Abstract
The effector cells and second messengers participating in nitrergic neuromuscular transmission (NMT) were investigated in the mouse internal anal sphincter (IAS). Protein expression of guanylate cyclase (GCα, GCβ) and cyclic GMP-dependent protein kinase I (cGKI) were examined in cryostat sections with dual-labeling immunohistochemical techniques in PDGFRα+ cells, interstitial cells of Cajal (ICC), and smooth muscle cells (SMC). Gene expression levels were determined with quantitative PCR of dispersed cells from Pdgfrαegfp/+, KitcopGFP/+, and smMHCCre-egfp mice sorted with FACS. The relative gene and protein expression levels of GCα and GCβ were PDGFRα+ cells > ICC ≫ SMC. In contrast, cGKI gene expression sequence was SMC = ICC > PDGFRα+ cells whereas cGKI protein expression sequence was neurons > SMC ≫ ICC = PDGFRα+ cells. The functional role of cGKI was investigated in cGKI−/− mice. Relaxation with 8-bromo (8-Br)-cGMP was greatly reduced in cGKI−/− mice whereas responses to sodium nitroprusside (SNP) were partially reduced and forskolin responses were unchanged. A nitrergic relaxation occurred with nerve stimulation (NS, 5 Hz, 60 s) in cGKI+/+ and cGKI−/− mice although there was a small reduction in the cGKI−/− mouse. Nω-nitro-l-arginine (l-NNA) abolished responses during the first 20–30 s of NS in both animals. The GC inhibitor ODQ greatly reduced or abolished SNP and nitrergic NS responses in both animals. These data confirm an essential role for GC in NO-induced relaxation in the IAS. However, the expression of GC and cGKI by all three cell types suggests that each may participate in coordinating muscular responses to NO. The persistence of nitrergic NMT in the cGKI−/− mouse suggests the presence of a significant GC-dependent, cGKI-independent pathway.
Keywords: enteric, interstitial cells of Cajal, PDGFRα+ cells, gastrointestinal, cGMP-dependent protein kinase
the internal anal sphincter (IAS) relaxes during defecation to permit evacuation of fecal contents. This relaxation is mediated by inhibitory motor neurons and NO plays a prominent role as a neurotransmitter in this response (7, 38). However, there is still uncertainty regarding the effector cells and second messengers that participate in nitrergic neuromuscular transmission (NMT) in the IAS.
Activation of nNOS in enteric motor neurons results in release of NO and smooth muscle relaxation (3, 46). A key receptor for NO in visceral muscles is soluble guanylate cyclase (GC), a heterodimeric enzyme containing α and β subunits that converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP) (12). An important downstream mediator of cGMP is cyclic GMP-dependent protein kinase I (cGKI; also referred to as PKGI), a serine/threonine kinase that exists in two isoforms cGKIα and cGKIβ produced by alternative splicing. cGKI has been shown to phosphorylate a number of different substrates, including ion channels and other proteins responsible for regulating the contractile state of smooth muscles (17, 39, 47, 50).
The pathway by which neurally released NO evokes relaxation in gastrointestinal (GI) muscles differs significantly from the pathways activated when NO is released from vascular endothelium. In the latter case, NO acts directly upon smooth muscle cells (SMC), activating second messenger pathways that cause relaxation. In contrast, there is substantial evidence that additional cells participate in nitrergic NMT in the GI tract, the most widely studied of these being interstitial cells of Cajal (ICC). ICC make very close contacts with varicose terminals of enteric motor neurons (10), express GC (26), synthesize cGMP (49), and generate hyperpolarization (4) in response to nitrergic stimulation. Recently, a new class of interstitial cells, identified by the expression of platelet-derived growth factor α (PDGFRα), has been described and become known as PDGFRα+ cells (24, 31). In contrast to ICC, little evidence links PDGFRα+ cells to nitrergic NMT, whereas these cells are thought to be responsible for the purinergic component of inhibitory NMT (30, 31).
ICC and PDGFRα+ cells are both closely associated with nNOS+ neurons in the IAS (8), so the present study examines whether effector proteins involved in nitrergic NMT are expressed predominantly by one or both of these interstitial cells. To do this, gene expression patterns of GC and cGKI were examined in purified populations of PDGFRα+ cells, ICC, and SMC. In addition we examined the protein expression patterns of GC and cGKI in PDGFRα+ cells, ICC, and SMC in intact muscles using immunohistochemical techniques. Finally, functional experiments were undertaken to examine the role of GC and cGKI in nitrergic NMT in the IAS. To do this we used cGKI−/− mice and a pharmacological inhibitor of GC. Some of the functional experiments on cGKI−/− mice were repeated on aortic smooth muscle to compare the phenotype of the cGKI−/− mouse used in this study to a strain of cGKI−/− mice studied previously (41). Our results suggest that nitrergic relaxation in the IAS is mediated by multiple effector cells and second messenger pathways, raising the possibility that unique targets can be identified that might aid in treating defecatory disorders.
METHODS
Animals
Mice (21–90 days old) were killed with isoflurane (Baxter, Deerfield, IL) followed by either cervical dislocation or decapitation (when aorta was required). All mice used in these studies were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments and procedures were performed with approval from the Institutional Animal Use and Care Committee at the University of Nevada, Reno. KitcopGFP/+ mice were generated and bred in house (42). C57BL/6 (wild-type, WT), Pdgfrαegfp/+, smMHCCre-egfp, and cGKI+/− mice (Prkg1Tn(sb-rtTA)2497B.SB2Ove; Fvb/N background) were purchased from Jackson Laboratories, Bar Harbor, ME.
cGKI+/− mice were bred in house to generate cGKI−/− and cGKI+/+ mice. Functional knockout of Prkg involves insertion of ∼14,000 bp into intron 10 of Prkg1 (https://www.mmrrc.org/catalog/sds.php?mmrrc_id=36283/036283.html). To test for mutation status, genomic DNA was examined with two different primer sets (i.e., 5′-ATTTGTCTAGCTCCCAATTCCA and 5′-TTGGCAGAAACAATGACATAGC) that flank the site in intron 10 where the transposon inserts. In cGKI+/+ and cGKI+/− mice these primers amplify a 750-bp band whereas no band is seen in the cGKI−/− mouse. Two additional primers were used to identify the transposon (i.e., 5′-ATTTGTCTAGCTCCCAATTCCA and 5′-GACTTGTGTCATGCACAAAGTAGATGTCC). In cGKI−/− and cGKI+/− mice these primers amplify a ∼500-bp band whereas no band is seen in cGKI+/+ mice.
cGKI−/− mice were smaller in size than cGKI+/+ and cGKI+/− littermates and die either before weaning or shortly thereafter (i.e., 4–6 wk of age, Paul Overbeek, Baylor College of Medicine, personal communication). Experiments were carried out shortly after weaning (i.e., 23 ± 0.7 days after birth). The stomach, intestine, cecum, and spleen of cGKI−/− mice were enlarged and the liver was pale (C. A. Cobine and K. D. Keef, personal observation). The average body weight of cGKI−/− mice was 86% of that of sex-matched cGKI+/+ littermates on the day of euthanasia (i.e., 11.0 ± 0.6 vs. 12.8 ± 0.7 g, n = 10 litters; P < 0.05, paired t-test).
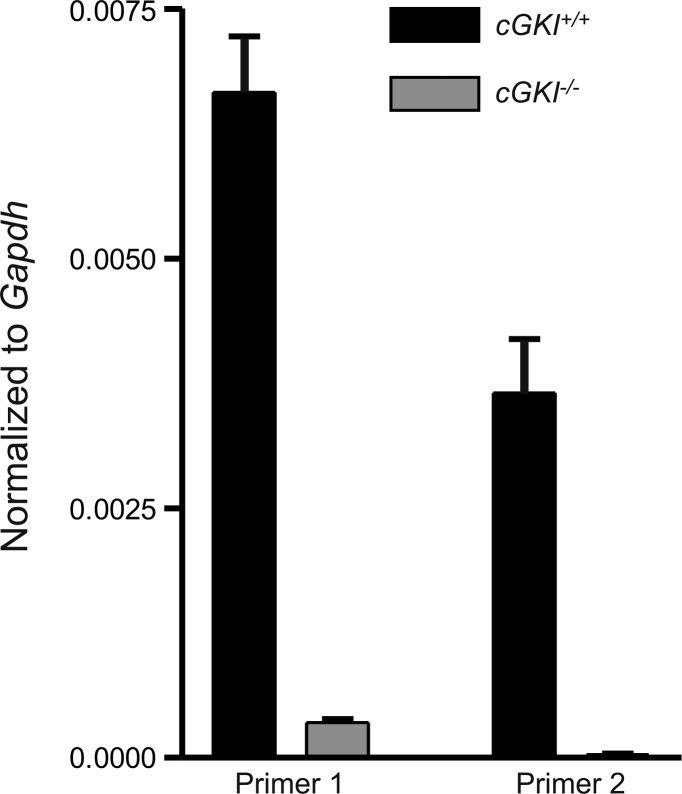
To verify cGKI expression in cGKI+/+ but not cGKI−/− mice RNA, transcripts were examined with two different primers sets. Primer 1 targeted a sequence spanning exons 5 and 6; a region preceding the insertion at intron 10 and primer 2 targeted a sequence spanning exons 11 to 13; a region subsequent to the insertion at intron 10 (see Table 1). Prkg1 expression was identified with primer 1 in cGKI+/+ mice and a small but detectible signal was seen in cGKI−/− mice. In contrast, primer 2 identified Prkg1 expression in cGKI+/+ but not in cGKI−/−, indicating disruption of Prkg1 in cGKI−/− mice (Fig. 1).
Table 1.
Primer sequences used for quantitative PCR
| Gene Name, Forward and Reverse Primers | Primer Sequence | Accession No. | Expected Size, base pairs | Exons Spanned |
|---|---|---|---|---|
| Gapdh-F | TGAACGGATTTGGCCGTATTG | NM_008084 | 200 bp | 2–3 |
| Gapdh-R | GATGGGCTTCCCGTTGATGA | |||
| Pdgfra-F | ATGACAGCAGGCAGGGCTTCAACG | NM_011058 | 195 bp | 4–5 |
| Pdgfra-R | CGGCACAGGTCACCACGATCGTTT | |||
| Myh11-F | CTACACTGCGCAATACCACG | NM_013607 | 200 bp | 16–17 |
| Myh11-R | TGCCAGGATCTCATAGCGTT | |||
| Kit-F | CGCCTGCCGAAATGTATGACG | NM_021099 | 162 bp | 19–21 |
| Kit-R | GGTTCTCTGGGTTGGGGTTGC | |||
| Uchl1-F | CGATGGAGATTAACCCCGAGATG | NM_011670 | 169 bp | 1–4 |
| Uchl1-R | TTTTCATGCTGGGCCGTGAG | |||
| Gucy1a3-F | TGTTCACCTCTGCAGGTCAT | NM_021896 | 115 bp | 6–7 |
| Gucy1a3-R | CCACACAATATGCATCCCCG | |||
| Gucy1b3-F | GATCCGCAATTATGGTCCCG | NM_017469 | 171 bp | 2–4 |
| Gucy1b3-R | AACATCTGCAGGATTTCGCC | |||
| Prkg1-F (primer 1) | TATCATCAGGCAGGGTGCAA | NM_011160 | 192 bp | 6–7 |
| Prkg1-R (primer 1) | GACAGCTTCTGCGGCAATAA | |||
| Prkg1-F (primer 2) | AGGGGGCTCATTCTGACTTC | NM_011160 | 178 bp | 11–13 |
| Prkg1-R (primer 2) | GGCGAATGCTTCTACCACAC |
Fig. 1.
Evaluation of Prkg1 gene expression in cGKI+/+ and cGKI−/− mice. Prkg1 expression was identified with 2 different primers (normalized to Gapdh) in whole small intestine of cGKI+/+ and cGKI−/− mice. Primer 1 targets a sequence spanning exons 5–6 and primer 2 targets a sequence spanning exons 11–13 (see Table 1). A small level of expression was observed in the first region of Prkg1 in the cGKI−/− mouse but not the second region; n = 4 cGKI+/+, n = 4 cGKI−/−.
Tissue Preparation
Tissues from the rectoanal region were obtained as previously described (21) and the distal most 1.5 mm of the GI tract was used. Thoracic aortas were obtained from the thoracic cavity after dissecting away the lungs and esophagus. Rectoanal tissues and aortas were subsequently pinned in a Sylgard bottomed dissection dish containing cold Krebs-Ringer bicarbonate solution (KRBS) of the following composition: 118.5 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 23.8 mM NaHCO3, 1.2 mM KH2PO4, 11.0 mM dextrose. This solution had a pH of 7.4 at 37°C when bubbled to equilibrium with 95% O2-5% CO2.
Immunohistochemistry
Rings of IAS muscle were fixed in ice-cold paraformaldehyde solution (4% wt/vol) and washed in 0.1 M phosphate buffer solution (PBS) overnight before dehydration in graded sucrose solutions (5, 10, and 15%, 15 min each; 20% overnight) and embedding in Tissue-Tek OTC compound (Sakura Finetek, Torrance, CA). Tissues were then frozen and stored at −80°C. Sections were cut parallel to the circular muscle layer at a thickness of 10–12 μm using a Leica CM 3050 cryostat (Leica Microsystems, Wetzlar, Germany). Tissues were preincubated with BSA (1% wt/vol) for 1 h at 20°C to minimize nonspecific labeling. Tissues were then incubated in the first primary antibody [i.e., either mSCFR (Kit) or PDGFRα] for 16 h at 4°C and then washed in 0.1 M PBS for 4 h to remove any unbound antibody. After washing, tissues were incubated with secondary antibodies (i.e., donkey anti-goat 594) for 1 h at 20°C and then washed with 0.1 M PBS overnight at 4°C. Tissues were incubated with BSA for a second time (1 h at 20°C) before labeling with the second primary antibody (i.e., GCα, GCβ, or cGKI) for 16 h at 4°C and then were washed in 0.1 M PBS for 4 h before labeling with secondary antibodies (i.e., donkey anti-rabbit 488) for 1 h at 20°C. After labeling with secondary antibodies, tissues were washed with 0.1 M PBS overnight at 4°C and subsequently covered with coverslips with use of Aquamount mounting medium (Lerner Laboratories, Pittsburgh, PA).
The following primary antibodies were used in these immunohistochemistry experiments: mSCFR (Kit), 1:500 (R&D Systems, Minneapolis, MN); PDGFRα, 1:1,000 (R&D Systems); GCα, 1:1,000 (Sigma-Aldrich, St. Louis, MO); GCβ (ER-19), 1:1,000 (Sigma-Aldrich); cGMP-dependent protein kinase (cGKI, PKGI), 1:100 to 1:4,000 (Enzo Life Sciences, Plymouth Meeting, PA). Alexa Fluor secondary antibodies were obtained from Molecular Probes, Eugene, OR.
Imaging.
Tissues were imaged via a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Thornwood, NY). Captured images are digital composites of Z-series of 0.25- to 1-μm optical sections through a depth of 0.5–10 μm. Final images were constructed by use of Zeiss LSM 510 Image Examiner Software, Adobe Photoshop CS5 Software, and CorelDRAW X4 Software.
Cell dispersion.
Strips of IAS from each of the various green fluorescent protein (eGFP or CopGFP)-expressing mice (i.e., Pdgfrαegfp/+, smMHCCre-egfp, and KitcopGFP/+ mice) were cut into four to five smaller pieces in the direction of the circular muscle. Tissues were dissected in Ca2+-free Hanks' solution consisting of (in mM) 125 NaCl, 5.36 KCl, 15.5 NaHCO3, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose, and 11 HEPES adjusted to pH 7.2 with NaOH. IAS pieces were incubated at 37°C for 30 min in an enzymatic cocktail containing 4 mg/ml collagenase type 2 (Worthington Biochemical, Lakewood, NJ), 8 mg/ml bovine serum albumin (Sigma-Aldrich), and 8 mg/ml trypsin inhibitor (Sigma-Aldrich). Tissues were then washed three times in Hanks' solution to remove all enzymes and triturated through a series of blunt pipettes of decreasing tip diameter in a final volume of ∼1.5 ml. Although eGFP is confined to the nucleus of cells dispersed from Pdgfrαegfp/+, we have referred to these cells as “PDGFRα+ cells” since we have previously shown that they are PDGFRα immunopositive (8).
Fluorescence-activated cell sorting.
Dispersed cells (see above) were filtered through 100-μm mesh filters (Partec, Swedesboro, NJ) before being loaded onto BD Bioscience FACSAria II SORP (BD Biosciences, San Jose, CA). The instrument was used to sort green fluorescent protein-positive (GFP+) cells using a blue laser (488 nm) and the GFP/fluorescein isothiocyanate emission detector (530/30 nm band-pass and 505 nm low-pass). A 130-μm nozzle was used at a sheath pressure of 12 psi and sort rate of 200 to 800 events/s. Live cells, gated on exclusion of Hoechst 33258 viability indicator dye were subsequently gated on GFP fluorescence intensity. FloJo software version 8.8.7 (Treestar, San Carlos, CA) were used to graphically plot the sort counts.
Gene expression.
The total RNA was isolated from collected GFP+ cells and unsorted cells (cells obtained from initial dispersions before cell sorting) using an illustra RNAspin Mini RNA Isolation Kit (GE Healthcare). qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) was used to synthesize first-strand cDNA. Since the number of cells in each sample was variable, a known quantity of total RNA (1 μg) was used from each sample according to the manufacturer's instructions. GoTaq DNA Polymerase (Promega, Madison, WI) was used to perform RT-PCR with the gene-specific primers listed in Table 1. Resulting PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers and first-strand cDNA as those used for RT-PCR. The reactions were carried out in 20-μl volumes and in technical duplicates containing 10 μl Fast Sybr green (Applied Biosystems), 125 nM of each primer, 6 μl molecular biology-grade water, and 2 μl of each template after cDNA synthesis. Thermal cycling consisted of denaturation (95°C for 1 s), annealing, and extension (60°C for 20 s), performed in 40 cycle steps in the 7900HT Fast Real-Time PCR System (Applied Biosystems). By the relative standard curve method, standard curves were generated for each set of primers using serially diluted solutions of cDNA. Unknown quantities relative to the standard curve for the gene-specific primers were calculated. These values were normalized to endogenous references glyceraldehyde-3-phosphate dehydrogenase (Gapdh), hypoxanthine guanine phosphoribosyl transferase (Hprt), actin β (Actb), and β-2 microglobulin (B2m). Since results obtained were very similar we chose Gapdh as our reference because it has proven from past experiments to be a good reference for GI tissues and the cell types used (40). Data are presented as means ± SE. Significance among groups was tested by Student's t-test. P values <0.05 were considered significant. Details of the primers used are listed in Table 1.
Whole tissue RT-PCR and qPCR.
Total RNA was isolated from whole tissue by using TRIzol Reagent (Life Technologies) following the manufacturer's protocol. qScript cDNA SuperMix (Quanta Biosciences) was used to synthesize first-strand cDNA according to the manufacturer's instructions. GoTaq DNA Polymerase (Promega) was used to perform RT-PCR with the gene-specific primers listed in Table 1. Resulting PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. qPCR was performed with the same primers as those used for RT-PCR as described above.
Contraction Experiments
Internal anal sphincter.
Muscle strips (1.5 mm × 8 mm) containing the IAS of cGKI−/− and cGKI+/+ mice were attached to a Gould strain gauge and a stable mount containing platinum stimulating electrodes as previously described (28). Spontaneous contractile activity developed over 20–30 min following immersion in warm oxygenated KRBS. Tissues were equilibrated for 60 min before the beginning of experiments that lasted 2.5–4 h. The few muscles that did not maintain consistent spontaneous contractile activity over this time period were discarded. Experiments from cGKI−/− and cGKI+/+ littermates were carried out at the same time. Concentration-response relationships were determined by cumulative addition of drug. For sodium nitroprusside (SNP) where cumulative additions were repeated a second time, at least 40-min reequilibration time was allotted between the first (control) and second [+1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ)] cumulative addition. Electrical field stimulation (EFS) of nerves (0.2-ms-duration pulses, 15 V, 5 Hz, 60 s) gave rise to tetrodotoxin (TTX, 1 μM) sensitive neural responses. All experiments were carried out in the presence of atropine (1 μM) and guanethidine (1 μM), i.e., nonadrenergic, noncholinergic (NANC) conditions. Data were collected and subsequently analyzed by use of AcqKnowledge data-acquisition software (AcqKnowledge 3.9.1; Biopac Systems, Goleta, CA).
Aorta.
Ring segments 4 mm long of cGKI−/− and cGKI+/+ mouse thoracic aorta were mounted on two wire triangles. The upper triangle was attached to a Gould strain gauge and the lower triangle to a stable mount. Rings were immersed in tissue baths containing KRBS maintained at 37°C. Aorta tissues were stretched initially to 0.3 g tension and then were allowed to equilibrate for 60 min. All experiments were carried out in the presence of the NOS inhibitor Nω-nitro-l-arginine (l-NNA; 100 μM) to eliminate the effects of release of NO from the endothelium. To evaluate responses to various nitrergic agents muscles were first contracted with 0.1 μM norepinephrine (NE). Data was collected and subsequently analyzed by use of AcqKnowledge data-acquisition software (AcqKnowledge 3.9.1; Biopac Systems).
Contraction Analysis
The contractile area during EFS was evaluated with AcqKnowledge software by determining the integral of the contractile trace in 5-s (i.e., 0–5 s, 5–10 s) or 10-s (i.e., 10–20 s, 20–30 s, 30–40 s, 40–50 s, 50–60 s) increments during trains of EFS (i.e., 60 s). The area during EFS was normalized to the prestimulus contractile area and expressed as “% change from control activity.” Baseline (i.e., zero active tension) was determined at the end of the experiment by the addition of 1 μM nifedipine and 10 μM SNP. Aortic relaxations were expressed as a percent of the initial contraction elicited with NE.
Statistics
Individual data points are expressed as means ± SE. Data sets for concentration-response relationships were fit with nonlinear regression by use of GraphPad Prism Software (3.02; San Diego, CA). IC50 values were obtained from these curves. Statistical analysis of multiple data sets was performed by one-way ANOVA followed by a post hoc Tukey multiple-comparison test. Data sets were considered significantly different when P < 0.05. Statistical analysis of two data sets was determined with two-tailed, paired or unpaired Student's t-test and considered significantly different when P < 0.05. N values indicate the number of animals used except for qPCR experiments on isolated cells where n indicates the number of samples analyzed with each sample containing tissues from three animals.
Drugs
Atropine sulfate, l-NNA, guanethidine, SNP, ODQ, 8-bromo (8-Br)-cGMP, forskolin, NE, TTX, and nifedipine were all purchased from Sigma-Aldrich. MRS2500, ODQ, 8-Br-cGMP, and forskolin were purchased from Tocris Bioscience (Ellisville, MO). Atropine, l-NNA, guanethidine, SNP, MRS2500, TTX, and NE were dissolved in deionized water. 8-Br-cGMP was dissolved in warm (37°C) KRBS. Forskolin and nifedipine were dissolved in ethanol and ODQ was dissolved in DMSO.
RESULTS
Guanylate cyclase Immunoreactivity in the Wild-Type Mouse IAS
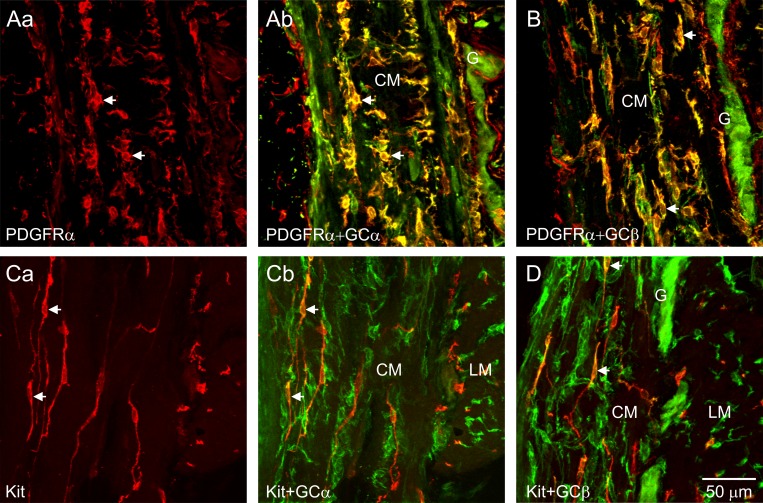
Dual-labeling immunohistochemistry was used to evaluate the expression patterns of GCα and GCβ (green) and interstitial cells (red) in the muscularis of the wild-type (WT) mouse IAS. Intense GCα and GCβ immunoreactivity was present in intramuscular PDGFRα+ cells (PDGFRα+-IM) (Fig. 2, A and B). Cellular colocalization is indicated in merged files by the color yellow. Intramuscular ICC (ICC-IM; Kit) also displayed weaker immunoreactivity for GCα and GCβ (Fig. 2, C and D). Strong GCα and GCβ immunoreactivity was also observed in enteric neurons within myenteric ganglia (Fig. 2, A, B, and D).
Fig. 2.
Immunohistochemical identification of guanylate cyclase (GC) in PDGFRα+ cells and interstitial cells of Cajal (ICC) in the wild-type (WT) mouse internal anal sphincter (IAS). The distribution of PDGFRα+ cells (Aa) and ICC (Ca) across the circular muscle (CM) layer of the WT mouse IAS are shown in red whereas the corresponding merged files of PDGFRα+GCα or Kit+GCα are shown in Ab and Cb, respectively. A and B: guanylate cyclase α (GCα; Ab; green) and guanylate cyclase β (GCβ; B; green) immunoreactivity was observed in PDGFRα+ cells (red) within the CM layer (arrows, yellow indicates colocalization) and in the myenteric region but not in PDGFRα+ cells located in the submucosa (left) (see also Fig. 3). C and D: weaker GCα (Cb; green) and GCβ (D; green) immunoreactivity was present in intramuscular ICC (ICC-IM; red) within the CM (see arrows). Strong GCα and GCβ immunoreactivity was also observed within enteric neurons in myenteric ganglia (G; A, B, D). LM, longitudinal muscle.
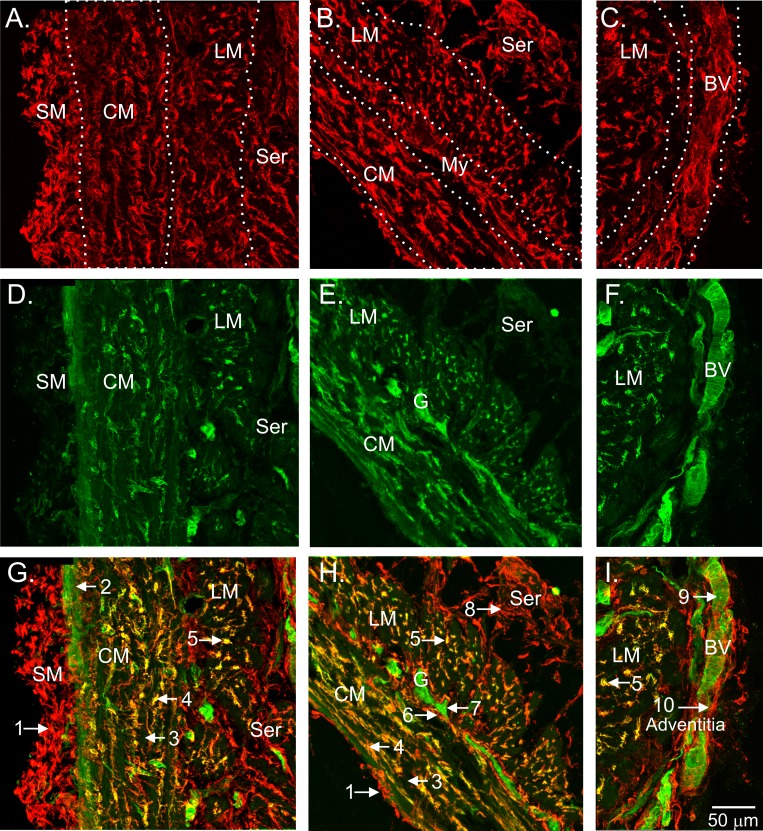
The relationship between PDGFRα+ cells and GC expression was further clarified in cryostat sections encompassing the entire muscular wall. PDGFRα+ cells were densely distributed throughout the thickness of the muscularis including the submucosa (SM), circular muscle (CM), myenteric region, longitudinal muscle (LM), serosa, and adjacent vascular structures. However, dual labeling of these sections with anti-GCβ antibody revealed that strong GCβ immunoreactivity was limited to PDGFRα+-IM in the CM (Fig. 3, A, B, D, E, G, H) and LM (Fig. 3, A–I) layers, suggesting that these cells could serve a specialized function in nitrergic NMT. Immunoreactivity for GCβ was also noted in spaces between PDGFRα+-IM (i.e., the location of SMC), but these occurrences were largely confined to the region adjacent to the submucosa (Fig. 3, D and G). In contrast to GI tissues, SMC in blood vessels adjacent to the serosa exhibited strong GCβ immunoreactivity, whereas GCβ was not detected in the PDGFRα+ cells associated with and surrounding these vessels (Fig. 3I). A similar relationship between PDGFRα+ cells and GCα immunoreactivity was observed (e.g., Fig. 2).
Fig. 3.
Cellular colocalization of GCβ with intramuscular PDGFRα+ cells of the WT mouse IAS. The distribution of PDGFRα+ cells across the muscularis of the WT mouse IAS is shown (red; A–C). The various divisions of the muscle wall are differentiated by dotted white lines for clarity. GCβ labeling of these specimens is shown in green (D–F). Merged images are shown in G–I. PDGFRα+ cells are present in the submucosa (SM; A, G), in CM and LM layers (A–C, G–I), in surrounding myenteric ganglia (My; B, H), in the serosa (Ser; A, B, G, H), and in the adventitia of blood vessels (BV; C, I). GCβ expression (green) is observed between PDGFRα+ cells within the CM near the SM (2; G) but not elsewhere in the CM (3; G, H). PDGFRα+ cells within the CM (4; G, H) and LM (5; G–I) exhibit GCβ immunoreactivity (yellow), as do enteric neurons within myenteric ganglia (7; E, H) and vascular smooth muscle cells (9; F, I). Weak expression of GCβ was also observed in some PDGFRα+ cells surrounding myenteric ganglia (6; E, H). GCβ-was not detected in PDGFRα+ cells in the SM (1; G), Ser (8; G, H) or adventitia (10; I). A, D, G and C, F, I are composite images from 2 separate confocal stacks assembled using Adobe Photoshop CS5 software.
cGMP-Dependent Protein Kinase Immunoreactivity in the WT Mouse IAS
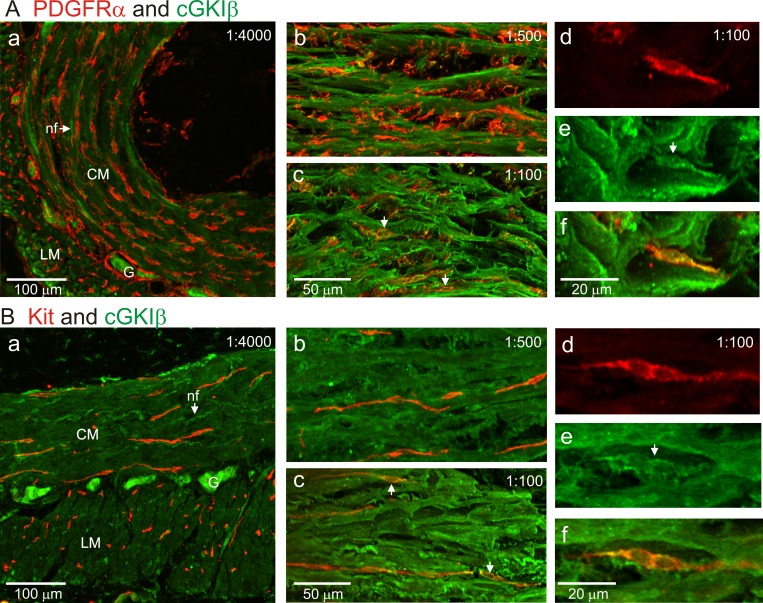
The expression levels of cGMP-dependent protein kinase Iβ (cGKIβ) were also examined in the muscularis with dual-labeling immunohistochemical techniques. Labeling with anti-cGKIβ antibody was carried out by serial dilutions ranging from 1:100 to 1:4,000. At 1:4,000, strong cGKIβ immunoreactivity was detected in myenteric neurons as well as in intramuscular nerve fibers (Fig. 4, Aa and Ba). Although some nerve fibers with cGKIβ immunoreactivity were located in close proximity to PDGFRα+-IM (Fig. 4Aa) and ICC-IM (Fig. 4Ba), cGKIβ immunoreactivity was not resolved in either type of interstitial cell at this antibody concentration. Weak cGKIβ immunoreactivity was detected in spaces between ICC-IM and PDGFRα+-IM cells where SMC are located. At an antibody dilution of 1:500, much stronger cGKIβ immunoreactivity was detected in cells located between ICC-IM and PDGFRα+-IM (Fig. 4, Ab and Bb), but it was still not resolved in interstitial cells. At the highest antibody concentration tested (i.e., 1:100 dilution), weak cGKIβ immunoreactivity was detected in PDGFRα+-IM (Fig. 4, Ac and Af) and ICC-IM (Fig. 4, Bc and Bf). These data suggest the following cGKIβ protein expression sequence: neurons > SMC ≫ PDGFRα+ cells = ICC.
Fig. 4.
cGKI immunoreactivity in the WT mouse IAS. Dual labeling is shown in the mouse IAS by use of various dilutions of an anti-cGKIβ antibody (i.e., 1:4,000, 1:500, and 1:100; green) with either an anti-PDGFRα antibody (red; A) or an anti-Kit antibody (red; B). At the greatest antibody dilution of 1:4,000 (Aa, Ba), strong cGKIβ immunoreactivity was observed in myenteric ganglia (G) and intramuscular nerve fibers (nf, indicated by arrows). Within the CM and LM layers weak cGKIβ immunoreactivity was observed between interstitial cells [i.e., where smooth muscle cells (SMC) are located]. cGKIβ immunoreactivity was not detected in either PDGFRα+ cells (Aa) or ICC-IM (Ba) at this antibody dilution. With an antibody dilution of 1:500, strong cGKIβ immunoreactivity was observed between interstitial cells but not in either PDGFRα+ cells (Ab) or ICC-IM (Bb). At an antibody dilution of 1:100, weak cGKIβ immunoreactivity was now observed in both PDGFRα+ cells (Ac–Af) and ICC-IM (Bc–Bf). Ad–Af and Bd–Bf are closeup excerpts taken from original 40× images with 1:100 cGKIβ antibody dilution. An intramuscular PDGFRα+ cell is shown (red, Ad) that displayed cGKIβ immunoreactivity (green, Ae) resulting in cellular colocalization (Af). An intramuscular ICC is shown (red, Bd) that displayed cGKIβ immunoreactivity (green, Be) resulting in cellular colocalization (Bf).
Gene Expression of GCα, GCβ, and cGKI in WT IAS and Aorta
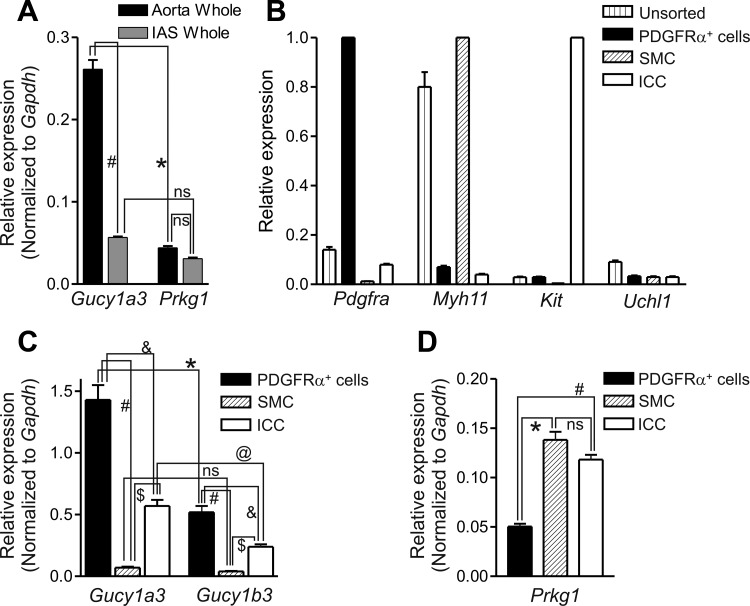
The relative expression levels of genes encoding GCα (Gucy1a3) and cGKI (Prkg1) were examined in whole IAS with qPCR (Fig. 5A). Gene expression levels were also determined in whole aorta, as a control and because this vessel was used later to evaluate the phenotype of cGKI−/− mice. Gucy1a3 expression in aorta was approximately five times greater than in IAS (P < 0.05). It was also approximately five times greater than Prkg1 expression in aorta (P < 0.05). In contrast, Prkg1 levels were not significantly different between muscles (Fig. 5A).
Fig. 5.
Gene expression of Gucy1 and Prkg1 in the mouse IAS and aorta. A: relative expression of Gucy1a3 (GCα gene) and Prkg1 in whole aorta and IAS tissues normalized to Gapdh. Gucy1a3 expression was significantly greater than Prkg1 expression in aorta (*P < 0.05) but not in IAS [not significant (ns)]. Gucy1a3 expression was also ∼5× greater in aorta (#P < 0.05) than in IAS. Prkg1 expression was not significantly different between muscles. B: gene expression patterns for GFP+ cells sorted from the IAS of Pdgfrαegfp/+, smMHCCre-egfp, and KitcopGFP/+ mice. Pdgfra, Myh11, and Kit levels were normalized to the cell exhibiting the greatest gene expression of these markers, i.e., PDGFRα+ cells, SMC, and ICC, respectively. Uchl1 was normalized to whole brain. The expression levels in unsorted cells is also shown. Pdgfra was most highly expressed in PDGFRα+ cells, Myh11 in SMC, and Kit in ICC. Uchl1 expression was low in all 4 cell samples. C: histogram of Gucy1a3 and Gucy1b3 (GCβ gene) expression in sorted PDGFRα+ cells, SMC, and ICC. Gucy1a3 levels were significantly greater than Gucy1b3 levels in PDGFRα+ cells (*P < 0.05) and in ICC (@P < 0.05) but not SMC (ns). Gucy1a3 and Gucy1b3 expression levels in PDGFRα+ cells were significantly greater than in ICC (&P < 0.05) and SMC (#P < 0.05) whereas Gucy1a3 and Gucy1b3 expression levels in ICC were significantly greater than in SMC ($P < 0.05). Thus the sequence of expression for both Gucy1a3 and Gucy1b3 was PDGFRα+ cells > ICC ≫ SMC. D: histogram of Prkg1 expression. Prkg1 expression was greatest in SMC and ICC and significantly less in PDGFRα+ cells (*,#P < 0.05), resulting in the sequence SMC = ICC > PDGFRα+ cells; n = 3 samples, each sample comprised of muscles from 3 animals.
Additional qPCR measurements were undertaken on cells isolated from the IAS using fluorescence-activated cell sorting (FACS). PDGFRα+ cells, ICC, and SMC were isolated from Pdgfrαegfp/+, KitcopGFP/+, and smMHCCre-egfp mice, respectively. Each cell population was evaluated for purity by determining expression levels of cell-specific genes [e.g., Pdgfra (PDGFRα+ cell marker), Kit (ICC marker gene), Myh11 (smooth muscle myosin heavy chain gene), and Uchl1 (protein gene product 9.5)] (Fig. 5B). Pdgfra was enriched in eGFP+ cells isolated from the Pdgfrαegfp/+ mouse IAS, Kit was enriched in copGFP+ cells isolated from the KitcopGFP/+ mouse, and Myh11 was enriched in eGFP+ cells isolated from the smMHCCre-egfp mouse, indicating that appropriate cells were obtained by the sorting strategies used. In contrast, transcripts for the neuronal marker Uch1 (normalized to brain) were present only in trace amounts, demonstrating little contamination of sorted cells by neurons.
The relative transcript expression of Gucy1a3 and Gucy1b3 was evaluated in extracts of isolated cells (Fig. 5C). PDGFRα+ cells exhibited the greatest levels of Gucy1a3 and Gucy1b3 expression. Significant transcript was also observed in ICC whereas the lowest expression levels were in SMC. Thus Gucy1 expression levels were PDGFRα+ cells > ICC ≫ SMC. The expression of genes encoding GCα and GCβ was therefore consistent with the expression of these proteins determined by immunohistochemistry (Fig. 2, 3).
The relative expression levels of Prkg1 transcript was also evaluated in extracts of isolated cells, using primer 1 that targets a sequence common to the alpha and beta isoforms of the gene (see Table 1 for details). Prkg1 transcript levels were the same in SMC and ICC but were significantly less (P < 0.05) in PDGFRα+ cells, giving rise to the sequence SMC = ICC > PDGFRα+ cells (Fig. 5D).
Comparison of Nitrergic-Mediated Relaxations in the IAS and Aorta of cGKI+/+ and cGKI−/− Mice
The role(s) of cGKI in nitrergic NMT in the IAS was investigated by evaluating responses to NO donors and second messengers that activate the NO pathway in the IAS of cGKI+/+ and cGKI−/− mice. Since previous studies examined the role of cGKI in nitrergic responses with a different strain of cGKI−/− mice (37, 41), we repeated some of the experiments previously performed on the aorta to confirm the phenotype of the strain of cGKI−/− mice used in the present study.
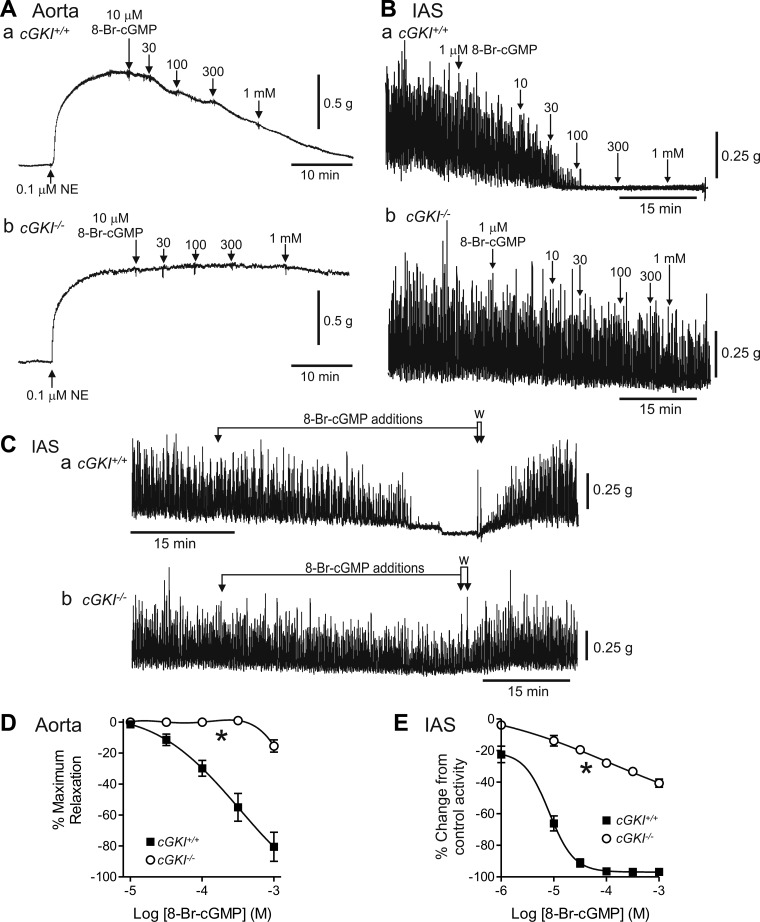
Relaxation responses to 8-Br-cGMP (a brominated membrane permeable derivative of cGMP that directly activates cGKI) were compared in cGKI+/+ and cGKI−/− mice (Fig. 6). In keeping with the protocol used in a previous study of cGKI−/− mice (41), aorta segments were precontracted with 0.1 μM NE before addition of relaxation agents. In the cGKI+/+ mouse, 8-Br-cGMP gave rise to a concentration-dependent relaxation, i.e., block of NE-induced contraction in the aorta (Fig. 6Aa) and block of the phasic activity that sums to create tone in IAS (Ref. 21; Fig. 6Ba). In cGKI−/− mice, relaxation responses were greatly attenuated (P < 0.05) and did not reach 50% relaxation at the highest concentration tested, i.e., 1 mM 8-Br-cGMP (Fig. 6, D and E). Removal of 8-Br-cGMP returned spontaneous activity in the IAS to the previous control level (Fig. 6C).
Fig. 6.
Comparison of 8-bromo (8-Br)-cGMP-mediated relaxations in the cGKI+/+ and cGKI−/− aorta and IAS. Sample traces showing relaxations elicited with 8-Br-cGMP in the cGKI+/+ aorta (Aa) and IAS (Ba) and in the cGKI−/− aorta (Ab) and IAS (Bb). 8-Br-cGMP responses were absent at concentrations up to 300 μM in the cGKI−/− aorta (Ab, D) whereas some relaxation persisted with ≥1 μM in the cGKI−/− IAS (Bb, E). Both differences were significant (*P < 0.05). The decline in spontaneous contractions in the IAS of cGKI+/+ (Ca) and cGKI−/− (Cb) mice during drug addition was not due to rundown since activity returned to the control level following washout (W, arrows). IC50 values for the relaxation in cGKI+/+ were 3.2 × 10−4 M and 8.1 × 10−6 M for aorta and IAS, respectively; n = 4 cGKI+/+, n = 5 cGKI−/− aorta; n = 4 cGKI+/+, n = 5 cGKI−/− IAS. NE, norepinephrine.
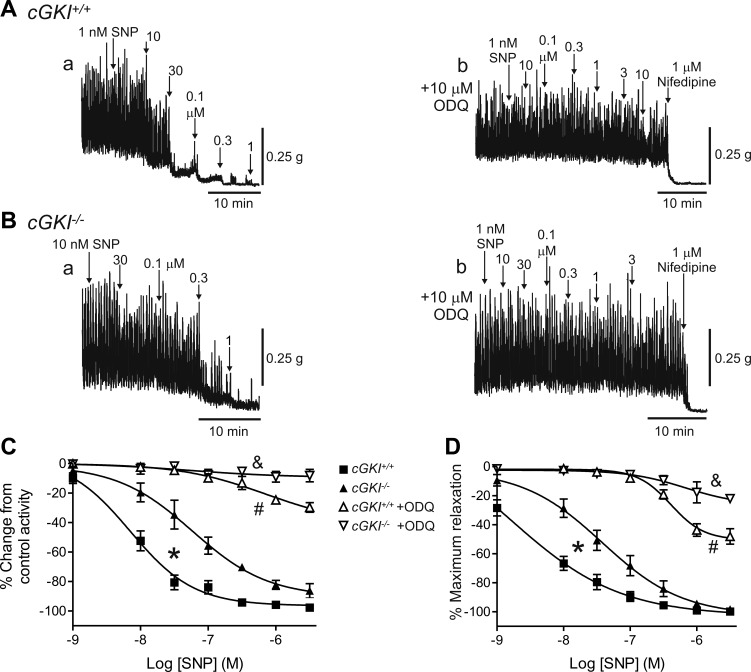
The concentration-response relationship for the NO donor SNP was also determined in the cGKI+/+ and cGKI−/− mouse IAS and aorta (Fig. 7). The SNP curve was shifted significantly (P < 0.05) to the right in the cGKI−/− IAS (Fig. 7, A–C) and aorta (Fig. 7D). The differences between cGKI+/+ and cGKI−/− muscles with SNP were smaller than those observed for 8-Br-cGMP. To clarify the role of GC in these responses the GC inhibitor ODQ (10 μM) was tested. ODQ abolished (P < 0.05) relaxations elicited with low concentrations of SNP in both cGKI+/+ and cGKI−/− IAS (Fig. 7, A–C) and aorta (Fig. 7D). However, with higher concentrations of SNP some relaxation persisted. This relaxation was significantly greater (P < 0.05) in cGKI+/+ than in cGKI−/− muscles.
Fig. 7.
Comparison of sodium nitroprusside (SNP)-induced relaxations in the cGKI+/+ and cGKI−/− IAS and aorta. Sample traces showing relaxations elicited with SNP in the cGKI+/+ (A) and cGKI−/− (B) IAS in the absence (Aa, Ba) and presence (Ab, Bb) of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μM). The SNP concentration-response curve was significantly (*P < 0.05) shifted to the right in the cGKI−/− IAS (C). Relaxations were almost completely abolished by ODQ in the cGKI−/− IAS (&P < 0.05; Bb, C), whereas in the cGKI+/+ IAS a significantly smaller relaxation persisted (Ab, C; #P < 0.05). Nifedipine produced additional relaxation in these muscles (Ab, Bb). The SNP concentration-response curve was also shifted significantly (*P < 0.05) to the right in the cGKI−/− mouse aorta (D). Relaxation was also almost completely abolished by ODQ in the cGKI−/− aorta (&P < 0.05; D) whereas in the cGKI+/+ aorta a significantly smaller relaxation persisted (#P < 0.05). IC50 values were 7.1 × 10−9 M and 1.6 × 10−9 M in IAS and aorta of cGKI+/+ mice, respectively, and 5.9 × 10−8 M and 3.8 × 10−8 M in IAS and aorta of cGKI−/− mice, respectively; n = 7 cGKI+/+, n = 8 cGKI−/− IAS for SNP; n = 5 cGKI+/+, n = 5 cGKI−/− IAS for SNP plus ODQ; n = 7 cGKI+/+, n = 7 cGKI−/− aorta for SNP; n = 6 cGKI+/+, n = 5 cGKI−/− aorta for SNP plus ODQ.
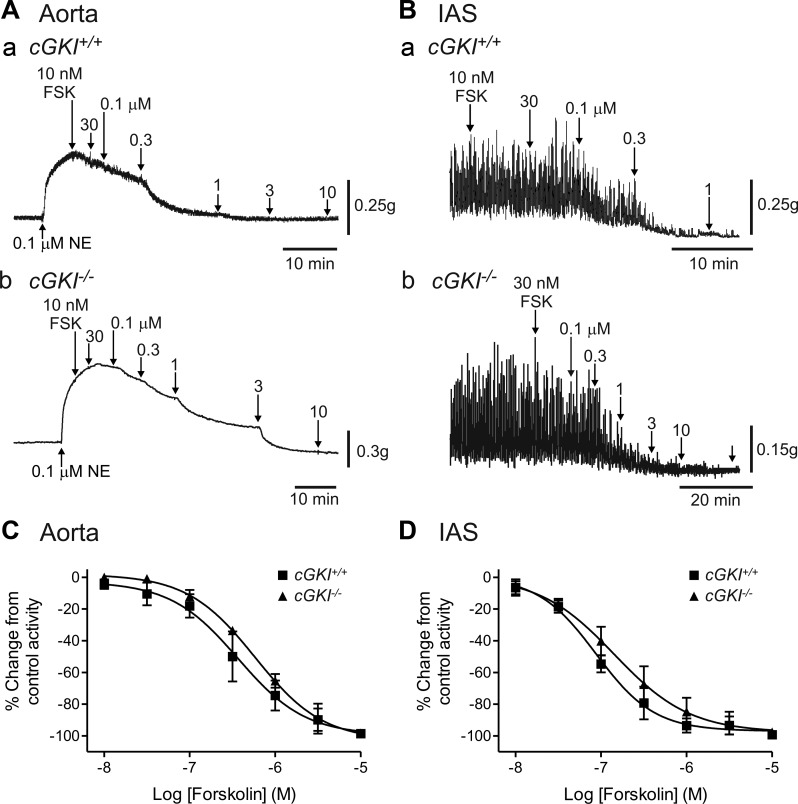
Responses to the adenylate cyclase activator forskolin (FSK) were also tested to determine whether the adenylate cyclase/cAMP/PKA pathway was somehow compromised in muscles of cGKI−/− mice. Concentration-dependent relaxations were observed in the aorta (Fig. 8A) and the IAS (Fig. 8B) of cGKI+/+ and cGKI−/− mice. Responses to FSK were not significantly different (P > 0.05) in muscles of cGKI+/+ vs. cGKI−/− mice (Fig. 8, C and D), indicating that this pathway was unaffected in cGKI−/− mice.
Fig. 8.
Comparison of forskolin (FSK)-induced relaxations in the cGKI+/+ and cGKI−/− aorta and IAS. Sample traces showing relaxations elicited with FSK in the cGKI+/+ aorta (Aa) and IAS (Ba) and in the cGKI−/− aorta (Ab) and IAS (Bb). The concentration-dependent responses to FSK were not significantly different between cGKI+/+ and cGKI−/− mice in either aorta (C) or IAS (D). IC50 values were 3.7 × 10−7 M and 8.7 × 10−8 M in aorta and IAS of cGKI+/+ mice, respectively, and 6.7 × 10−7 M and 1.4 × 10−7 M in aorta and IAS of cGKI−/− mice, respectively; n = 4 cGKI+/+, n = 4 cGKI−/− aorta; n = 4 cGKI+/+, n = 4 cGKI−/− IAS.
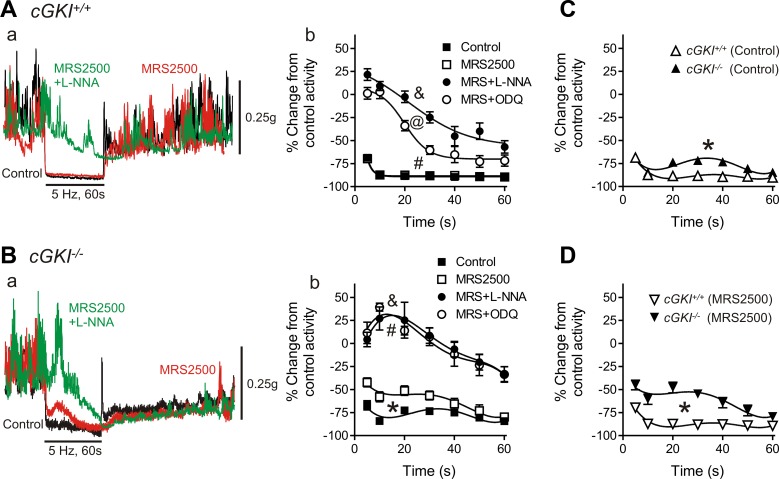
Comparison of Nerve-Evoked Relaxations in cGKI+/+ and cGKI−/− Mice
Relaxation responses were elicited in the IAS of cGKI+/+ and cGKI−/− mice with EFS (5 Hz, 60 s). Under control NANC conditions EFS caused profound inhibition of phasic contractions and loss of tone in the IAS of cGKI+/+ and cGKI−/− mice (Fig. 9, A–C) although there was a small, but significant (P < 0.05), reduction in relaxation in cGKI−/− mice (Fig. 9C). The selective P2Y1 receptor antagonist MRS2500 (1 μM) was then added to eliminate the influence of purinergic responses. The relaxations observed in the cGKI+/+ mouse were not different in the presence or absence of MRS2500 (Fig. 9Ab), whereas MRS2500 significantly reduced (P < 0.05) relaxation in the cGKI−/− mouse (Fig. 9Bb). The nonpurinergic relaxation in the cGKI−/− IAS was also significantly less (P < 0.05) than in the cGKI+/+ IAS (Fig. 9D). In both mice MRS2500 also greatly reduced the poststimulus contraction as previously described (14, 28).
Fig. 9.
Comparison of nerve-mediated relaxations in the cGKI+/+ and cGKI−/− IAS. Superimposed contractile traces are shown for responses to nerve stimulation [5 Hz electrical field stimulation (EFS), 60 s] in the cGKI+/+ (Aa) and cGKI−/− (Ba) IAS under control conditions (black) in the presence of MRS2500 (MRS; red) and with MRS2500 plus Nω-nitro-l-arginine (l-NNA; green). Relaxation in the cGKI+/+ IAS was not reduced with MRS2500 alone but was significantly reduced with MRS2500 plus l-NNA (&P < 0.05; Aa, Ab). In the cGKI−/− IAS relaxation was significantly reduced with MRS2500 (*P < 0.05) and further reduced with MRS2500 plus l-NNA (&P < 0.05; Ba, Bb). MRS2500 plus ODQ also reduced relaxation significantly more than MRS2500 alone in both mice (#P < 0.05). The effect of ODQ differed in that it reduced relaxation in combination with MRS2500 to a lesser extent than MRS2500 plus l-NNA in the cGKI+/+ IAS (@P < 0.05), whereas in the cGKI−/− IAS relaxation was reduced to the same extent by either drug combination. C and D: comparison of neural responses in the cGKI+/+ and cGKI−/− IAS. Small but significant differences were observed in neural responses in the cGKI−/− IAS under control conditions (C, *P < 0.05) and in the presence of MRS2500 (D, *P < 0.05); n = 13 cGKI+/+, n = 11 cGKI−/− control; n = 13 cGKI+/+, n = 11 cGKI−/− MRS2500; n = 6 cGKI+/+, n = 4 cGKI−/− MRS2500 plus l-NNA; n = 7 cGKI+/+, n = 5 cGKI−/− MRS2500 plus ODQ.
To determine which portion of the nonpurinergic response was nitrergic, EFS was repeated after addition of MRS2500 plus l-NNA (100 μM). Under these conditions relaxation was abolished during the first 20–30 s of EFS in both cGKI+/+ and cGKI−/− mice usually revealing a contractile response after initiation of EFS (Fig. 9, A and B). Thus a substantial nitrergic response persists in the absence of cGKI.
We also tested the combined effects of MRS2500 plus ODQ (10 μM) to determine the role of GC in nerve-mediated responses. In the cGKI−/− mouse the responses observed with combined MRS2500 plus ODQ were not different from those observed with MRS2500 plus l-NNA (Fig. 9Bb). In contrast, in cGKI+/+ mice, MRS2500 plus ODQ caused significantly less (P < 0.05) inhibition of relaxation than MRS2500 plus l-NNA (Fig. 9Ab).
DISCUSSION
The effector cells and second messenger pathways underlying nitrergic NMT in the mouse IAS were examined. Subunits necessary for functional GC (i.e., GCα, GCβ) were identified in SMC, ICC, and PDGFRα+ cells. The kinase most commonly thought to activate responses, i.e., cGKI, was also identified in each of these cell types. Expression of nitrergic effectors in the PDGFRα+ cells suggests the novel hypothesis that these cells could be involved in transduction of nitrergic responses in the IAS. Functional experiments indicate that GC plays a central role transducing nitrergic signals in the IAS whereas cGKI is responsible for only part of nitrergic relaxation. These data demonstrate that nitrergic responses in the IAS are far more complicated than typically considered. Further studies should therefore look deeper into the cells and mechanisms activated by NO and cGMP that give rise to relaxation during nitrergic NMT. Developing this topic may identify unique targets for the treatment of defecatory disorders.
Relative Protein and Gene Expression Levels of GCα, GCβ, and cGKIβ in IAS Tissue and Cells
This is the first study in which cell-specific expression patterns of nitrergic pathway genes have been evaluated in purified cell populations in the IAS. The expression of genes encoding GCα, GCβ, and cGKIβ were examined in PDGFRα+ cells, SMC, and ICC obtained by sorting dispersed cells from the IAS with FACS. The effectiveness of our sorting techniques was confirmed by quantitative evaluations of cell-specific biomarkers, such as Pdgfra, Myh11, and Kit. Transcripts of these genes were all highly enriched in the appropriate cell populations.
Gucy1a3 (GCα gene) and Gucy1b3 (GCβ gene) expression was observed in each cell population. However, expression levels differed markedly between cell types, with GCα and GCβ gene transcript and protein levels being greatest in PDGFRα+ cells, followed by ICC with only weak expression observed in SMC. Indeed, Gucy1a3 and Gucy1b3 transcript levels in SMC were only 5 and 8%, respectively, of those observed in PDGFRα+ cells. This expression sequence was highly specific for IAS tissues, since GC labeling of SMC in adjacent vascular tissues was robust whereas GC was not detectable in PDGFRα+ cells associated with these vascular structures (see Fig. 3). It is also interesting to note that qPCR studies revealed that Gucy1a3 expression was approximately five times greater in whole aorta than in whole IAS. This might reflect the fact that SMC are the major cellular component in smooth muscle tissues whereas PDGFRα+ cells represent only a fraction of the total cell population [e.g., PDGFRα+ cell cross-sectional area in sections of the mouse IAS is estimated to be ∼18% of the total area (8)].
Stimulation of GC has generally been recognized as a key initiator of nitrergic responses in various organs (12). Thus the substantial differences we observed in expression levels of GC in SMC and PDGFRα+ cells in IAS vs. blood vessels suggests that there are important differences in the mechanisms of nitrergic relaxation in response to NO released from the endothelium vs. NO released from enteric motor neurons. In previous studies of the mouse IAS nNOS+ fibers were found in close association with intramuscular PDGFRα+ cells (PDGFRα+-IM; Ref. 8). The present study further clarifies this relationship by identifying intense immunoreactivity for GC in PDGFRα+-IM but not in serosal or submucosal PDGFRα+ cells (weak labeling was observed in some myenteric PDGFRα+ cells). After finding that PDGFRα+-IM express the major receptor for NO, it is tempting to conclude that these cells are innervated by nitrergic motor neurons and are possibly participants in the motor responses to nitrergic NMT. This would be a novel function for this class of interstitial cells, since previous studies have suggested only involvement in purinergic NMT in the GI tract (30, 31). Alternatively, GC in PDGFRα+-IM may not couple to effectors that influence the contractile state of the IAS. Rather, GC may serve as a buffer for NO released from nerves, confining its actions to a restricted volume close to the site of release. This type of action has previously been proposed for GC in ICC (29). Finally, GC in PDGFRα+-IM may serve some as-yet-unrecognized function.
GC expression in ICC was intermediate between that of PDGFRα+ cells and SMC. ICC have long been recognized as participants in nitrergic NMT (4, 43, 44) and expression of GC protein by ICC has been reported in other GI muscles (19, 25, 26, 43). In a previous study, GC immunoreactivity was compared in ICC and PDGFRα+ cells in the guinea pig esophagus, stomach, small intestine, cecum, and colon (25). This study identified only one region (i.e., the small intestine deep muscular plexus) where intense GC immunoreactivity was observed in PDGFRα+ cells along with weak expression in ICC (i.e., equivalent to the mouse IAS). In all other regions, GC expression in ICC was equal to or greater than that of PDGFRα+ cells. Thus just as there are organ-specific differences in GC expression by PDGFRα+ cells and SMC (e.g., IAS vs. vascular structures), there are also region-specific differences in GC expression by PDGFRα+ cells and ICC in the GI tract. These region-specific differences may in turn be associated with region-specific differences in the role(s) that these cells play in nitrergic NMT.
We have previously shown that nitrergic inhibitory junction potentials (IJPs) elicited with EFS (5 Hz, 10 s) are abolished by ODQ in the mouse IAS, emphasizing the importance of GC in the generation of IJPs in this muscle (14). This conclusion is in agreement with studies of the colon and fundus that found that nitrergic IJPs were absent in the global GC−/− mouse (32). We have also reported that nitrergic IJPs elicited by single pulse stimuli are absent in the IAS of W/WV mice, which lack ICC-IM, whereas IJPs elicited with trains of stimuli (5 Hz, 10 s) were reduced by only ∼50% (8, 14). Taken together these data suggest that ICC-IM, through the action of GC, contribute to the generation of nitrergic IJPs in the mouse IAS. However, persistence of significant nitrergic relaxation and hyperpolarization with longer stimulus trains in the IAS of W/WV mice suggests that other cell types (e.g., PDGFRα+ cells and SMC) also contribute to nitrergic responses (14). This conclusion is in agreement with recent studies of the fundus and colon (20, 32) from mice lacking GC in either ICC or SMC. These studies concluded that the nitrergic IJP consists of two components, i.e., an initial phase dependent on ICC and a second phase dependent on SMC (32). Likewise, contractile studies of the fundus suggest that nitrergic NMT is mediated by both ICC and SMC since relaxation is not abolished in the ICC-GCKO or the SMC-GCKO mouse but is abolished in the combined SMC/ICC-GCKO mouse (20). Complicating these studies is the fact that GC is unlikely to be entirely knocked out.
The relative expression of cGKIβ in ICC, SMC, and PDGFRα+ cells was not strictly aligned with GC expression. Whereas GC expression was highest in PDGFRα+ cells, cGKI expression was greatest in SMC. This observation is in keeping with the important role described for cGKI in the actions of NO on GI SMC (36). Gene expression of Prkg1 in ICC was not significantly different from that of SMC while expression in PDGFRα+ cells was significantly less (P < 0.05). In contrast, cGKIβ immunoreactivity was detected in ICC and PDGFRα+ cells with only the highest concentration of anti-cGKIβ antibody tested (i.e., 1:100 dilution), whereas robust cGKIβ immunoreactivity was detected in neurons with an antibody dilution of 1:4,000 and in SMC with a 1:2,000 dilution. From these data we propose the following sequence for cGKI protein expression: neurons > SMC > ICC = PDGFRα+ cells. Despite the apparently low levels of cGKIβ in ICC, cGKI may still play a physiological role in the generation of electrical events in ICC. Indeed, in studies of the mouse colon (where cGKIβ was also identified in ICC with a 1:100 dilution of cGKIβ antibody) nitrergic IJPs were absent in mice lacking cGKIβ in ICC (29).
Our expression studies have identified GC and cGKIβ in all three cell types although expression levels of these signaling molecules differed significantly. SMC form gap junctions with adjacent ICC and PDGFRα+ cells (18, 22, 23, 27), giving rise to an electrical syncytium referred to as the “SIP syncytium.” The SIP syncytium has been proposed to be the functional unit underlying integration of motor responses in the GI tract (45). Identification of nitrergic signaling molecules in all three of these cells in the IAS along with previous morphological findings showing close associations between ICC-IM, PDGFRα+-IM, and nNOS+ neurons (8) support the hypothesis that nitrergic responses in the IAS result from integration of signals within the SIP syncytium.
Role of cGKI in Nitrergic Responses
To further examine the role of cGKI in nitrergic responses in the IAS we undertook functional studies using a cGKI−/− mouse. The cGKI−/− mouse used in this study (i.e., Prkg1Tn(sb-rtTA)2497B.SB2Ove) contains a ∼14,000-bp insert in intron 10 to disrupt expression of functional cGKIα and cGKIβ. Primer pairs targeting a 178-bp section within exons 11–13 identified message in cGKI+/+ littermates but not in cGKI−/−, showing that functional cGKI expression was absent in this mouse. Previous studies have used a different strain of cGKI−/− mouse (cGKIL−/L−) to examine the role of cGKI in nitrergic signaling in aorta and colon (13, 41). This transgenic mouse was generated with a similar strategy but a different insertion site (i.e., a ∼19,000-bp insert in intron 3). Despite this difference, the phenotypes of Prkg1Tn(sb-rtTA)2497B.SB2Ove and cGKIL−/L− mice appear to be indistinguishable. Both cGKI−/− transgenic mice were smaller than littermate controls, died near the time of weaning, and had enlarged stomachs, intestines, and cecums, as well as other abnormalities in liver and spleen. The phenotype of our cGKI−/− was further established by repeating some of the studies on aorta performed on cGKIL−/L− mice.
The relaxant effects of 8-Br-cGMP were greatly reduced in the IAS and aorta of our cGKI−/− whereas responses to the adenylate cyclase activator FSK were unchanged. These data are in agreement with previous studies of the colon and aorta using the cGKIL−/L− mouse (37, 41). Although the response to SNP was also reduced in cGKI−/− mice it was reduced to a lesser extent than 8-Br-cGMP. Since complete relaxation still occurred in the IAS and aorta with SNP, cGKI-independent pathways appear to importantly contribute to nitrergic relaxations in both muscles. cGMP is known to affect the activity of targets other than cGKI, including PKA, cGMP-gated channels, and phosphodiesterases (i.e., PDE3; Refs. 2, 17, 34). One or more of these targets may have a role in nitrergic responses. It should be noted that there is evidence that cGKI negatively feeds back onto GC, reducing cGMP formation (16, 51). This effect would tend to strengthen cGKI-independent pathways in the cGKI−/− mouse. The fact that 8-Br-cGMP responses were attenuated to a much greater degree than SNP in cGKI−/− mice may be due to the limited ability of 8-Br-cGMP to activate other proteins that cGMP modulates (1, 5), e.g., the IC50 for cGMP inhibition of PDE3 is 0.4 μM whereas for 8-Br-cGMP it is 28 μM.
Responses to SNP in the IAS and aorta were substantially reduced or abolished by ODQ confirming a key role for GC/cGMP in nitrergic relaxations in both muscles, as reported previously in studies of other GI muscles (11, 19) and blood vessels (9, 35). Interestingly, ODQ produced a more complete block of SNP-induced relaxations in the cGKI−/− IAS and aorta than in cGKI+/+ mice. Furthermore, whereas l-NNA and ODQ caused equivalent reductions of nerve-mediated relaxations in the cGKI−/− IAS, l-NNA was a more effective blocker than ODQ in cGKI+/+ mice. The most likely explanation for the incomplete block of nitrergic responses in the cGKI+/+ IAS is that ODQ (10 μM) produces an incomplete block of GC. This conclusion is supported by recent studies of the mouse aorta in which 10 μM ODQ also produced an incomplete block of diethylamine (DEA)-NONOate-induced relaxations. This study went on to show that higher concentrations of DEA-NONOate still significantly increased cGMP levels in the presence of 10 μM ODQ in HEK-GC cell homogenates (33). Furthermore, in studies of the gastric fundus DEA-NONOate-mediated relaxation was reduced significantly more in the GC−/− fundus than in WT muscles bathed in 10 μM ODQ, again suggesting incomplete block of GC by 10 μM ODQ (19). The more complete block of NO-mediated effects by ODQ in the cGKI−/− IAS may be because these animals lack an important downstream mediator of cGMP (i.e., cGKI), making them more susceptible to the reduction in cGMP caused by ODQ.
Role of cGKI in Nitrergic Neuromuscular Transmission in the IAS
Relaxation responses caused by EFS in the IAS of WT Fvb/N mice (present study) were similar to those described previously in muscles of WT C57BL/6 mice (14). Under NANC conditions, EFS (5 Hz, 60 s) gave rise to near-maximum relaxation. Selective removal of the purinergic component with MRS2500 did not significantly reduce this relaxation (present study and Ref. 14), nor does selective removal of the nitrergic pathway with l-NNA (14). In contrast, blockade of both pathways with MRS2500 plus l-NNA eliminates relaxation during the first 20–30 s of EFS at 5 Hz uncovering an initial tachykinergic contraction (present study; Refs. 14, 28). The redundancy of purinergic and nitrergic pathways arises because each hyperpolarizes membrane potential below the threshold for activation of l-type calcium channels. Thus either pathway alone is sufficient to cause near-maximum relaxation whereas blockade of both abolishes relaxation (14). With longer stimulus times (>20–30 s) a third component of inhibitory NMT develops (present study and Refs. 14, 28). This component has been studied in detail and is due to VIP (28).
Nerve-evoked nitrergic relaxation in the IAS was greatly reduced by ODQ in cGKI−/− and cGKI+/+ mice, further emphasizing the importance of GC in these responses. This agrees with studies of the GC−/− fundus (19). In contrast, nerve-mediated nitrergic relaxation persisted in the cGKI−/− mouse although it reached a significantly smaller amplitude providing evidence that cGKI significantly contributes to nitrergic NMT. It should be noted that there is evidence that P2Y receptor expression is increased in the W/Wv mouse fundus, which has diminished nitrergic NMT (48). This effect would tend to counteract any loss of inhibitory NMT under control conditions in the cGKI−/− mouse IAS.
As discussed above for responses to SNP, the persistence of EFS-induced nitrergic relaxations in cGKI−/− mice in the absence of purinergic NMT suggests that cGKI-independent pathway(s) such as modulation of PKA, cGMP-gated channels, or PDE3 (2, 17, 34) are likely to contribute to nitrergic NMT in the IAS. In contrast, previous studies of the cGKI−/− mouse reported that nerve-mediated nitrergic responses were absent in the fundus (15, 37, 41). These studies differ in that only shorter periods of EFS were examined (i.e., 5–10 s). However, we also observed cGKI-independent nitrergic relaxation during the initial period of EFS. Thus cGKI-independent pathways appear to play a more important role in the IAS than in fundus.
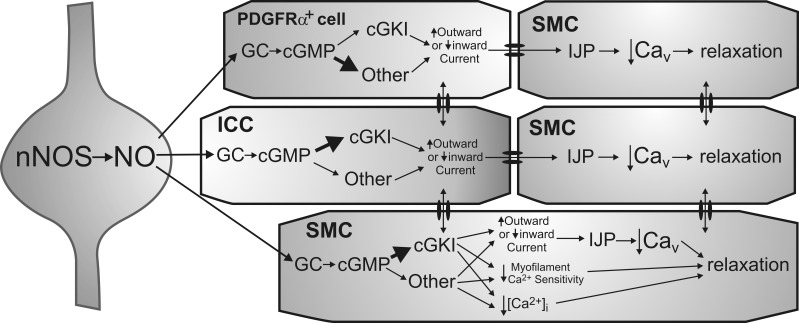
In summary, we have identified GC and cGKIβ in the three cell types of the SIP syncytium, although expression levels differed significantly. These data in combination with our previous morphological findings showing close associations between nNOS+ neurons, ICC, and PDGFRα+ cells (8) lend support to the hypothesis that nitrergic responses in the IAS result from integration of signals within the SIP syncytium. Functional studies demonstrated that GC plays a central role in nitrergic relaxation in the IAS, but cGKI and its downstream targets are responsible for less than half of this relaxation. Thus other, as-yet-undescribed cGMP-dependent mechanisms in the SIP syncytium of the IAS are responsible for a significant portion of the relaxation response. The possible pathways underlying nitrergic NMT in the mouse IAS are depicted in Fig. 10. As these pathways are explored and described in detail, new ways to regulate IAS contraction may be revealed and become potential targets for the treatment of defecation disorders.
Fig. 10.
Diagram depicting proposed pathways underlying nitrergic neuromuscular transmission in the mouse IAS. Shown is an inhibitory motor neuron that synthesizes and releases nitric oxide (NO) via nNOS (left). Postjunctional effector cells include PDGFRα+ cells (top), ICC (middle), and SMC (bottom and right), which constitute the “SIP” syncytium. Both cGKI-dependent and -independent pathways are depicted. The immediate receptor for NO is GC resulting in cGMP formation. Numerous downstream mediators may then be involved in the actions of cGMP. In ICC, cGKI is suggested to be the initial downstream mediator (thick arrow) since single nitrergic inhibitory junction potentials (IJPs) are absent from the colon of mice lacking cGKI in ICC (29). In contrast, cGKI-independent pathways may predominate in PDGFRα+ cells (thick arrow) since gene expression of cGKI was least in these cells. Both cell types are shown coupled to SMC via gap junctions as previously described (18, 22, 23, 27). There is also evidence that ICC and PDGFRα+ cells may couple to one another (27). Hyperpolarizing currents generated by interstitial cells (via either an increase in outward current or a decrease in inward current) are transmitted to adjacent SMC. This results in IJPs that cause relaxation via the closing of voltage-dependent calcium channels (Cav). SMC are shown at the greatest distance from nerve terminals to depict their role in more slowly developing responses that require multiple stimuli. cGKI is proposed to be the predominant mediator of cGMP effects in SMC (thick arrow) since gene and protein expression of cGKI was greatest in these cells. Unlike ICC and PDGFRα+ cells, relaxation may also be initiated in SMC via mechanisms that do not require a change in membrane potential, e.g., changes in myofilament sensitivity to Ca2+ as well as other mechanisms that reduce intracellular Ca2+ concentration ([Ca2+]i) in a voltage-independent manner. However, since both phasic activity and tone are blocked in the mouse IAS with the Cav blocker nifedipine (6), membrane potential-dependent mechanisms clearly play a pivotal role in the regulation of contraction in this muscle.
GRANTS
Grant funding included DK078736 to K. D. Keef and S. M. Ward and P01DK041315 to K. M. Sanders, S. M. Ward, and K. D. Keef. Support for the Zeiss LSM510 confocal microscope was provided by 1 S10 RR16871.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.A.C., K.M.S., S.M.W., and K.D.K. conception and design of research; C.A.C., A.G.S., L.E.P., and K.D.K. performed experiments; C.A.C., A.G.S., L.E.P., and K.D.K. analyzed data; C.A.C., L.E.P., K.M.S., S.M.W., and K.D.K. interpreted results of experiments; C.A.C., A.G.S., L.E.P., and K.D.K. prepared figures; C.A.C. and K.D.K. drafted manuscript; C.A.C., K.M.S., S.M.W., and K.D.K. edited and revised manuscript; C.A.C., A.G.S., L.E.P., K.M.S., S.M.W., and K.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We extend our appreciation to Byoung Koh for FACS, to Yulia Bayguinov for assistance with immunohistochemistry, and to Nancy Horowitz for assistance with breeding and maintenance of transgenic mice.
REFERENCES
- 1.Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA. Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Mol Pharmacol 47: 330–339, 1995. [PubMed] [Google Scholar]
- 2.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 93: 12008–12013, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt E, Nolte C, Schulz S, Beltman J, Beavo JA, Jastorff B, Walter U. Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-para-chlorophenylthio-cGMP. Biochem Pharmacol 43: 2591–2600, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil 19: 937–945, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cobine CA, Hennig GW, Bayguinov YR, Hatton WJ, Ward SM, Keef KD. Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves. Am J Physiol Gastrointest Liver Physiol 298: G643–G656, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res 344: 17–30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogolludo AL, Perez-Vizcaino F, Zaragoza-Arnaez F, Ibarra M, Lopez-Lopez G, Lopez-Miranda V, Tamargo J. Mechanisms involved in SNP-induced relaxation and [Ca2+]i reduction in piglet pulmonary and systemic arteries. Br J Pharmacol 132: 959–967, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 246: G305–G315, 1984. [DOI] [PubMed] [Google Scholar]
- 11.De Man JG, De Winter BY, Herman AG, Pelckmans PA. Study on the cyclic GMP-dependency of relaxations to endogenous and exogenous nitric oxide in the mouse gastrointestinal tract. Br J Pharmacol 150: 88–96, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Desch M, Sigl K, Hieke B, Salb K, Kees F, Bernhard D, Jochim A, Spiessberger B, Hocherl K, Feil R, Feil S, Lukowski R, Wegener JW, Hofmann F, Schlossmann J. IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc Res 86: 496–505, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Duffy AM, Cobine CA, Keef KD. Changes in neuromuscular transmission in the W/Wv mouse internal anal sphincter. Neurogastroenterol Motil 24: e41–e55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertl C, Lukowski R, Sigl K, Schlossmann J, Hofmann F, Wegener JW. Kinetics of relaxation by cGMP/cGKI signaling in fundus smooth muscle. Eur J Pharmacol 670: 266–271, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero R, Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Nitric oxide-sensitive guanylyl cyclase activity inhibition through cyclic GMP-dependent dephosphorylation. J Neurochem 75: 2029–2039, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci 92: 35–42, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Groneberg D, Konig P, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology 140: 1608–1617, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Groneberg D, Lies B, Konig P, Jager R, Seidler B, Klein S, Saur D, Friebe A. Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology 145: 188–196, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Hall KA, Ward SM, Cobine CA, Keef KD. Spatial organization and coordination of slow waves in the mouse anorectum. J Physiol 592: 3813–3829, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiguchi K, Komuro T. Ultrastructural characterization of interstitial cells of Cajal in the rat small intestine using control and Ws/Ws mutant rats. Cell Tissue Res 293: 277–284, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/Wν mouse small intestine. J Auton Nerv Syst 80: 142–147, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131: 691–702, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience 152: 437–448, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil 21: 542–543, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa K, Komuro T, Hirota S, Kitamura Y. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res 289: 137–143, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Keef KD, Saxton SN, McDowall RA, Kaminki RE, Duffy AM, Cobine CA. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J Physiol 591: 1489–1506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun 4: 1630, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 307: C561–C570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jimenez M, Friebe A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 307: G98–G106, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Lies B, Groneberg D, Gambaryan S, Friebe A. Lack of effect of ODQ does not exclude cGMP signalling via NO-sensitive guanylyl cyclase. Br J Pharmacol 170: 317–327, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lincoln TM, Komalavilas P, Boerth NJ, MacMillan-Crow LA, Cornwell TL. cGMP signaling through cAMP- and cGMP-dependent protein kinases. Adv Pharmacol 34: 305–322, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Lovren F, Triggle C. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br J Pharmacol 131: 521–529, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Ny L, Pfeifer A, Aszodi A, Ahmad M, Alm P, Hedlund P, Fassler R, Andersson KE. Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP-dependent protein kinase I. Br J Pharmacol 129: 395–401, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut 34: 689–693, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orstavik S, Natarajan V, Tasken K, Jahnsen T, Sandberg M. Characterization of the human gene encoding the type I alpha and type I beta cGMP-dependent protein kinase (PRKG1). Genomics 42: 311–318, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRalpha-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil 25: e609–e620, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology 138: 1068–1078, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmhofer H, Neuhuber WL, Ruth P, Huber A, Russwurm M, Allescher HD. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res 305: 331–340, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 262: G379–G392, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Schlossmann J, Feil R, Hofmann F. Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci 10: 1279–1289, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics 11: 1–9, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM. Immunohistochemical localization of 3′,5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience 56: 513–522, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Wernet W, Flockerzi V, Hofmann F. The cDNA of the two isoforms of bovine cGMP-dependent protein kinase. FEBS Lett 251: 191–196, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Z, Sayed N, Pyriochou A, Roussos C, Fulton D, Beuve A, Papapetropoulos A. Protein kinase G phosphorylates soluble guanylyl cyclase on serine 64 and inhibits its activity. Arterioscler Thromb Vasc Biol 28: 1803–1810, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]