possible relation to modulation of chemotaxis. Agents Actions 29: 299–307, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda-Sceppa C, Subramanian S, Castaneda F. Protein kinase C mediated intracellular signaling pathways are involved in the regulation of sodium-dependent glucose co-transporter SGLT1 activity. J Cell Biochem 109: 1109–1117, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chan CC, Ecclestone P, Nicholson DW, Metters KM, Pon DJ, Rodger IW. Leukotriene D4-induced increases in cytosolic calcium in THP-1 cells: dependence on extracellular calcium and inhibition with selective leukotriene D4 receptor antagonists. J Pharmacol Exp Ther 269: 891–896, 1994. [PubMed] [Google Scholar]
- 7.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem 278: 13061–13068, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Deiss K, Kisker C, Lohse MJ, Lorenz K. Raf kinase inhibitor protein (RKIP) dimer formation controls its target switch from Raf1 to G protein-coupled receptor kinase (GRK) 2. J Biol Chem 287: 23407–23417, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George AJ, Holsinger RM, McLean CA, Tan SS, Scott HS, Cardamone T, Cappai R, Masters CL, Li QX. Decreased phosphatidylethanolamine binding protein expression correlates with Abeta accumulation in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Aging 27: 614–623, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Grönroos E, Thodeti CK, Sjölander A. Leukotriene D4 induces a rapid increase in cAMP in the human epithelial cell line, Int 407: a potential role for this signal in the regulation of calcium influx through the plasma membrane. Cell Calcium 24: 9–16, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Hammerbeck DM, Brown DR. Presence of immunocytes and sulfidopeptide leukotrienes in the inflamed guinea pig distal colon. Inflammation 20: 413–425, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Han HJ, Park SH, Lee JC, Lee HB, Park HS. Leukotriene D4 inhibits Na+ uptake through cAMP and PLC pathways in primary cultured renal proximal tubular cells. Kidney Blood Press Res 22: 106–113, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C betaII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem 278: 28644–28650, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Hu CJ, Zhou L, Cai Y. Dihydroartemisinin induces apoptosis of cervical cancer cells via upregulation of RKIP and downregulation of bcl-2. Cancer Biol Ther 15: 279–288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Mahavadi S, Sriwai W, Grider JR, Murthy KS. Cross-regulation of VPAC2 receptor desensitization by M3 receptors via PKC-mediated phosphorylation of RKIP and inhibition of GRK2. Am J Physiol Gastrointest Liver Physiol 292: G867–G874, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Keller ET, Fu Z, Yeung K, Brennan M. Raf kinase inhibitor protein: a prostate cancer metastasis suppressor gene. Cancer Lett 207: 131–137, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Li FF, Song SJ, Zhang RN. [Phosphatidylethanolamine-binding protein (PEBP) in basic and clinical study]. Sheng Li Ke Xue Jin Zhan 40: 214–218, 2009. [PubMed] [Google Scholar]
- 18.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426: 574–579, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Massoumi R, Larsson C, Sjölander A. Leukotriene D(4) induces stress-fibre formation in intestinal epithelial cells via activation of RhoA and PKCdelta. J Cell Sci 115: 3509–3515, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Massoumi R, Sjölander A. The role of leukotriene receptor signaling in inflammation and cancer. ScientificWorldJournal 7: 1413–1421, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun 15: 243–250, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Mezhybovska M, Wikström K, Ohd JF, Sjölander A. The inflammatory mediator leukotriene D4 induces beta-catenin signaling and its association with antiapoptotic Bcl-2 in intestinal epithelial cells. J Biol Chem 281: 6776–6784, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Nissen-Meyer LS, Popescu MC, Hamdani el H, Chaudhry FA. Protein kinase C-mediated phosphorylation of a single serine residue on the rat glial glutamine transporter SN1 governs its membrane trafficking. J Neurosci 31: 6565–6575, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohd JF, Wikström K, Sjölander A. Leukotrienes induce cell-survival signaling in intestinal epithelial cells. Gastroenterology 119: 1007–1018, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Pan M, Meng QH, Wolfgang CL, Lin CM, Karinch AM, Vary TC, Souba WW. Activation of intestinal arginine transport by protein kinase C is mediated by mitogen-activated protein kinases. J Gastrointest Surg 6: 876–882, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Pan M, Wolfgang CA, Karinch AM, Lin C, Meng Q, Vary TC, Souba WW. Protein kinase C activation of intestinal glutamine transport is mediated by mitogen-activated protein kinases. J Surg Res 106: 137–144, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Paruchuri S, Broom O, Dib K, Sjölander A. The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J Biol Chem 280: 13538–13544, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Paruchuri S, Hallberg B, Juhas M, Larsson C, Sjölander A. Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. J Cell Sci 115: 1883–1893, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Saha P, Arthur S, Kekuda R, Sundaram U. Na-glutamine co-transporters B(0)AT1 in villus and SN2 in crypts are differentially altered in chronically inflamed rabbit intestine. Biochim Biophys Acta 1818: 434–442, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology 86: 453–460, 1984. [PubMed] [Google Scholar]
- 31.Subramanian S, Glitz P, Kipp H, Kinne RK, Castaneda F. Protein kinase-A affects sorting and conformation of the sodium-dependent glucose co-transporter SGLT1. J Cell Biochem 106: 444–452, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Sundaram U, West AB. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am J Physiol Gastrointest Liver Physiol 272: G732–G741, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Sundaram U, Wisel S, Coon S. Neutral Na-amino acid cotransport is differentially regulated by glucocorticoids in the normal and chronically inflamed rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 292: G467–G474, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352: 628–631, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Talukder JR, Kekuda R, Saha P, Sundaram U. Mechanism of leukotriene D4 inhibition of Na-alanine cotransport in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G1–G6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thodeti CK, Sjölander A. Leukotriene D4-induced calcium signaling in human intestinal epithelial cells. Adv Exp Med Biol 507: 187–191, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Vayro S, Silverman M. PKC regulates turnover rate of rabbit intestinal Na+-glucose transporter expressed in COS-7 cells. Am J Physiol Cell Physiol 276: C1053–C1060, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Wardle TD, Hall L, Turnberg LA. Inter-relationships between inflammatory mediators released from colonic mucosa in ulcerative colitis and their effects on colonic secretion. Gut 34: 503–508, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol 200: 287–293, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401: 173–177, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol 21: 7207–7217, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousuf S, Duan M, Moen EL, Cross-Knorr S, Brilliant K, Bonavida B, LaValle T, Yeung KC, Al-Mulla F, Chin E, Chatterjee D. Raf kinase inhibitor protein (RKIP) blocks signal transducer and activator of transcription 3 (STAT3) activation in breast and prostate cancer. PLoS One 9: e92478, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zeng L, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin Ther Targets 12: 1275–1287, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XM, Gu H, Yan L, Zhang GY. RKIP inhibits the malignant phenotypes of gastric cancer cells. Neoplasma 60: 196–202, 2013. [DOI] [PubMed] [Google Scholar]