Abstract
Dysfunction of macrophages (MΦs) in diabetic wounds impairs the healing. MΦs produce anti-inflammatory and pro-resolving neuroprotectin/protectin D1 (NPD1/PD1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid); however, little is known about endogenous NPD1 biosynthesis by MΦs and the actions of NPD1 on diabetic MΦ functions in diabetic wound healing. We used an excisional skin wound model of diabetic mice, MΦ depletion, MΦs isolated from diabetic mice, and mass spectrometry-based targeted lipidomics to study the time course progression of NPD1 levels in wounds, the roles of MΦs in NPD1 biosynthesis, and NPD1 action on diabetic MΦ inflammatory activities. We also investigated the healing, innervation, chronic inflammation, and oxidative stress in diabetic wounds treated with NPD1 or NPD1-modulated MΦs from diabetic mice. Injury induced endogenous NPD1 biosynthesis in wounds, but diabetes impeded NPD1 formation. NPD1 was mainly produced by MΦs. NPD1 enhanced wound healing and innervation in diabetic mice and promoted MΦs functions that accelerated these processes. The underlying mechanisms for these actions of NPD1 or NPD1-modulated MΦs involved 1) attenuating MΦ inflammatory activities and chronic inflammation and oxidative stress after acute inflammation in diabetic wound, and 2) increasing MΦ production of IL10 and hepatocyte growth factor. Taken together, NPD1 appears to be a MΦs-produced factor that accelerates diabetic wound healing and promotes MΦ pro-healing functions in diabetic wounds. Decreased NPD1 production in diabetic wound is associated with impaired healing. This study identifies a new molecular target that might be useful in development of more effective therapeutics based on NPD1 and syngeneic diabetic MΦs for treatment of diabetic wounds.
Keywords: pro-healing and neurotrophic function, anti-inflammatory and pro-resolving mediators, diabetic wound healing, macrophage
wound healing occurs in four separate and overlapping phases: hemostasis, inflammation, proliferation, and remodeling (9, 20). Diabetic wounds are characterized by prolonged chronic inflammation and MΦ inflammatory activities after acute inflammation, and by altered generation of cytokines and lipid mediators (38, 50), which contributes to the impairment of pro-healing functions carried out by macrophages (MΦs) (53, 70). These features contribute to the lack of healing (38, 52, 70) and repair of injured peripheral nerves. The latter results in peripheral neuropathy and insensitivity to injury, and ultimately the poor healing of diabetic wounds such as foot ulcers (18).
MΦs play a critical role in epithelialization and innervation in wound healing, as well as in immune responses. MΦs also produce growth factors, including hepatocyte growth factor (HGF) (7, 73, 80–82), pro-resolving, anti-inflammatory lipid mediators (20, 39, 57, 70), and IL-10 (3, 24, 27, 43). MΦs or monocytes from blood or injury sites convert docosahexaenoic acid (DHA) to pro-resolving lipid mediators such as neuroprotectin/protectin D1 (NPD1/PD1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) (34, 49, 63, 64), maresins (65), and resolvins (64), as well as 14,21-dihydroxy-DHAs (47). NPD1/PD1 stereostructure and potent actions have been thoroughly studied (63). NPD1 promotes resolution of inflammation by regulating leukocyte infiltration, increasing MΦ phagocytosis and evacuation from inflammation site to lymphatic system after efferocytosis (60). NPD1, resolvin D1 (29, 32, 68), and 14,21-diHDHAs (33, 47, 69, 70) accelerate wound healing. In addition, NPD1 also increases corneal nerve area and neural cell survival (5, 6, 11, 16). Moreover, 14S,21R-diHDHA recovers the reparative functions of diabetic MΦs (70). Therefore, lipid mediators may be partly responsible for the roles of MΦs in wound healing.
These previous findings inspired the three hypotheses in the present study: 1) wounding induces NPD1 biosynthesis in skin, but this process is disturbed by diabetes; 2) MΦs are the key producers of NPD1; and 3) NPD1 promotes MΦs in accelerating diabetic wound healing and nerve regrowth. The putative mechanism involves 1) attenuating inflammation and oxidative stress after acute inflammation (9) in diabetic wounds, and suppressing MΦ inflammatory activities, and 2) augmenting the expression of pro-healing molecules. We tested these hypotheses using a combination of a splinted excisional-wound model on diabetic mice, in vitro assays, and targeted-lipidomic analysis by aqueous reversed-phase chiral liquid chromatography with ultraviolet and tandem mass spectrometry (aR chiral LC-UV-MS/MS). The results support our hypotheses.
MATERIALS AND METHODS
Diabetic mouse models.
Mice were handled following protocols approved by the IACUC of our institute. We used, Type 2 diabetic db/db (BKS.Cg-m+/+leprdb) and non-diabetic db/+ mice (26 wk old, female). The blood glucose levels were 18–22 mM for db/db mice and 4–6 mM for non-diabetic db/+ mice after 6 h of fasting, as measured in tail blood.
MΦ isolation and culture.
Briefly (28, 70, 83), 3 days after mice were injected with thioglycolate (ip), MΦs were collected by peritoneal lavage and cultured in RPMI 1640 medium containing 25 mM glucose in unprocessed plastic bacterial Petri dishes. More than 95% of adherent cells were F4/80+ MΦs, as our laboratory reported previously (70). NPD1/PD1 was prepared by total organic synthesis (63). The adherent MΦs were incubated with 200 nM NPD1 (37°C, 5% CO2, 24 h), washed with warm PBS without Ca2+/Mg2+ and cold PBS without Ca2+/Mg 2+, then incubated with a nonenzymatic cell dissociation solution (Sigma-Aldrich) (5 min at 37°C) (83). The cells were detached by a gentle PBS stream formed from pipetting, washed with PBS without Ca2+/Mg2+, then resuspended in RPMI 1640, and used as NPD1-treated MΦs; this final MΦ suspension did not contain detectable NPD1. Trypan blue exclusion confirmed that these NPD1-treated MΦs were ≥95% viable.
Mouse splinted excisional wound-healing model and administration of NPD1-treated db/db-MΦs or NPD1.
Briefly (71), two full-thickness wounds (5 mm circular) were made symmetrically across the dorsal midline of db/db mice. A donut-shaped silicone splint was adhered around the wound (25). Two days post-wounding (dpw), wounds were either instilled once with 106 cells/wound of NPD1-treated db/db-MΦs or vehicle-treated db/db-MΦs, or from 2 dpw onward, wounds received a daily injection of NPD1 (50 ng/wound) or RPMI 1640 (vehicle control). Injections were applied to the wound bed (10 μl/bed) and intradermally at four points (10 μl/site) distributed evenly near the wound edge (50 μl total/wound).
Wound healing analysis.
Skin wounds rimmed with 3-mm edges were analyzed (70). Serial cryosections (10 μm thick/section) were made through the center zone or region of the widest wound bed on each wound specimen. The section of each wound representing the widest wound bed or the center of the wound will be chosen for the following analysis. The epithelial gap and granulation tissue area were measured from this section selected after H&E staining. The relative epithelial gap was equal to the advancing epithelial gap (advancing edges of migrated keratinocytes/original epithelial gap × 100%). Granulation tissue was new connective tissue and blood vessel capillaries growing from the base of the wound. Collagen deposition in skin sections was analyzed by a Masson's trichrome stain kit (72), where collagen was stained blue.
Skin nerve-fiber density measurement.
Nerve fibers were detected in skin cryosections with a rabbit antibody against the nerve-fiber pan-axonal marker, protein gene product 9.5 (PGP9.5), and a secondary antibody (goat anti-rabbit FITC-conjugated IgG) (Millipore, Billerica, MA) (36). Nuclei were stained with Hoechst 33342, and images were acquired using a Zeiss AxioImager-M1 deconvolution microscope. Nerve-fiber density was measured as % PGP9.5-positive area per field, using ImageJ software.
Targeted lipidomics analysis of skin wounds using aR chiral LC-UV-MS/MS.
Lipidomics were performed as reported previously (4, 47, 75). Briefly, wounded skin was extracted three times with cold methanol:butylated hydroxytoluene (1:0.005%). Deuterium-labeled internal standards (prostanglandin-D2-d4 and DHA-d5, 5 ng/each, Cayman Chemical, Ann Arbor, MI) were added to each sample. Part of the pooled supernatants for each sample was cleaned up by C18 solid-phase extraction and then analyzed for the targeted lipidomics study using aR chiral LC-UV-MS/MS with a ChiralPak-IA column (150 mm long × 2.1 mm inner diameter × 5 μm; Chiral Technologies, West Chester, PA). The analysis of 8-isoprostane was conducted by hydrolysis of the esterified fatty acids in another portion of the extract (4, 35). The procedures were as follows: 1) the lipid extract was suspended in 50 μl of methanol; 2) 8 μl of 1 M sodium hydroxide and 42 μl of H2O were added; 3) the mixture was then incubated at 42°C for 3 h; 4) the pH was adjusted to 4 with 0.05 M HCl, and the aqueous phase was extracted with 2 ml of hexane:isopropanol (3:2 vol/vol); 5) the tube was centrifuged at 3,000 g for 5 min, the organic phase was removed from the top of the aqueous phase, and the procedure was repeated by washing with 1 ml more of solvent mixture; and 6) the organic extracts were dried and resuspended in methanol for LC-UV-MS/MS analysis. The LC mobile-phase gradient was the same as described previously (70). Standards of 17S/R-hydroxy-DHA (17S/R-HDHA), leukotriene B4 (LTB4), thromboxane B2 (TXB2), and 8-isoprostane were obtained from Cayman Chemical.
Analysis of IL-10 and TNF-α in skin wounds.
Skin wounds were pulverized in liquid nitrogen, extracted for cytokines in a buffer compatible with ELISA, and then analyzed using ELISA kits for quantification of IL-10 and TNF-α (R & D Systems) following established procedures (51).
MΦ depletion.
MΦs were depleted in mice by administration of dichloromethylene-diphosphonate (Clodronate)-loaded liposomes (CL) (Encapsula Nano-Sciences, Nashville, TN) following the established procedures for similar animal conditions (23, 40, 74). CL was injected at 51 mg/kg ip (200 μl/mouse) and subcutaneously at 50 μl/wound (in RPMI 1640 as above or site to be wounded), 5 days before wounding and then every 3 days until the mice were killed at 7 dpw.
Effects of NPD1 on production of HGF, IL-10, TNF-α, LTB4, and TXB2 by MΦs under conditions simulating diabetic wounds.
MΦs (3 × 105) were resuspended in fresh RPMI 1640 containing 100 ng/ml LPS, 25 mM glucose, and NPD1 at 0, 20, or 200 nM, and then incubated in hypoxic chamber (24 h, 95% N2, 5% CO2). MΦ-produced HGF, TNF-α, IL-10, LTB4, and TXB2 in the medium supernatant were quantified using ELISA kits (R & D Systems). Hypoxia, LPS stimulation, and high glucose in vitro were used to simulate low oxygen levels, infection potential, and hyperglycemia in diabetic wounds, respectively (12, 22, 37, 42, 61).
Statistical analysis.
Data were analyzed by Mann-Whitney's ANOVA or t-test. A P value of <0.05 was considered statistically significant. Results are means ± SE.
RESULTS
Injury induced NPD1 biosynthesis in wounds, but this was disrupted by diabetes.
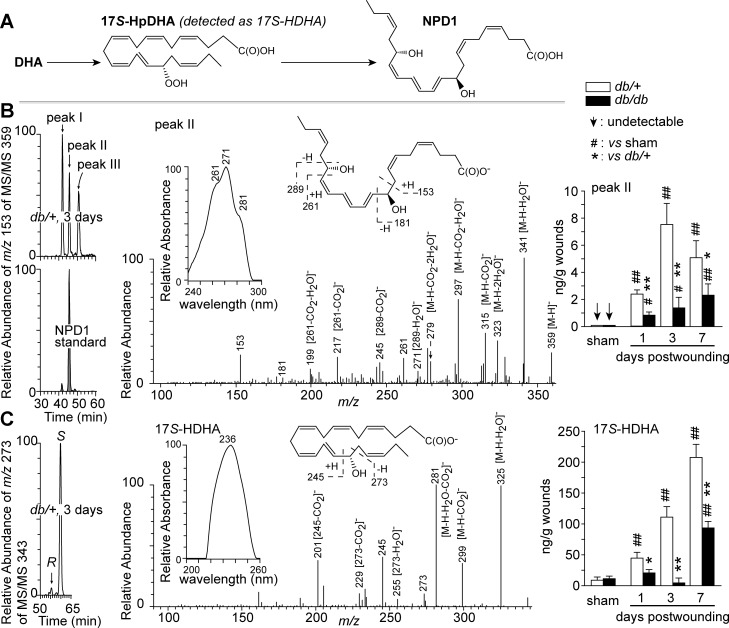
We tested the first hypothesis by using aR chiral LC-UV-MS/MS to analyze wounds of non-diabetic db/+ and diabetic db/db mice for NPD1 and its biosynthesis marker 17S-HDHA (Fig. 1A). NPD1 was found in wounds at 1, 3, and 7 dpw but not in the uninjured sham skin (Fig. 1B). 17S-HDHA levels were generally several times higher than those of NPD1, consistent with the upstream position of 17S-HDHA in NPD1 biosynthesis (Fig. 1, A and C). NPD1 and 17S-HDHA reached maximal levels at 3 and 7 dpw, respectively, in db/+ wounds, but their kinetics differed in db/db wounds; NPD1 levels increased continuously from 1 to 7 dpw, whereas 17S-HDHA levels dropped at 3 dpw and then increased again. NPD1 levels were lower in diabetic wounds than in non-diabetic wounds at the same time points. This was also the case for 17S-HDHA. In general, injury induced NPD1 biosynthesis in wounds, whereas diabetes suppressed this biosynthesis, consistent with our first hypothesis.
Fig. 1.
Diabetes altered endogenous NPD1 levels in wounds of diabetic db/db mice compared with non-diabetic db/+ mice. A: brief biosynthetic pathways for NPD1. B: NPD1. C: 17S-HDHA (a marker of NPD1 biosynthetic intermediate 17S-hydroperoxy DHA). Left: selective ion MS/MS aR chiral LC chromatograms. Middle: MS/MS spectra with insets of UV spectra and structure elucidation, which are representative for aR chiral LC-UV-MS/MS analysis, and acquired from wounds collected from db/+ mice at 3 days post-wounding (dpw). Right: quantitative kinetic levels of each compound in wounds. Sham mice underwent no wounding. Excisional wounds were collected at 1, 3, or 7 dpw after death, extracted and analyzed by chiral LC-UV-MS/MS. Data are means ± SE (n = 5). Significant difference vs. db/+: *P < 0.05; **P < 0.01. Significant difference vs. sham: #P < 0.05; ##P < 0.01; vs. sham.
Endogenous NPD1, 17S-HDHA, and 17R-HDHA in wounds were identified based on their aR chiral LC-UV-MS/MS spectra and chromatographic retention times, which matched those of known standards (Fig. 1). For example, the NPD1 from mouse wounds, appearing as peak II in the chromatogram of Fig. 1B, showed MS/MS ions at m/z 359 [M-H]−, 341 [M-H-H2O]−, 323 [M-H-2H2O]−, 315 [M-H-CO2]−, 297 [M-H-H2O-CO2]−, and 279 [M-H-2H2O-CO2]−, consistent with the NPD1 molecular weight (M) of 360 Da (Fig. 1B). The MS/MS ions consistent with the C10 and C17 alcohol-containing positions of NPD1 were observed at m/z 153, 181, 199 [261-CO2-H2O]−, 217 [261-CO2]−, 245 [289-CO2]−, and 261. The triplet bands of the UV spectrum [wavelength of maximum absorbance (λmax) of 271 nm, and two shoulders at 261 and 281 nm] revealed the conjugated-triene structure of NPD1 (Fig. 1B, inset). 17R-HDHA, the epimer of 17S-HDHA, accounted for ∼3% of 17S-HDHA (Fig. 1C). Two NPD1 isomers were also found in mouse wounds (Fig. 1B, peak I and III) with LC-UV-MS/MS spectra consistent with those of NPD1 (data not shown).
Diabetes caused sustained inflammation and oxidative stress after acute inflammation in wounds.
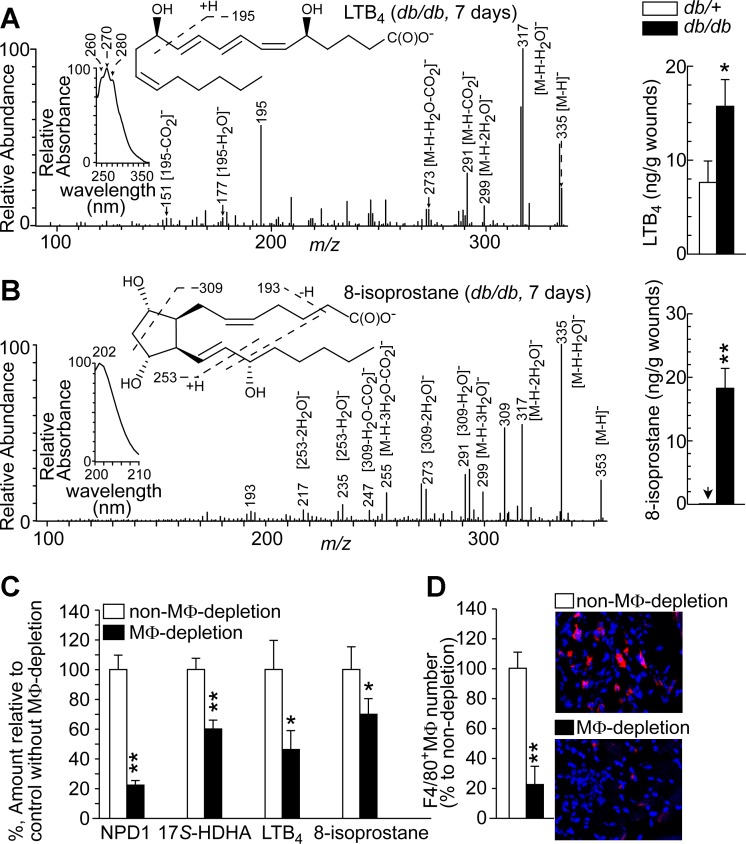
The acute inflammation induced by injury can occur up to 5 dpw. During this phase, the inflammatory cytokines, lipid mediators, neutrophils, and other leukocytes reach maximal levels (9, 20, 48, 58), and then inflammatory factors in the wounds recede to basal levels as the inflammation resolves. However, diabetic wounds show sustained inflammation, which becomes chronic, and resolution of inflammation is impaired (53, 66); this is a major mechanism underlying the observed failure to heal (38, 52, 70). We identified the inflammation marker LTB4 and oxidative stress marker 8-isoprostane by matching their aR chiral LC-UV-MS/MS characteristics to those of known standards. At 7 dpw, after acute wound inflammation (48), higher levels of LTB4 and 8-isoprostane were detected in diabetic than in non-diabetic wounds (Fig. 2, A and B), which was opposite to the trend seen for NPD1 and 17S-HDHA (Fig. 1). The LTB4 extracted from wounds (Fig. 2A) had MS/MS ions m/z 335 [M-H]−, 317 [M-H-H2O]−, 299 [M-H-2H2O]−, 291 [M-H-CO2]−, 273 [M-H-H2O-CO2]−, 195, 177 [195-H2O]−, and 151 [195-CO2]−, consistent with its M of 336 Da, and 12-hydroxy and 5-hydroxy of LTB4. The LTB4 also had an UV triplet band with λmax 270 nm for its conjugated triene. The 8-isoprostane extracted from wounds (Fig. 2B) had MS/MS ions m/z 353 [M-H]−, 335 [M-H-H2O]−, 317 [M-H-2H2O]−, 299 [M-H-3H2O]−, 255 [M-H-3H2O-CO2]−, 309, 291 [309-H2O]−, 273 [309–2H2O]−, 247 [309-H2O-CO2]−, 235[253-H2O]−, 217 [253–2H2O]−, and 193, consistent with its M of 354 Da and three hydroxyls, and had a UV singlet with λmax 202 nm, typical of a cyclopentyl eicosanoid containing non-conjugated double bands.
Fig. 2.
Higher levels of inflammatory LTB4 and oxidative-stress marker 8-isoprostane were observed after acute inflammation of wounds in diabetic db/db mice compared with non-diabetic db/+ mice. Macrophage (MΦ) depletion diminished the levels of NPD1, 17S-HDHA, LTB4, and 8-isoprostane in wounds. A: LTB4. B: 8-isoprostane. Left: aR chiral LC-MS/MS spectra with insets of aR chiral LC-UV spectra and MS/MS fragment interpretation. Right: LTB4 and 8-isoprostane levels in wounds collected at 7 dpw when wounds passed acute inflammation. C: amount (%), with MΦ-depletion, of NPD1 and 17S-HDHA in db/+ wounds or of LTB4 and 8-isoprostane in db/db wounds at 7 dpw relative to non-MΦ-depletion. D: MΦs in wounds at 7 dpw after depletion by Clodronate liposomes. Left: percentage of F4/80+ MΦs relative to non-depletion in wound sections. Right: immunohistological images. Data are means ± SE (n = 5). Significant difference: *P < 0.05; **P < 0.01.
MΦs were the key producers of NPD1 in wounds and significantly contributed to inflammation and oxidative stress after acute inflammation in diabetic wounds.
We determined the role of skin-wound MΦs in NPD1 biosynthesis by treating mice with chlondrate liposomes. Following clodronate-liposome treatment, besides slightly reduced activity, mice continued consuming food and water normally and showed no obvious illness or weight loss, consistent with previous reports using similar procedures and doses of CL (23, 40). MΦ-depletion by CL treatment reduced F4/80+ MΦs by 78% in the skin wounds of db/db mice at day 7 post-wounding based on immunohistological analysis of F4/80+ MΦs in the skin wound sections (Fig. 2D). Corresponding to MΦ-depletion in wounds, the levels of NPD1 and its biosynthesis marker 17S-HDHA also decreased (Fig. 2C). This finding supported our second hypothesis that MΦs are the key producers of NPD1. Levels of LTB4 and 8-isoprostane at 7 dpw, after acute wound inflammation (9), were also reduced by MΦ depletion in diabetic wounds, suggesting that MΦs were significant contributors to the chronic inflammation and oxidative stress occurring after acute inflammation in diabetic wounds.
NPD1 accelerated healing and nerve-fiber growth of skin wounds of diabetic mice.
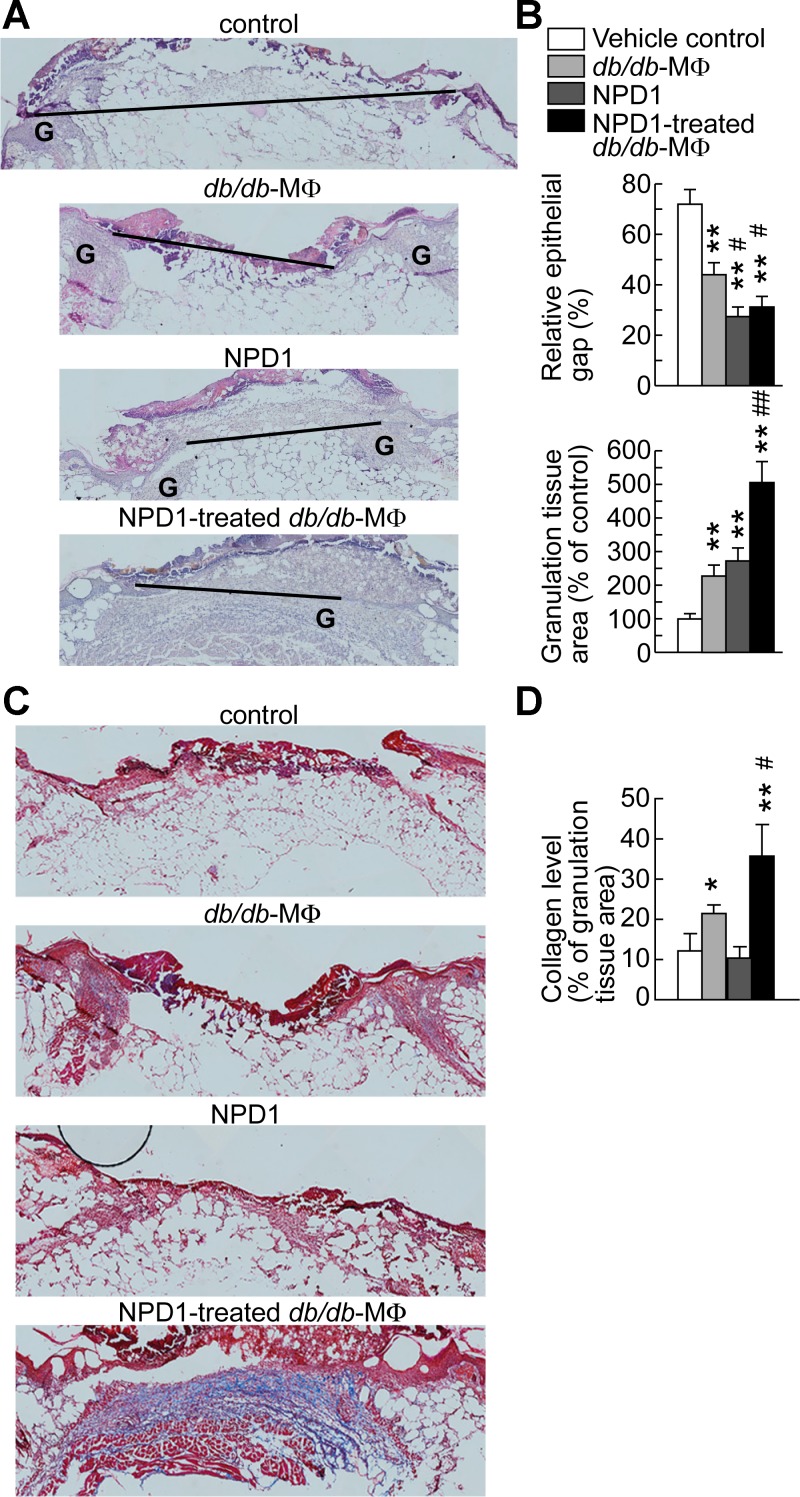
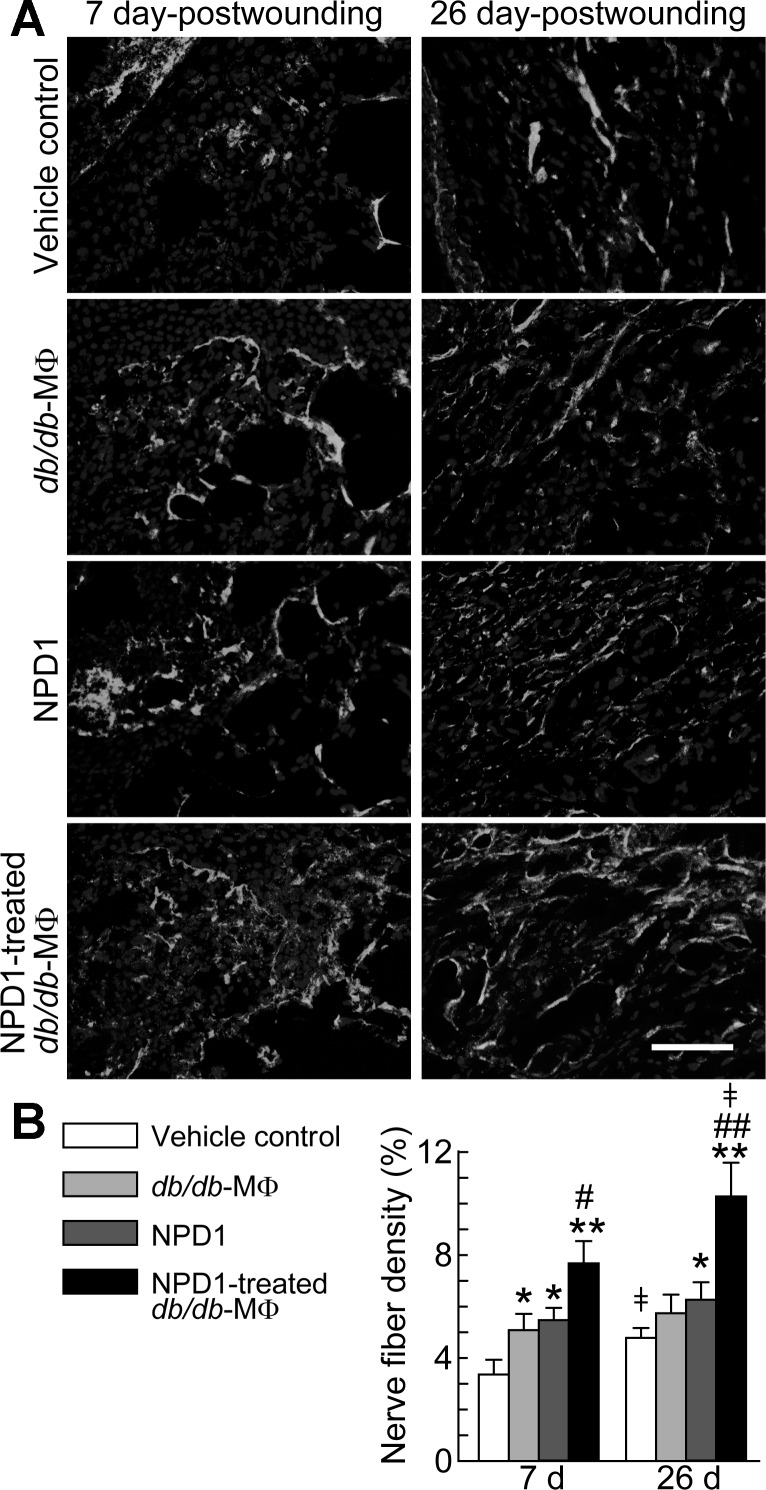
Biosynthesis of NPD1, a lipid mediator known to resolve inflammation and enhance nerve regrowth in injured corneas (5, 6, 11, 16), was induced by wounding in skin (Fig. 1). We therefore examined whether NPD1 could promote wound healing and nerve regrowth in diabetic mice. After the administration of NPD1 to the wounds of diabetic mice, HE staining of sections from wounds collected at 7 dpw showed a reduction in the epithelial gap from 72% (control) to 27% (NPD1-treated) (Fig. 3, A and B, top) and an increase in granulation tissue formation from 100% (control) to 272% (NPD1-treated) (Fig. 3, A and B, bottom). Administration of NPD1 to wounds resulted in higher densities of PGP9.5+ nerve fibers in the wounds of diabetic db/db mice than in wounds treated with the vehicle control at 7 dpw (5.4% vs. 3.3%) and at 26 dpw (6.2% vs. 4.8%) (Fig. 4). Overall, NPD1 accelerated reepithelialization, granulation tissue formation, and nerve-fiber regrowth.
Fig. 3.
NPD1 increased MΦ promotion of reepithelialization, granulation tissue formation, and collagen deposition in wounds of diabetic db/db mice. Treatment with NPD1 alone also accelerated the reepithelialization and granulation tissue formation but did not affect collagen level. At 2 dpw of db/db mice, skin excisional wounds were treated with db/db-MΦs, NPD1-treated db/db-MΦs, NPD1, or vehicle control. A: micrographs of HE-stained wound sections. Black bar, epithelial gap; G, granulation tissue. B: relative epithelial gap (top) and granulation tissue area (bottom). C: micrographs of wound sections show Masson-trichrome-stained collagen (blue). D: collagen level (% of granulation tissue area). Original magnification: ×100. Wounds were collected at 7 dpw. Data are means ± SE (n = 8). Significant difference vs. vehicle control: *P < 0.05; **P < 0.01. Significant difference vs. db/db-MΦs: #P < 0.05; ##P < 0.01.
Fig. 4.
NPD1 treatment of wounds of diabetic mice promoted nerve fiber regrowth. NPD1 also enhanced neurotrophic function of MΦs in diabetic wounds. The treatment of excisional wounds of db/db diabetic mice was the same as described in Fig. 3. A: micrographs of cryosections of wounds collected at 7 and 26 dpw show PGP9.5+ nerve fibers (white). B: relative density (%) of nerve fiber area in wounds. Original magnification, ×200; scale bar, 100 μm. Nuclei (dark gray) were stained with Hoechst 33342. Data are means ± SE (n = 8). Significant difference vs. vehicle control: *P < 0.05; **P < 0.01. Significant difference vs. db/db-MΦs: #P < 0.05; ##P < 0.01. ‡Significant difference vs. 7 dpw (P < 0.05).
NPD1 promoted MΦ functions that accelerate diabetic wound healing and nerve-fiber regrowth.
We tested part of our third hypothesis that NPD1 promotes MΦ functions that accelerate diabetic wound healing and nerve-fiber regrowth by treating the db/db-MΦs with or without NPD1 and then injecting these cells into db/db wounds. The injections were performed at 2 dpw, when endogenous MΦs are considerably recruited to wounds (9). The injection suspensions themselves did not contain detectable NPD1, indicating that db/db MΦs metabolized NPD1 during the 24-h incubation. The epithelial gap relative to controls was significantly decreased in the wounds injected with NPD1-treated db/db-MΦs (Fig. 3). Administration of NPD1-treated db/db-MΦs promoted robust increases in granulation tissue area (4.0-fold or 1.2-fold vs. controls or vehicle-treated db/db-MΦs, respectively). Wounds injected with NPD1-treated db/db-MΦs were filled with more collagen, displayed as blue Masson-trichrome stain, and possessed a better organized extracellular matrix compared with control wounds or wounds injected with vehicle-treated db/db-MΦs (Fig. 3). The control wounds showed poor collagen deposition and an immature extracellular matrix. Interestingly, collagen deposition was also increased in the wounds of vehicle-treated db/db-MΦs relative to controls but not in the wounds treated with NPD1 alone. Therefore, better wound healing was obtained by the administration of NPD1-treated db/db-MΦs compared with the administration of vehicle-treated db/db-MΦs or vehicle control. Higher densities of PGP9.5+ nerve fibers were found in the wounds of diabetic db/db mice injected with NPD1-treated db/db-MΦs than with db/db-MΦs or vehicle control at 7 dpw (7.7% vs. 5.1% or 3.3%) and at 26 dpw (10.3% vs. 5.7 or 4.8%) (Fig. 4). PGP9.5+ nerve-fiber densities increased for all three groups from 7 to 26 dpw, but, interestingly, a significant increase was only observed for control and NPD1-treated db/db-MΦs. These results indicate that NPD1 augments MΦ neurotrophic functions in diabetic wounds. Together, these data support our third hypothesis proposing that NPD1 promotes MΦ pro-healing activities and neurotrophic functions in diabetic wounds.
Mechanistic insights: actions of NPD1 and NPD1-treated MΦs involve attenuation of chronic inflammation and oxidative stress, and promotion of MΦ production of pro-healing factors.
We then tested the part of our third hypothesis. The NPD1-treatment reduced wound levels of TNF-α, LTB4, and 8-isoprostane at 7 dpw by 48%, 22%, and 70%, respectively, compared with the control (Fig. 5). The NPD1-treated db/db-MΦs also reduced levels of TNF-α, LTB4, and 8-isoprostane by 74%, 45%, and 69%, respectively, compared with the control. Administration of NPD1 or the NPD1-treated db/db-MΦs increased IL-10 in diabetic wounds by 136% or 243%, respectively. IL-10 is a pro-healing and anti-inflammatory cytokine (38, 70). Therefore, administration of NPD1 or NPD1-treated db/db-MΦs dampened chronic inflammation and oxidative stress in diabetic wounds after acute inflammation (9).
Fig. 5.
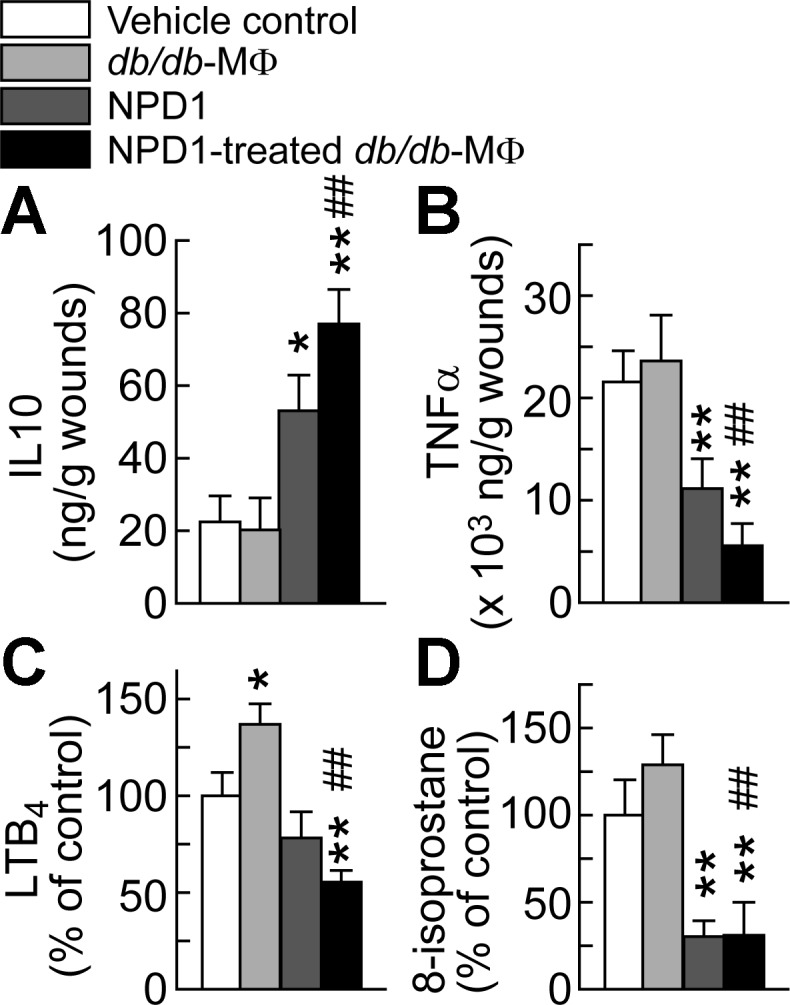
Administration of NPD1 or NPD1-treated MΦs to diabetic wounds increased pro-healing IL-10 levels and attenuated inflammation and oxidative stress after acute inflammation of wounds of diabetic db/db mice. A: IL-10 levels. The inflammation status of wounds is represented by levels of TNF-α (B) and LTB4 (C); the oxidative stress was represented by 8-isoprostane levels (D). The treatment of wounds of db/db diabetic mice was the same as described in Fig. 3. Skin wounds were collected at 7 dpw and analyzed by ELISA for IL-10 and TNF-α, and by aR LC-UV-MS/MS for LTB4 and 8-isoprostane. Data are means ± SE (n = 4). Significant difference vs. vehicle control: *P < 0.05; **P < 0.01. Significant difference vs. db/db-MΦs: #P < 0.05; ##P < 0.01.
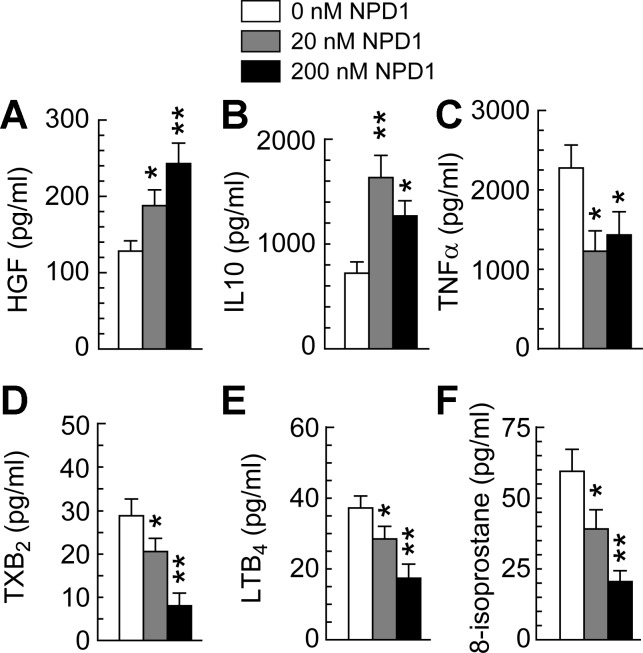
MΦs significantly contribute to chronic inflammation in diabetic wounds (see LTB4 in Fig. 2C); NPD1 is anti-inflammatory (34); and MΦs produce growth factors and cytokines that promote wound healing (39). For these reasons, we also investigated the effects of NPD1 on db/db-MΦs cultured in a simulated diabetic wound microenvironment (hypoxia, LPS-stimulation, and high glucose). NPD1 markedly promoted HGF production by db/db-MΦs in a dose-dependent manner (Fig. 6A). At 20 nM, NPD1 increased the production of anti-inflammatory, pro-healing IL-10 by ∼1.3-fold in db/db-MΦs (Fig. 6B). It also reduced production of inflammatory TNF-α by 0.5-fold in db/db-MΦs (Fig. 6C). NPD1 suppressed production of the inflammatory lipid mediators LTB4 and TXA2 (detected as TXB2) in a dose-dependent manner in db/db-MΦs. Treatment with 200 nM NPD1 decreased production of TXB2 by 72% and LTB4 by 54%, as well as 8-isoprotane by 65%. Therefore, NPD1 treatment reduced the inflammatory and oxidative activities of db/db-MΦs (Fig. 6, D–F). The promotion of HGF and IL-10 production and the attenuation of inflammatory activities in diabetic MΦs by NPD1 may represent novel mechanistims that underline how NPD1 promotes MΦ pro-healing and neurotrophic functions in diabetic wounds.
Fig. 6.
NPD1 promoted pro-healing HGF and IL-10 expression but suppressed formation of inflammatory markers TNF-α, LTB4, and TXB2, and oxidative stress marker 8-isoprostane by diabetic db/db-MΦs. A: HGF. B: IL-10. C: TNF-α. D: TXB2. E: LTB4. F: 8-isoprostane. MΦs (3 × 105) of db/db mice were incubated under hypoxia with 25 mM glucose, 100 ng/ml LPS, and NPD1 at 0, 20, or 200 nM (24 h). The supernatant of the final medium was analyzed by ELISA. Data are expressed as means ± SE (n = 4). Significant difference vs. db/db-MΦs without NPD1 treatment: *P < 0.05; **P < 0.01.
DISCUSSION
Diabetes impairs the formation or actions of reparative factors, including those involving MΦs, leading to the delay or failure of wound healing (8, 38, 52, 70, 78). This study demonstrates for the first time that NPD1 and activation of MΦs by NPD1 are among these factors. MΦs and injured tissue are known to produce NPD1 (34, 49, 63), and our data show that the NPD1 formation is induced in wounded skin. However, diabetes disturbed NPD1 kinetics, as indicated by lower levels of NPD1 at 1, 3, or 7 dpw. A similar finding was reported in adipose tissue, adjacent to injury sites, of diabetic patients who had lower levels of NPD1 and 17S-HDHA than found in non-diabetic controls (15), suggesting the operation of a similar mechanism involving NPD1 deficiency (53). The present finding that NPD1 levels in mouse wounds were diminished after MΦs depletion suggests that MΦs are the key cells involved in NPD1 generation in wounds.
The promotion of diabetic wound healing by NPD1 suggests that the wound-induced formation of NPD1 is a reparative response of the skin. NPD1 is potently anti-inflammatory and pro-resolving (63), and clearly reduced chronic inflammation in diabetic wounds after acute inflammation (Fig. 5). There are multiple types of intracellular mechanisms that could underline NPD1 promotion of reparative functions of diabetic MΦs in addition to the involvement of the expression of IL-10 and HGF and the suppression of chronic inflammation and oxidative stress we observed in this report. MΦ efferocytosis of apoptotic cells may be involved. NPD1 enhances the MΦ phagocytosis capability (60, 62), and the MΦ engulfment of apoptotic cells leads to the production of HGF with concomitant activation of PI3K, MAPK, and Rho A pathways (54, 55). MΦ efferocytosis also induces microRNA-21 by silencing PTEN and GSK3β, triggers release of IL-10, and suppresses LPS-induced TNF-α expression (17). NPD1 actions on diabetic MΦs may include the activation of peroxisome proliferator-activated receptor (PPAR) γ. NPD1 upregulates PPARγ transcriptional activity in primary human neuronal-glial cells (84). It is likely that such activation could also take place in MΦs. The PPARγ pathway regulates MΦ-increased expression and secretion of both IL-10 (1, 14, 59) and HGF (19, 44). Alternatively, NPD1 could have restored diabetic MΦ pro-healing functions by activating/phosphorylating the mTOR, AKT, and/or p70S6K, similar to the mechanism described in retinal pigment epithelial cells and neutrophils under oxidative stress (21, 26, 30, 31). The mTOR regulates cell growth and cell proliferation (26, 31), AKT signaling is essential in sifting inflammatory MΦ phenotype to anti-inflammatory phenotype (10), and p70S6K are associated with cell movement in the phagocytic process (41). These pathways may underline the NPD1 rescue of the pro-healing functions of diabetic MΦs and should be systematically investigated in the future.
In this report, we found that NPD1 accelerates wound healing and promotes the pro-healing function of MΦs by enhancing wound epithelialization and granulation tissue formation similar to our previous reports utilizing 14S,21R-diHDHA (69, 70), and by promoting collagen deposition and regrowth of nerve fibers. Interestingly, NPD1 appears to be more potent than 14S,21R-diHDHA in inducing the increase of granulation area by db/db MΦs (70). DHA-derived pro-resolving lipid mediators are likely to act in concert to regulate wound healing when they are generated together in the body or administered together. For example, both NPD1 and 14S,21R-diHDHA are induced in wounds (69), and they could act together in promoting the healing. We should comprehensively assay the efficacies of various prohealing lipid mediators, including NPD1 and 14S,21R-diHDHA, under the same experimental conditions in the future for therapeutic intervention.
The promotion of innervation in diabetic wounds by NPD1 treatment, as shown here, demonstrates a further neurotrophic property of NPD1 in rescuing diabetes-damaged peripheral nerve fibers in skin. This is consistent with previously observed enhancement of innervation in corneal injury by NPD1 (16). The functional outcome of NPD1-promoted innervation in diabetic wounds warrants further study.
We observed that the presence of diabetes also corresponded to a greater degree of chronic inflammation and oxidative stress in wounds after acute inflammation, as indicated by higher levels of inflammatory TNF-α and LTB4 (13, 56, 67), and of the oxidative stress marker 8-isoprostane (22, 46, 79) at 7 dpw (Figs. 2, A and B, and 5). Depletion of MΦs in diabetic wounds suppressed the levels of these molecules after acute inflammation (Fig. 2C), confirming previous reports that endogenous diabetic MΦs contribute significantly to chronic inflammation and oxidative stress in diabetic wounds (39). Thus MΦs are a “double-edge sword”; they can show impaired pro-healing functions or can even deteriorate healing if not well regulated, as happens in diabetic wounds. In diabetic mice, MΦs display dysregulated production of growth factors and lipid mediators, which contributes to impairment of diabetic wound healing (22, 70). Therefore, the treatment of diabetic wounds should aim at restoring MΦ pro-healing functions as a potential strategy for achieving adequate healing, as suggested by the findings reported here.
As observed by other researchers (50) and our laboratory previously (70), administration of exogenous db/db-MΦs to wounds at 2 dpw also improved wound healing (Fig. 3). The MΦ density in wounds of db/db mice at 2 dpw is lower compared with non-diabetic db/+ mice as reported previously (76, 77). Adding exogenous db/db-MΦs supplemented the MΦ deficit in db/db wounds, which might contribute to the improvement of db/db wound healing. The db/db-MΦs administrated to the wounds at 2 dpw, a time point of the acute inflammatory phase of healing, are likely to directly act on non-inflammatory aspects of the early phase of wound healing. They did not significantly increase the levels of TNF-α or oxidative stress marker 8-isoprostane but increased the level of inflammation marker LTB4 in db/db wounds after the acute inflammatory phase (at 7 dpw) (Fig. 5). It is reported that MΦs derived from bone marrow cells of db/db mice cross-bred with C57BL/6 GFP-transgenic mice increased TNF-α level in wounds at 5 dpw when these MΦs were transplanted to wounds at 3 dpw (2). Different animal strains, organs, timing of MΦ transplantation, and/or healing phases might contribute to this macrophage difference on the pro-inflammatory marker expression. The db/db MΦs used in this manuscript are likely to consist of diversified populations, including M1-like and M2-like phenotypes (38, 45). The db/db MΦs without incubation with NPD1 can still produce reparative HGF and IL-10 (Fig. 6) (38) as well as VEGF (50, 70), although at lower levels compared with db/db MΦs incubated with NPD1, which might contribute to the db/db-MΦ improvement of wound healing.
NPD1 reduced inflammatory and oxidative activities, as manifested by dampened production of TNF-α, LTB4, TXB2, and 8-isoprostane in db/db-MΦs treated in vitro with NPD1 (Fig. 6). Endogenous diabetic MΦs also contributed substantially to chronic inflammation and oxidative stress in diabetic wounds (Fig. 2C) (39). Therefore, the reduction of chronic inflammation and oxidative stress in diabetic wounds by NPD1 or NPD1-treated MΦs at 7 dpw after acute wound inflammation could largely result from the attenuation of these inflammatory, oxidative activities of MΦs. NPD1 promoted healing, at least in part, by reducing chronic inflammation due to a MΦ-associated mechanism.
Treatment of db/db-MΦs with NPD1 increased the secretion of HGF, a factor that regulates the major cellular processes responsible for wound repair, including reepithelialization and collagen deposition (20, 39, 57, 70). It also increased secretion of anti-inflammatory, pro-healing cytokine IL-10 from db/db-MΦs (38). These observations suggest that the mechanism by which NPD1 promotes MΦ reparative functions in diabetic wounds also includes the promotion of MΦ paracrine/autocrine functions, such as the production of HGF and IL-10, which can promote the pro-healing functions of neighboring cells or of the MΦs themselves (39). NPD1 alone did not affect collagen deposition; therefore, it is possible that NPD1 alone, in our experimental conditions, was insufficient in activating endogenous MΦs for collagen deposition, whereas in vitro pretreatment with NPD1 did. This implies that, in regard to collagen deposition, exogenous MΦs respond better than endogenous MΦs to activation by NPD1, suggesting that the microenvironment during the activation might affect the outcome, since there are many other cells and extracellular molecules surrounding MΦs in vivo, whereas there are only MΦs and limited molecular species in the medium in vitro. These results could indicate that, in diabetic skin wounds, there is a potential therapeutic advantage to using NPD1-treated MΦs over using NPD1 directly. The findings of NPD-treated db/db MΦs in this report are of translational significance for therapeutic intervention on diabetic wound treatment. However, their implication for endogenous wound db/db-MΦs needs to be systematically confirmed in the future.
The bioactions and mechanisms for NPD1 in diabetic wound healing were not studied previously. Our new data advances the field as follows over what was already published. First, they unbiasedly show, with full-scan MS/MS and UV spectra and chiral LC chromatograms, the existence of endogenous NPD1 in mouse wounds and the diabetic diminishment of NPD1 in the wounds. The NPD1 deficiency in diabetic wounds could be, at least partly, responsible for the diabetic impairment of skin wound healing. Second, they provide the time courses of NPD1 in diabetic and non-diabetic wounds, which are important to the field in studying NPD1-related mechanisms and therapeutic intervention in diabetic wound healing. These results are consistent with the recent discovery by Claira et. al (15) that subcutaneous adipose tissues from patients with peripheral vascular disease exhibited a marked deficit in PD1/NPD1 and wounding induces NPD1 formation in adipose tissue peri-wound from patients with peripheral vascular disease. Third, in wounds, NPD1 was mainly produced by MΦs. Fourth, NPD1 enhanced diabetic wound healing and innervation and promoted MΦ functions that accelerated these processes. Fifth, the underlying mechanisms for the actions of NPD1 or NPD1-modulated MΦs involved 1) attenuating MΦ inflammatory activities, wound chronic inflammation, and oxidative stress after acute inflammation in diabetic wound; and 2) increasing MΦ production of IL-10 and HGF. At present, no studies have reported the modulation of MΦ actions in skin wound healing by NPD1. This study shows that NPD1 treatment of db/db-MΦs markedly augments their pro-healing functions in diabetic wounds. Our data suggest that MΦs are likely to be an important target for the promotion of wound healing and innervation by NPD1.
In conclusion, our data clearly show that skin injury induces NPD1 biosynthesis in wounds and that MΦs are the key cells producing NPD1. Diabetes disrupts the time course of NPD1 biosynthesis in wounds. NPD1 treatment not only accelerates diabetic wound healing and innervation but also promotes the functioning of diabetic MΦs to further accelerate healing processes in diabetic wounds. NPD1 promotion of HGF and IL-10 production and attenuation of inflammatory activities of diabetic MΦs may represent the underlying mechanism responsible for NPD1 induction of pro-healing and neurotrophic functions of MΦs in diabetic wounds. NPD1 could be a factor produced and released by skin wound MΦs to accelerate wound healing by promoting the pro-healing function of the MΦs and surrounding neighbor cells. The mechanistic insight revealed in this study points to a new target that can be used to develop more effective therapies based on the NPD1 molecular template and MΦ function for the treatment of diabetic wounds.
GRANTS
This work is supported by the grants from the National Institutes of Health (R01-DK-087800 to S. Hong, P30-GM-103340 to N. G. Bazan, and 1P01 GM-095467 to C. N. Serhan), and Research to Prevent Blindness (New York, NY).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H., H.T., and Y.L. conception and design of research; S.H., H.T., Y.L., J.M.L., F.A.M., Q.W., and B.V.A. performed experiments; S.H., H.T., Y.L., J.M.L., F.A.M., Q.W., B.V.A., C.N.S., and N.G.B. analyzed data; S.H., H.T., Y.L., J.M.L., F.A.M., Q.W., B.V.A., C.N.S., and N.G.B. interpreted results of experiments; S.H., Y.L., F.A.M., and B.V.A. drafted manuscript; S.H., H.T., Y.L., F.A.M., and C.N.S. edited and revised manuscript; S.H., H.T., Y.L., J.M.L., F.A.M., Q.W., B.V.A., C.N.S., and N.G.B. approved final version of manuscript; H.T., Y.L., Q.W., and B.V.A. prepared figures.
ACKNOWLEDGMENTS
We sincerely appreciate Professor Nicos A. Petasis (Department of Chemistry and the Loker Hydrocarbon Research Institute, University of Southern California, Los Angeles, CA) for providing neuroprotectin/protectin D1 prepared by organic synthesis. Many thanks to Ryan Labadens, Shirley N. Hong, and Yue-Liang Brewerton for editorial assistance and proofreading.
REFERENCES
- 1.Ayoub SS, Botting RM, Joshi AN, Seed MP, Colville-Nash PR. Activation of macrophage peroxisome proliferator-activated receptor-gamma by diclofenac results in the induction of cyclooxygenase-2 protein and the synthesis of anti-inflammatory cytokines. Mol Cell Biochem 327: 101–110, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, Mace KA. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Disease Models Mech 6: 1434–1447, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC., Jr Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg 39: 912–915, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bazan HA, Lu Y, Thoppil D, Fitzgerald TN, Hong S, Dardik A. Diminished omega-3 fatty acids are associated with carotid plaques from neurologically symptomatic patients: implications for carotid interventions. Vasc Pharmacol 51: 331–336, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 29: 263–271, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol 44: 216–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevan D, Gherardi E, Fan TP, Edwards D, Warn R. Diverse and potent activities of HGF/SF in skin wound repair. J Pathol 203: 831–838, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219–1222, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 117: 12S–34S, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro-inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol 664: 663–670, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Calandria JM, Mukherjee PK, de Rivero Vaccari JC, Zhu M, Petasis NA, Bazan NG. Ataxin-1 poly(Q)-induced proteotoxic stress and apoptosis are attenuated in neural cells by docosahexaenoic acid-derived neuroprotectin D1. J Biol Chem 287: 23726–23739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Gajendrareddy PK, DiPietro LA. Differential expression of HIF-1alpha in skin and mucosal wounds. J Dental Res 91: 871–876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christman JW, Christman BW, Shepherd VL, Rinaldo JE. Regulation of alveolar macrophage production of chemoattractants by leukotriene B4 and prostaglandin E2. Am J Respir Cell Mol Biol 5: 297–304, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27: 952–964, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol 304: C1141–C1149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci 54: 4109–4116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 192: 1120–1129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Intl J Lower Extremity Wounds 4: 154–159, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Doi S, Masaki T, Arakawa T, Takahashi S, Kawai T, Nakashima A, Naito T, Kohno N, Yorioka N. Protective effects of peroxisome proliferator-activated receptor gamma ligand on apoptosis and hepatocyte growth factor induction in renal ischemia-reperfusion injury. Transplantation 84: 207–213, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127: 514–525, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res 90: 718–725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLos One 6: e24358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filep JG. Resolution of inflammation: leukocytes and molecular pathways as potential therapeutic targets. Front Immunol 4: 256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galiano RD, Michaels Jt Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12: 485–492, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Gangoiti P, Arana L, Ouro A, Granado MH, Trueba M, Gomez-Munoz A. Activation of mTOR and RhoA is a major mechanism by which Ceramide 1-phosphate stimulates macrophage proliferation. Cell Signal 23: 27–34, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Gordon A, Kozin ED, Keswani SG, Vaikunth SS, Katz AB, Zoltick PW, Favata M, Radu AP, Soslowsky LJ, Herlyn M, Crombleholme TM. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen 16: 70–79, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Gregory SH. Substratum-dependent proliferation and survival of bone marrow-derived mononuclear phagocytes. J Leukoc Biol 43: 67–79, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem 280: 15267–15278, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Halapin NA, Bazan NG. NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res 35: 1944–1947, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann J, Tang Y, Spite M. Proresolving lipid mediators and diabetic wound healing. Curr Opin Endocrinol Diabetes Obes 19: 104–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocking AM. Mesenchymal stem cell therapy for cutaneous wounds. Adv Wound Care 1: 166–171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278: 14677–14687, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Jenner A, Ren M, Rajendran R, Ning P, Huat BT, Watt F, Halliwell B. Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic Biol Med 42: 559–566, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PC, Beggs JL, Olafsen AG, Watkins CJ. Unmyelinated nerve fiber estimation by immunocytochemistry. Correlation with electron microscopy. J Neuropathol Exp Neurol 53: 176–183, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Kang S, Lee D, Theusch BE, Arpey CJ, Brennan TJ. Wound hypoxia in deep tissue after incision in rats. Wound Repair Regen 21: 730–739, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLos One 5: e9539, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13: e23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Szilagyi E, Chen L, Premanand K, Dipietro LA, Ennis W, Bartholomew AM. Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J Surg Res 181: 20–24, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Lehman JA, Gomez-Cambronero J. Molecular crosstalk between p70S6k and MAPK cell signaling pathways. Biochem Biophys Res Commun 293: 463–469, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Ollague Sierra J, Zhu L, Tang L, Rahill K, El-Sabawi B, Liu-Mares W, Mertz PM, Davis SC. Effects of a topical aqueous oxygen emulsion on collagen deposition and angiogenesis in a porcine deep partial-thickness wound model. Exp Dermatol 22: 674–676, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Li SL, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, Natarajan R. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes 55: 2611–2619, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol 17: 54–65, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu HF, Zhang HJ, Hu QX, Liu XY, Wang ZQ, Fan JY, Zhan M, Chen FL. Altered polarization, morphology, and impaired innate immunity germane to resident peritoneal macrophages in mice with long-term type 2 diabetes. J Biomed Biotechnol 2012: 867023, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loo AE, Wong YT, Ho R, Wasser M, Du T, Ng WT, Halliwell B. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLos One 7: e49215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Tian H, Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res 51: 923–932, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem 278: 43807–43817, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170: 1178–1191, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matalka KZ, Tutunji MF, Abu-Baker M, Abu Baker Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: application to lipopolysaccharide-induced differential milieu of cytokines. Neuro Endocrinol Lett 26: 231–236, 2005. [PubMed] [Google Scholar]
- 52.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in Type 2 diabetic humans and mice. Diabetes 63: 1103–1114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, Hochbrugger EE, Fraisl P, Cinti S, Serhan CN, Stulnig TM. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 62: 1945–1956, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park HJ, Baen JY, Lee YJ, Choi YH, Kang JL. The TAM-family receptor Mer mediates production of HGF through the RhoA-dependent pathway in response to apoptotic cells. Mol Biol Cell 23: 3254–3265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park HJ, Choi YH, Cho YJ, Henson PM, Kang JL. RhoA-mediated signaling up-regulates hepatocyte growth factor gene and protein expression in response to apoptotic cells. J Leukoc Biol 89: 399–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parlapiano C, Danese C, Marangi M, Campana E, Pantone P, Giovanniello T, Zavattaro E, Sanguigni S. The relationship between glycated hemoglobin and polymorphonuclear leukocyte leukotriene B4 release in people with diabetes mellitus. Diabetes Res Clin Practice 46: 43–45, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, Chun M, Auster ME, Nabzdyk C, Deso S, Andersen N, Gnardellis C, LoGerfo FW, Veves A. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J Vasc Surg 58: 766–775 e712, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics 34: 162–184, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, Suganami T, Hasegawa K, Ogawa Y. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care 35: 2631–2639, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447: 869–874, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 17: 1–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered DHA pathway. Chem Biol 18: 976–987, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol 176: 1848–1859, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206: 15–23, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 19: 21–36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci 51: 1699–1708, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 62: 618–627, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian H, Lu Y, Shah SP, Hong S. 14S,21R-Dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem 286: 4443–4453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian H, Lu Y, Shah SP, Hong S. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am J Pathol 179: 1780–1791, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian H, Lu Y, Shah SP, Hong S. Novel 14S,21-dihydroxy-docosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure. J Cell Biochem 111: 266–273, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiganescu A, Tahrani AA, Morgan SA, Otranto M, Desmouliere A, Abrahams L, Hassan-Smith Z, Walker EA, Rabbitt EH, Cooper MS, Amrein K, Lavery GG, Stewart PM. 11beta-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J Clin Invest 123: 3051–3060, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toyoda M, Takayama H, Horiguchi N, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett 509: 95–100, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994. [DOI] [PubMed] [Google Scholar]
- 75.Wang M, Han RH, Han X. Fatty acidomics: global analysis of lipid species containing a carboxyl group with a charge-remote fragmentation-assisted approach. Analytical Chem 85: 9312–9320, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 115: 245–253, 2000. [DOI] [PubMed] [Google Scholar]
- 77.Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Qin S, Dipietro LA, Zloza A, Zhang C, Shafikhani SH. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLos One 2014: e91574, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeh J, Green LM, Jiang TX, Plikus M, Huang E, Chang RN, Hughes MW, Chuong CM, Tuan TL. Accelerated closure of skin wounds in mice deficient in the homeobox gene Msx2. Wound Repair Regen 17: 639–648, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeoh-Ellerton S, Stacey MC. Iron and 8-isoprostane levels in acute and chronic wounds. J Invest Dermatol 121: 918–925, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Yin J, Lee JH, Zhang J, Gao Z, Polotsky VY, Ye J. Regulation of hepatocyte growth factor expression by nf-kb and pparggamma in adipose tissue. Am J Physiol Endocrinol Metab 306: E929–E936, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 22: 111–119, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida S, Yamaguchi Y, Itami S, Yoshikawa K, Tabata Y, Matsumoto K, Nakamura T. Neutralization of hepatocyte growth factor leads to retarded cutaneous wound healing associated with decreased neovascularization and granulation tissue formation. J Invest Dermatol 120: 335–343, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 14: unit 14.1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLos One 6: e15816, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]